Abstract
The human ether-a-go-go-related gene (hERG) channel, a member of a family of voltage-gated potassium (K+) channels, plays a critical role in the repolarization of the cardiac action potential. The reduction of hERG channel activity as a result of adverse drug effects or genetic mutations may cause QT interval prolongation and potentially lead to acquired long QT syndrome. Thus, screening for hERG channel activity is important in drug development. Cardiotoxicity associated with the inhibition of hERG channels by environmental chemicals is also a public health concern. To assess the inhibitory effects of environmental chemicals on hERG channel function, we screened the National Toxicology Program (NTP) collection of 1408 compounds by measuring thallium influx into cells through hERG channels. Seventeen compounds with hERG channel inhibition were identified with IC50 potencies ranging from 0.26 to 22 μM. Twelve of these compounds were confirmed as hERG channel blockers in an automated whole cell patch clamp experiment. In addition, we investigated the structure-activity relationship of seven compounds belonging to the quaternary ammonium compound (QAC) series on hERG channel inhibition. Among four active QAC compounds, tetra-n-octylammonium bromide was the most potent with an IC50 value of 260 nM in the thallium influx assay and 80 nM in the patch clamp assay. The potency of this class of hERG channel inhibitors appears to depend on the number and length of their aliphatic side-chains surrounding the charged nitrogen. Profiling environmental compound libraries for hERG channel inhibition provides information useful in prioritizing these compounds for cardiotoxicity assessment in vivo.
Keywords: cardiotoxicity, hERG, long QT syndrome, NTP 1408 library, patch clamp, qHTS, tetra-n-octylammonium bromide, thallium influx
Introduction
The human ether-a-go-go-related gene (hERG) channel belongs to a family of voltage-gated potassium (K+) channels (KV) (Vandenberg et al., 2004). It was first identified and cloned in 1994 (Warmke and Ganetzky, 1994). The functional KV channels are comprised of four subunits, each containing six transmembrane domains (S1-S6), with a pore (P) between S5 and S6 (Wray, 2004). The S4 domain, which has multiple positive charges, constitutes the voltage sensor. The S5 and S6 regions connecting with the pore loop (P) contribute to the channel pore (Vandenberg et al., 2004). To date, approximately 80 different K+ channel genes have been identified in humans (Szabo et al., 2010). In the KV channel family, there are 12 distinct subfamilies based on their amino acid sequence homology (KV1 to KV12) (Gutman et al., 2005). According to the current KV channel nomenclature, the gene name of the hERG channel is KCNH2 and the channel protein is KV11.1 (Gutman et al., 2005). hERG channels are mainly expressed in heart, but are also expressed in many other tissues including the brain, kidney, liver, and lung (Gutman et al., 2005).
By conducting the rapid delayed rectifier K+ current (Tseng, 2001), the hERG channel plays an essential role in the proper repolarization of action potential in normal heart as well as in prevention of arrhythmias induced by ectopic depolarizations (Vandenberg et al., 2004). Functional block of the hERG channel due to either genetic defects or adverse drug effects can cause the abnormal action potentials and prolongation of the QT interval (i.e., the portion of an electrocardiogram between the onset of the Q wave and the end of the T wave, representing the total time for ventricular depolarization and repolarization), which may lead to long QT syndrome (LQTS) (Tseng, 2001). Drug-induced LQTS (also referred to as acquired LQTS) has become the most common cause of drug-induced cardiac arrhythmia and sudden death (Vandenberg et al., 2001). During 1990–2006, 29% of the drugs withdrawn from approval was due to their potential to prolong the QT interval and/or induce a unique and potentially fatal ventricular tachyarrhythmia known as torsade de pointes (TdP) (Shah, 2006).
Although an assessment of hERG channel activity is an important step in early drug development, it has not been a focus in the evaluation of environmental chemicals for toxicity. To date, more than 100,000 chemicals have been introduced into commerce without sufficient toxicological testing (Belpomme et al., 2007). These synthetic chemicals are widely used in the energy, transportation, agriculture, food, and pharmaceutical industries, and they may cause environmental pollution via contamination of air, soil, water, and food. To meet the needs of toxicity testing in the 21st century, the National Research Council (NRC) developed a long-term vision and strategic plan for the toxicological evaluation of chemicals (NRC, 2007). In response to this NRC report, the National Toxicology Program (NTP), the NIH Chemical Genomics Center (NCGC), and the U.S. Environmental Protection Agency (EPA) Office of Research and Development formed the Tox21 partnership; the U.S. Food and Drug Administration (FDA) has recently joined this partnership (http://yosemite.epa.gov/opa/admpress.nsf/d0cf6618525a9efb85257359003fb69d/571f805f9c4ff71385257765004cdb78!OpenDocument). The goals of Tox21 are to identify mechanisms of compound action at the cellular level, prioritize chemicals for further toxicological evaluation, and develop useful predictive models of in vivo biological response (Collins et al., 2008; Kavlock et al., 2009)
To assess the effect of environmental chemicals on hERG channels as part of the Tox21 program, we screened a collection of 1408 compounds (provided by the NTP) using a cell-based thallium influx assay (Titus et al., 2009) in a quantitative high throughput screening (qHTS) format (Inglese et al., 2006; Xia et al., 2008). Using this assay, we identified 17 compounds that reproducibly inhibited the hERG channel at IC50 concentrations below 10 μM in the primary screen; 12 of these compounds were confirmed using an automated patch clamp electrophysiological method. The selectivity of hERG channel inhibition of these 12 compounds was demonstrated using a voltage gated KV1.3 thallium assay. In addition, the cytotoxicity of these 12 compounds in the cell line used for the thallium influx assay was evaluated. This tiered screening approach appears useful for the detection of environmental chemicals that merit more extensive evaluation for cardiotoxicity and provides useful information that might be used to develop computational models for predicting the ability of new chemical entities to induce LQTS.
Materials and Methods
Cell line and culture condition
The U-2 OS cell line (HTB-96), a human osteosarcoma cell line, was purchased from the American Type Culture Collection (ATCC; Manassas, VA, USA). U-2 OS cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM; Invitrogen, Carlsbad, CA, USA) with Glutamax containing 10% fetal bovine serum (FBS; HyClone, Logan, UT, USA), and 50 U/mL penicillin/50 μg/mL streptomycin (Invitrogen). CHO-K1 cells (from ATCC) stably transfected with hERG cDNA (GenBank sequence NM_000238) were constructed at Cerep, Inc. (Redmond, WA, USA) and used in the automated patch clamp experiments. The cells were cultured in F-12 Kaighn’s Nutrient Mixture medium (Invitrogen) plus 10% FBS at 37°C for one to three days before conducting the patch clamp experiment. A CHO DUKX cell line (Perkin Elmer, Shelton, CT, USA) stably expressing the human KV1.3 voltage gated K+ channel was maintained in MEM Alpha media containing glutamine (Invitrogen), 0.4 mg/mL Geneticin (Invitrogen), and 10% FBS (Hyclone). All the cells were maintained at 37°C under a humidified atmosphere and 5% CO2.
Compounds
A library of 1408 compounds (1353 unique compounds, 55 compounds in duplicate) was provided by the NTP. The compounds were dissolved in dimethyl sulfoxide (DMSO; Fisher Scientific, Pittsburgh, PA, USA) at a stock concentration of 10 mM. The completed list of compounds in this library is provided at (http://www.ncbi.nlm.nih.gov/sites/entrez?db=pcsubstance&term=NTPHTS).
For the follow-up studies, all the compounds (see Table 1) were purchased from Sigma-Aldrich (St. Louis, MO, USA) except trixylenyl phosphate was purchased from ChemBridge (San Diego, CA, USA) and verapamil from Tocris (Ellisville, MO, USA). The purity of these compounds was provided by the vendors.
Table 1.
Compounds used in the confirmation and follow-up studies
| Compound | CASRN | Purity (%) |
|---|---|---|
| 1,3-diphenylguanidine | 102-06-7 | 99.5 |
| 2-aminoanthracene | 613-13-8 | 96 |
| 3,3,5-trimethylcyclohexyl salicylate | 118-56-9 | NP |
| 4′-N-hexyl-4-biphenylcarbonitrile | 41122-70-7 | 98 |
| astemizole | 68844-77-9 | 98 |
| benzethonium chloride | 121-54-0 | 99 |
| benzyltrimethylammonium chloride | 56-93-9 | 97 |
| cetyltrimethylammonium bromide | 57-09-0 | 100 |
| curcumin | 458-37-7 | 80 |
| D&C red dye 27 | 18472-87-2 | NP |
| decamethonium bromide | 541-22-0 | 99 |
| didecyl dimethyl ammonium chloride | 2390-68-3 | 98 |
| digitonin | 11024-24-1 | 63 |
| domiphen bromide | 538-71-6 | 97 |
| econazole nitrate | 24169-02-6 | 99 |
| malachite green oxalate | 2437-29-8 | NP |
| o,p′-DDT | 789-02-6 | NP |
| quinidine | 56-54-2 | 89 |
| reserpine | 50-55-5 | 98 |
| tamoxifen, citrate salt | 54965-24-1 | 99 |
| tetra-n-octylammonium bromide | 14866-33-2 | 98 |
| tricresyl phosphate | 1330-78-5 | 90 |
| trixylenyl phosphate | 25653-16-1 | 90 |
| verapamil | 152-11-4 | 99 |
| zinc pyrithione | 13463-41-7 | 95 |
Abbreviations: CASRN = Chemical Abstracts Services Registry Number; DDT = 1,1,1-trichloro-2-(2-chlorophenyl)-2-(4-chlorophenyl)ethane; NP = not provided by vendors
Transduction of hERG in U-2 OS cells
For viral transduction of hERG, U-2 OS cells were transduced using BacMam-hERG K+ channel kit (Invitrogen) as described previously (Titus et al., 2009). The cells used in all experiments had been passaged no more than 25 times. Briefly, after cells grew to approximately 70– 80% confluence in a T225 flask, the culture medium in the flask was removed and replaced with 2.5 mL of hERG–BacMam virus plus 12.5 mL of phosphate buffered saline (PBS, Invitrogen) (corresponding roughly to a multiplicity of infection ratio of 100 virus particles/cell). After the cell flask was incubated for 4 hr at room temperature in the dark, the hERG–BacMam viruses in the flask were removed and the flask was washed once with 25 mL of PBS. Then, 35 mL of complete culture medium was added and the cell flask was cultured at 37°C overnight. The next day, the cells were detached and re-suspended in Opti-MEM medium (Invitrogen) containing 2% fetal calf serum (FCS; HyClone) at a density of 6.7 × 105 cells/mL. At this stage, cells were ready for thallium influx and cell viability assays.
FluxOR thallium influx assay and qHTS
Two thousand hERG transduced cells were dispensed in a volume of 3 μL per well in 1536-well, black wall/clear bottom plates (Greiner Bio-One North America, NC, USA) using a Multidrop Combi 8 channel dispenser (Thermo Fisher, Waltham, MA, USA). After plates were incubated at 37°C for 4 hr to allow the cells to adhere to the plates, 1 μL of loading buffer in Hanks Balanced Salt Solution (HBSS) [10 mM Red 40 (Spectrum Chemicals, Gardena, CA, USA)], 2.5 mM probenecid (Invitrogen), FluxOR dye at 0.7X final concentration prepared according to the manufacturer’s instructions (Invitrogen), and 20 mM HEPES [4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid] was added to each well using a Multidrop Combi 8 channel dispenser. The plates were then incubated at room temperature in the dark for 1 hr. Following incubation, 23 nL of each compound, dissolved in DMSO at the top stock concentration of 10 mM, was transferred to the assay plate by a Pintool (Kalypsys, San Diego, CA, USA). To achieve a concentration of 92 μM, the maximum concentration tested, 23 nL was transferred twice from the highest concentration of the compound plate into each well of the assay plate; control plates using DMSO only at this higher concentration were also included. The plates were incubated at room temperature for 10 min in the presence of compounds and the fluorescence intensity (488 nm excitation and 520 nm emission) from each well was recorded for 10 sec, in 1 sec intervals, using an Functional Drug Screening System (FDSS) 7000 kinetic plate reader (Hamamatsu, Hamamatsu City, Japan). Next, 1 μL of stimulation buffer containing 5 mM Tl2SO4 (Invitrogen) and 25 mM K2SO4 (Invitrogen) was added into all the wells using the tip head in the FDSS 7000 kinetic plate reader and fluorescence intensity was continuously measured for 2 min at 1 sec intervals.
The final compound concentrations tested ranged from 0.59 nM to 92 μM, in 14 concentrations. The total number of plates was 18, including four DMSO-only plates (two at each final DMSO concentration of 0.46% and 0.92%). Each treatment plate included concurrent DMSO and positive control wells; the positive control was astemizole, a known hERG blocker (Maxwell and Salnikow, 2004). The controls were arrayed as follows: Column 1, concentration-response titration of astemizole from 1.4 nM to 46 μM; Column 2, 10 μM astemizole; Column 3, DMSO only; and Column 4, 5 μM astemizole. The concentration-response titration for astemizole was used to evaluate plate-to-plate consistency, based on the calculated effective concentration that inhibited a half-maximal response (IC50). The NTP 1408 compound library was screened twice for hERG inhibition to identify compounds exhibiting a consistent response.
To confirm the results of the initial study, compounds with an IC50 of less than 10 μM were retested three times in the FluxOR thallium influx assay either in hERG transduced or wild type cells. The assay protocol was the same as described above, except that the concentration titrations were all within one 1536-well plate and the compounds were tested at 24 concentrations ranging from 11 pM to 92 μM in duplicate.
Data analysis
Primary data analysis was performed as previously described (Titus et al., 2009). Briefly, the slope of fluorescence intensities versus time during the first 30 sec after addition of the stimulation buffer was calculated from the kinetic results. The slopes for each titration point were first normalized relative to the astemizole positive control (10 μM, response set at -100%) and DMSO only wells (0%), and then corrected by applying a pattern correction algorithm using compound-free control plates (DMSO plates). Concentration response titration points for each compound were fitted to the Hill equation yielding IC50 concentrations and maximal inhibition (efficacy) values. The concentration response curves were sorted into four major classes (1–4) using previously published criteria (Xia et al., 2009). Curve classes were further subdivided to provide more detailed classification. Briefly, the highest confidence data are from compounds in curve classes 1.1, 1.2, and 2.1; compounds with class 1.3, 1.4, 2.2, 2.3, 2.4 and 3 curves have lower confidence data. Curve class 4 compounds showed no concentration-related response and were defined as inactive in this assay. Class 3 compounds display significant activity only at the highest concentration tested; class 2 compounds have incomplete curves (i.e., no low-concentration asymptote) and class 1 compounds have complete response curves (i.e., two asymptotes). Class 1 or 2 compounds were further divided into subclasses based on efficacy and quality of fit (R2). Compounds with high (> 80%) efficacy were designated as subclass 1.1 or 2.1 (R2 > 0.9) or 1.3 or 2.3 (R2 < 0.9), and compounds with low efficacies (30–80%) as subclass 1.2 or 2.2 (R2 > 0.9) or 1.4 or 2.4 (R2 < 0.9).
Automated patch clamp
hERG CHO-K1 cells were harvested by trypsinization and kept in CHO-S-SFM II Medium (Invitrogen) for up to 4 hours at room temperature before recording. The cells were washed and resuspended in extracellular solution (for components, see below) before being applied to the patch clamp experiments. K+ currents of these cells were recorded using the automated whole-cell patch clamp by Qpatch16 (Sophion Biosciences Inc, North Brunswick, NJ, USA). After whole cell configuration was achieved, the cell was held at −80 mV. A 50 millisecond pulse to −40 mV was delivered to measure the leak current, which was subtracted from the tail current on-line. Then, the cell was depolarized to +20 mV for 2 sec, followed by a one-second pulse to −40 mV to reveal the hERG tail current. This treatment pattern was delivered once every 5 seconds to monitor the tail current amplitude. Intracellular solutions included (in mM): 130 KCl, 10 NaCl, 1 MgCl2, 10 EGTA (ethylene glycol tetraacetic acid), 5 Mg-ATP (magnesium-adenosine 5′ triphosphate), and 10 HEPES (pH adjusted to 7.2 with KOH). Extracellular solutions included (in mM): 137 NaCl, 4 KCl, 1.8 CaCl2, 1 MgCl2, 10 D(+)-glucose, and 10 HEPES (pH adjusted to 7.4 with NaOH). All the chemicals in these solutions were purchased from Sigma-Aldrich. After the whole cell configuration was achieved, the extracellular solution (control) was applied first and the cell was stabilized in extracellular solution for 5 minutes. Then, the test compound was applied from low concentrations to high concentrations cumulatively. The cell was incubated with each test concentration for 5 minutes. During the incubation, the cell was repeatedly stimulated using the voltage protocol described above, and the tail current amplitude was continuously monitored. All electrophysiological recordings were performed at room temperature.
The inhibition (%) of hERG tail current is calculated by the equation of 100 × (Control-Test Compound) / Control. Control is the mean hERG tail current amplitude from the data collected over 24 seconds in the presence of control. Test Compound is the mean hERG tail current amplitudes from the data collected over 24 seconds in the presence of the test compound at each concentration.
IC50 values of compounds were obtained by fitting the following equation:
where y is the mean inhibition (%) of hERG tail current amplitudes, n is Hill slope, C is the test compound concentration, and IC50 is the test compound concentration producing 50% inhibition of hERG tail current.
KV1.3 assay
Stably transfected KV1.3 cells were plated at a density of 1000 cells/well in 3 μL of media in 1536-well, black wall/clear bottom plates. After the assay plates were incubated overnight at 37°C, 1 μL of the FluxOR dye loading buffer was added into each well of these assay plates using a Multidrop Combi 8 channel dispenser. After 1 hr of incubation with the loading buffer, 23 nL of each compound dissolved in DMSO was transferred into the assay plate by a Pintool. The FDSS kinetic reader was used to measure changes in fluorescence intensity using a standard Fluo-4 filter set (488 nm excitation, 520 nm emission). After 2 min of baseline measurement in the presence of compounds, 1 μL of stimulation buffer (final concentration 4 mM Tl2SO4, 10 mM K2SO4) was delivered to each well using the tip head in the FDSS 7000 kinetic plate reader. Fluorescence intensity was measured for an additional 1 min in 1 sec intervals and 2 min in 10 sec intervals. Psoralen (CASRN: 66-97-7, Sigma-Aldrich) was used as a positive control in the assay. Raw data from maximum minus minimum fluorescence reading was normalized relative to the psoralen control (1 μM, -100%) and DMSO only wells (0%). Concentration response titration points for each compound were fitted to the Hill equation yielding IC50 concentrations and maximal inhibition (efficacy) values.
Cell viability assay
Cell viability after treatment with the compounds identified as consistent hERG channel inhibitors was measured using a luciferase-coupled ATP quantitation assay (CellTiter-Glo® viability assay, Promega, Madison, USA) in hERG transduced U-2 OS cells. Changes in intracellular ATP content are directly proportional to the number of metabolically competent cells after compound treatment. The cells were dispensed at 2,000 cells/5 μL/well in 1,536-well white/solid bottom assay plates using a Flying Reagent Dispenser (FRD) (Aurora Discovery, San Diego, CA, USA). The cells were incubated a minimum of 5 h at 37°C prior to the addition of compounds using the pin tool. After compound addition, plates were incubated for 30 min at 37°C, followed by the addition of 5 μL per well of CellTiter-Glo reagent. After an additional 30 min of incubation at room temperature, the luminescence intensity of the plates was measured using a ViewLux plate reader (Perkin Elmer, Shelton, CT, USA).
Results
Identification of hERG channel blockers using cell-based qHTS
To profile the NTP library of 1408 compounds (1353 unique compounds) for potential activity on the hERG channel, we used a cell-based FluxOR thallium influx assay in hERG transduced cells. In this assay, thallium ions are used as surrogates for K+ ions to monitor the activity of the hERG K+ channel. The screen was carried out over 14 concentrations ranging from 0.59 nM to 92 μM in a qHTS format. Astemizole, a known hERG channel blocker, was used as the positive control to monitor assay performance and plate-to-plate variations during the screen. In both primary runs, the astemizole concentration response curves reproduced well in all 18 plates, including the four DMSO control plates. In the first run, the astemizole IC50 value was 56 ± 15 nM (mean ± standard deviation), the average signal-to-background ratio was 4.6 ± 0.2, the average coefficient of variation (CV) was 7.6 ± 3.3 (%), and the average Z′ factor (Zhang et al., 1999) was 0.77 ± 0.04. Assay performance was similar in the second run (data not presented).
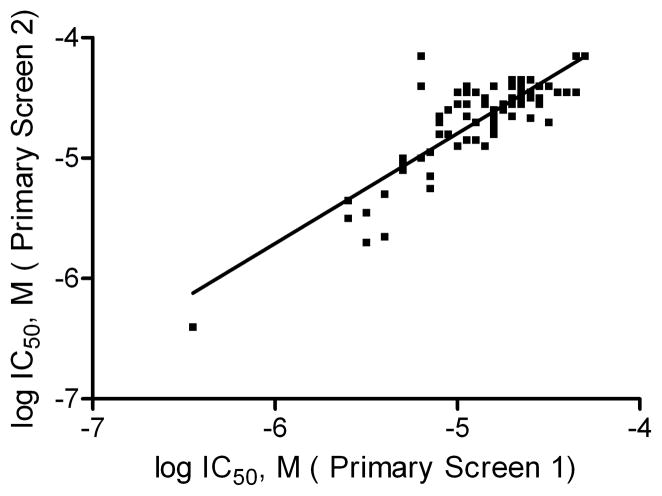
To evaluate assay and data reproducibility, the NTP 1408 compound library was screened in this thallium influx assay twice, on two different days. Among the 55 duplicate compounds in the library, the 10 duplicate compounds that showed hERG channel inhibition in the first run did so also in the second run, resulting in a 100% concordance rate. Of the 1353 unique NTP compounds, 88 (6.5%) hERG channel inhibitors with potent and moderate activity (curve classes 1.1, 1.2, and 2.1 in at least one run) were identified (Table 2). The IC50 values for these 88 unique compounds in these two runs correlated well (R = 0.85) (Fig 1). The distributions of curve class and potency for these compounds are listed in Table 2. Of these 88 compounds, 19 (1.4% of the 1353 unique NTP compounds) had an IC50 <10 μM, including one compound that had an IC50 less than 1 μM, in the first run of primary screening. These 19 compounds (Table 3) were purchased from commercial vendors for further study.
Table 2.
Potency (IC50) distribution of hERG inhibitors in the primary qHTS
| IC50 (μM) | Number of inhibitors in curve class | ||
|---|---|---|---|
| 1.1 | 1.2 | 2.1 | |
| <1.0 | 1 | 0 | 0 |
| 1.0 – <10.0 | 11 | 7 | 0 |
| 10.0 – 100.0 | 15 | 47 | 7 |
| Total | 27 | 54 | 7 |
IC50 values (concentration of half the maximal inhibition) and efficacy (inhibition of thallium influx as a % of positive control) were calculated from the concentration response titration points for individual compound. There were four major classes (1–4) based on the concentration response curves using previously published criteria (Xia et al., 2009). Curve classes were further subdivided to provide more detailed classification. Briefly, the highest confidence data are from compounds in curve classes 1.1, 1.2, and 2.1; compounds with class 1.3, 1.4, 2.2, and 3 curves have lower confidence data. Curve class 4 compounds showed no concentration-related response and were defined as inactive in this assay.
Fig 1.
qHTS reproducibility of the FluxOR thallium influx assay. The NTP 1408 compound library was screened twice in hERG transduced cells at two separate times. Linear correlation of IC50 values from 88 compounds with concentration response curves in two independent screenings yielded an average R of 0.85.
Table 3.
Potencies and efficacies of hERG blockers identified from primary qHTS (thallium influx), confirmation (thallium influx), and patch clamp assays
| Compound | qHTS (two runs) | Confirmation | Patch clamp | ||
|---|---|---|---|---|---|
| IC50 | Efficacy | IC50 | Efficacy | IC50 | |
| 1,3-diphenylguanidine | 3.4 ± 0.3 | 56 ± 7 | 6.9 ± 3 | 39 ± 12 | 13 |
| 2-aminoanthracene* | 6.5 ± 2.1 | 61 ± 3 | 17.5 ± 26 | 67 ± 19 | >100 |
| 3,3,5-trimethylcyclohexylsalicylate | 23 ± 23 | 52 ± 6 | Inactive | ND | |
| 4-N-hexyl-4′-cyanobiphenyl | 8.2 ± 2.6 | 39 ± 6 | Inactive | ND | |
| benzethonium chloride | 2.8 ± 0.5 | 91 ± 1 | 3.6 ± 1.4 | 77 ± 9 | 0.98 |
| curcumin* | 2.6 ± 0.9 | 129 ± 4 | 4.4 ± 1.4 | 140 ± 4 | 22 |
| D&C red dye 27* | 4.5 ± 0.7 | 119 ± 5 | 7.0 ± 2.5 | 173 ± 41 | >100 |
| domiphen bromide | 7.0 ± 2.8 | 78 ± 0.6 | 4.3 ±1.0 | 62 ± 11 | 1.5 |
| econazole nitrate | 9.1 ± 2.9 | 109 ± 8 | 7.2 ± 2.8 | 112 ± 11 | 3.5 |
| malachite green oxalate | 7.1 ± 0 | 97 ± 3 | 4.8 ± 0.8 | 108 ± 7 | 1.9 |
| o,p′-DDT | 17 ± 11 | 56 ± 2 | 22.2 ± 4.1 | 58 ± 8 | 2.6 |
| quinidine | 11 ± 5.6 | 107 ± 25 | 13.6 ± 5.4 | 110 ± 13 | 2.1 |
| reserpine | 7.5 ± 3.5 | 125 ± 23 | 4.9 ± 1.7 | 118 ± 7 | 1.9 |
| tamoxifen, citrate salt | 6.4 ± 1.0 | 106 ± 22 | 8.3 ± 0.8 | 110 ± 4 | 4.2 |
| tetra-n-octylammonium bromide | 0.38 ± 0.03 | 102 ± 4 | 0.26 ± 0.1 | 100 ± 3 | 0.082 |
| tricresyl phosphate | 14 ± 9 | 82 ± 11 | 9.7 ± 1.6 | 68 ± 16 | 8.8 |
| trixylenyl phosphate | 39 ± 45 | 67 ± 12 | 15.9 ± 11 | 43 ± 15 | >100 |
| verapamil | 3.1 ± 1.2 | 105 ± 1 | 3.4 ± 0.8 | 100 ± 8 | 0.2 |
| zinc pyrithione* | 3.5 ± 1.4 | 155 ± 9 | 3.4 ± 0.3 | 178 ± 7 | 33 |
Abbreviations: DDT = 1,1,1-trichloro-2-(2-chlorophenyl)-2-(4-chlorophenyl)ethane; ND = not determined
Inhibition was found in wild type cells.
Each value of potency (IC50, μM) and efficacy (inhibition of thallium influx as a % of positive control) is the mean ± SD of the results from two runs of primary screening and from three experiments, with each concentration tested in duplicate, in the confirmation assay.
Confirmation of hERG channel blockers
Of the 19 compounds retested in the cell-based FluxOR thallium influx assay, 17 showed activity in the confirmation study that was similar to the activity observed in the primary screen, and two did not (Table 3). These two unconfirmed compounds had low efficacy (42–47%) in the primary screen (Table 3). The concentration response curves of these 19 compounds are provided in Supplementary Fig 1. For the 17 confirmed compounds, IC50 values in the primary screen (average of both runs) and confirmation studies were well correlated (R=0.88, Table 3). The most potent compound among the 17 confirmed compounds was tetra-n-octylammonium bromide, which had average IC50 values of 0.38 μM in the primary qHTS and 0.26 μM in the confirmation study. The next most potent compounds were verapamil (average IC50 = 3.4 μM), zinc pyrithione (average IC50 = 3.4 μM), and benzethonium chloride (average IC50 = 3.6 μM); IC50 values provided are from the confirmation study. These 17 compounds were also tested for thallium influx in the parental cells, which have few endogenous K+ channels (Titus et al., 2009); all were inactive in the parental cells except 2-aminoanthracene, curcumin, D&C red dye 27, and zinc pyrithione, suggesting that the inhibitory effect of these four compounds on thallium influx may not be specific to hERG channels.
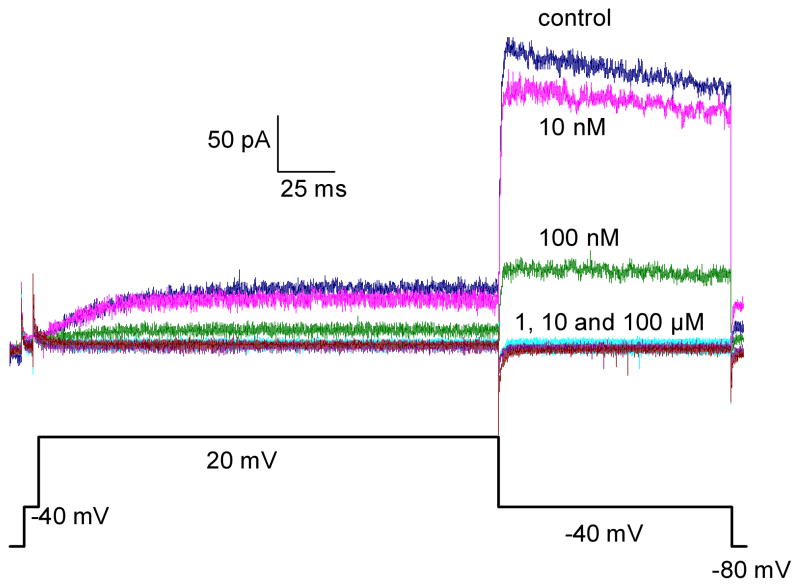
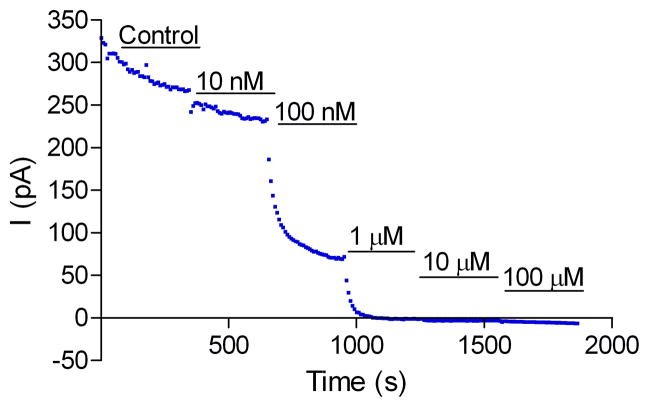
To confirm the inhibitory effect of these 17 compounds on the hERG channel, we tested them using an automated whole cell patch clamp electrophysiology method, a method that is comparable to the conventional patch clamp method (gold standard) for characterization of hERG channel activity in vitro (Kiss et al., 2003; Tao et al., 2004). Among the compounds tested in the automated whole cell patch clamp experiment (Table 3 and Supplementary Fig 2), tetra-n-octylammonium bromide remained the most potent with an IC50 of 0.08 μM (Fig 2), followed by verapamil (IC50 0.2 μM), and benzethonium chloride (IC50 0.98 μM). As expected, the four compounds that showed a non-specific inhibitory effect in untransduced parental cells had much lower potency in the whole cell patch clamp experiment. For example, 2-aminoanthracene and D&C red dye 27 had IC50 values greater than 100 μM in the patch clamp assay compared to IC50 values of 17 and 7 μM, respectively, in the thallium influx assay. Curcumin and zinc pyrithione had IC50 values of 22 and 33 μM, respectively, in the patch clamp assay, but they had IC50 values of 4.4 and 3.4 μM, respectively, in the thallium influx assay. The IC50 values of 12 of the other 13 compounds correlated well (R = 0.77) between the thallium influx assay and the patch clamp experiment, confirming their inhibitory effect on the hERG channel. Only one compound, trixylenyl phosphate, did not inhibit hERG channel activity in the patch clamp experiment; the potency (IC50 of 16 μM) of this compound was relatively low in the thallium influx assay and, therefore, trixylenyl phosphate may have weak and inconsistent activity across these assays. The discordance between the potency of these compounds in the thallium influx assay and the patch clamp assay might be due to the color of these compounds. Colored compounds in solution will absorb light, which will reduce the fluorescence signal generated in the thallium influx assay. Results of these experiments indicate that the thallium influx assay can be used as a primary screen and false positives can be eliminated by the electrophysiological experiment in the confirmation stage.
Fig 2.
Inhibitory effect of tetra-n-octylammonium bromide on hERG tail current measured in an automated whole cell patch clamp experiment. A. Representative electrophysiology recording from one automated patch clamp experiment. The voltage protocol used to induce the hERG current is shown at the bottom. B. The current vs. time plot (I–T plot) of the experiment from A.
In addition, the cytotoxicity of these 12 compounds, after a 30-minute treatment period, was evaluated in a cell viability assay that measures intracellular ATP content. Four of the 12 compounds -- benzethonium chloride, domiphen bromide, malachite green oxalate, and tetra-n-octylammonium bromide -- showed low levels of cytotoxicity, with IC50 values of 79, 65, 31, and 34 μM, respectively, and maximum inhibition of cell viability of 34%, 33%, 72%, and 50%, respectively. However, these compounds were much more potent in blocking hERG channel, with IC50 values ranging from 0.26 to 4.8 μM, suggesting that the ability of these compounds to inhibit the hERG channel is not due to cytotoxicity. The other eight compounds were not cytotoxic at concentrations up to 92 μM.
Inhibition of quaternary ammonium compounds on hERG channel
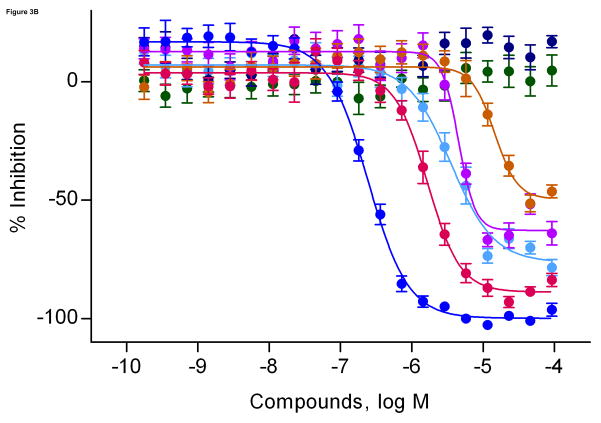
In this study, we found that benzethonium chloride, domiphen bromide, and tetra-n-octylammonium bromide significantly inhibited hERG channel activity in both the thallium influx assay and the patch clamp experiment. Notably, all three compounds are quaternary ammonium compounds (QACs). Therefore, to further investigate the effect of QACs on the hERG channel activities, we purchased four more QAC analogs: benzyltrimethylammonium chloride, cetyltrimethylammonium bromide, decamethonium dibromide, and didecyl dimethyl ammonum chloride. We found the greatest potency in hERG channel blocking activity for QACs with at least two long aliphatic side chains surrounding the charged nitrogen (Fig 3), such as tetra-n-octylammonium bromide (IC50, 0.24 μM in the thallium influx assay, and 0.08 μM in the patch clamp experiment) and didecyl dimethyl ammonum chloride (IC50, 1.7 μM in thallium influx assay, and 0.65 μM in patch clamp experiment). In contrast, benzyltrimethylammonium chloride and decamethonium dibromide, both of which are trimethyl ammonium ions with the charged nitrogen(s) at the end of the structure, were inactive in both the thallium influx assay and the patch clamp experiment (Fig 3).
Fig 3.
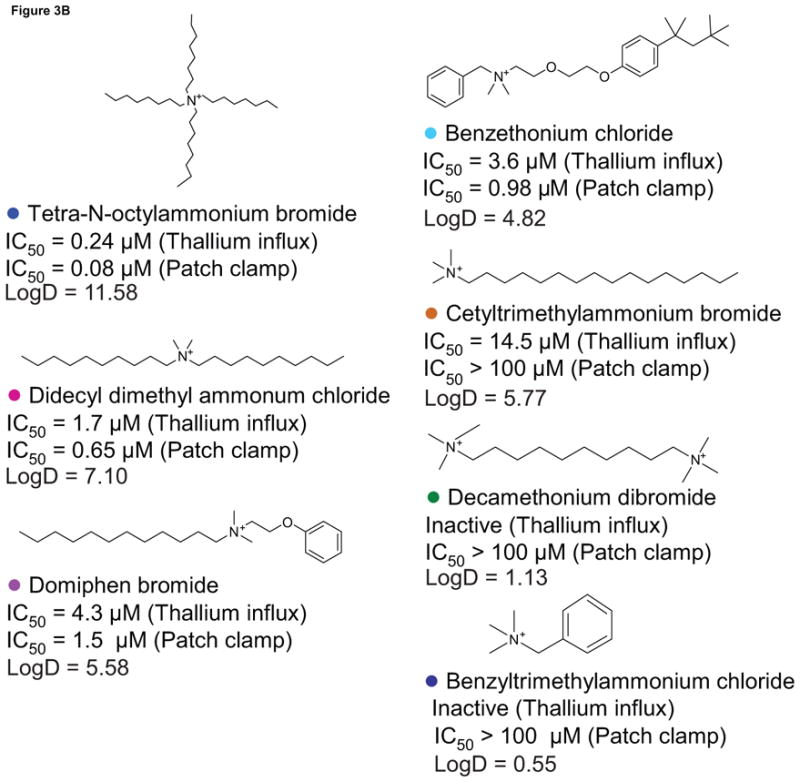
Quaternary ammonium compounds. A. Concentration response curves of the compounds from the ammonium series in the thallium influx assay in hERG cells. B. Structures of these compounds are shown with compound names, IC50 values, and logD values in the thallium influx assay and the whole cell patch clamp experiment.
QACs are often used as powerful disinfectants in medical and food industries (Heir et al., 1999; McDonnell and Russell, 1999) due to their detergent (surfactant) properties. To investigate if the inhibitory action of the QACs on the hERG channel was related to their cell membrane disruption capability, we included digitonin, a well characterized detergent, as a control in this study. We found that digitonin had no inhibitory effect on hERG channel activity at 1 μM (the highest noncytotoxic concentration) in the patch clamp experiment. In the thallium influx assay, digitonin had minimal inhibition on hERG channel activity, with an IC50 of 40 μM. These results suggest that the inhibition of the QACs on the hERG channel is likely unrelated to their detergent action.
Compound selectivity for hERG channel blockers
To further examine the selectivity of the 12 hERG channel blockers identified in the thallium influx assay and confirmed in the patch clamp experiment in the current study, we tested compound activity on the voltage gated KV1.3 K+ channel by measuring thallium influx in KV1.3 stably transfected cells. We found that 11 of the 12 compounds tested had low potency (IC50 > 11 μM) or no activity for KV1.3 channel inhibition (Table 4). Among these were five compounds -- 1.3-diphenylguanidine, malachite green oxalate, o,p′-DDT, quinidine, and reserpine -- that had either IC50 values >30 μM or were inactive in the KV1.3 assay. Among the compounds that showed KV1.3 inhibition, tetra-n-octylammonium bromide had the highest selectivity for the hERG channel over the KV1.3 channel (42-fold potency difference), followed by reserpine (11.8-fold), verapamil (8.8-fold), malachite green oxalate (6.5-fold), and benzethonium chloride (5-fold). In contrast, domiphen bromide and o,p′ –DDT showed no selectivity between channel types, and tamoxifen (2.7-fold), and tricresyl phosphate (2.7-fold) had low selectivity.
Table 4.
Effect of hERG channel blockers on thallium influx in hERG-transduced and KV1.3 cells
| Compound | hERG | KV 1.3 | Selectivity | ||
|---|---|---|---|---|---|
| IC50 | Efficacy | IC50 | Efficacy | Fold | |
| 1,3-diphenylguanidine | 6.9 ± 3 | 39 ± 12 | Inactive | ||
| benzethonium chloride | 3.6 ± 1.4 | 77 ± 9 | 18±6 | 93 ± 20 | 5 |
| domiphen bromide | 4.3 ± 1.0 | 62 ± 11 | 5.2 ± 0.6 | 102 ± 22 | 1.2 |
| econazole nitrate | 7.2 ± 2.8 | 112 ± 11 | 30 ± 2 | 104 ± 10 | 4.2 |
| malachite green oxalate | 4.8 ± 0.8 | 108 ± 7 | 31 | 89 | 6.5 |
| o,p′ –DDT | 22.2 ± 4.1 | 58 ± 8 | 35 | 61 | 1.6 |
| quinidine | 13.6 ± 5.4 | 110 ± 13 | Inactive | ||
| reserpine | 4.9 ± 1.7 | 118 ± 7 | 58 ± 18 | 78 ± 7 | 11.8 |
| tamoxifen, citrate salt | 8.3 ± 0.8 | 110 ± 4 | 22 ± 3 | 108 | 2.7 |
| tetra-n-octylammonium bromide | 0.26 ± 0.1 | 100 ± 3 | 11 ± 8 | 72 ± 16 | 42 |
| tricresyl phosphate | 9.7 ± 1.6 | 68 ± 16 | 26 ± 7 | 68 ± 17 | 2.7 |
| verapamil | 3.4 ± 0.8 | 100 ± 8 | 30 ± 3 | 88 ± 12 | 8.8 |
Abbreviations: DDT = 1,1,1-trichloro-2-(2-chlorophenyl)-2-(4-chlorophenyl)ethane
Each value of potency (IC50, μM) and efficacy (inhibition of thallium influx as a % of positive control) from thallium influx assays in hERG-transduced and KV1.3 cells is the mean ± SD from two to three experiments.
Discussion
In the present study, we used a cell-based thallium influx assay in qHTS format to evaluate the effect of a large set of environmental chemicals on hERG channel activity. The processes involved in conducting the screening, confirmation, and follow-up studies are summarized as a flow chart in Fig 4. Seventeen unique compounds were identified in the NTP 1408 compound library that strongly inhibited hERG channel activity, with IC50 values determined in the confirmation study ranging from 0.26 to 22 μM. Twelve of these compounds were confirmed as hERG blockers in an automated whole cell patch clamp assay; these 12 compounds were negative for thallium influx activity in the non-hERG transduced cells. Among these 12 confirmed hERG channel blockers, tetra-n-octylammonium bromide, a quaternary ammonium compound (QAC), was the most potent. Therefore, we investigated the structure-activity relationship of a QAC series on hERG inhibition and found that the potency of hERG inhibition activity for this class of compounds depended on the number and length of the aliphatic side-chains surrounding the charged nitrogen. Lastly, we found that 11 of these 12 hERG blockers had either low potency (IC50 > 11 μM) or no activity in the KV1.3 channel inhibition assay, indicating the selectivity of these compounds for the hERG channel.
Fig 4.
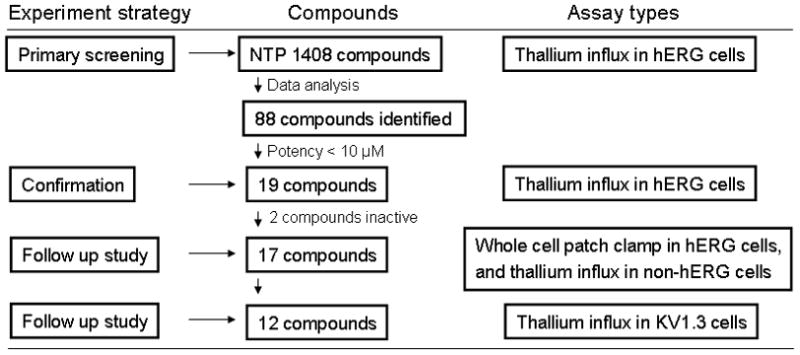
A flowchart of identification of hERG channel blockers. Eighty-eight compounds with class 1.1, 1.2, and 2.1 curves were identified from primary screening. Nineteen compounds with IC50 values less than 10 μM were selected based on the first run of primary screening for further studies. Seventeen of nineteen compounds were confirmed in thallium influx assay, and further tested in a whole cell patch clamp experiment. Twelve of these compounds were confirmed as hERG channel blockers in a whole cell patch clamp experiment and had no off target activity in the non-hERG transduced cells. Nine of the twelve compounds had either low potency or no activity in the KV1.3 channel inhibition assay.
Drug-induced QT interval prolongation is a major health concern for both cardiac and non-cardiac drugs. Among the 12 confirmed hERG channel blockers in the NTP 1408 compound library, three compounds -- quinidine (Perrin et al., 2008), verapamil (Chouabe et al., 1998), and tamoxifen (Thomas et al., 2003; Titus et al., 2009) -- have previously been reported to inhibit the hERG channel. Quinidine is used to treat a variety of cardiac arrhythmias primarily by blocking rapid inward sodium current (Grace and Camm, 1998), but clinical use of quinidine has been linked to cardiac QT prolongation through inhibition of the hERG K+ channel (Grace and Camm, 1998). Verapamil, an L-type Ca2+ channel antagonist, has been clinically used in the treatment of cardiovascular diseases including hypertension (Richard, 2005). In the previous study, we found that verapamil effectively blocked L-type Ca2+ channels (IC50 of 0.7 μM) in HEK 293 cells stably transfected with L-type Ca2+ channels (Xia et al., 2004). In addition to blocking L-type Ca2+ channels, verapamil has been found to block the hERG channel by binding to the helix residue Y652 and F656 in the S6 transmembrane domain (Duan et al., 2007). However, it has been shown that verapamil did not affect QT prolongation because the block of the L-type Ca2+ channel compensates the effect from the block of hERG channel (Ca2+ channels conduct inward current, whereas hERG channels conduct outward current). Therefore, the verapamil induced TdP in humans has not been reported (Redfern et al., 2003). Tamoxifen is a well-known estrogen receptor antagonist that is used in the treatment of breast cancer. However, in clinical trials, tamoxifen was linked to QT interval prolongation (Trump et al., 1992). Also, tamoxifen has been shown to inhibit hERG current in electrophysiology voltage clamp experiments (Thomas et al., 2003) and to block hERG channels in thallium influx assays (Titus et al., 2009).
In the present study, we identified two drugs, econazole nitrate (IC50 of 7.2 μM in the thallium influx assay and 3.5 μM in the patch clamp assay) and reserpine (IC50 of 4.9 μM in the thallium influx assay and 1.9 μM in the patch clamp assay), as hERG channel blockers, which to our knowledge has not been reported previously, but is consistent with their therapeutic action. Econazole nitrate, an imidazole derivative, is one of the topical antifungal medications used clinically in the treatment of superficial mycoses of the skin (Veraldi and Milani, 2008). Previous studies have shown that econazole blocks the Ca2+-dependent K+ channel in human red blood cells (Alvarez et al., 1992) and inhibits receptor-operated calcium channels in human neutrophils (Montero et al., 1991). Econazole has also been reported to relax phenylephrine- and KCl-induced contraction in rat isolated aorta rings by inhibiting calcium entry via L-type Ca2+ channels (Tunctan et al., 2000). Reserpine, an indole alkaloid, has been used as an antihypertensive drug (Shamon and Perez, 2009).
QACs, including tetraethylammonium (TEA), have been widely used for decades as classic voltage-gated K+ channel blockers, with IC50 values ranging from 0.6 to 129 mM in electrophysiological studies (Stanfield, 1983; Pongs, 1992). The block of voltage-gated K+ channels by TEA and other QACs occurs not only at the external region of the pore, but also via an internal TEA-binding site in the S5 region of the channel (Pongs, 1992). Due to their high lipophilicity, these compounds can easily cross the hydrophobic core of the plasma membrane to interact with the internal S5 region of the K+ channel. However, to date, block of the hERG channel by these compounds has not been reported. In the present study, we found in our initial screen that the QACs, such as benzethonium chloride, domiphen bromide, and tetra-n-octylammonium bromide, significantly blocked the hERG channel. The binding of these compounds to the hERG channel may occur via the internal binding site because we found that the potency of the QACs for hERG channel inhibition is directly related to the number and length of the aliphatic side-chains. Among the QACs we screened, tetra-n-octylammonium bromide, with four long, aliphatic side-chains, was the most potent. Potency decreased in compounds that contain short, aromatic side chains or trimethylammonium heads, where the charged nitrogen is “exposed” at the end of the structure. For example, decamethonium dibromide and benzyltrimethylammonium chloride did not inhibit the hERG channel, presumably because the charged nitrogen(s) in these compounds are exposed. Compounds with more and longer aliphatic side chains, where the charged nitrogen is hidden in the middle of the structure, are more lipophilic and thus, they can more easily cross the plasma membrane and bind to the internal site of the hERG channel. To validate our QAC structure-activity hypothesis, we calculated the Log D values (Figure 3B) using Pipeline Pilot 7.0 (Accelrys, Inc., San Diego, CA, USA) for the seven QACs and found a significant correlation (R = 0.95, p = 0.001) between lipophilicity and their potency for inhibiting the hERG channel.
This study identified several compounds, including 1,3-diphenylguanidine, malachite green oxalate, o,p′-DDT, and tricresyl phosphate, as hERG channel blockers. Some of these compounds have been widely used in agriculture and industry, and have significant human exposure potential, although there have been no reports associating exposure to these compounds with QT interval prolongation. For example, 1,3-diphenylguanidine has been used as a primary and secondary accelerator in the vulcanization of rubber (NTP, 1995). Bempong and Hall (1983) reported that this compound decreased sperm count and altered sperm morphology in rodents, although these observations were not confirmed in an independent study (Koeter et al., 1992). Recently, 1,3-diphenylguanidine was reported to be involved in the development of allergic contact dermatitis (Piskin et al., 2006). Malachite green, a triarylmethane dye, is widely used as a parasiticide in the aquaculture industry and is also used as a food and clothing coloring agent (Srivastava et al., 2004). It has been reported to be carcinogenic, mutagenic, and teratogenic, and to induce respiratory toxicity (Srivastava et al., 2004). We found, using a qHTS cell viability assay, that malachite green oxalate is highly cytotoxic to a number of different human and rodent cell types (Xia et al., 2008). DDT (dichlorodiphenyltrichloroethane) is one of the most well-known synthetic pesticides. DDT and related chlorinated pesticides have been banned from agricultural use in the United States since 1972, but due to their persistence in the environment, they remain a significant public health concern. Due to the lipophilic nature of these chemicals, they are stored in lipid-rich tissues such as liver, brain, and adipose tissue. DDT is highly toxic to various organ systems including neurological, immunological, endocrinological, cardiovascular, respiratory, gastrointestinal, and other systems (Crinnion, 2009). It has been reported that DDT also causes cardiac arrhythmias, such as Q-T prolongation and repetitive ventricular tachyarrhythmias of the TdP type, after patients were poisoned with this chemical (Ludomirsky et al., 1982). DDT is also known to modulate Na+ channels of nerve cell membranes at exposure levels that cause hyperexcitatory symptoms in animals (Song et al., 1996). Results with DDT in the current study suggest that hERG channel inhibition by DDT (IC50 values of 22.2 μM in the thallium influx assay and 2.6 μM in the patch clamp assay) may be a factor in DDT-associated cardiac arrhythmias. Tricresyl phosphate (TCP), an organophosphate compound, has been used as a plasticizer, lubricant, hydraulic fluid, paint additive, oil additive, dust suppressant, and in other commercial applications. However, most commercial uses of TCP have been halted due to its toxicity, particularly neurotoxicity (Winder and Balouet, 2002).
In summary, we have identified several known and novel hERG channel blockers in the NTP 1408 library. In the present study, we used a cell-based thallium influx assay as the primary screen, in combination with secondary assays including the whole cell patch clamp assay. In the next steps, the characterization of the hERG blockers identified in the current study would likely include QT prolongation studies in animals and investigations of the mechanisms of action (e.g., effects on ionic permeation/selectivity and/or activation, deactivation, or inactivation kinetics of hERG channels). We conclude that the approach used in this study will allow for the efficient identification and profiling of hERG channel blockers in anticipated future screenings of large collections of environmental chemicals and drugs.
Supplementary Material
Acknowledgments
This work was supported by the Intramural Research Programs (Interagency agreement #Y2-ES-7020-01) of the National Toxicology Program, National Institute of Environmental Health Sciences, and the National Human Genome Research Institute, National Institutes of Health (NIH), and the NIH Roadmap for Medical Research Molecular Libraries Program. This article is the work product of employees of the National Institute of Environmental Health Sciences and the National Human Genome Research Institute, NIH. The statements, opinions, or conclusions contained therein do not necessarily represent the statements, opinions, or conclusions of NIH or the United States government.
Footnotes
Conflict of interest statement
The authors declare that there are no conflicts of interest, related to this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alvarez J, Montero M, Garcia-Sancho J. High affinity inhibition of Ca(2+)-dependent K+ channels by cytochrome P-450 inhibitors. J Biol Chem. 1992;267:11789–11793. [PubMed] [Google Scholar]
- Belpomme D, Irigaray P, Hardell L, Clapp R, Montagnier L, Epstein S, Sasco AJ. The multitude and diversity of environmental carcinogens. Environ Res. 2007;105:414–429. doi: 10.1016/j.envres.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Chouabe C, Drici MD, Romey G, Barhanin J, Lazdunski M. HERG and KvLQT1/IsK, the cardiac K+ channels involved in long QT syndromes, are targets for calcium channel blockers. Mol Pharmacol. 1998;54:695–703. [PubMed] [Google Scholar]
- Collins FS, Gray GM, Bucher JR. Toxicology. Transforming environmental health protection. Science. 2008;319:906–907. doi: 10.1126/science.1154619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crinnion WJ. Chlorinated pesticides: threats to health and importance of detection. Altern Med Rev. 2009;14:347–359. [PubMed] [Google Scholar]
- Duan JJ, Ma JH, Zhang PH, Wang XP, Zou AR, Tu DN. Verapamil blocks HERG channel by the helix residue Y652 and F656 in the S6 transmembrane domain. Acta Pharmacol Sin. 2007;28:959–967. doi: 10.1111/j.1745-7254.2007.00562.x. [DOI] [PubMed] [Google Scholar]
- Grace AA, Camm AJ. Quinidine. N Engl J Med. 1998;338:35–45. doi: 10.1056/NEJM199801013380107. [DOI] [PubMed] [Google Scholar]
- Gutman GA, Chandy KG, Grissmer S, Lazdunski M, McKinnon D, Pardo LA, Robertson GA, Rudy B, Sanguinetti MC, Stuhmer W, Wang X. International Union of Pharmacology. LIII. Nomenclature and molecular relationships of voltage-gated potassium channels. Pharmacol Rev. 2005;57:473–508. doi: 10.1124/pr.57.4.10. [DOI] [PubMed] [Google Scholar]
- Heir E, Sundheim G, Holck AL. Identification and characterization of quaternary ammonium compound resistant staphylococci from the food industry. Int J Food Microbiol. 1999;48:211–219. doi: 10.1016/s0168-1605(99)00044-6. [DOI] [PubMed] [Google Scholar]
- Inglese J, Auld DS, Jadhav A, Johnson RL, Simeonov A, Yasgar A, Zheng W, Austin CP. Quantitative high-throughput screening: a titration-based approach that efficiently identifies biological activities in large chemical libraries. Proc Natl Acad Sci U S A. 2006;103:11473–11478. doi: 10.1073/pnas.0604348103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavlock RJ, Austin CP, Tice RR. Toxicity testing in the 21st century: implications for human health risk assessment. Risk Anal. 2009;29:485–487. doi: 10.1111/j.1539-6924.2008.01168.x. discussion 492–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss L, Bennett PB, Uebele VN, Koblan KS, Kane SA, Neagle B, Schroeder K. High throughput ion-channel pharmacology: planar-array-based voltage clamp. Assay Drug Dev Technol. 2003;1:127–135. doi: 10.1089/154065803321537845. [DOI] [PubMed] [Google Scholar]
- Koeter HB, Regnier JF, van Marwijk MW. Effect of oral administration of 1,3-diphenylguanidine on sperm morphology and male fertility in mice. Toxicology. 1992;71:173–179. doi: 10.1016/0300-483x(92)90064-l. [DOI] [PubMed] [Google Scholar]
- Ludomirsky A, Klein HO, Sarelli P, Becker B, Hoffman S, Taitelman U, Barzilai J, Lang R, David D, DiSegni E, Kaplinsky E. Q-T prolongation and polymorphous (“torsade de pointes”) ventricular arrhythmias associated with organophosphorus insecticide poisoning. Am J Cardiol. 1982;49:1654–1658. doi: 10.1016/0002-9149(82)90242-9. [DOI] [PubMed] [Google Scholar]
- Maxwell P, Salnikow K. HIF-1: an oxygen and metal responsive transcription factor. Cancer Biol Ther. 2004;3:29–35. doi: 10.4161/cbt.3.1.547. [DOI] [PubMed] [Google Scholar]
- McDonnell G, Russell AD. Antiseptics and disinfectants: activity, action, and resistance. Clin Microbiol Rev. 1999;12:147–179. doi: 10.1128/cmr.12.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero M, Alvarez J, Garcia-Sancho J. Agonist-induced Ca2+ influx in human neutrophils is secondary to the emptying of intracellular calcium stores. Biochem J. 1991;277 (Pt 1):73–79. doi: 10.1042/bj2770073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NRC. Toxicity Testing in the 21st Century: A Vision and A Strategy. National Academy Press; Washington, DC: 2007. [Google Scholar]
- NTP. Toxicity Studies of 1,3-Diphenylguanidine (CAS No. 102-06-7) Administered in Feed to F344/N Rats and B6C3F1 Mice. Toxic Rep Ser. 1995;42:1–D6. [PubMed] [Google Scholar]
- Perrin MJ, Kuchel PW, Campbell TJ, Vandenberg JI. Drug binding to the inactivated state is necessary but not sufficient for high-affinity binding to human ether-a-go-go-related gene channels. Mol Pharmacol. 2008;74:1443–1452. doi: 10.1124/mol.108.049056. [DOI] [PubMed] [Google Scholar]
- Piskin G, Meijs MM, van der Ham R, Bos JD. Glove allergy due to 1,3-diphenylguanidine. Contact Dermatitis. 2006;54:61–62. doi: 10.1111/j.0105-1873.2006.0729d.x. [DOI] [PubMed] [Google Scholar]
- Pongs O. Structural basis of voltage-gated K+ channel pharmacology. Trends Pharmacol Sci. 1992;13:359–365. doi: 10.1016/0165-6147(92)90109-j. [DOI] [PubMed] [Google Scholar]
- Redfern WS, Carlsson L, Davis AS, Lynch WG, MacKenzie I, Palethorpe S, Siegl PK, Strang I, Sullivan AT, Wallis R, Camm AJ, Hammond TG. Relationships between preclinical cardiac electrophysiology, clinical QT interval prolongation and torsade de pointes for a broad range of drugs: evidence for a provisional safety margin in drug development. Cardiovasc Res. 2003;58:32–45. doi: 10.1016/s0008-6363(02)00846-5. [DOI] [PubMed] [Google Scholar]
- Richard S. Vascular effects of calcium channel antagonists: new evidence. Drugs. 2005;65(Suppl 2):1–10. doi: 10.2165/00003495-200565002-00002. [DOI] [PubMed] [Google Scholar]
- Shah RR. Can pharmacogenetics help rescue drugs withdrawn from the market? Pharmacogenomics. 2006;7:889–908. doi: 10.2217/14622416.7.6.889. [DOI] [PubMed] [Google Scholar]
- Shamon SD, Perez MI. Blood pressure lowering efficacy of reserpine for primary hypertension. Cochrane Database Syst Rev. 2009:CD007655. doi: 10.1002/14651858.CD007655.pub2. [DOI] [PubMed] [Google Scholar]
- Song JH, Nagata K, Tatebayashi H, Narahashi T. Interactions of tetramethrin, fenvalerate and DDT at the sodium channel in rat dorsal root ganglion neurons. Brain Res. 1996;708:29–37. doi: 10.1016/0006-8993(95)01239-7. [DOI] [PubMed] [Google Scholar]
- Srivastava S, Sinha R, Roy D. Toxicological effects of malachite green. Aquat Toxicol. 2004;66:319–329. doi: 10.1016/j.aquatox.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Stanfield PR. Tetraethylammonium ions and the potassium permeability of excitable cells. Rev Physiol Biochem Pharmacol. 1983;97:1–67. doi: 10.1007/BFb0035345. [DOI] [PubMed] [Google Scholar]
- Szabo I, Zoratti M, Gulbins E. Contribution of voltage-gated potassium channels to the regulation of apoptosis. FEBS Lett. 2010;584:8. doi: 10.1016/j.febslet.2010.01.038. [DOI] [PubMed] [Google Scholar]
- Tao H, Santa Ana D, Guia A, Huang M, Ligutti J, Walker G, Sithiphong K, Chan F, Guoliang T, Zozulya Z, Saya S, Phimmachack R, Sie C, Yuan J, Wu L, Xu J, Ghetti A. Automated tight seal electrophysiology for assessing the potential hERG liability of pharmaceutical compounds. Assay Drug Dev Technol. 2004;2:497–506. doi: 10.1089/adt.2004.2.497. [DOI] [PubMed] [Google Scholar]
- Thomas D, Gut B, Karsai S, Wimmer AB, Wu K, Wendt-Nordahl G, Zhang W, Kathofer S, Schoels W, Katus HA, Kiehn J, Karle CA. Inhibition of cloned HERG potassium channels by the antiestrogen tamoxifen. Naunyn Schmiedebergs Arch Pharmacol. 2003;368:41–48. doi: 10.1007/s00210-003-0766-8. [DOI] [PubMed] [Google Scholar]
- Titus SA, Beacham D, Shahane SA, Southall N, Xia M, Huang R, Hooten E, Zhao Y, Shou L, Austin CP, Zheng W. A new homogeneous high-throughput screening assay for profiling compound activity on the human ether-a-go-go-related gene channel. Anal Biochem. 2009;394:30–38. doi: 10.1016/j.ab.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trump DL, Smith DC, Ellis PG, Rogers MP, Schold SC, Winer EP, Panella TJ, Jordan VC, Fine RL. High-dose oral tamoxifen, a potential multidrug-resistance-reversal agent: phase I trial in combination with vinblastine. J Natl Cancer Inst. 1992;84:1811–1816. doi: 10.1093/jnci/84.23.1811. [DOI] [PubMed] [Google Scholar]
- Tseng GN. I(Kr): the hERG channel. J Mol Cell Cardiol. 2001;33:835–849. doi: 10.1006/jmcc.2000.1317. [DOI] [PubMed] [Google Scholar]
- Tunctan B, Altug S, Uludag O, Abacioglu N. Effects of econazole on receptor-operated and depolarization-induced contractions in rat isolated aorta. Life Sci. 2000;67:2393–2401. doi: 10.1016/s0024-3205(00)00822-5. [DOI] [PubMed] [Google Scholar]
- Vandenberg JI, Torres AM, Campbell TJ, Kuchel PW. The HERG K+ channel: progress in understanding the molecular basis of its unusual gating kinetics. Eur Biophys J. 2004;33:89–97. doi: 10.1007/s00249-003-0338-3. [DOI] [PubMed] [Google Scholar]
- Vandenberg JI, Walker BD, Campbell TJ. HERG K+ channels: friend and foe. Trends Pharmacol Sci. 2001;22:240–246. doi: 10.1016/s0165-6147(00)01662-x. [DOI] [PubMed] [Google Scholar]
- Veraldi S, Milani R. Topical fenticonazole in dermatology and gynaecology: current role in therapy. Drugs. 2008;68:2183–2194. doi: 10.2165/00003495-200868150-00007. [DOI] [PubMed] [Google Scholar]
- Warmke JW, Ganetzky B. A family of potassium channel genes related to eag in Drosophila and mammals. Proc Natl Acad Sci U S A. 1994;91:3438–3442. doi: 10.1073/pnas.91.8.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winder C, Balouet JC. The toxicity of commercial jet oils. Environ Res. 2002;89:146–164. doi: 10.1006/enrs.2002.4346. [DOI] [PubMed] [Google Scholar]
- Wray D. The roles of intracellular regions in the activation of voltage-dependent potassium channels. Eur Biophys J. 2004;33:194–200. doi: 10.1007/s00249-003-0363-2. [DOI] [PubMed] [Google Scholar]
- Xia M, Huang R, Sun Y, Semenza GL, Aldred SF, Witt KL, Inglese J, Tice RR, Austin CP. Identification of chemical compounds that induce HIF-1alpha activity. Toxicol Sci. 2009;112:153–163. doi: 10.1093/toxsci/kfp123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia M, Huang R, Witt KL, Southall N, Fostel J, Cho MH, Jadhav A, Smith CS, Inglese J, Portier CJ, Tice RR, Austin CP. Compound cytotoxicity profiling using quantitative high-throughput screening. Environ Health Perspect. 2008;116:284–291. doi: 10.1289/ehp.10727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia M, Imredy JP, Koblan KS, Bennett P, Connolly TM. State-dependent inhibition of L-type calcium channels: cell-based assay in high-throughput format. Anal Biochem. 2004;327:74–81. doi: 10.1016/j.ab.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Zhang JH, Chung TD, Oldenburg KR. A Simple Statistical Parameter for Use in Evaluation and Validation of High Throughput Screening Assays. J Biomol Screen. 1999;4:67–73. doi: 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.