Abstract
Yersinia pestis (Y. pestis) is the causative pathogen of plague, a highly fatal disease for which an effective vaccine, especially against mucosal transmission, is still not available. Like many bacterial infections, antigen-specific antibody responses have been traditionally considered critical, if not solely responsible, for vaccine-induced protection against Y. pestis. Studies in recent years have suggested the importance of T cell immune responses against Y. pestis infection but information is still limited about the details of Y. pestis antigen-specific T cell immune responses. In current report, studies are conducted to identify the presence of CD8+ T cell epitopes in LcrV protein, the leading antigen of plague vaccine development. Furthermore, depletion of CD8+ T cells in LcrV DNA vaccinated Balb/C mice led to reduced protection against lethal intranasal challenge of Y. pestis. These findings establish that an LcrV DNA vaccine is able to elicit CD8+ T cell immune responses against specific epitopes of this key plague antigen and that a CD8+ T cell immune response is involved in LcrV DNA vaccine-elicited protection. Future studies in plague vaccine development will need to examine if the presence of detectable T cell immune responses, in particular CD8+ T-cell immune responses, will enhance the protection against Y. pestis in higher animal species or humans.
Keywords: Y. pestis, CD8+ T-cell immune responses, LcrV DNA vaccine-elicited protection
1. Introduction
Plague is one of the world’s most feared infectious diseases throughout recorded history. Infection with the Gram negative bacterium Y. pestis, which is the etiological agent of plague, remains a serious public health threat in many parts of the world and it also has the potential to be used as a bioterrorism agent [1–3]. Infection with Y. pestis can take several forms. For example, the bubonic plague usually results from transmission of the bacteria via the bite of an infected flea; the bacillus may spread to the blood stream and lungs and progress to the more deadly septicemic and pneumonic forms of Y. pestis infection. The pneumonic plague is also transmissible from person to person via airborne transmission and is almost 100% fetal when left untreated. Vaccines are effective in preventing the infection in humans. Previous studies demonstrated that killed whole-cell (KWC) vaccine could protect the less deadly bubonic plague, and a live attenuated plague vaccine has been tested in certain countries. However, the limited efficacy against pneumonic plague and safety concerns surrounding these vaccines have limited their use in the prevention of plague in humans [4–7]. Most critically, after many years of research efforts, there are still no licensed vaccines that are effective against pneumonic plague.
Recently, subunit-based plague vaccines have received much attention. Recombinant protein vaccines using two known protective Y. pestis antigens, F1 and V, have been shown to be immunogenic and protective against both bubonic and pneumonic plague challenges in mouse models [8–16]. Early phase clinical trials examining safety and immunogenicity have been reported for a recombinant protein plague vaccine comprising of F1 and V antigens [17]. Newer vaccine forms, such as DNA vaccines, have also been tested and have been shown quite effective in eliciting protective immunity in small animal models with V, F1, and other new or less studied antigens [18–24]. However, these studies mainly focused on the induction of protective antibody responses.
At the same time, basic research studies have started to indicate that both humoral and cellular immunity may contribute to vaccine efficacy against plague [1, 25–29][30]. The critical role of humoral immunity in protection against plague is well established and evidenced by the demonstration that passive transfer of convalescent-phase sera, monoclonal antibodies specific to F1 or V antigen, or immune animal sera generated by recombinant F1 and LcrV vaccination could confer protection to mice from pneumonic and bubonic plague challenge[27, 31–34]. The role of T cell immunity has only been described more recently. In one report, B cell-deficient μMT mice, which lack the capacity to mount antibody responses, were used to investigate the cellular immunity against plague [26]. The μMT mice vaccinated with live Y. pestis could elicit protective immunity against a lethal pulmonary challenge and the observed protection was abrogated by treatment with T cell-depleting monoclonal antibodies (mAb). Moreover, the transfer of Y. pestis-primed T cells to naive μMT mice could protect against lethal intranasal Y. pestis challenge. Although the reports did not specify which specific bacterial antigen(s) may be involved in such T cell responses against plague, the findings demonstrated that vaccine-induced cell-mediated immune responses, in the absence of protective antibodies, may contribute to the protective immunity against pulmonary Y. pestis infection.
Humoral immunity relies upon antigen-specific B cell development and antibody production to effectively block the infection. Cellular immunity relies upon the cytolytic and cytokine-producing capacities of T cells to effectively restrict or even eradicate intracellular pathogens, in addition to its helper function for the development of antigen-specific B cells. Vaccination approach plays a key role in eliciting T cell immune responses. Neither killed organism vaccines nor recombinant protein vaccines are effective in eliciting T cell immune responses due to the exogenous antigen processing and presentation pathways used by these vaccines. In contrast, vaccines comprised of antigens produced in vivo, such as live attenuated vaccines, and gene-based vaccines (DNA or viral vector vaccines) are more effective in generating antigen-specific cellular immune responses, especially class I MHC molecule mediated CD8+ T cell responses [35–38].
In the current study, we used a highly immunogenic DNA vaccine expressing Y. pestis LcrV antigen as a model immunogen to investigate the involvement of T cell-mediated immune responses in this V DNA vaccine-elicited protection in mouse. Potential T cell epitopes in the LcrV antigen were predicted and tested in V DNA vaccinated mice. Depletion of CD8+ or CD4+ T cells was also conducted to determine if any of these T cell subsets contribute to protection elicited by the V DNA vaccine against lethal mucosal challenge of Y. pestis. Our data provided clear evidence that a CD8+ T cell immune response is, indeed, involved in eliciting protection against Y. pestis challenge in mice immunized with the LcrV DNA vaccine and our findings indicate the need to design and develop next generation plague vaccines with optimal humoral and T cell-mediated immunities.
2. Materials and Methods
2.1 CD 8+ T cell epitope prediction
H2d class I epitopes in the LcrV protein were predicted according to a computational system (PRED(BALB/c)) as previously described [39]. This prediction system utilizes quantitative matrices, which were rigorously validated using experimentally-determined binders and non-binders and also by in vivo studies using viral proteins. The initial quantitative matrices, using logarithmic equations based on the frequency of amino acids at specific positions within the training set of 9-mer peptides, followed by refinement to include information on the consensus and other binding motifs. The anchor positions were assigned higher weights than other positions. All amino acids at the anchor positions, other than the permissible ones, were assigned low scores to exclude peptides with non-permissible amino acids. The final binding scores were normalized as 1–9 for each amino acid. Eight predicted peptides from LcrV with high H2-Kd scores (Table 1) were synthesized by EZBiolab (Westfield, IN) for in vitro T cell studies.
Table 1.
List of predicted LcrV peptides
| Peptides | Sequence | Position* | H2-kd score |
|---|---|---|---|
| LcrV-1 | AYFLPEDAIL | 76–85 | 19 |
| LcrV-2 | KGGHYDNQL | 86–94 | 12 |
| LcrV-3 | NGIKRVKEFL | 96–105 | 6 |
| LcrV-4 | FMAVMHFSLTADRI | 118–131 | 14 |
| LcrV-5 | LYGYTDEEIF | 198–207 | 23 |
| LcrV-6 | FKASAEYKI | 207–215 | 14 |
| LcrV-7 | SYNKDNNEL | 258–266 | 24 |
| LcrV-8 | IYSVIQAEI | 166–174 | 23 |
Amino acid positions in LcrV protein
2.2 IFN-γ ELISPOT
ELISPOT (Enzyme-linked immunospot) assays were performed on fresh mouse splenocytes, as previously described [40–42]. Immobilon P membrane White Sterile 96-well plates (Millipore, MA) were coated with 5 μg/ml of purified rat anti-mouse IFN-γ IgG1 (clone R4-6A2, BD Biosciences, CA) in PBS at 4°C overnight. After the plates were washed three times with PBS, each plate was blocked by 200 μl of R10 medium per well for 2–3 h at 37°C. The peptides employed were the predicted LcrV epitope peptides and the negative control HIV-1 Env V3 (HIV-V3, IGPGRAFYT) peptide. The peptides, at a final concentration of 4 μg/ml, were added to the wells which contained 100 μl of freshly isolated splenocytes (500,000 or 100,000 cells/well in R10 medium) in duplicate. The plates were incubated for 20 –24 h overnight at 37°C in 5% CO2. The plates were then washed, incubated with 100 μl of biotinylated rat anti-mouse IFN-γ IgG1 (clone XMG1.2, BD Biosciences) at 1 μg/ml in dilution buffer (PBS with 0.005% Tween20 and 5% FBS), and incubated at 4°C overnight. After additional washes, 100 μl of AP-conjugated streptavidin complex (BD Bioscience) was added to each well in above dilution buffer for 2 h at room temperature. The plates were washed, and spots representing individual IFN-γ-producing cells were detected after a 7-min color reaction using 1-STEP NBT/BCIP. IFN-γ spot-forming cells (SFC) were counted. The results were expressed as the number of SFC per 106 input cells. The number of peptide-specific IFN-γ-secreting T cells was calculated by subtracting the background (no-peptide) control value from the established SFC count.
2.3 Intracellular-cytokine staining
Intracellular IFN-γ staining was performed similar to previously described [43]. The freshly isolated splenocytes (106 cells in 200 μl) were cultured at 37°C for five hours in 96-well round-bottom plates in completed R10 medium supplemented with 20 U/ml of human IL-2 and 0.4 μg/ml of Golgi-plug (BD Biosciences, CA). Stimulatory conditions included 5 μg/ml of the LcrV peptides or a non-relevant HIV-1 Env V3 peptide (HIV-V3) as negative control. After incubation, the following procedures were performed at 4°C. First, cells were washed with FACS buffer (PBS with 2% FBS and 0.01% sodium azide) and then incubated for 10 min in 100 μl of FcBlock (2.4 G2 MAb, BD Biosciences). After washes, the FITC-conjugated anti-CD8 mAb (clone 53-6.7, BD Biosciences) was used for the cell surface staining for 30 min at 4°C and then washed. For intracellular cytokine staining, the cells were fixed and permeabilized using the Cytofix/Cytoperm kit, in accordance with the manufacturer’s recommendations (BD Pharmingen). Then the cells were stained using PE-conjugated rat anti-mouse IFN-γ MAb (clone XMG1.2) for 30 min at 4°C. Flow cytometry analysis was performed using FACScalibur (Becton-Dickinson), and data were analyzed using FlowJo software (TreeStar, Inc).
2.4 LcrV DNA vaccines and Immunization
The codon optimized DNA vaccine (V.opt) expressing LcrV protein of Y. pestis was constructed as previously described (Wang Vaccine 2010). Synthetic lcrV gene was cloned into DNA vaccine vector pSW3891 [44] at PstI and BamHI sites down stream of cytomegalovirus (CMV) immediate early (IE) promoter and its adjacent Intron A. Two versions of V.opt were produced: one using the original full length LcrV amino acid sequences and the other with an additional tPA leader [18]. The DNA plasmids used in this study were prepared by a Mega purification kit (QIAGEN).
Female Balb/C mice of 6–8 weeks old were purchased from Taconic Farms (Germantown, NY) and housed in the animal facility managed by the Department of Animal Medicine at the University of Massachusetts Medical School (UMMS) in accordance with IACUC approved protocol. The animals received three DNA immunizations at Week 0, 2, and 4 by a Helios gene gun (Bio-Rad) and were boosted at Week 8 prior to the lethal Y. pestis challenge at Week 9. The two version of V.opt or the pSW3891 vector plasmid were coated onto the 1.0-micron gold beads at 2 μg DNA/mg gold. Each shot delivered 1 μg of DNA and a total of six non-overlapping shots were delivered to shaved abdominal skin at each immunization after animals were anesthetized. Sera were collected at Weeks 0, 2, 4, 6, 8, and 9 (prior to challenge).
2.5 ELISA (Enzyme-linked immunosorbent assay)
Mouse sera were tested for V-specific IgG antibody responses by ELISA. Microtiter plates were coated with 100 μl/well of transiently produced recombinant V antigen (1 μg/ml in PBS, pH7.2) at 4°C overnight and then washed five times with washing buffer (PBS at pH 7.2 with 0.1% Triton X-100). Blocking was done with 200 μl/well of 4% milk-whey blocking buffer for 1 hour at room temperature. After removal of the blocking buffer and another five washes, 100 μl of serially diluted mouse sera were added and incubated for 1 hour. The plates were washed five times and incubated with 100 μl of biotinylated anti-mouse IgG (Vector Laboratories, Burlingame, CA) diluted at 1:1000 for 1 hour followed with washes. Horseradish peroxidase-conjugated streptavidin (Vector Laboratories), diluted at 1:2000, was added (100 μl/well) and incubated for 1 hour. After the final washes, 100 μl of fresh TMB substrate (Sigma) was added to each well and incubated for 3.5 min. The reaction was stopped by adding 25 μl of 2 M H2SO4, and the plate was measured at OD 450 nm.
2.6 Depletion of CD4+ or CD8+ T cells
CD4+ or CD8+ T cells depletions were conducted after the DNA immunization and prior to Y. pestis intranasal challenge following well-established protocols in the literature [45, 46]. Mice were injected (intraperitoneally) with a monoclonal antibody specific to either CD4+ (clone GK 1.5) or CD8+ (clone 2.43) T cells, as previously described [47]. Indicated monoclonal antibodies (200 μg each) in 200 μl PBS or PBS alone was administered daily for five days just before the day of challenge. On day −1, prior to the challenge, mouse peripheral blood samples were collected to detect the CD4+ and CD8+ T cell counts by FACS analysis.
2.7 Animal challenge
KIM 1001 strain of Y. pestis was prepared by growing inocula for 18 h at 37°C on Tryptose Blood Agar Base (Difco) supplemented with 2.5 mM CaCl2 without the addition of blood. Bacteria were removed from the plate with an inoculating loop and suspended in injection-grade PBS. The bacteria count in the suspension was correlated to its optical density (OD600). The number of bacteria in the final inocula was confirmed by colony counts.
Immunized Balb/C mice were challenged with Y. pestis by an intranasal instillation of 50 μl saline containing lethal doses of Y. pestis into the nostril of ketamine-anesthethetized mice. This method leads to rapid infections and is lethal to 100% of non-immunized mice in 3–4 days. The LD50 (median lethal dose) of this challenge model was previously determined as 80,000 cfu equal to 240 LD50. Individual mice were challenged one week after the fourth immunization and observed twice daily to monitor both morbidity and mortality after challenge for two weeks. All the studies were conducted in a Biosafety Level 3 containment facility at UMMS.
2.8 Statistical analysis
Fisher’s exact test was conducted to analyze differences in protective immunity induced by V DNA vaccines with and without T cell depletions. Student’s t-test was performed to evaluate the differences of V-specific antibody and T cell responses.
3. Results
3.1 Prediction of CD8+ T cell epitopes in LcrV
Our previous studies have demonstrated that high level antibody responses can be elicited in mice by DNA vaccines expressing several known protective antigens [18–20]. In order to further understand whether T cell immune responses, particularly CD8+ T cells, are also elicited by these protective DNA vaccines, epitope prediction analysis was conducted to identify potential T cell epitopes in LcrV protein, the leading protective antigen in Y. pestis. Because our previous plague DNA vaccine studies used Balb/C mice as the intranasal animal challenge model for Y. pestis, binding scores of potential LcrV peptides to the class I major histocompatibility complex (MHC) H2-Kd molecule in Balb/C mouse were calculated and eight peptides with high binding scores were identified as possible CD8+ T cell epitopes (Table 1). These peptides were chemically synthesized and tested for their ability to stimulate LcrV-specific T cell immune responses in vitro.
3.2 Two CD8+ T cell epitopes were confirmed by cytokine analysis
Two groups of Balb/C mice (5 mice/group) were immunized with either V-opt DNA vaccine or empty vector at Weeks 0, 2, 4, and 8. Mouse splenocytes were isolated for ELISPOT and ICS assays at one week after the last DNA immunization. Splenocytes were stimulated for 24 hours with one of eight predicted T cell epitope peptides, as listed in Table 1, and positive T cell responses were identified by the secretion of IFN-γ in an ELISPOT assay. High levels of IFN-γ responses were observed when cells were stimulated with peptides LcrV-7 (SYNKDNNEL) or LcrV-8 (IYSVI QAEI) but not with the other six predicted LcrV peptides. Stimulation with the negative control HIV-1 Env V3 (HIV-V3) peptide also failed to induce detectable IFN-γ secretion. Mice that received the empty DNA vector did not have LcrV peptide-specific IFN-γ spots after stimulation. LcrV-7 elicited higher IFN-γ ELISPOT responses (500–700 spot forming cells/million splenocytes) than LcrV-8 peptide (200–400 spot forming cells/million splenocytes) (p<0.05). These results clearly indicated the presence of potential T cell epitopes in LcrV protein and that the LcrV DNA vaccine was effective in eliciting antigen-specific T cell responses in immunized Balb/C mice.
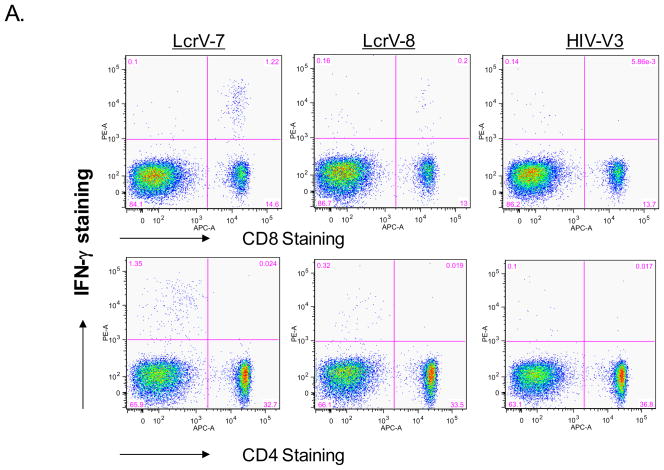
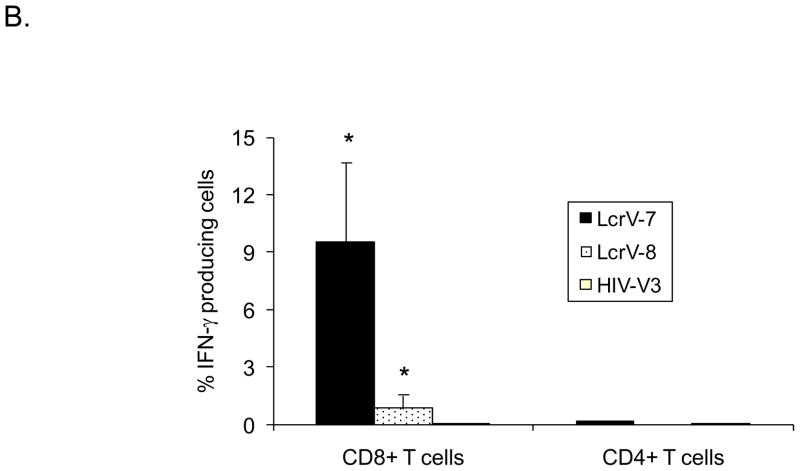
In order to further dissect what subsets of T cell populations were responsible for LcrV-7 and LcrV-8 peptide-stimulated IFN-γ ELISPOT responses, intracellular cytokine staining (ICS) assay was conducted by FACS analysis (Fig. 2). Strong IFN-γ staining in cells stimulated by both LcrV-7 and LcrV-8 peptides was observed in CD8+ T cells but not in CD4+ T cells (Fig. 2-A), confirming that both peptides are indeed CD8+ T cell epitopes. Similar to the ELISPOT results, the LcrV-7 peptide stimulated stronger IFN-γ ICS responses (average of 9.6% of total CD8+ T cells) compared with the LcrV-8 peptide (average of 1.2 % of total CD8+ T cells) (p<0.01) (Fig. 2-B). Stimulation with HIV-1 Env V3 peptide did not elicit high level IFN-γ staining.
Fig 2.
Intracellular cytokine staining (ICS) analysis of IFN-γ production in CD8+ and CD4+ T cells isolated from mouse spleens immunized with V.opt DNA vaccine. (A) A set of sample FACS data, showing IFN-γ production in CD8+ or CD4+ T cells with LcrV-7, LcrV-8, or HIV-V3 peptide stimulation, as indicated. (B) Percent of peptide-specific IFN-γ production cells in CD8+ or CD4+ T cells following LcrV-7, LcrV-8, or HIV-V3 peptide stimulation. Data represent the average of 5 mice/group ± standard deviation. “*” shows the statistical difference, p<0.05. Splenocytes were collected at 1 week after the last (4th) DNA immunization.
3.3 Protection against lethal intranasal challenge of Y. pestis in LcrV DNA vaccinated Balb/C mice with or without T cell depletion
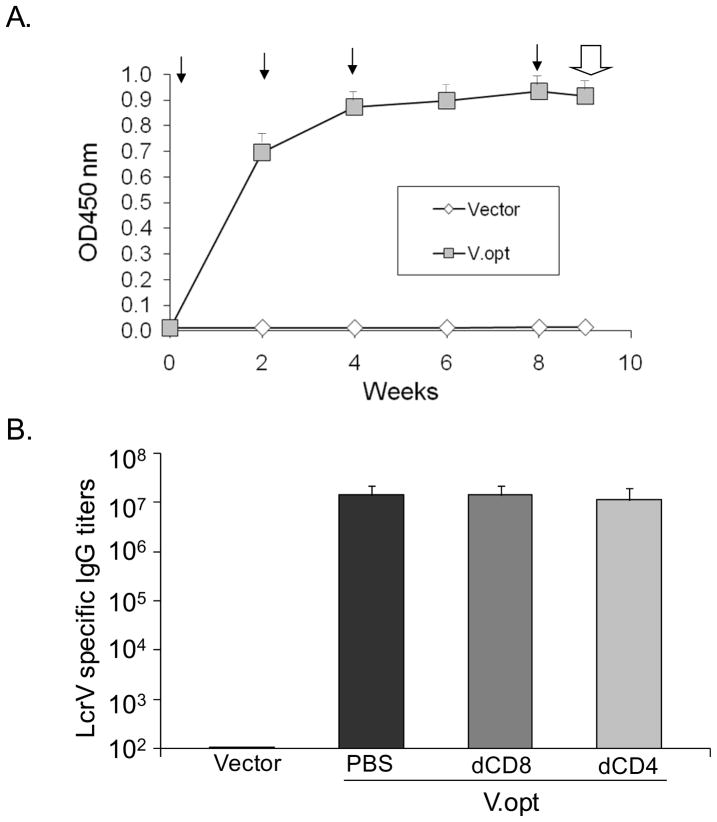
Next we attempted to determine the involvement of CD8+ T cells in protection against Y. pestis challenge in LcrV DNA vaccinated Balb/C mice. Forty-five mice received V-opt DNA vaccine at Weeks 0, 2, 4, and 8. The control group included 20 mice receiving only empty DNA vector pSW3891 at the same time points. LcrV-specific IgG antibody responses were measured by ELISA using pooled sera at each time point for both groups at the dilution of 1:1000 (Fig. 3-A). Quick and high level V-specific antibody responses were elicited in V-opt DNA vaccine immunized Balb/C mice, similar to our previous reports [18–20].
Figure 3.
(A). Temporal LcrV-specific antibody responses induced by V.opt DNA vaccine or empty vector in Balb/C mice. The LcrV antigen-specific IgG responses were measured by ELISA at 1:1000 serum dilution against recombinant LcrV protein produced from E. coli expression. The solid arrows indicate the time points of DNA immunizations and the open arrow indicates the time of Y. pestis intranasal challenge. The OD values are expressed as the average with standard deviation of 15 mice/group. (B). LcrV-specific antibody titers in the serum samples collected at Week 9, prior to Y. pestis intranasal challenge. “PBS”, “dCD8”, or “dCD4” signify groups immunized with V.opt DNA vaccine and treated with PBS, anti-CD8a, or anti-CD4 monoclonal antibody, respectively, for 5 days prior to challenge. For “Vector”, the group received empty DNA vector. Antibody titers are expressed as the geometric means with standard deviation of 10–20 mice/group.
At two days after the 4th DNA immunization, mice that had received the V-opt DNA vaccination were divided into three groups to receive one of the following treatments: CD4-specific mAb (clone GK 1.5) (N=10 mice), CD8-specific mAb (clone 2.43) (N=15 mice), or PBS (N=20 mice). The mice were treated daily for five days and also received 200 μg T cell depleting mAb or 200 μl PBS, intraperitoneally. At the end of the 5-day treatment, mouse peripheral blood cells were collected and numbers of CD4+ or CD8+ T cells were determined by FACS. The results demonstrated that over 98% CD4+ or CD8+ T cells were depleted in respective mAb-treated groups compared to control mice (no mAb treatment) (data not shown). V-specific antibody titers of individual mice in each group at the end of treatment were also measured by ELISA. High levels of LcrV-specific antibody were maintained despite the treatment with either anti-CD4 or anti-CD8 mAbs (Fig. 3-B).
The above immunized mice, with or without T cell depletion mAb treatment, along with mice that received the empty DNA vector were then challenged with 80,000 cfu (~240 LD50) of Y. pestis KIM1001 strain by intranasal inoculation on the day immediately after 5-day T cell depletion treatment. Survival was monitored in challenged mice for two weeks (Fig. 4). Mice that received the empty DNA vector all died by Day 4 after challenge, as expected in this highly lethal mucosal challenge model. V-opt DNA vaccinated mice with PBS mock treatment survived following challenge and achieved 100% protection. One of 10 V-opt DNA vaccinated mice that received the anti-CD4 mAb treatment for five days before challenge died but the remaining nine mice (90%) survived, which was not statistically different from the V-opt DNA vaccinated group with PBS treatment. Interestingly, four out of 15 V-opt DNA vaccinated mice that received anti-CD8 treatment died, reducing the total survival to 73.3%. This drop in protection was statistically significant when compared to the V-opt immunized group with PBS mock treatment (p=0.026). These results indicated that CD8+ T cell responses, in addition to the antibody responses, may also contribute to the protective immunity against plague in this lethal mucosal challenge model.
Fig 4.
In vivo protection of V.opt DNA-vaccinated mice treated with PBS, anti-CD8a (dCD8), or anti-CD4 (dCD4) monoclonal antibody prior to lethal challenge. Balb/C mice were challenged with a lethal dose of 80,000 cfu Y. pestis (KIM strain) by intranasal inoculation at one week after the 4th DNA immunization and treated for 5 days with PBS (20 mice), anti-CD8a (15 mice), or anti-CD4 (10 mice) monoclonal antibody. Cumulative survival curves were plotted to show the protection for each group as indicated. The vector control group (20 mice) was included as a negative control. “*” shows the statistical difference, p<0.05 compared to the PBS-treated group.
The roles of CD8+ T cells were confirmed in a separate Balb/C mouse study when another version of the codon optimized V DNA vaccine (tPA-V-opt) was tested. tPA-V-opt has an additional tPA signal peptide upstream of the coding V gene. Similar to V-opt, it is also highly immunogenic in eliciting V-specific antibody responses (Wang, 2010). In this study, 20 mice received tPA-V-opt DNA vaccine and five mice received empty DNA vector. High levels of V-specific antibody responses were elicited after four DNA immunizations (data not shown) and the vaccinated mice were treated daily with either anti-CD4 (N=5 mice) or anti-CD8 (N=5 mice) mAbs or PBS (N=10 mice) for five days, as described above. At the end of the 5-day treatment, mice were challenged with 80,000 cfu of Y. pestis by intranasal inoculation and monitored for 12 days (Fig. 5). None of the mice that received the empty vector survived from the challenge while nine out of 10 mice (90%) in tPA-V-opt DNA vaccinated group with PBS mock treatment survived. Four out of five tPA-V-opt vaccinated mice (80%) with anti-CD4 mAb treatment were protected from the lethal challenge, which is not significantly different from the tPA-V-opt group with PBS treatment. However, only two out of five mice (40%) survived from the tPA-V-opt vaccinated group with anti-CD8 mAb treatment, While a large reduction in survival was observed following treatment with anti-CD8 mAb, this difference was not statistically significant, due to the small number of animals included in each group.
Fig 5.
In vivo protection of tPA-V.opt DNA-vaccinated mice treated with PBS, anti-CD8a (dCD8), or anti-CD4 (dCD4) monoclonal antibody prior to lethal challenge. Balb/C mice were challenged with a lethal dose of 80,000 cfu Y. pestis (KIM strain) by intranasal inoculation at one week after the 4th DNA immunization and treated for 5 days with PBS (10 mice), anti-CD8a (5 mice), or anti-CD4 (5 mice) monoclonal antibody. Cumulative survival curves were plotted to show the protection for each group as indicated. The vector control group (5 mice) was included as a negative control. “*” shows the statistical difference, p<0.05 compared to the PBS-treated group.
The results from the above two Y. pestis challenge experiments demonstrated that depletion of CD8+ T cells in V DNA vaccine immunized Balb/C mice greatly reduced the level of protection, pointing to a possible involvement of CD8+ T cells and antibody responses induced by LcrV DNA vaccines.
4. Discussion
Our previous studies demonstrated that LcrV DNA vaccines could elicit protective immunity against lethal intranasal Y. pestis challenge in a Balb/C model [18–20]. Significant levels of LcrV-specific antibodies in serum were detected in immunized animals. Preliminary analysis of antibody isotypes has suggested that a Th1-type antibody response may be important in providing better protection [18, 20]. In the current study, we further examined whether T cell immune responses could be elicited against any of the predicted T cell epitopes. Two positive CD8+ T cell epitopes were identified from animals immunized with the LcrV DNA vaccine. T cell immune responses against these two epitopes were very high, as confirmed by both ELISPOT and ICS assays. More importantly, depletion of CD8+ T cells in LcrV DNA vaccine-immunized mice led to reduced levels of protection when a Y. pestis intranasal challenge was conducted. This would be the first time that CD8+ T cell epitopes have been identified for the LcrV antigen when CD8+ T cell immune responses were elicited by the Y. pestis DNA vaccine and also that these epitopes play an important role in protection against lethal Y. pestis.
It was somewhat unexpected to see the limited effect of CD4+ T cell depletion on the outcome of the challenge. CD4+ T cells must play a key role in the development of LcrV-specific antibody responses. It is possible that once such antibody responses are established, CD4+ T cells may not directly contribute to the protection of immunized mice at challenge. In the current study, mainly CD8+ T cell epitopes were studied. A more specific analysis on CD4+ T cell epitopes may yield information related to the epitope specificity of CD4+ T cell immune responses.
The role of T cell immune responses in protective immunity against bacterial infections is less understood particularly compared to their role against viral infections. Antibody responses against bacterial toxins are classical examples of the critical protective immunity against infectious diseases caused by exotoxin-producing microorganisms, such as tetanus and diphtheria. For intracellular bacterial infections, such as Listeria and Mycobacterium, protective immunity often relies upon the development of cell-mediated immune responses to control the intracellular bacteria [48]. Although animal tissues obtained at the late stages of the infection revealed aggregates of extracellular bacteria, Y. pestis has long been considered a facultative intracellular pathogen that replicates within the macrophage phagolysosome in the mammalian host [49–51]. Therefore, it is not unexpected that cell-mediated immune responses may be generated during Y. pestis infection. However, as an acute virulent disease, antibody protection has received much attention as the main mechanism of protective immunity while Y. pestis-specific T cell responses have been less studied in the past.
In 1970s, Wong et al. demonstrated, for the first time, that T cells isolated from immune mice could produce soluble factors protecting phagocytes from cytolysis upon subsequent encounters with Y. pestis in vitro [52]. Later studies reported that these soluble factors may include the Th1 cytokines, IFN-γ and tumor necrosis factor alpha (TNF-α) [25, 27, 53]. The pre-treatment of phagocytes with these cytokines could reduce bacterial intracellular replication and pre-injecting mice with IFN-γ and TNF-α protects against septicemic plague [25, 54, 55]. Recent studies demonstrated that cell-mediated immune responses, induced in B cell deficient mice using live Gram-negative Y. pestis, contributed to the protection against Y. pestis intranasal infection and transfer of T cells from immune mice to naïve mice could also confer protection [26]. Reports also demonstrated that T cell responses might participate in vaccine-mediated protection against subcutaneous Y. pestis challenge [56]. Collectively, these results strongly support the hypothesis that cell-mediated immunity can contribute to the protection against plague.
Results from the current report provide further evidence to demonstrate the direct link between CD8+ T cells and protection in a well-established mucosal challenge model. Identification of two CD8+ T cell epitopes proved the effectiveness of DNA vaccines to elicit high-quality T cell immune responses and provided useful biomarkers to monitor CD8+ T cell responses in future plague vaccine development. Our data justify more studies using gene-based vaccination approaches, such as DNA vaccines, to elicit protective CD8+ T cell responses, which are difficult to elicit by either killed virus vaccines or recombinant protein-based plague vaccines. A more complete profile of cytokines should be mapped to understand whether Y. pestis-specific CD8+ T cell responses are polyfunctional, i.e., whether multiple cytokines are elicited with the LcrV antigen. Protection studies should also be conducted in non-human primates to establish if gene-based plagues vaccines can elicit better protection against pneumonic plague challenges, an important benchmark for plague vaccine development. T cell immunity, especially epitope-specific CD8+ T cell immune responses should be measured in such non-human primate studies to provide guidance to later human studies.
Fig. 1.
ELISPOT analysis for IFN-γ secretion in mouse splenocytes immunized with V.opt DNA vaccine or empty vector. (A) Actual sample wells of IFN-γ ELISPOT with LcrV peptide stimulations, HIV-V3, or no stimulation, as indicated. (B) Frequency of LcrV peptide-specific IFN-γ spots per million splenocytes in mice immunized with the various V.opt DNA vaccines or empty vector. Data represent the average number of spot forming cells (SFCs) per million of splenocytes from 5 mice/group ± standard deviation. “*” shows the statistical difference, p<0.05. Splenocytes were collected at 1 week after the last (4th) DNA immunization.
Acknowledgments
This work was supported in part by US NIH/NIAID grants 5U01AI078073. Authors would like to thank Dr. Jill M. Grimes Serrano for critical reading and editing of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Williamson ED. Plague. Vaccine. 2009 Nov 5;27( Suppl 4):D56–60. doi: 10.1016/j.vaccine.2009.07.068. [DOI] [PubMed] [Google Scholar]
- 2.Perry RD, Fetherston JD. Yersinia pestis--etiologic agent of plague. Clinical microbiology reviews. 1997 Jan;10(1):35–66. doi: 10.1128/cmr.10.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stenseth NC, Atshabar BB, Begon M, Belmain SR, Bertherat E, Carniel E, et al. Plague: past, present, and future. PLoS medicine. 2008 Jan 15;5(1):e3. doi: 10.1371/journal.pmed.0050003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meyer KF, Cavanaugh DC, Bartelloni PJ, Marshall JD., Jr Plague immunization. I. Past and present trends. J Infect Dis. 1974 May;129(Suppl):S13–8. doi: 10.1093/infdis/129.supplement_1.s13. [DOI] [PubMed] [Google Scholar]
- 5.Reisman RE. Allergic reactions due to plague vaccine. J Allergy. 1970 Jul;46(1):49–55. doi: 10.1016/0021-8707(70)90061-4. [DOI] [PubMed] [Google Scholar]
- 6.Marshall JD, Jr, Bartelloni PJ, Cavanaugh DC, Kadull PJ, Meyer KF. Plague immunization. II. Relation of adverse clinical reactions to multiple immunizations with killed vaccine. J Infect Dis. 1974 May;129(Suppl):S19–25. doi: 10.1093/infdis/129.supplement_1.s19. [DOI] [PubMed] [Google Scholar]
- 7.Williams JE, Altieri PL, Berman S, Lowenthal JP, Cavanaugh DC. Potency of killed plague vaccines prepared from avirulent Yersinia pestis. Bull World Health Organ. 1980;58(5):753–6. [PMC free article] [PubMed] [Google Scholar]
- 8.Jones SM, Griffin KF, Hodgson I, Williamson ED. Protective efficacy of a fully recombinant plague vaccine in the guinea pig. Vaccine. 2003 Sep 8;21(25–26):3912–8. doi: 10.1016/s0264-410x(03)00379-7. [DOI] [PubMed] [Google Scholar]
- 9.Eyles JE, Williamson ED, Spiers ID, Alpar HO. Protection studies following bronchopulmonary and intramuscular immunisation with yersinia pestis F1 and V subunit vaccines coencapsulated in biodegradable microspheres: a comparison of efficacy. Vaccine. 2000 Aug 1;18(28):3266–71. doi: 10.1016/s0264-410x(00)00128-6. [DOI] [PubMed] [Google Scholar]
- 10.Leary SE, Williamson ED, Griffin KF, Russell P, Eley SM, Titball RW. Active immunization with recombinant V antigen from Yersinia pestis protects mice against plague. Infect Immun. 1995 Aug;63(8):2854–8. doi: 10.1128/iai.63.8.2854-2858.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heath DG, Anderson GW, Jr, Mauro JM, Welkos SL, Andrews GP, Adamovicz J, et al. Protection against experimental bubonic and pneumonic plague by a recombinant capsular F1-V antigen fusion protein vaccine. Vaccine. 1998 Jul;16(11–12):1131–7. doi: 10.1016/s0264-410x(98)80110-2. [DOI] [PubMed] [Google Scholar]
- 12.Williamson ED, Eley SM, Griffin KF, Green M, Russell P, Leary SE, et al. A new improved sub-unit vaccine for plague: the basis of protection. FEMS Immunol Med Microbiol. 1995 Dec;12(3–4):223–30. doi: 10.1111/j.1574-695X.1995.tb00196.x. [DOI] [PubMed] [Google Scholar]
- 13.Williamson ED, Eley SM, Stagg AJ, Green M, Russell P, Titball RW. A sub-unit vaccine elicits IgG in serum, spleen cell cultures and bronchial washings and protects immunized animals against pneumonic plague. Vaccine. 1997 Jul;15(10):1079–84. doi: 10.1016/s0264-410x(96)00303-9. [DOI] [PubMed] [Google Scholar]
- 14.Williamson ED, Eley SM, Stagg AJ, Green M, Russell P, Titball RW. A single dose sub-unit vaccine protects against pneumonic plague. Vaccine. 2000 Oct 15;19(4–5):566–71. doi: 10.1016/s0264-410x(00)00159-6. [DOI] [PubMed] [Google Scholar]
- 15.Glynn A, Freytag LC, Clements JD. Effect of homologous and heterologous prime-boost on the immune response to recombinant plague antigens. Vaccine. 2005 Mar 14;23(16):1957–65. doi: 10.1016/j.vaccine.2004.10.025. [DOI] [PubMed] [Google Scholar]
- 16.Glynn A, Roy CJ, Powell BS, Adamovicz JJ, Freytag LC, Clements JD. Protection against aerosolized Yersinia pestis challenge following homologous and heterologous prime-boost with recombinant plague antigens. Infect Immun. 2005 Aug;73(8):5256–61. doi: 10.1128/IAI.73.8.5256-5261.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williamson ED, Flick-Smith HC, Lebutt C, Rowland CA, Jones SM, Waters EL, et al. Human immune response to a plague vaccine comprising recombinant F1 and V antigens. Infect Immun. 2005 Jun;73(6):3598–608. doi: 10.1128/IAI.73.6.3598-3608.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang S, Heilman D, Liu F, Giehl T, Joshi S, Huang X, et al. A DNA vaccine producing LcrV antigen in oligomers is effective in protecting mice from lethal mucosal challenge of plague. Vaccine. 2004 Sep 3;22(25–26):3348–57. doi: 10.1016/j.vaccine.2004.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang S, Joshi S, Mboudjeka I, Liu F, Ling T, Goguen JD, et al. Relative immunogenicity and protection potential of candidate Yersinia Pestis antigens against lethal mucosal plague challenge in Balb/C mice. Vaccine. 2008 Mar 20;26(13):1664–74. doi: 10.1016/j.vaccine.2008.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang S, Mboudjeka I, Goguen JD, Lu S. Antigen engineering can play a critical role in the protective immunity elicited by Yersinia pestis DNA vaccines. Vaccine. 2010 Feb 23;28(8):2011–9. doi: 10.1016/j.vaccine.2009.10.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garmory HS, Freeman D, Brown KA, Titball RW. Protection against plague afforded by immunisation with DNA vaccines optimised for expression of the Yersinia pestis V antigen. Vaccine. 2004 Feb 25;22(8):947–57. doi: 10.1016/j.vaccine.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 22.Grosfeld H, Bino T, Flashner Y, Ber R, Mamroud E, Lustig S, et al. Vaccination with plasmid DNA expressing the Yersinia pestis capsular protein F1 protects mice against plague. Advances in experimental medicine and biology. 2003;529:423–4. doi: 10.1007/0-306-48416-1_84. [DOI] [PubMed] [Google Scholar]
- 23.Grosfeld H, Cohen S, Bino T, Flashner Y, Ber R, Mamroud E, et al. Effective protective immunity to Yersinia pestis infection conferred by DNA vaccine coding for derivatives of the F1 capsular antigen. Infect Immun. 2003 Jan;71(1):374–83. doi: 10.1128/IAI.71.1.374-383.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamanaka H, Hoyt T, Yang X, Bowen R, Golden S, Crist K, et al. A parenteral DNA vaccine protects against pneumonic plague. Vaccine. 2010 Apr 19;28(18):3219–30. doi: 10.1016/j.vaccine.2010.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smiley ST. Immune defense against pneumonic plague. Immunological reviews. 2008 Oct;225:256–71. doi: 10.1111/j.1600-065X.2008.00674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parent MA, Berggren KN, Kummer LW, Wilhelm LB, Szaba FM, Mullarky IK, et al. Cell-mediated protection against pulmonary Yersinia pestis infection. Infect Immun. 2005 Nov;73(11):7304–10. doi: 10.1128/IAI.73.11.7304-7310.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parent MA, Wilhelm LB, Kummer LW, Szaba FM, Mullarky IK, Smiley ST. Gamma interferon, tumor necrosis factor alpha, and nitric oxide synthase 2, key elements of cellular immunity, perform critical protective functions during humoral defense against lethal pulmonary Yersinia pestis infection. Infect Immun. 2006 Jun;74(6):3381–6. doi: 10.1128/IAI.00185-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Philipovskiy AV, Smiley ST. Vaccination with live Yersinia pestis primes CD4 and CD8 T cells that synergistically protect against lethal pulmonary Y. pestis infection. Infect Immun. 2007 Feb;75(2):878–85. doi: 10.1128/IAI.01529-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smiley ST. Cell-mediated defense against Yersinia pestis infection. Advances in experimental medicine and biology. 2007;603:376–86. doi: 10.1007/978-0-387-72124-8_35. [DOI] [PubMed] [Google Scholar]
- 30.Smiley ST. Current challenges in the development of vaccines for pneumonic plague. Expert review of vaccines. 2008 Mar;7(2):209–21. doi: 10.1586/14760584.7.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wake A, Morita H, Wake M. Mechanisms of long and short term immunity to plague. Immunology. 1978 Jun;34(6):1045–52. [PMC free article] [PubMed] [Google Scholar]
- 32.Green M, Rogers D, Russell P, Stagg AJ, Bell DL, Eley SM, et al. The SCID/Beige mouse as a model to investigate protection against Yersinia pestis. FEMS Immunol Med Microbiol. 1999 Feb;23(2):107–13. doi: 10.1111/j.1574-695X.1999.tb01229.x. [DOI] [PubMed] [Google Scholar]
- 33.Eyles JE, Butcher WA, Titball RW, Hill J. Concomitant administration of Yersinia pestis specific monoclonal antibodies with plague vaccine has a detrimental effect on vaccine mediated immunity. Vaccine. 2007 Oct 16;25(42):7301–6. doi: 10.1016/j.vaccine.2007.08.021. [DOI] [PubMed] [Google Scholar]
- 34.Anderson DM, Ciletti NA, Lee-Lewis H, Elli D, Segal J, DeBord KL, et al. Pneumonic plague pathogenesis and immunity in Brown Norway rats. The American journal of pathology. 2009 Mar;174(3):910–21. doi: 10.2353/ajpath.2009.071168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Letvin NL. Progress in the development of an HIV-1 vaccine. Science (New York, NY) 1998 Jun 19;280(5371):1875–80. doi: 10.1126/science.280.5371.1875. [DOI] [PubMed] [Google Scholar]
- 36.Whalen RG, Leclerc C, Deriaud E, Schirmbeck R, Reimann J, Davis HL. DNA-mediated immunization to the hepatitis B surface antigen. Activation and entrainment of the immune response. Annals of the New York Academy of Sciences. 1995 Nov 27;772:64–76. doi: 10.1111/j.1749-6632.1995.tb44732.x. [DOI] [PubMed] [Google Scholar]
- 37.Siegrist CA, Lambert PH. DNA vaccines: what can we expect? Infectious agents and disease. 1996 Jan;5(1):55–9. [PubMed] [Google Scholar]
- 38.Spooner RA, Deonarain MP, Epenetos AA. DNA vaccination for cancer treatment. Gene therapy. 1995 May;2(3):173–80. [PubMed] [Google Scholar]
- 39.Zhang GL, Srinivasan KN, Veeramani A, August JT, Brusic V. PREDBALB/c: a system for the prediction of peptide binding to H2d molecules, a haplotype of the BALB/c mouse. Nucleic acids research. 2005 Jul 1;33(Web Server issue):W180–3. doi: 10.1093/nar/gki479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taguchi T, McGhee JR, Coffman RL, Beagley KW, Eldridge JH, Takatsu K, et al. Detection of individual mouse splenic T cells producing IFN-gamma and IL-5 using the enzyme-linked immunospot (ELISPOT) assay. Journal of immunological methods. 1990 Mar 27;128(1):65–73. doi: 10.1016/0022-1759(90)90464-7. [DOI] [PubMed] [Google Scholar]
- 41.Miyahira Y, Murata K, Rodriguez D, Rodriguez JR, Esteban M, Rodrigues MM, et al. Quantification of antigen specific CD8+ T cells using an ELISPOT assay. Journal of immunological methods. 1995 Apr 12;181(1):45–54. doi: 10.1016/0022-1759(94)00327-s. [DOI] [PubMed] [Google Scholar]
- 42.Favre N, Bordmann G, Rudin W. Comparison of cytokine measurements using ELISA, ELISPOT and semi-quantitative RT-PCR. Journal of immunological methods. 1997 May 12;204(1):57–66. doi: 10.1016/s0022-1759(97)00033-1. [DOI] [PubMed] [Google Scholar]
- 43.Badovinac VP, Harty JT. Intracellular staining for TNF and IFN-gamma detects different frequencies of antigen-specific CD8(+) T cells. Journal of immunological methods. 2000 Apr 21;238(1–2):107–17. doi: 10.1016/s0022-1759(00)00153-8. [DOI] [PubMed] [Google Scholar]
- 44.Wang S, Chou TH, Sakhatskyy PV, Huang S, Lawrence JM, Cao H, et al. Identification of two neutralizing regions on the severe acute respiratory syndrome coronavirus spike glycoprotein produced from the mammalian expression system. J Virol. 2005 Feb;79(3):1906–10. doi: 10.1128/JVI.79.3.1906-1910.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McCurdy LH, Rutigliano JA, Johnson TR, Chen M, Graham BS. Modified vaccinia virus Ankara immunization protects against lethal challenge with recombinant vaccinia virus expressing murine interleukin-4. Journal of virology. 2004 Nov;78(22):12471–9. doi: 10.1128/JVI.78.22.12471-12479.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu R, Johnson AJ, Liggitt D, Bevan MJ. Cellular and humoral immunity against vaccinia virus infection of mice. J Immunol. 2004 May 15;172(10):6265–71. doi: 10.4049/jimmunol.172.10.6265. [DOI] [PubMed] [Google Scholar]
- 47.Graham BS, Bunton LA, Wright PF, Karzon DT. Role of T lymphocyte subsets in the pathogenesis of primary infection and rechallenge with respiratory syncytial virus in mice. The Journal of clinical investigation. 1991 Sep;88(3):1026–33. doi: 10.1172/JCI115362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kaufmann SH. Immunity to intracellular bacteria. Annual review of immunology. 1993;11:129–63. doi: 10.1146/annurev.iy.11.040193.001021. [DOI] [PubMed] [Google Scholar]
- 49.Pujol C, Bliska JB. Turning Yersinia pathogenesis outside in: subversion of macrophage function by intracellular yersiniae. Clinical immunology (Orlando, Fla. 2005 Mar;114(3):216–26. doi: 10.1016/j.clim.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 50.Titball RW, Hill J, Lawton DG, Brown KA. Yersinia pestis and plague. Biochemical Society transactions. 2003 Feb;31(Pt 1):104–7. doi: 10.1042/bst0310104. [DOI] [PubMed] [Google Scholar]
- 51.Cavanaugh DC, Randall R. The role of multiplication of Pasteurella pestis in mononuclear phagocytes in the pathogenesis of flea-borne plague. J Immunol. 1959 Oct;83:348–63. [PubMed] [Google Scholar]
- 52.Wong JF, Elberg SS. Cellular immune response to Yersinia pestis modulated by product(s) from thymus-derived lymphocytes. J Infect Dis. 1977 Jan;135(1):67–78. doi: 10.1093/infdis/135.1.67. [DOI] [PubMed] [Google Scholar]
- 53.Nakajima R, Brubaker RR. Association between virulence of Yersinia pestis and suppression of gamma interferon and tumor necrosis factor alpha. Infect Immun. 1993 Jan;61(1):23–31. doi: 10.1128/iai.61.1.23-31.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lukaszewski RA, Kenny DJ, Taylor R, Rees DG, Hartley MG, Oyston PC. Pathogenesis of Yersinia pestis infection in BALB/c mice: effects on host macrophages and neutrophils. Infect Immun. 2005 Nov;73(11):7142–50. doi: 10.1128/IAI.73.11.7142-7150.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pujol C, Grabenstein JP, Perry RD, Bliska JB. Replication of Yersinia pestis in interferon gamma-activated macrophages requires ripA, a gene encoded in the pigmentation locus. Proceedings of the National Academy of Sciences of the United States of America. 2005 Sep 6;102(36):12909–14. doi: 10.1073/pnas.0502849102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Williamson ED, Stagg AJ, Eley SM, Taylor R, Green M, Jones SM, et al. Kinetics of the immune response to the (F1+V) vaccine in models of bubonic and pneumonic plague. Vaccine. 2007 Jan 22;25(6):1142–8. doi: 10.1016/j.vaccine.2006.09.052. [DOI] [PubMed] [Google Scholar]