Abstract
It has been almost two decades since dietary restriction was first shown to increase Drosophila lifespan. Since then, understanding this phenomenon advanced as groups worked to identify what quality of restricted diet matters: calories or a specific nutrient. The problem is complex because is it difficult to measure what a fly actually consumes. A powerful solution uses the geometric framework of nutrition where diets in many combinations can be tested for their effects on lifespan and reproduction while measuring intake. Applied to Drosophila, it is now clear that specific nutrients, not calories, mediate longevity. The geometric framework also reveals a nutritional basis for the trade-off between reproduction and lifespan. This complements a stable isotope analysis that tracked the allocation of nitrogen, carbon and essential amino acids into eggs versus reproduction. Together these studies show this is not possible to explain how DR extends lifespan through a mechanism were resources are simply reallocated to somatic maintenance away from reproduction. Although promising in principle, genetic analysis of DR mechanisms has had limited success. To be productive studies must include enough diets at appropriate concentrations. In reviewing the best data, there is little evidence to date for any gene to be required for DR to increase Drosophila lifespan, including insulin signaling or 4eBP. Strong analyses of genes required for DR should be a priority in future research with Drosophila and this may be made most robust by considering the effect of mutants in the context of the geometric framework.
Keywords: dietary restriction, geometric nutritional analysis, trade-off, Drosophila, insulin
Since Drosophila was introduced as a model genetic system a century ago, researchers have dabbled with laboratory diets to optimize rearing and to explore basic concepts of nutrition. Remarkably given this history, the first work to increase lifespan by dietary restriction (DR) was presented only in 1993 [1]. Longevity was extended (10–30%) and reproduction was reduced by maintaining adults on cornmeal-sugar-agar diet topped with a dilute concentration of yeast. Subsequent work showed why the efficiency of DR in Drosophila had been overlooked [2]: adults maintained on very restricted diets were short lived and infertile. Longevity was maximized at an intermediate diet; dietary restriction occurs across the range where food intake is reduced without malnutrition. Advances in analytical approaches with dietary restriction have now helped define the specific dietary conditions required for DR to extend lifespan but as discussed in this review, only limited progress has been made toward understanding its mechanisms.
Methods of nutrient analysis in Drosophila
One reason for this state of affairs has been the necessity to first work out the appropriate methods of nutrient analysis. To understand how DR extends lifespan we must understand what aspect of the diet must be restricted. Work from rodents set a historical precedence that reduced intake of calories is the operational quality, suggesting that the mechanisms of longevity extension might involve protection from damage associated with energy metabolism. Alternatively, aging may be slowed by limitation of specific nutrient components, as suggested by the observation where Drosophila lifespan was extended simply by manipulating dietary yeast [1]. Most of the recent progress on Drosophila DR has focused on finding the specific quality of diet that affects aging. This problem turns out to be remarkably complex.
Adult Drosophila in the laboratory feed from the surface of an agar media. Flies are dietary restricted by presenting them with media containing different concentrations of sugar and yeast ([3] comprehensively reviews details of diet composition). Because it is used in media for larvae, cornmeal is sometimes included in adult media where it may provide nutrient value or simply help suspend yeast particles toward the media surface. The use of agar media for adults has two implications. We do not know if flies on dilute diets actually consume fewer nutrients; they may compensate for a poor diet by eating more of it. Second, all nutrients are not equally available. Flies drink or sponge nutrients from the media surface and in practice adults feed upon a sugar solution (in agar matrix) with (potentially) insoluble yeast particles exposed on the surface. What is mixed into media may not equal what the fly actually consumes.
How much a fly eats from agar media has been estimated in various ways: dye uptake, fecal deposits, proboscis extension behavior, body weight, radiotracers and calorimetry [4–9]. Test conditions have also varied, using virgin, once-mated or continuously mated adults. Diets have varied both sugar and yeast, or just yeast. Many results show there can be compensatory feeding on diluted diets although these adults still acquire fewer total nutrients that those on richer diets. Consumption therefore is not proportional to the nutrients in the diet. One consequence is illustrated in the first studies aimed to determine if DR extends lifespan by reducing caloric intake or by limiting a specific nutrient [7, 9]. In this design, adults were fed diets with similar caloric value while differing in proportion of yeast and sugar. Median lifespan was greatest on diets with relatively low yeast even while diet caloric value differed because of sugar content (figure 1A). On the other hand, lifespan varied among adults fed equal calorie diets that differed in concentration of both yeast and sugar. If food intake were proportional to diet nutrient concentration, these data would suggest that longevity was extended by limiting yeast nutrients and not calories. However, estimates of actual assimilated calories showed this was not the case [7]. Flies on diets with low yeast assimilated fewer calories and were longer lived than flies on high yeast regardless of sugar concentration (figure 1B). Thus, nutrient quality and caloric intake were confounded such that it was not possible to determine which feature affected longevity during dietary restriction.
Figure 1.
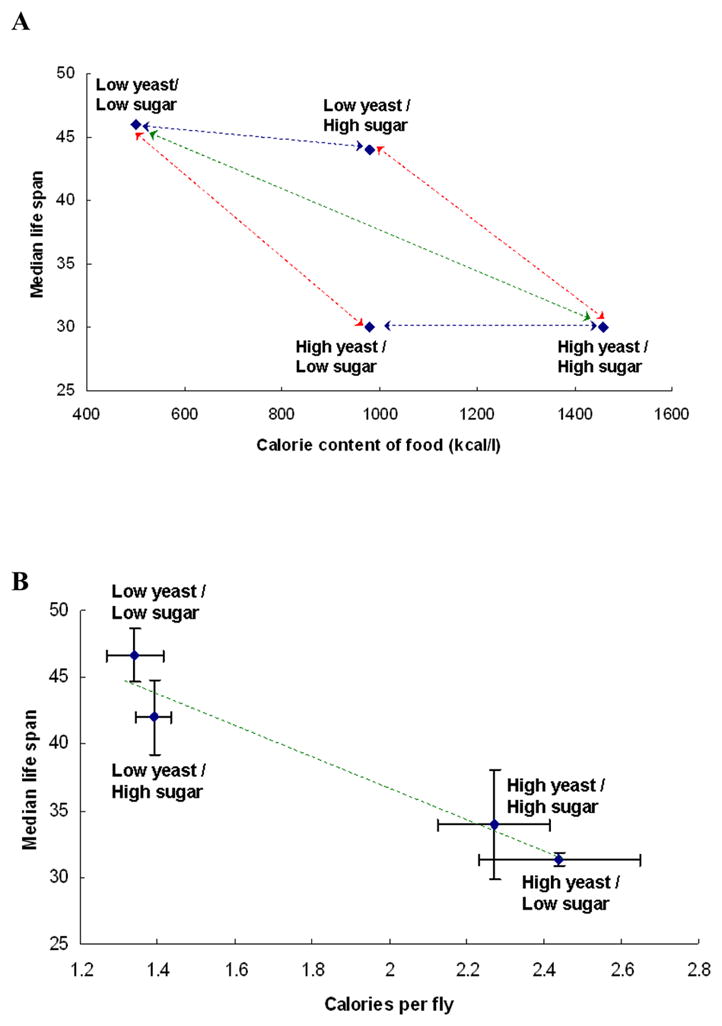
An early study design to disentangle the effects of calories and specific nutrients by maintaining flies on complementary diets. Data and figures from Min et al [7]. A) Lifespan plotted relative to the caloric value of the diet, following the presentation of Mair et al. [9]. B) Lifespan plotted relative to the measured assimilation of calories estimated for each treatment group from micro-bomb calorimetry of adult bodies and all deposited eggs.
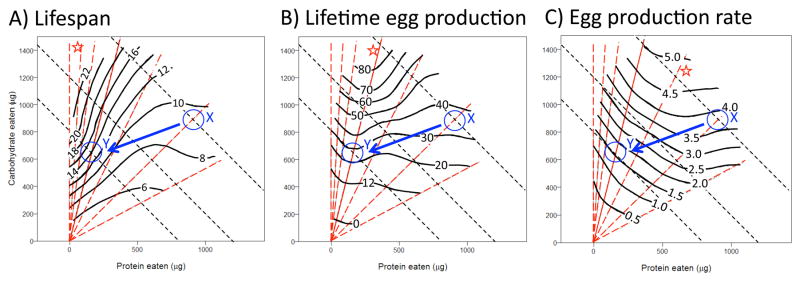
These problems have been recently addressed by studies based on the theory of nutritional geometry [10, 11]. Phenotypes are measured from individuals when fed one of a number of diets that span a range of nutrient composition and total concentration. A state-space plot is made with the types of nutrients consumed (protein versus sugar) on the x- and y-axis and phenotype on the z-axis. This forms a parametric response surface visualized by isoclines of a thin-plate spline (Figure 2). Vectors connect diets of different total concentration with the same nutrient ratio, such as protein to carbohydrate (P:C) of 1:16 or 1:2. Similarly, lines can be drawn to denote clines with identical caloric intake. Food intake for each individual is measured throughout the study. To do this with Drosophila, adults are given a liquid diet delivered in a calibrated capillary tube. An essential task for this framework is to also conduct a food choice analysis. Sugar and yeast solutions are presented to adults in separate tubes and at various concentrations to determine their regulated intake for each nutrient, their target diet. Together these methods analytically partition the effects of nutrients and energy, and measure phenotypes relative to actual nutrient consumption.
Figure 2.
Geometric nutrient analysis of Drosophila lifespan and reproduction. Figures are redrawn and modified from Lee et al. [11]. Observed lifespan and reproduction from 1008 females are regressed against protein and carbohydrate eaten and represented by a response surface where clines (solid black lines) connect responses of similar value. Radiating from the origin, arrays delineate food intake with specified ratios of protein to carbohydrate (dashed red lines). A star (*) denotes the array along which each trait is optimized: P:C 1:16, Lifespan in Panel A; P:C 1:4, Lifetime Egg Production in Panel B; P:C 1:2, Egg Production Rate in Panel C. Isocaloric lines A, B and C (dashed black lines) connect intakes with the same caloric value but differing ratios of P:C. The circled regions X and Y are hypothetical intake and corresponding life history traits for adults respectively fed an agar based, high-yeast diet or low-yeast diet (containing the same dietary sugar). Region X sits at a higher total caloric intake than Region Y. Among the panels, the arrow connecting these regions illustrates the negative correlation between reproduction and lifespan that are commonly observed when DR modulates life histories in response to dilution of nutrients in media without controlling total caloric intake.
Two projects with Drosophila have applied this approach [11, 12], most formally by Lee et al. [11] where 1008 individual, mated females were maintained on one of 28 diets while measuring life span, eggs production and food intake (figure 2). Mean lifespan was greatest (although, only 22 d) for females on low yeast, high carbohydrate diets. Along an isocaloric cline, lifespan was maximized at the P:C ratio 1:16. In contrast, increasing or decreasing the total caloric intake had little effect on survival. Egg production showed a qualitatively different pattern. The daily rate of egg production was maximum on diets of P:C 1:2 and increased with diet caloric value. Lifetime egg production, which is a function of daily egg production and survival, was maximized on diets of P:C 1:4. The diet choice analysis revealed females regulated their uptake to acquire P:C 1:4, this was their preferred uptake target. When maintained on very dilute sugar, however, females could overcome the net lack of carbohydrates by increasing their total food intake. These females would consequently eat more yeast (which contains some carbohydrates) and thus increased their P:C toward values as high as 1:1.
These data unambiguously demonstrate that reduction of a specific nutrient from dietary yeast and not caloric restriction extends Drosophila lifespan. Although yeast contains micronutrients and essential lipids and sterols, protein is the likely regulatory quality of diet. Indeed, Drosophila lifespan can be extended when adults are fed synthetic agar-based diets where total protein or specific amino acids are decreased [13, 14]. The data also reveal a basis for the commonly observed trade-off between longevity and reproduction. Lifetime egg production is a good index of evolutionary fitness. In the Lee study, this was maximized when females fed at P:C 1:4, which was their preferred dietary target [11]. Fitness, therefore, was gained at the expense of longevity alone, which would be maximized by feeding at P:C 1:16. Finally, the work shows how the quality of the diet can affect feeding behavior. Adult Drosophila adults sense nutrient quality and adjust their food intake [4]. Flies compensate for a dilute sugar diet by eating more of this component only up to a point, after which they also consume more yeast and thus drive their P:C away from the vector that maximizes fitness and survival.
Resources, reproduction and dietary restriction
These studies reveal what part of the diet modulates aging during DR. It involves not just the net intake of a specific nutrient, but also the balance between carbohydrates and proteins. One potential mechanism by which the quality of nutrients could affect aging could be through their effects on reproduction. In a classic model, resources are thought to competitively allocated between the demands of reproduction and somatic maintenance. Testing this idea, however, is complicated because studies have rarely measured the actual uptake of nutrients, they have not defined a measurable currency for ‘resources’ in order to track allocation, and they have lacked an operational measure of ‘somatic maintenance’ independent of the outcome variable, survival.
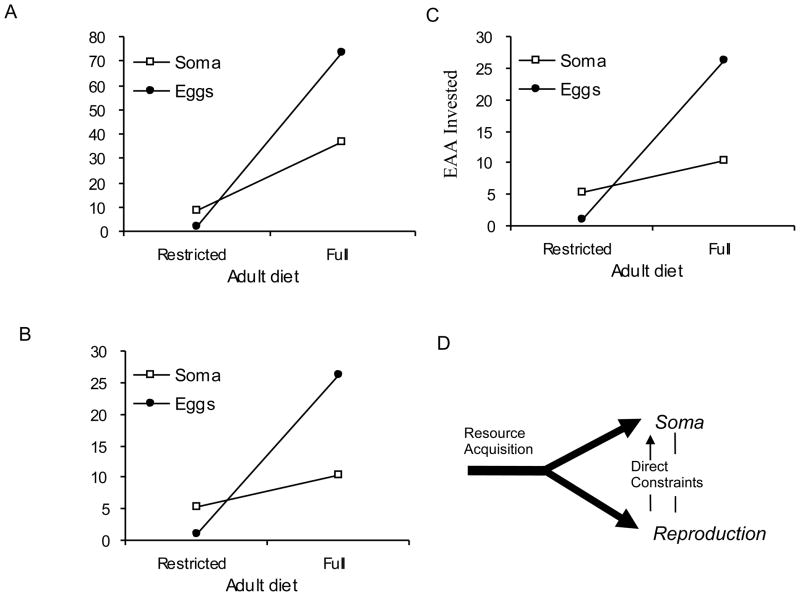
These constraints were addressed in a study where dietary yeast was labeled with stable isotopes of carbon and nitrogen [15]. Larvae where reared on either a light- or heavy-labeled media. Adults were then maintained on media with the alternative label, and upon restricted (4% yeast, 10% sugar) or full-diet (16% yeast, 10% sugar). By measuring isotopes in tissues with mass spectrometry, it was possible to estimate the lifetime differences in the acquisition and allocation of carbon, nitrogen and essential amino acids (EAA) as nutrient currencies invested into eggs and to somatic tissue. Investment into somatic maintenance was measured as the rate of N, C and EAA turnover and their net replacement in somatic tissue. Females acquired nearly all the nutrients needed to make eggs from their adult diets. Relative to restricted females, females on full-diet acquired and invested more nutrients into eggs, but unexpectedly these females also had a higher somatic turnover rate of C, N and EAA leading to a two- to four-fold greater net replacement of adult soma (figure 3A–C). The assimilation of EAA into somatic tissue from adult diet, in particular, provided a direct index of protein turnover because it was not confounded by events of de novo synthesis. Contrary to expectation from the resource trade-off model, dietary restricted adults had less rather than more absolute investment of EAA into somatic maintenance; net somatic investment was greater in the shorter-lived full-diet females.
Figure 3.
Net allocation of resources acquired from dietary yeast to adult somatic tissue and eggs from females maintained on restricted-diet (4% yeast, 10% sugar) or full-diet (16% yeast, 10% sugar). Redrawn from O’Brien et al [15]. Females on the restricted diet were 37% longer lived (based on median lifespan) than those on restricted diet and produced 11-fold less eggs. A) Nitrogen. B) Carbon. C) Essential amino acids (list). D) Competitive resource allocation and direct costs of reproduction are complementary mechanisms for the trade-off between reproduction and lifespan (Redrawn from [16]).
Resource allocation, however, may still help explain why dietary restriction extends lifespan: egg production may incur direct somatic costs either by repressing defense systems or by damaging tissues (figure 3D) [15, 16]. In this view, full-diet females with high fecundity driven by abundant resources produce more damage than can be repaired by their active somatic maintenance. Restricted females, in contrast, may require little somatic maintenance to overcome their negligible direct reproductive costs. The framework of nutrient geometry provides a different interpretation of these trade-offs. In the work of Lee et al. [11], along any isocaloric line there is little change in egg production (rate, lifetime) as P:C increases toward the optimum for these traits (figure 2). But along these same isocaloric lines, median lifespan monotonically decreases (figure 2), and the rate egg production is therefore decoupled from variation in longevity, as has been seen in other instances of diet manipulation with Drosophila [14, 17]. Thus, producing more eggs does not necessarily increase direct somatic damage (or divert resources) that is then expressed as reduced survival. These ideas of the geometric framework may be applied to the isotope study data of O’Brien et al. [15]. The observed negative correlation between reproduction and lifespan could occur because females on the full-diet had high caloric consumption as well as increased assimilation of protein. In a nutrient state space such females might be placed on the right end of a high isocaloric line (figure 2, Region X for instance) while females on a yeast-restricted diet would be placed on the left side of a relatively low isocaloric line (figure 2, Region Y, for instance). Females at Region X would have high fecundity with short lifespan while those at Y would have low fecundity with long lifespan. Based on these interpretations, data from isotope studies and geometric analysis are not compatible with explanations based on resource allocation or the notion of direct reproductive costs. Rather, trade-offs are commonly observed because egg production and survival are optimized at different intake ratios of protein to carbohydrate, and to date we lack an adequate mechanistic model to explain this phenomenon.
The view that nutrients are a complex variable is reinforced by new insights on the way Drosophila responds to their diet. Smelling dietary yeast was sufficient to block the longevity benefits of feeding upon a restricted diet [18]. The cue for this perception may be carbon dioxide, which is emitted by live yeast and is sensed by olfactory neurons expressing the receptor Gr63a [19]. Blocking Gr63a was sufficient to extend lifespan and increase egg production (again defying the expectations of nutrient mediated life history trade-offs). Yet, longevity induction by DR must also involve more than the perception of carbon dioxide because lifespan was readily extended when adults were fed dilute media containing autolysed yeast. Carbon dioxide must be an accurate predictor of yeast concentration and thus an anticipatory ‘cue’ sufficient to start a physiological response that will later be induced directly in response to the consumption of yeast.
Genetic analyses of dietary restriction
While understanding the features of food that mediate dietary restriction has advanced, there has been only minimal progress toward understanding the mechanism by which DR extends lifespan. Drosophila should provide a very powerful tool to dissect the pathways through which DR controls aging by looking for mutants that fail to increase longevity on restricted diets. Putting this into practice, however, requires appropriate methods to make strong inferences from the analysis of gene-by-diet interactions (reviewed in [3]). A gene can be said to affect the process by which DR extends lifespan if its loss changes the slope of the diet-restriction response plot relative to that of the wildtype allele. To make this test one must generate demographic data across a range of diets where limited food does not cause malnutrition. These points are to the right of the diet that maximizes survival; diets at lower dilutions presumably reduce survival by starvation and malnutrition. One must test enough nutrient levels to identify and exclude diets from the malnutrition side of the function and to provide sufficient data in the DR range to accurately estimate response functions for each genotype. In practice this means evaluating at least four or five diets for each genotype in the range where DR affects lifespan. Finally, appropriate statistical analysis must be used to make informed inferences; one method is proportional hazard analysis with gene and diet as main effects, and a formal test for a gene-by-diet interaction.
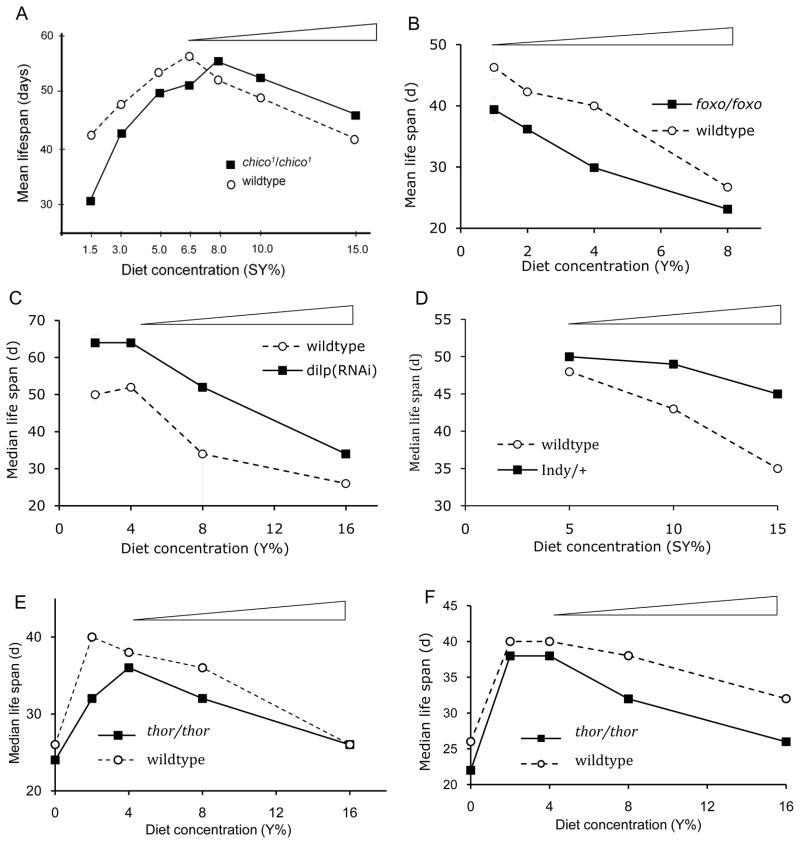
Several genes using only two diet levels have been tested for their potential role in the mechanism of dietary restriction ([14] and cases reviewed in [3]), but with so few diets these results are at best tentative. In fact, only a few genes have been studied with an appropriate range of diets. The insulin receptor substrate encoded by chico provides the first case (figure 4A) [20]. In the range where reduced diet increases lifespan, the plot of mean lifespan relative to diet level is remarkably parallel between wildtype and chico mutant, suggesting that chico does not affect the mechanism by which DR extends lifespan. Rather, chico appears to shift the entire diet response plot suggests that it affects how flies respond to diet during all conditions, including starvation. The insulin responsive transcription factor FOXO provides the second case (figure 4B). Two groups showed that dietary restriction was equally efficient in foxo-null mutants and their wildtype controls [21, 22]. A related instance involves Drosophila insulin-like peptides (dilp) [21]. Among the insulin-like peptides, dilp5 mRNA alone is reduced when diluted dietary yeast extends lifespan. RNAi against dilp3 reduces mRNA of dilp2, dilp3 and dilp5 and prevents the change in dilp5 message in response to reduced diet. Flies with reduce insulin like-peptides are long-lived on all diets but the loss of dilp5 response to dietary restriction does not impede the ability of DR to extend lifespan (figure 4C). All together these results suggest that dietary restriction may not function through insulin signaling.
Figure 4.
Genotype-by-diet interaction plots. In each, the tapered bar indicates the range where data is available to analyze the effect of genes upon dietary restriction (lifespan increases as diet is diluted). A) chico, (chico1/chico1), redrawn from [20]. B) foxo null mutants (foxo21/foxo24), redrawn from [21]. C) dilp (dilp3(RNAi)) reducing mRNA of dilp2, dilp3 and dilp5. Redrawn from [21]. D) Indy, modified and redrawn from [23]. E) Females and F) Males for thor (4eBP), unpublished data of K.J. Min and M. Tatar, Brown University. Strains provided by D. A. Kimbrell (UC Davis, [25] and as used in Zid et al. [24]: wildtype is a clean excision of a P-element restoring the native thor sequence; thor/thor is a viable homozygous, null mutant produced by excision of the P-element.
The gene Indy, encoding a transporter of Krebs cycle intermediaries, provides an encouraging case but with only three diet levels [23]. Lifespan increased monotonically from rich to dilute diets in both the heterozygotes (Indy206/+) and wildtype (figure 4D). Critically, while the heterozyogotes were longer lived on all diets their response to dietary restriction was markedly less than that of the wildtype. Indy homozyogotes, on the other hand, were ambiguous because it was not possible to accurately estimate the range were dietary restriction was operational (see [23]). Overall, a clearer picture emerged when information on mRNA abundance was combined with all the survival data. Lifespan was greatest when Indy mRNA was at an intermediate level, which was achieved by DR and by the Indy206 insertion mutant [23].
A last case involves the translational inhibitor 4eBP, encoded in Drosophila by thor. A recent study tested the ability of thor null mutants to extend lifespan on diets that varied yeast extract or Brewer’s yeast concentration [24]. The data from Brewer’s yeast are most informative because this is the nutrient used in other Drosophila DR studies. With data from three levels of Brewer’s yeast with Zid et al. [24] argured that 4eBP was essential for DR to extend lifespan. However, these data confound regions of malnutrition and dietary restriction, and the criteria used to select data for the inferences were not uniformly applied to all genotypes. Previously unpublished data from our laboratory may clarify the role of 4eBP in dietary restriction (figure 4E, F). Using the same genotypes as Zid et al. [24] and a dilution series of autolysed Brewer’s yeast, we found that dietary restriction extends lifespan in 4eBP mutants just as well as in wildtype controls.
Whether a gene is required for DR to extend lifespan is likely to be an even more complex question once we consider the lessons from the geometric framework. Genes could affect the shape of the response surface in a protein-carbohydrate nutrient state space such that along one vector of diets there appears to be no effect of the gene upon lifespan while upon another vector there is a strong gene-by-diet interaction. Nonetheless, Drosophila provides an opportunity to explore genetic mechanisms by which DR extends lifespan. With the fly we can combine screens of genetic mutants with an accurate framework of dietary restriction. Given the progress made in recent years on what DR means to a fly and how we manipulate fly nutrition, a stage is set to dig deeply into the mechanisms of longevity control by nutrition.
Acknowledgments
Thanks to Stephen Simpson (The University of Sydney) for providing figure elements, and to Stephen Simpson and Mathew Piper for insights on ways to think about nutrients, to Deborah O’Brien (University of Alaska) for collaboration on the stable-isotope study and to Kyung-Jin Min (Inha University, Korea) both for his work on the stable-isotope study and for conducting the thor-by-diet lifespan experiment while a postdoctoral fellow at Brown University. The Ellison Medical Foundation and by NIH R01 AG024360 provided support for our research on dietary restriction in Drosophila.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chippindale AK, Leroi AM, Kim SB, Rose MR. Phenotypic plasticity and selection in Drosophila life-history evolution. I. Nutrition and the cost of reproduction. Journal of Evolutionary Biology. 1993;6:171–193. [Google Scholar]
- 2.Chapman T, Partridge L. Female fitness in Drosophila melanogaster: An interaction between the effect of nutrition and of encounter rate with males. Proc Biol Sci. 1996;263:755–759. doi: 10.1098/rspb.1996.0113. [DOI] [PubMed] [Google Scholar]
- 3.Tatar M. Diet restriction in Drosophila melanogaster: Design and analysis. Interdiscip Top Gerontol. 2007;35:115–136. doi: 10.1159/000096559. [DOI] [PubMed] [Google Scholar]
- 4.Ja WW, Carvalho GB, Mak EM, de la Rosa NN, Fang AY, Liong JC, Brummel T, Benzer S. Prandiology of Drosophila and the CAFE assay. Proc Natl Acad Sci U S A. 2007;104:8253–8256. doi: 10.1073/pnas.0702726104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carvalho GB, Kapahi P, Benzer S. Direct quantification of food intake reveals compensatory ingestion upon dietary restriction in Drosophila. Nature Methods. 2005;2:813–815. doi: 10.1038/nmeth798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Min KJ, Tatar M. Drosophila diet restriction in practice: Do flies consume fewer nutrients. Mechanisms Ageing Development. 2005;127:93–96. doi: 10.1016/j.mad.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Min KJ, Flatt T, Kulaots I, Tatar M. Counting calories in Drosophila diet restriction. Exp Gerontol. 2007;42:247–251. doi: 10.1016/j.exger.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong R, Piper MD, Blanc E, Partridge L. Pitfalls of measuring feeding rate in the fruit flyDrosophila melanogaster. Nat Methods. 5:214–215. doi: 10.1038/nmeth0308-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mair W, Piper MDW, Partridge L. Calories do not explain extension of life span by dietary restriction in Drosophila. PLoS Biology. 2005;3:e223. doi: 10.1371/journal.pbio.0030223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simpson SJ, Raubenheimer D. Caloric restriction and aging revisited: The need for a geometric analysis of the nutritional bases of aging. J Gerontol A Biol Sci Med Sci. 2007;62:707–713. doi: 10.1093/gerona/62.7.707. [DOI] [PubMed] [Google Scholar]
- 11.Lee KP, Simpson SJ, Clissold FJ, Brooks R, Ballard JW, Taylor PW, Soran N, Raubenheimer D. Lifespan and reproduction in Drosophila: New insights from nutritional geometry. Proc Natl Acad Sci U S A. 2008;105:2498–2503. doi: 10.1073/pnas.0710787105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Skorupa DA, Dervisefendic A, Zwiener J, Pletcher SD. Dietary composition specifies consumption, obesity, and lifespan in Drosophila melanogaster. Aging Cell. 2008;7:478–490. doi: 10.1111/j.1474-9726.2008.00400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Min KJ, Tatar M. Restriction of amino acids extends lifespan in Drosophila melanogaster. Mech Ageing Dev. 2006;127:643–646. doi: 10.1016/j.mad.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 14.Grandison RC, Piper MD, Partridge L. Amino-acid imbalance explains extension of lifespan by dietary restriction in Drosophila. Nature. 2009;462:1061–1064. doi: 10.1038/nature08619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Brien DM, Min KJ, Larsen T, Tatar M. Use of stable isotopes to examine how dietary restriction extends Drosophila lifespan. Curr Biol. 2008;18:R155–156. doi: 10.1016/j.cub.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 16.Tatar M, Carey JR. Nutrition mediates reproductive trade-offs with age-specific mortality in the beetle Callosobruchus maculatus. Ecology. 1995;76:2066–2073. [Google Scholar]
- 17.Tu MP, Tatar M. Juvenile diet restriction and the aging and reproduction of adult Drosophila melanogaster. Aging Cell. 2003;2(6):327–333. doi: 10.1046/j.1474-9728.2003.00064.x. [DOI] [PubMed] [Google Scholar]
- 18.Libert S, Zwiener J, Chu X, Vanvoorhies W, Roman G, Pletcher SD. Regulation of Drosophila life span by olfaction and food-derived odors. Science. 2007;315:1133–1137. doi: 10.1126/science.1136610. [DOI] [PubMed] [Google Scholar]
- 19.Poon PC, Kuo TH, Linford NJ, Roman G, Pletcher SD. Carbon dioxide sensing modulates lifespan and physiology in Drosophila. PLoS Biol. 2010;8:e1000356. doi: 10.1371/journal.pbio.1000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clancy DJ, Gems D, Hafen E, Leevers SJ, Partridge L. Dietary restriction in long-lived dwarf flies. Science. 2002;296:319. doi: 10.1126/science.1069366. [DOI] [PubMed] [Google Scholar]
- 21.Min KJ, Yamamoto R, Buch S, Pankratz M, Tatar M. Drosophila lifespan control by dietary restriction independent of insulin-like signaling. Aging Cell. 2008;7:199–206. doi: 10.1111/j.1474-9726.2008.00373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giannakou ME, Goss M, Partridge L. Role of dfoxo in lifespan extension by dietary restriction in Drosophila melanogaster: Not required, but its activity modulates the response. Aging Cell. 2008;7:187–198. doi: 10.1111/j.1474-9726.2007.00362.x. [DOI] [PubMed] [Google Scholar]
- 23.Wang P-Y, Neretti N, Whitaker R, Hosier S, Chang C, Lu D, Rogina B, Helfand SL. Long-lived indy and calorie restriction interact to extend life span. Proceedings of the National Academy of Sciences. 2009;106:9262–9267. doi: 10.1073/pnas.0904115106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zid BM, Rogers AN, Katewa SD, Vargas MA, Kolipinski MC, Lu TA, Benzer S, Kapahi P. 4e-bp extends lifespan upon dietary restriction by enhancing mitochondrial activity in Drosophila. Cell. 2009;139:149–160. doi: 10.1016/j.cell.2009.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bernal A, Kimbrell DA. Drosophila thor participates in host immune defense and connects a translational regulator with innate immunity. Proc Natl Acad Sci U S A. 2000;97:6019–6024. doi: 10.1073/pnas.100391597. [DOI] [PMC free article] [PubMed] [Google Scholar]