Abstract
Human adult bone marrow-derived skeletal stem cells a.k.a mesenchymal stem cells (hMSCs) have been shown to be precursors of several different cellular lineages, including osteoblast, chondrocyte, myoblast, adipocyte, and fibroblast. Several studies have shown that cooperation between transforming growth factor β (TGF-β) and Wnt/β-catenin signaling pathways plays a role in controlling certain developmental events and diseases. Our previous data showed that agents like TGF-β, cooperation with Wnt signaling, promote chondrocyte differentiation at the expense of adipocyte differentiation in hMSCs. In this study, we tested mechanisms by which TGF-β activation of β-catenin signaling pathway and whether these pathways interact during osteoblast differentiation of hMSCs. With selective small chemical kinase inhibitors, we demonstrated that TGF-β1 requires TGFβ type I receptor ALK-5, Smad3, PI3K and PKA to stabilize β-catenin, and needs ALK-5, PKA and JNK to inhibit osteoblastogenesis in hMSCs. Knockdown of β-catenin with siRNA stimulated alkaline phosphatase activity and antagonized the inhibitory effects of TGF-β1 on bone sialoprotein expression, suggested that TGF-β1 cooperated with β-catenin signaling in inhibitory of osteoblastogenesis in hMSCs. In summary, TGF-β1 activates β-catenin signaling pathway via ALK-5, Smad3, PKA and PI3K pathways, and modulates osteoblastogenesis via ALK5, PKA and JNK pathways in hMSCs; the interaction between TGF-β and β-catenin signaling supports the view that β-catenin signaling is a mediator of TGF-β’s effects on osteoblast differentiation of human mesenchymal stem cells.
Keywords: TGF-β, Wnt, β-catenin, hMSCs, osteoblastogenesis
INTRODUCTION
Bone marrow-derived skeletal stem cells a.k.a mesenchymal stem cells or marrow stromal cells (MSCs) are multipotent, self-renewing, mesodermal-origin stem cells that are sequestered in the endosteal compartment. MSCs are maintained in a relative state of quiescence in vivo but in response to a variety of physiological and pathological stimuli, proliferate and differentiate into osteoblasts, chondrocytes, adipocytes, or hematopoiesis-supporting stromal cells [Pittenger et al., 1999; Bianco et al., 2001; Friedman et al., 2006]. Transforming growth factor-β (TGF-β) family proteins have emerged as key players in self-renewal and maintenance of embryonic stem cells and somatic stem cells in their undifferentiated state, the selection of cell fate and the progression of differentiation along a lineage [James et al., 2005]. The canonical Wnt is not only a general stem cell growth factor but can also influence cell lineage decisions in certain stem cell types [Kléber and Sommer, 2004; Reya and Clevers, 2005]. Several studies have shown that cooperation between TGF-β and Wnt/wingless signaling pathways plays a role in controlling certain developmental events [Letamendia et al., 2001] and diseases [Minoo and Li, 2010]. In previous studies, we demonstrated that a variety of signal pathways, such as TGFβ/Smad and Wnt/β-catenin, are involved in stimulation of skeletogenesis and inhibition of adipogenesis of human marrow stromal cells [Zhou et al. 2004]. In this study, we hypothesize that β-catenin signaling is one of the mechanisms by which TGF-β regulates osteoblastogenesis of human bone marrow-derived skeletal stem cells or mesenchymal stem cells. To test our hypothesis, we used chemical biology and RNAi approaches to elucidate the mechanisms by which TGF-β1 regulates β-catenin signaling and osteoblastogenesis in hMSCs.
MATERIALS AND METHODS
CLINICAL MATERIAL AND CHEMICALS
Femoral bone marrow was obtained as discarded materials from subjects undergoing total hip replacement for osteoarthritis. Those subjects did not take medications (e.g. hormone replacement therapy, thyroid hormone, glucocorticoids) or have comorbid conditions that could affect skeletal metabolism, including renal insufficiency, alcoholism, active liver disease, malabsorption, hyperthyroidism, rheumatoid arthritis, ankylosing spondylitis, hyperparathyroidism, or diabetes. ALK-5 inhibitor SB431542, PI-3 kinase inhibitor LY294002, p38 MAPK inhibitor SB203580, p42/44 MAPK inhibitor PD098059, JNK inhibitor SP600125, PKC inhibitor Chelerythrine Chloride (CHE), PKA inhibitor H-89, Smad3 inhibitor SIS3 and lithium chloride (LiCl) were purchase from Sigma (Sigma, St. Louis, MO). Recombinant human TGF-β1 was purchased from R&D Systems (Minneapolis, MN).
CELL CULTURE OF MESENCHYMAL STEM CELLS
Adherent human MSCs were prepared from femoral bone marrow as our previous described [Zhou et al., 2010]. Low-density mononuclear cells were isolated by density centrifugation with Ficoll/Histopaque 1077 (Sigma, MO). The adherent fraction was expanded in monolayer culture with phenol red-free α-MEM medium (Gibco BRL, Invitrogen, Carlsbad, CA), 10% heat-inactivated fetal bovine serum (FBS-HI, Invitrogen) and antibiotics (100 U/ml penicillin, and 100 µg/ml streptomycin) (Gibco BRL). KM101, a human marrow stromal cell line [Harigaya and Handa, 1985], was maintained in Iscove’s modified Dulbecco’s medium (IMDM) (Gibco BRL) with 10% FBS-HI until they reached 80% confluence.
ANALYSES OF OSTEOBLAST DIFFERENTIATION
After confluence in 12-well or 24-well plates, hMSCs were transferred to 1% FBS-HI with osteogenic supplements (10 nM dexamethasone, 5 mM β-glycerophosphate and 50 µg/ml ascorbate-2-phosphate) with or without treatments. Alkaline phosphatase (ALP) activity was analyzed at 14 days by histochemical staining or biochemical enzyme activity assays as our previously described [Zhou et al., 2001; Zhou et al., 2008].
WESTERN BLOT ANALYSES OF PROTEIN LEVELS OF β-CATENIN
Human MSCs were cultured in 100 mm dishes. After confluence, the medium was changed to MEM-α with 1% FBS-HI for 2 days. The cells were incubated with kinase inhibitors for 1 hour before treated with 1 ng/mL of TGF-β1 for 48 hours. The appreciated concentrations of kinase chemical inhibitors were determined by previously published doses that have been demonstrated to inhibit their kinase activity. MSCs were treated with 10 µM of ALK-5 inhibitor SB431542 [Inman et al., 2002], 40 µM of PI-3 kinase inhibitor LY294002 [Vlahos et al., 1994; Zhou et al., 2005], 10 µM of p38 MAPK inhibitor SB203580 [Cuenda et al., 1995; Zhou et al., 2005], 50 µM of p42/44 MAPK inhibitor PD098059 [Dudley et al., 1995; Zhou et al., 2005], 20 µM of JNK inhibitor SP600125 [Han et al., 2001], 1 µM of PKC inhibitor Chelerythrine Chloride [Herbert et al., 1990], 30 µM of PKA inhibitor H-89 [Lee and Lorenzo, 2002], or 3 µM of Smad3 inhibitor SIS3 [Jinnin et al., 2006]. The whole-cell lysates were prepared with lysis buffer containing 150 mM NaCl, 3 mM NaHCO3, 0.1% of Triton X-100 and a mixture of protease inhibitors (Roche Diagnostics, CA). The whole-cell lysates were homogenized with a Kontes’ Pellet Pestle and separated from insoluble cell materials by centrifugation at 16,000 g in a bench-top Eppendorf centrifuge at 4 °C. Protein concentration was determined with the BCA system (Pierce, Rockford, IL). The Western blotting was performed as our previously described [Zhou et al., 2005]. The primary antibody anti-β-catenin (E5) was purchased from Santa Cruz Biotechnology; anti-β-actin was purchased from Sigma (St. Louis, MO). The second antibody anti-mouse IgG-HRP was purchased from Santa Cruz Biotechnology. The antibody-associated protein bands will be revealed with the ECL-plus Western blotting system (Amersham Biosciences, UK).
TRANSIENT TRANSFECTION AND LUCIFERASE REPORTER ASSAYS
Transient transfection for Luciferase reporter assays were performed by electroporation as previously described [Zhou et al., 2003; Peister et al., 2004]. In brief, KM101 cells were maintained in IMDM with 10% FBS-HI until they reached 80% confluence, cells were harvested with trypsin-EDTA and centrifuged at 1000 rpm for 5 min. after washing once with PBS, cell pellets were resuspended wih 100 µL PBS. It was demonstrated that PBS can support equally transfection efficiency compared with commercial buffers [Kang et al., 2009]. The mixtures of 5×106 cells in PBS with 5 µg of TOPFlash luciferase reporter plasmids (Upstate, NY) and 0.5 µg of pRL-CMV Renilla luciferase control reporter plasmids (Promega, Madison, WI) were electroporated at 600 V and 100 µs in a 2-mm gap curvette with Eppendorf Multiporator as described [Peister et al., 2004]. After electroporation, cells were cultured overnight in IMDM with 10% FBS-HI, cell media were changed to MEM-α with 1% FBS-HI and treated with or without TGF-β1, NaCl or LiCl for 24 hours. The cells in 24-well plates were washed once with PBS and lysed with 1x passive lysis buffer (Promega). The luciferase activities were measured by Turner 20/20 luminometer with Promega Dual-luciferase reporter assay kit according to the manufacturer’s instruction. Protein concentrations were measured with Bio-Rad protein assay reagent (Bio-Rad, CA), and luciferase activity was normalized to protein concentration as described [Rahmani et al., 2005], or normalized to Renilla luciferase. Luciferase activity was shown as relative luminescence units per microgram protein (RLU/µg) or ratio of firefly luciferase over Renilla luciferase.
TRANSIENT TRANSFECTION OF β-CATENIN siRNA
Transient transfection of β-catenin siRNA (Stealth RNAi duplex siRNA, Invitrogen) or control siRNA (SiRNA-A, Santa Cruz Biothech., Inc.) into hMSCs was performed by electroporation with the Human MSC Nucleofector Kit (Lonza) according to the manufacturer’s instruction and as described [Aslan et al., 2006]. In briefly, hMSCs were harvested by trypsinization, and resuspended one million cells in 100 µL of human MSC nucleofector solution with 100 pmole of β-catenin siRNA or control siRNA. Electroporation was performed in Nucleofector™ II with program U-23 provided by Lonza/Amaxa Biosystems. Immediately after electroporation, the cells were transferred into 6 or 24-well plates in MEM-α with 10% FBS-HI. After confluence, cells were used for western blot assays (6-well plates) or the medium was changed into osteogenic medium for 7 days to analyze osteogenic marker genes (6-well plates) or ALP enzyme activity (24-well plates).
RNA ISOLATION AND SEMI-QUANTITATIVE RT-PCR
Total RNA was isolated from hMSCs with Trizol reagent (Invitrogen). For semi-quantitative RT-PCR, 2 µg of total RNA was reverse-transcribed into cDNA with M-MLV reverse transcriptase (Promega), following the manufacturer’s instructions. Concentration of cDNA and amplification conditions were optimize to reflect the exponential phase of amplification. In general, one-twentieth of the cDNA was used in each 50 µL PCR reaction (30–35 cycles of 94° C for 1 minute, 60° C for 1 minute, and 72° C for 2 minutes) as described [Zhou et al., 2008]. The gene-specific primers for human ALP [Winn et al., 1999] and bone sialoprotein (BSP) [D'Ippolito, et al., 2006] were used for amplification with Promega GoTaq Flexi DNA Polymerase. PCR products were quantified by densitometry of captured gel images with KODAK Gel Logic 200 Imaging System and measured by KODAK Molecular Imaging Software, following the manufacturer’s instructions (KODAK, Molecular Imaging Systems, New Haven, CT, USA). Quantitative data were expressed by normalizing the densitometric units to GAPDH (internal control).
STATISTICAL ANALYSES
The experiments were performed three or more times independently. Data are presented as mean values ± SD of all experiments or a representative result of three or more experiments. There was at least triplicate per group in each of three or more ALP activity or luciferase reporter assays. Quantitative data were analyzed by GraphPad InStat software with either one-way ANOVA or Student’s t-test. A value of p<0.05 was considered significant.
RESULTS
TGF-β ACTIVATION OF β-CATENIN SIGNALING PATHWAY
To evaluate the effect of TGF-β on activation of Wnt/β-catenin pathway, the stabilization of β-catenin, a key member in the canonical Wnt signaling, was analyzed by Western blot in hMSCs obtained from a 42-year-old female subject. TGF-β1 (1 ng/mL) increased β-catenin protein levels, 4.1 and 8.8-fold over control at 24 and 48 hours respectively, in hMSCs (Fig. 1A). To test whether the stimulation of TGF-β1 increases transcriptional activity, a β-catenin/TCF/LEF responsive vector (TOPFlash luciferase reporter plasmid) and a pRL-CMV Renilla luciferase transfection control plasmid were electroporated into human marrow stromal cell line KM101 cells. After 24 hours, TGF-β1 (1 ng/mL) significantly enhanced β-catenin/TCF/LEF transcription in KM101 cells (p<0.05, t-test) (Fig. 1B). As a positive control, Wnt mimic LiCl stimulated TOPFlash luciferase activity in a dose-dependent manner, and the same dose of NaCl did not stimulate TOPFlash luciferase activity (p<0.01, LiCl vs. the same dose of control NaCl, t-test) (Fig. 1C). There were similar results after firefly luciferase activity was normalized to Renilla luciferase activity.
Fig. 1.
TGF-β activation of β-catenin signaling pathway. (A) TGF-β (1 ng/mL) increased β-catenin protein levels in a time-dependent manner in hMSCs as shown with Western blot; β-actin was used as an internal loading control. (B) TGF-β1 (1 ng/mL) enhanced β-catenin/TCF/LEF transcription in human marrow stromal cells line KM101 cells as shown with TOPFlash luciferase reporter assays (*p<0.05, t-test); (C) the Wnt mimic LiCl stimulated TOPFlash luciferase activity in a dose-dependent manner and the same dose of NaCl did not stimulate TOPFlash luciferase activity in KM101 cells (**p<0.01, LiCl vs. NaCl, ANONA). Luciferase activity was shown as relative luminescence units per microgram protein (RLU/µg).
THE SMAD PATHWAY IN TGF-β1 STABILIZATION OF β-CATENIN
TGF-β1 acts through the TGF-β type I and type II receptors to activate intracellular mediators, such as Smad proteins. As shown by Western blot, at 48 hrs, a specific inhibitor (SB431542, 10 µM) of TGF-β type I receptor (ALK-5) antagonized the stimulatory effects of 1 ng/mL of TGF-β1 on stabilization of β-catenin protein in hMSCs obtained from a 42-year-old female subject (Fig. 2A). The up-regulation of β-catenin protein by TGF-β1 in hMSCs was diminished with 3 µM of Smad3 inhibitor SIS3 (Fig. 2B). Those results suggested that TGF-β/ALK-5/Smad3 axis is necessary for stabilization of canonical Wnt intracellular molecule β-catenin in hMSCs.
Fig. 2.
The Smad pathway in TGF-β1 stabilization of β-catenin. (A) After 48 hours treatment, TGF-β type I receptor inhibitor SB431542 (10 µM) antagonized the stimulatory effects of TGF-β1 (1 ng/mL) on β-catenin in hMSCs; (B) The stabilization of β-catenin by TGF-β1 in hMSCs was diminished with Smad3 inhibitor SIS3 (3 µM). β-actin was used as an internal loading control in Western blot.
THE NON-SMAD PATHWAYS IN TGF-β1 STABILIZATION OF β-CATENIN
To evaluate the non-smad mechanism by which TGF-β activates Wnt pathway, we used small chemical molecule kinase inhibitors. As shown by Western blot, PI-3 kinase inhibitor (LY294002, 40 µM) antagonized, and p42/44 MAPK inhibitor (PD098059, 50 µM) or p38 MAPK inhibitor (SB203580, 10 µM) had no affects on TGF-β up-regulation of β-catenin levels in hMSCs obtained from a 42-year-old female subject (Fig. 3A). PKC inhibitor (Chelerythrine Chloride, CHE, 1 µM) or JNK inhibitor (SP600125, 20 µM) had no affects, and PKA inhibitor H-89 (30 µM) antagonized the stabilization of β-catenin by TGF-β1 in hMSCs (Fig. 3B). This result demonstrated that PI-3 kinase and PKA pathways were required for stabilization of canonical Wnt intracellular molecule β-catenin by TGF-β in hMSCs.
Fig. 3.
The non-Smad pathways in TGF-β1 stabilization of β-catenin. (A) The effects of PI-3 kinase inhibitor (LY294002, 40 µM), p42/44 MAPK inhibitor (PD098059, 50 µM) or p38 MAPK inhibitor (SB203580, 10 µM) on TGF-β (1 ng/mL) up-regulation of β-catenin protein levels in hMSCs. (B) The effects of PKC inhibitor (Chelerythrine Chloride, CHE, 1 µM), PKA inhibitor (H-89, 30 µM) or JNK inhibitor (SP600125, 20 µM) on the stabilization of β-catenin by TGF-β1 in hMSCs. β-actin was used as an internal loading control in Western blot.
COOPERATION BETWEEN TGF-β AND WNT/β-CATENIN SIGNALS DURING OSTEOBLAST DIFFERENTIATION
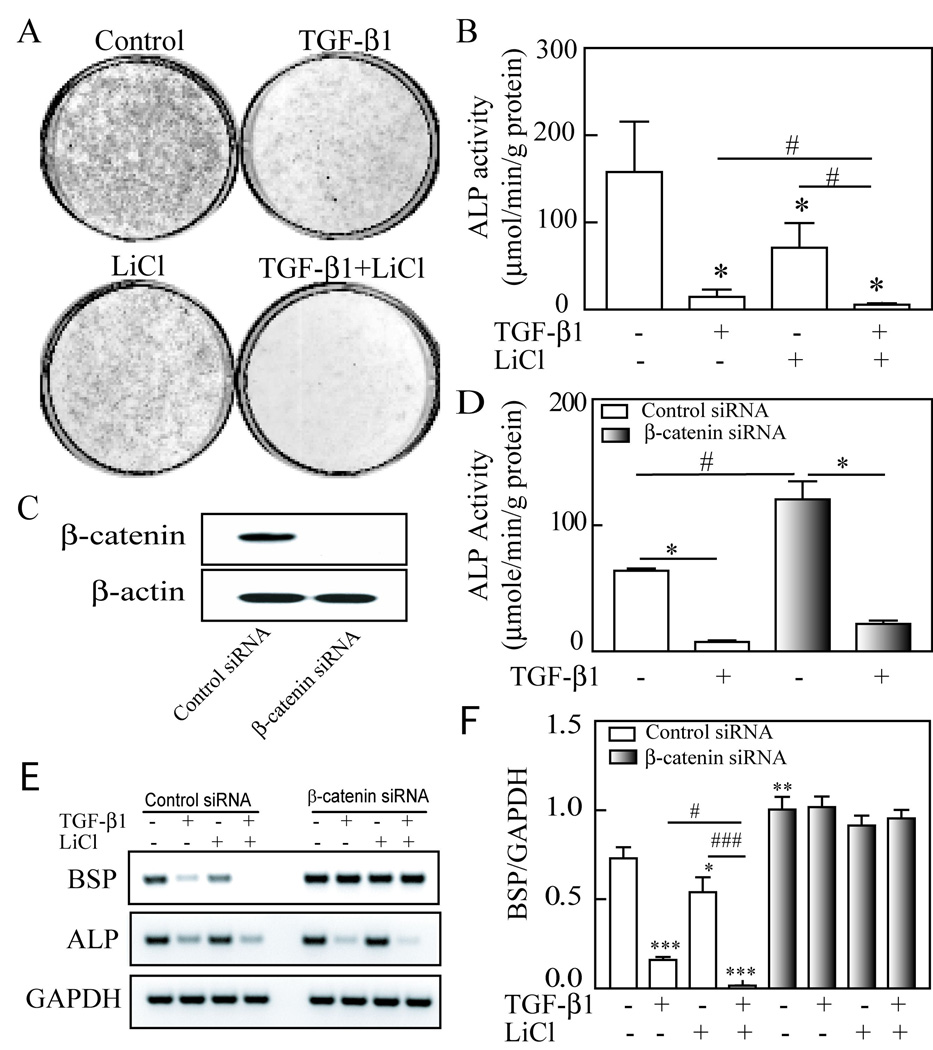
To examine cooperation of TGF-β and Wnt/β-catenin signal pathways in osteoblastogenesis, we studied the effects of TGF-β1 and/or LiCl (a surrogate for Wnt treatment) on alkaline phosphatase (ALP) and osteogenic marker genes in hMSCs. After confluence in 12-well plates, hMSCs obtained from a 42-year-old female subject were cultured for 14 days in MEM-α with 1% FBS-HI plus osteogenic supplements (10 nM dexamethasone, 5 mM β-glycerophosphate and 50 µg/ml ascorbate-2-phosphate) and were treated with 1 ng/mL TGF-β1 and/or 5 mM LiCl. After 14 days treatment, alkaline phosphatase activity was analyzed by histochemical staining (Fig. 4A) and biochemical enzyme activity assays (Fig. 4B). Our data showed that ALP activities of hMSCs in treated groups with 1 ng/mL TGF-β1 (n=15 wells of 12-well-plates in 5 different experiments), 5 mM LiCl (n=9 in 3 experiments) or TGF-β1 plus LiCl (n=6 in 2 experiments) were significantly lower than controls (n=18 in 6 experiments) (*p<0.001, treatments vs. control, ANOVA). Thus TGF-β inhibited osteoblast differentiation of hMSCs in osteogenic medium in vitro, and synergistically cooperated with Wnt signaling as indicated that the ALP activity of TGF-β1 plus LiCl group was significantly lower than TGF-β1 or LiCl alone (p<0.05, TGF-β1 plus LiCl vs. TGF-β1 or LiCl alone; t-test).
Fig. 4.
Cooperation between TGF-β and Wnt signaling in osteoblastogeneis of hMSCs. (A) Alkaline Phosphatase (ALP) histochemical staining in hMSCs with/without 1 ng/mL TGF-β1 and/or 5 mM LiCl. (B) Biochemical assays of ALP enzyme activity of hMSCs in control or treated with 1 ng/mL TGF-β1, 5 mM LiCl or TGF-β1 plus LiCl in 12-well plates (*p<0.001, treatments vs. control, ANOVA; #p<0.05, TGF-β1 plus LiCl vs. TGF-β1 or LiCl alone; t-test). (C) Western blot showed that β-catenin proteins were knockdown by β-catenin siRNA, but not control siRNA. (D) β-catenin siRNA increased ALP activity in hMSCs (#p<0.001 vs. control siRNA, ANOVA); blocking of β-catenin in hMSCs did not affects the inhibitory on ALP activity by TGF-β1 (*p<0.001, TGF-β1 vs. vehicle control). (E) RT-PCR showed that knockdown β-catenin dismished the inhibitory effect of TGF-β and/or LiCl on BSP gene, but not ALP gene expression. (F) Quantitative results were expressed by normalizing the densitometric units of BSP to GAPDH (internal control). Asterisk indicated TGF-β1 and/or LiCl treatments or β-catenin siRNA vs. control siRNA (*p<0.05, **p<0.01, ***p<0.001, ANOVA); number symbol indicated TGF-β1 plus LiCl vs. either TGF-β1 or LiCl alone (#p<0.05, ###p<0.001, ANVOA).
To assess the role of β-catenin in osteoblast differentiation of hMSCs, we transfected β-catenin siRNA (Stealth RNAi duplex siRNA, Invitrogen) or control siRNA (SiRNA-A, Santa Cruz Biothech.) into hMSCs obtained from a 54-year-old male subject. Western blot showed that β-catenin proteins were knockdown by 100 pmole siRNA per million cells, but not by control siRNA (Fig. 4C). Our results showed that knockdown of β-catenin with siRNA increased osteoblast differentiation of hMSCs (Fig. 4D, p<0.001 vs. control siRNA, n=4, ANOVA), indicated that there was an inhibitory effect of β-catenin on differentiation of hMSCs into osteoblasts. The inhibition of osteoblast differentiation by TGF-β1 was only partially blocked in cells with knockdown of β-catenin, 81.9% decline in β-catenin siRNA vs. 88.1% of decline in control siRNA group (Fig. 4D) (p<0.001, TGF-β1 vs. vehicle control, n=4). This shows that β-catenin is not a critical factor for TGF-β1 to inhibit early-stage of osteoblast differentiation in hMSCs. Our RT-PCR data of ALP gene expression (Fig. 4E) confirmed the results of biochemical enzyme activity assay, TGF-β1 down-regulated ALP gene expression in both control siRNA and β-catenin siRNA groups.
The effects of TGF-β1 and/or LiCl on bone sialoprotein (BSP) gene expression in hMSCs were analyzed with semi-quantitative RT-PCR. Knockdown β-catenin dismissed the inhibitory effect of TGF-β and/or LiCl on BSP gene expression in hMSCs obtained from a 49-year-old male subject (Fig. 4E). Quantitative results of 3 different experiments (Fig. 4F) showed that in control siRNA group, BSP expression of TGF-β1 (p<0.001), LiCl (p<0.05) or TGF-β1 plus LiCl (p<0.001) treatments are significantly lower than control (n=3, ANOVA), and the BSP expression in TGF-β1 plus LiCl treatment was significantly lower than either TGF-β1 (p<0.05) or LiCl alone (p<0.001, n=3, ANVOA), indicated a cooperation between TGF-β1 and LiCl. Knockdown of β-catenin with siRNA increased BSP gene expression of hMSCs (Fig. 4F, p<0.01 vs. control siRNA, n=3, ANOVA), and diminished the effects of TGF-β1 and/or LiCl on BSP expression, indicated that the down-regulation of BSP gene by TGF-β1 and/or LiCl requires β-catenin.
THE SMAD AND NON-SMAD PATHWAYS IN TGF-β1 REGULATION ON OSTEOBLASTOGENESIS
To assess the role of Smad and non-smad pathways in the inhibitory effects of TGF-β1 on osteoblastogenesis, hMSCs obtained from a 42-year-old female subject were cultured in 12 or 24-well plates in MEM-α with 1% FBS-HI plus osteogenic supplements for 14 days with or without 1 ng/mL of TGF-β1 and/or kinase inhibitors. SB431542 (10 µM), a small molecule inhibitor of TGF-β type I receptor activin receptor-like kinase (ALK) 5 stimulated ALP activity (12 fold increase vs. control, n=12 wells in 4 experiments, p<0.001, ANOVA) and antagonized the inhibitory effects of TGF-β1 (n=9) on ALP activity as shown that there is no significantly difference between SB431542 (n=3) and TGF-β1 plus SB431542 (n=3) treatments (Fig. 5A). These data indicated that TGFβ type I receptor ALK-5 is required the inhibitory effect of TGF-β on osteoblast differentiation of hMSCs. To test whether Smad3 is necessary in the inhibitory effects of TGF-β1 on ALP activity, hMSCs was treated with SIS3 (2 µM), a inhibitor of Smad3, and the results showed that SIS3 has no effect on the inhibition of ALP activity by TGF-β1 (p<0.01, TGF-β1 vs. control; p<0.001, SIS3 plus TGF-β1 vs. SIS3; n=4, ANOVA) (Fig. 5B), suggested that the Smad pathway is not involved in the inhibitory of ALP activity by TGF-β1 in hMSCs.
Fig. 5.
The Smad and non-smad pathways in the inhibitory effects of TGF-β1 (1 ng/mL) on osteoblastogenesis in hMSCs. (A) Blocking TGF-β type I receptor with SB431542 (10 µM) enhanced ALP activity of hMSCs (**p<0.01, TGF-β1 vs. control; ***p<0.001, SB431542 vs. control; ANOVA), and there was no significantly (NS) difference between SB431542 and SB431542 plus TGF-β1. (B) Blocking Smad3 with SIS3 (2 µM) had no effect on the inhibition of ALP activity by TGF-β1 (***p<0.01, TGF-β1 vs. control; ***p<0.001, SIS3 plus TGF-β1 vs. SIS3; ANOVA). (C) Blocking PI3K pathway with LY294002 (1 µM) had no effect on the inhibition of ALP activity by TGF-β1 (***p<0.001, TGF-β1 vs. control; **p<0.01, LY plus TGF-β1 vs. LY; ANOVA). (D) Blocking p42/44 MAPK pathway with 10 µM PD098059 had no effect on the inhibition of ALP activity by TGF-β1 (***p<0.001, TGF-β1 vs. control; ***p<0.001, PD plus TGF-β1 vs. PD; ANOVA). (E) Blocking p38 MAPK pathway with 10 µM SB203580 had no effect on the inhibition of ALP activity by TGF-β1 (***p<0.001, TGF-β1 or SB203580 vs. control; ***p<0.001, SB203580 plus TGF-β1 vs. SB203580; ANOVA). (F) Blocking PKC pathway with 0.5 µM CHE had no effect on the inhibition of ALP activity by TGF-β1 (***p<0.001, TGF-β1 vs. control; ***p<0.001, CHE plus TGF-β1 vs. CHE; ANOVA). (G) Blocking PKA pathway with 10 µM H-89 diminished the inhibition of ALP activity by TGF-β1 (***p<0.001, TGF-β1 or H-89 vs. control; *p<0.05, H-89 plus TGF-β1 vs. H-89; ANOVA). (H) Blocking JNK pathways with 2 µM SP600125 diminished the inhibition of ALP activity by TGF-β1 (***p<0.001, TGF-β1 or SP vs. control; no significantly difference between SP plus TGF-β1 and SP; ANOVA).
To identify the pathways by which TGF-β1 inhibitory osteoblast differentiation in hMSCs, non-smad pathways were examined with specific kinase inhibitors. Our data (Fig. 5C) showed that blocking phosphoinositide 3-kinases (PI3K) pathway with LY294002 (1 µM) has no effect on the inhibition of ALP activity by TGF-β1 (p<0.001, TGF-β1, n=12, vs. control, n=18; p<0.01, LY plus TGF-β1 vs. LY, n=4; ANOVA); LY294002 treatment declined ALP activity, but there was no significantly different with vehicle control. We tested the effects of MAPK pathways on the regulation of TGF-β1 in osteoblastogenesis of hMSCs, and showed that blocking p42/44 MAPK pathway with 10 µM of PD098059 has no effect on the inhibition of ALP activity by TGF-β1 (p<0.001, TGF-β1, n=12, vs. control, n=18; p<0.001, PD plus TGF-β1 vs. PD, n=4; ANOVA) (Fig. 5D) and blocking p38 MAPK pathway with 10 µM SB203580 has also no effect on the inhibition of ALP activity by TGF-β1 (p<0.001, TGF-β1 vs. control, n=4; p<0.001, SB203580 plus TGF-β1 vs. SB203580, n=4; ANOVA) (Fig. 5E); SB203580 treatment had a significantly higher of ALP activity than vehicle control (p<0.001, n=4), suggested that p38 MAPK pathway blocks osteoblastogenesis of hMSCs, but not synergize the inhibitory effects of TGF-β1. We assessed the role of PKC pathway in the modulation of ALP activity in hMSCs by TGF-β1, and showed that blocking PKC pathway with 0.5 µM CHE has no effect on the inhibition of ALP activity by TGF-β1 (p<0.001, TGF-β1 vs. control, n=4; p<0.001, CHE plus TGF-β1 vs. CHE, n=4; ANOVA) (Fig. 5F). We examined the effects of PKA pathway on the regulation of ALP activity by TGF-β1 in hMSCs with PKA inhibitor H-89. Our data showed that blocking PKA pathway with 10 µM H-89 diminishes the inhibition of ALP activity by TGF-β1 (p<0.001, TGF-β1 vs. control; p<0.05, H-89 plus TGF-β1 vs. H-89; n=4, ANOVA) (Fig. 5G), and PKA alone also blocked ALP activity (p<0.001, H-89 vs. control), suggested that PKA is necessary for TGF-β1 regulation on osteoblastogenesis in hMSCs. Finally, we tested the role of c-Jun N-terminal kinases (JNKs) in the inhibitory effects of TGF-β1 on ALP activity, and showed that blocking JNK pathways with 2 µM of SP600125 decreases ALP activity (p<0.001, SP, n=4, vs. control, n=18, ANOVA) and diminishes the inhibition of ALP activity by TGF-β1 (p<0.001, TGF-β1, n=12, vs. control, n=18, ANOVA) and there was no significantly difference between SP plus TGF-β1 and SP (p<0.001, n=4; ANOVA) (Fig. 5H), suggested that JNKs are necessary for TGF-β1 regulation on osteoblastogenesis in hMSCs. In sum, those data suggested that TGF-β1 needs PKA or JNK pathway, but not PI3K, p42/44 MAPK, p38 MAPK or PKC, to inhibit ALP activity in hMSCs.
DISCUSSION
Signaling cross talk between the TGF-β pathway and Wnt pathway through transcription factors Tcf/Lef1 and Smad3 has been reported [Labbe et al., 2000]. Facilitation of Wnt signaling has also been shown to occur through the interaction of Smad4 with β-catenin and Tcf/Lef1 [Nishita et al., 2000]. Axin, a negative regulator involved in the Wnt signaling pathway, also participates in the regulation of TGF-β signaling [Furuhashi et al., 2001]. Our previous data indicated that TGF-β activates Wnt signaling in hMSCs and there is cooperation between TGF-β and Wnt pathways during chondrocyte and adipocyte differentiation of human marrow stromal cells [Zhou et al., 2004]. In this study, we test the mechanisms by which TGF-β regulates β-catenin signaling and their cross-talk in osteoblastogenesis of human bone marrow-derived mesenchymal stem cells.
To evaluate the activation of Wnt/β-catenin pathway by TGF-β in hMSCs, we analyzed the stabilization of β-catenin by TGF-β1 with Western blotting, and the β-catenin/TCF/LEF transcription with luciferase activity of a TCF/LEF reporter vector. Our data indicated that TGF-β1 increased β-catenin protein levels and enhanced β-catenin/TCF/LEF transcription activities in hMSCs. Smad3-dependent nuclear translocation of β-catenin is required for TGF-β1-induced proliferation of human MSCs [Jian et al., 2006]. Smad3 prevents β-catenin degradation and facilitates its nuclear translocation in rat mesenchymal chondrogenic cell line [Zhang et al., 2010]. SB431542 is a selective and potent inhibitor of the phylogenetically related subset of ALK-4 (activin type I receptor), ALK-5 (TGF-β type I receptor), and ALK -7 (nodal type I receptor) [Inman et al., 2002]. SIS3, a specific inhibitor of Smad3, attenuated the TGF-β1-induced phosphorylation of Smad3 and interaction of Smad3 with Smad4, and did not affect the phosphorylation of Smad2 [Jinnin et al., 2006]. By using SB431542 and SIS3, we demonstrated that TGF-β/ALK-5/Smad3 axis is necessary for stabilization of β-catenin in hMSCs.
TGF-β activates Smad-dependent and Smad-independent pathways [Derynck and Zhang, 2003]. The non-Smad pathways include various branches of MAP kinases (MAPK), Rho-like GTPase signaling, phosphatidylinositol-3-kinase (PI3K)/AKT, c-Jun N-terminal kinases (JNKs), protein kinase C (PKC), and protein kinase A (PKA) [Moustakas and Heldin, 2005; Zhang, 2009]. To evaluate the non-Smad mechanisms by which TGF-β activates Wnt/β-catenin signaling pathway, we used small chemical molecule kinase inhibitors. Our data showed that TGF-β1 stabilized Wnt signaling molecule β-catenin via PI3K and PKA, but did not require p38 MAPK, p42/44 MAPK, JNK, and PKC in hMSCs. These results indicated that TGF-β regulates Wnt/β-catenin signaling in hMSCs via both Smad and non-Smad pathways. Our previous results showed that TGF-β up-regulated Wnt2, Wnt4, Wnt5a, Wnt7a, Wnt10a and Wnt co-receptor LRP5 in hMSCs [Zhou et al., 2004], suggested that Wnt proteins secreted from hMSCs may serve as autocrine/paracrine factors in TGF-β1 stabilization of β-catenin.
Many seemingly contradictory data have been reported on the exact functioning of TGF-β1 in the bone milieu [Janssens et al., 2005]. It was reported that endogenous TGF-β signaling suppresses osteoblast differentiation in mouse C2C12 cells [Maeda et al., 2004]. TGF-β can provide competence for early stages of chondroblastic and osteoblastic differentiation, but it inhibits myogenesis, adipogenesis, and late-stage osteoblast [Roelen and ten Dijke, 2003]. Clinical investigations show that mutations in LRP-5, a Wnt co-receptor, are associated with bone mineral density and fractures [Gong et al., 2001; Little et al., 2002; Boyden et al., 2002]. Studies of knockout and transgenic mouse models for Wnt pathway components, such as Wnt-10b, LRP-5/6, secreted frizzled-related protein-1, dickkopf-2, Axin-2 and β-catenin, demonstrated that canonical Wnt signaling modulates most aspects of osteoblast physiology including proliferation, differentiation, bone matrix formation, mineralization and apoptosis as well as coupling to osteoclastogenesis and bone resorption [Bodine and Komm, 2006]. Canonical Wnt signaling appears to either suppress or promote osteoblastogenesis of MSCs that may depend on differences in the cellular background, the species employed, the experimental conditions and stimuli applied, the level of Wnt activity, or the stage of target MSCs [Ling et al., 2009]. Our data indicated that TGF-β1, required TGFβ type I receptor ALK-5, PKA and JNK, inhibited osteoblast differentiation of human MSCs in osteogenic medium in vitro, and the inhibition was synergistic with Wnt/β-catenin signaling, which has an inhibitory effect on differentiation of hMSCs into osteoblasts as demonstrated by increasing osteoblast differentiation of hMSCs after knockdown of β-catenin with siRNA. Bone sialoprotein (BSP) is thought to function in the initial mineralization of bone, selectively expressed by differentiated osteoblast [Ganss et al., 1999]. Our data showed that knockdown of β-catenin with siRNA diminished the effects of TGF-β1 on BSP gene expression, but not on ALP transcription as well as activity that is a well-established early marker of osteoblast differentiation [Hoemann et al., 2009], demonstrated that Wnt/β-catenin may involve the inhibitory effects of TGF-β1 on late-stage, but not early-stage, osteoblast differentiation of hMSCs. PKA activation induces in vitro osteogenesis and in vivo bone formation by hMSCs [Siddappa et al., 2008]. Our data confirmed that PKA is necessary in osteoblastogenesis as shown by the inhibitory effects of PKA inhibitor H-89 on ALP activity in hMSCs, and TGF-β1 needs PKA to inhibit osteoblastogenesis in hMSCs. c-jun N-terminal kinase (JNK) was specifically required for the late-stage differentiation events of murine osteoblasts [Matsuguchi et al., 2009], and was associated with extracellular matrix synthesis and calcium deposition in hMSCs [Jaiswal et al., 2000]. Our data showed that JNK pathways are necessary in osteoblastogenesis as shown by the inhibitory effects of JNK inhibitor SP600125 on ALP activity in hMSCs. It needs further study on the mechanism by which TGF-β1 regulates osteoblastogenesis via JNK signaling pathways.
This study reveals the mechanisms by which TGF-β affects Wnt/β-catenin signaling and osteoblastogenesis in human mesenchymal stem cells (Fig. 6). Wnts signal across the plasma membrane by interacting with receptors of the Frizzled (Fz) family and members of the low-density-lipoprotein-related protein (LRP) family that promotes the stability and nuclear localization of β-catenin by either degradation of Axin or inhibitory of GSK3β activity, and β-catenin activates transcription in conjunction with co-transcription factors Lefs/Tcfs [Moon et al., 2002; Cadigan and Liu, 2006; Ling et al., 2009]. Lithium activates Wnt signaling pathway by inhibitory of GSK3β to accumulation of β-catenin protein, it has been used to mimic Wnt signals [Hedgepeth et al., 1997]. TGF-β ligand initiates signaling by binding to a heteromeric receptor complex, type I and type II receptor serine/threonine kinases on the cell surface [Shi and Massague, 2003]. Upon phosphorylation by the receptors, Smad complexes translocate into the nucleus, where they cooperate with sequence-specific transcription factors to regulate gene expression [Feng and Derynck, 2005]. Signaling cross talk between the TGF-β pathway and Wnt pathway through transcription factors Tcf/Lef1 and Smad3 has been reported [Labbe et al., 2000]. Facilitation of Wnt signaling was also shown to occur through the interaction of Smad4 with β-catenin and Tcf/Lef1 [Nishita et al., 2000], Dvl-1 with Smad1 [Liu et al., 2006], and Axin with Smad3 [Furuhashi et al., 2001; Dao et al., 2007]. TGF-β utilizes a multitude of intracellular signaling pathways in addition to Smads to regulate a wide array of cellular functions. These non-Smad pathways include MAPK, Rho-like GTPase signaling, PI3K/AKT, JNKs, PKC, and PKA [Moustakas and Heldin, 2005; Zhang, 2009]. Among the non-Smad pathways, PI3K/Akt and PKA signaling inactivated GSK-3 and increased β-catenin translocation in osteocyte-like cell line [Xia et al., 2010]. Our data showed the activation of β-catenin signal pathway via ALK-5/Smad3, PKA and PI3K by TGF-β in hMSCs, and TGF-β1 needs ALK-5, PKA and JNK pathways, but not PI3K, p42/44 MAPK, p38 MAPK and PKC, to inhibit osteoblastogenesis of hMSCs. In conclusion, our studies and previous data [Zhou et al., 2004] demonstrated that Wnt/β-catenin signaling is one of the mechanisms of TGF-β’s effects on cell fate of human bone marrow-derived mesenchymal stem cells.
Fig. 6.
A summary scheme showed the mechanisms by which TGF-β regulation of β-catenin signaling and osteoblastogenesis. TGF-β1 requires TGF-β type I receptor ALK-5, Smad3, PI3K and PKA pathways to stimulate β-catenin signaling, and need ALK-5, PKA and JNK to inhibit osteoblastogenesis in human bone marrow-derived mesenchymal stem cells.
ACKNOWLEGMENTS
The author greatly appreciates help and supporting from Dr. Julie Glowacki for aspects of these experiments, and Dr. Joel S. Greenberger for KM101 human marrow stromal cells. This study was supported by grants from the American Federation for Aging Research A09052, BRI Musculoskeletal Translational Research, and National Institutes of Health AG 025015 and AG 028114. The discarded marrow was obtained from subjects undergoing total hip replacement for osteoarthritis. This study was found to be exempt from the need for written informed consent by the Institutional Review Board for Biomedical Research of the Partners Healthcare system, Inc. The IRB considered and granted a waiver of consent/authorization, which is allowed by both the federal regulations governing human subject research and HIPPA.
Grant sponsor: the American Federation for Aging Research (A09052), Brigham and Women’s Hospital BRI Musculoskeletal Translational Research Award, and National Institutes of Health (AG025015, AG028114 and AG034254).
The abbreviations used are
- TGF-β
Transforming Growth Factor β
- Wnt
Wingless/Int
- hMSCs
human Marrow Stromal Cells or Mesenchymal Stem Cells
- ALK-5
Activin receptor-like Kinase 5
- PI3K
Phosphoinositide 3-kinases
- PKA
Protein Kinase A
- PKC
Protein Kinase C
- MAPK
Mitogen-Activated Protein Kinase
- JNKs
c-Jun N-terminal Kinases
- ALP
Alkaline phosphatase
- siRNA
short interfering RNA
REFERENCES
- Aslan H, Zilberman Y, Arbeli V, Sheyn D, Matan Y, Liebergall M, Li JZ, Helm GA, Gazit D, Gazit Z. Nucleofection-based ex vivo nonviral gene delivery to human stem cells as a platform for tissue regeneration. Tissue Eng. 2006;12:877–889. doi: 10.1089/ten.2006.12.877. [DOI] [PubMed] [Google Scholar]
- Bianco P, Riminucci M, Gronthos S, Robey PG. Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells. 2001;19:180–192. doi: 10.1634/stemcells.19-3-180. [DOI] [PubMed] [Google Scholar]
- Bodine PV, Komm BS. Wnt signaling and osteoblastogenesis. Rev Endocr Metab Disord. 2006;7:33–39. doi: 10.1007/s11154-006-9002-4. [DOI] [PubMed] [Google Scholar]
- Boyden LM, Mao J, Belsky J, Mitzner L, Farhi A, Mitnick MA, Wu D, Insogna K, Lifton RP. High bone density due to a mutation in LDL-receptor-related protein 5. N Engl J Med. 2002;346:1513–1521. doi: 10.1056/NEJMoa013444. [DOI] [PubMed] [Google Scholar]
- Cadigan KM, Liu YI. Wnt signaling: complexity at the surface. J Cell Sci. 2006;119:395–402. doi: 10.1242/jcs.02826. [DOI] [PubMed] [Google Scholar]
- Cuenda A, Rouse J, Doza YN, Meier R, Cohen P, Gallagher TF, Young PR, Lee JC. SB 203580 is a specific inhibitor of a MAP kinase homologue which is stimulated by cellular stresses and interleukin-1. FEBS Lett. 1995;364:229–233. doi: 10.1016/0014-5793(95)00357-f. [DOI] [PubMed] [Google Scholar]
- Dao DY, Yang X, Chen D, Zuscik M, O'Keefe RJ. Axin1 and Axin2 are regulated by TGF-β and mediate cross-talk between TGF-β and Wnt signaling pathways. Ann N Y Acad Sci. 2007;1116:82–99. doi: 10.1196/annals.1402.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-β family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- D'Ippolito G, Diabira S, Howard GA, Roos BA, Schiller PC. Low oxygen tension inhibits osteogenic differentiation and enhances stemness of human MIAMI cells. Bone. 2006;39:513–522. doi: 10.1016/j.bone.2006.02.061. [DOI] [PubMed] [Google Scholar]
- Dudley DT, Pang L, Decker SJ, Bridges AJ, Saltiel AR. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc Natl Acad Sci USA. 1995;92:7686–7689. doi: 10.1073/pnas.92.17.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng XH, Derynck R. Specificity and versatility in TGF-β signaling through Smads. Annu Rev Cell Dev Biol. 2005;21:659–693. doi: 10.1146/annurev.cellbio.21.022404.142018. [DOI] [PubMed] [Google Scholar]
- Friedman MS, Long MW, Hankenson KD. Osteogenic differentiation of human mesenchymal stem cells is regulated by bone morphogenetic protein-6. J Cell Biochem. 2006;98:538–554. doi: 10.1002/jcb.20719. [DOI] [PubMed] [Google Scholar]
- Furuhashi M, Yagi K, Yamamoto H, Furukawa Y, Shimada S, Nakamura Y, Kikuchi A, Miyazono K, Kato M. Axin facilitates Smad3 activation in the transforming growth factor beta signaling pathway. Mol Cell Biol. 2001;21:5132–5141. doi: 10.1128/MCB.21.15.5132-5141.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganss B, Kim RH, Sodek J. Bone sialoprotein. Crit Rev Oral Biol Med. 1999;10:79–98. doi: 10.1177/10454411990100010401. [DOI] [PubMed] [Google Scholar]
- Gong Y, Slee RB, Fukai N, Rawadi G, Roman-Roman S, Reginato AM, Wang H, Cundy T, Glorieux H, Lev D, et al. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell. 2001;107:513–523. doi: 10.1016/s0092-8674(01)00571-2. [DOI] [PubMed] [Google Scholar]
- Han Z, Boyle DL, Chang L, Bennett B, Karin M, Yang L, Manning AM, Firestein GS. c-Jun N-terminal kinase is required for metalloproteinase expression and joint destruction in inflammatory arthritis. J Clin Invest. 2001;108:73–81. doi: 10.1172/JCI12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harigaya K, Handa H. Gene expression of functional clonal cell lines from bone marrow stroma. Proc Natl Acad Sci USA. 1985;83:3477–3480. doi: 10.1073/pnas.82.10.3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedgepeth CM, Conrad LJ, Zhang J, Huang HC, Lee VM, Klein PS. Activation of the Wnt signaling pathway: a molecular mechanism for lithium action. Dev Biol. 1997;185:82–91. doi: 10.1006/dbio.1997.8552. [DOI] [PubMed] [Google Scholar]
- Herbert JM, Augereau JM, Gleye J, Maffrand JP. Chelerythrine is a potent and specific inhibitor of protein kinase C. Biochem Biophys Res Commun. 1990;172:993–999. doi: 10.1016/0006-291x(90)91544-3. [DOI] [PubMed] [Google Scholar]
- Hoemann CD, El-Gabalawy H, McKee MD. In vitro osteogenesis assays: influence of the primary cell source on alkaline phosphatase activity and mineralization. Pathol Biol. 2009;57:318–323. doi: 10.1016/j.patbio.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Inman GJ, Nicolas FJ, Callahan JF, Harling JD, Gaster LM, Reith AD, Laping NJ, Hill CS. SB-431542 is a potent and specific inhibitor of transforming growth factor-β superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Mol Pharmacol. 2002;62:65–74. doi: 10.1124/mol.62.1.65. [DOI] [PubMed] [Google Scholar]
- Jaiswal RK, Jaiswal N, Bruder SP, Mbalaviele G, Marshak DR, Pittenger MF. Adult human mesenchymal stem cell differentiation to the osteogenic or adipogenic lineage is regulated by mitogen-activated protein kinase. J Biol Chem. 2000;275:9645–9652. doi: 10.1074/jbc.275.13.9645. [DOI] [PubMed] [Google Scholar]
- James D, Levine AJ, Besser D, Hemmati-Brivanlou A. TGFβ/activin/nodal signaling is necessary for the maintenance of pluripotency in human embryonic stem cells. Development. 2005;132:1273–1282. doi: 10.1242/dev.01706. [DOI] [PubMed] [Google Scholar]
- Janssens K, ten Dijke P, Janssens S, Van Hul W. Transforming growth factor-β1 to the bone. Endocr Rev. 2005;26:743–774. doi: 10.1210/er.2004-0001. [DOI] [PubMed] [Google Scholar]
- Jian H, Shen X, Liu I, Semenov M, He X, Wang XF. Smad3-dependent nuclear translocation of β-catenin is required for TGF-β1-induced proliferation of bone marrow-derived adult human mesenchymal stem cells. Genes Dev. 2006;20:666–674. doi: 10.1101/gad.1388806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinnin M, Ihn H, Tamaki K. Characterization of SIS3, a novel specific inhibitor of Smad3, and its effect on transforming growth factor-β1-induced extracellular matrix expression. Mol Pharmacol. 2006;69:597–607. doi: 10.1124/mol.105.017483. [DOI] [PubMed] [Google Scholar]
- Kang J, Ramu S, Lee S, Aguilar B, Ganesan SK, Yoo J, Kalra VK, Koh CJ, Hong YK. Phosphate-buffered saline-based nucleofection of primary endothelial cells. Anal Biochem. 2009;386:251–255. doi: 10.1016/j.ab.2008.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kléber M, Sommer L. Wnt signaling and the regulation of stem cell function. Curr Opin Cell Biol. 2004;16:681–687. doi: 10.1016/j.ceb.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Labbe E, Letamendia A, Attisano L. Association of Smads with lymphoid enhancer binding factor 1/T cell-specific factor mediates cooperative signaling by the transforming growth factor-β and Wnt pathways. Proc Natl Acad Sci USA. 2000;97:8358–8363. doi: 10.1073/pnas.150152697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SK, Lorenzo JA. Regulation of receptor activator of nuclear factor-κ B ligand and osteoprotegerin mRNA expression by parathyroid hormone is predominantly mediated by the protein kinase a pathway in murine bone marrow cultures. Bone. 2002;31:252–259. doi: 10.1016/s8756-3282(02)00804-9. [DOI] [PubMed] [Google Scholar]
- Letamendia A, Labbé E, Attisano L. Transcriptional regulation by Smads: crosstalk between the TGF-β and Wnt pathways. J Bone Joint Surg Am. 2001;83-A:S31–S39. [PubMed] [Google Scholar]
- Ling L, Nurcombe V, Cool SM. Wnt signaling controls the fate of mesenchymal stem cells. Gene. 2009;433:1–7. doi: 10.1016/j.gene.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Little RD, Carulli JP, Del Mastro RG, Dupuis J, Osborne M, Folz C, Manning SP, Swain PM, Zhao SC, Eustace B, et al. A mutation in the LDL receptor-related protein 5 gene results in the autosomal dominant high-bone-mass trait. Am J Hum Genet. 2002;70:11–19. doi: 10.1086/338450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Tang Y, Qiu T, Cao X, Clemens TL. A dishevelled-1/Smad1 interaction couples WNT and bone morphogenetic protein signaling pathways in uncommitted bone marrow stromal cells. J Biol Chem. 2006;281:17156–17163. doi: 10.1074/jbc.M513812200. [DOI] [PubMed] [Google Scholar]
- Maeda S, Hayashi M, Komiya S, Imamura T, Miyazono K. Endogenous TGF-β signaling suppresses maturation of osteoblastic mesenchymal cells. EMBO J. 2004;23:552–563. doi: 10.1038/sj.emboj.7600067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuguchi T, Chiba N, Bandow K, Kakimoto K, Masuda A, Ohnishi T. JNK activity is essential for Atf4 expression and late-stage osteoblast differentiation. J Bone Miner Res. 2009;24:398–410. doi: 10.1359/jbmr.081107. [DOI] [PubMed] [Google Scholar]
- Minoo P, Li C. Cross-talk between transforming growth factor-β and Wingless/Int pathways in lung development and disease. Int. J Biochem Cell Biol. 2010;42:809–812. doi: 10.1016/j.biocel.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon RT, Bowerman B, Boutros M, Perrimon N. The promise and perils of Wnt signaling through β-catenin. Science. 2002;296:1644–1646. doi: 10.1126/science.1071549. [DOI] [PubMed] [Google Scholar]
- Moustakas A, Heldin CH. Non-Smad TGF-β signals. J Cell Sci. 2005;118:3573–3584. doi: 10.1242/jcs.02554. [DOI] [PubMed] [Google Scholar]
- Nishita M, Hashimoto MK, Ogata S, Laurent MN, Ueno N, Shibuya H, Cho KW. Interaction between Wnt and TGF-β signalling pathways during formation of Spemann's organizer. Nature. 2000;403:781–785. doi: 10.1038/35001602. [DOI] [PubMed] [Google Scholar]
- Peister A, Mellad JA, Wang M, Tucker HA, Prockop DJ. Stable transfection of MSCs by electroporation. Gene Ther. 2004;11:224–228. doi: 10.1038/sj.gt.3302163. [DOI] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Rahmani M, Read JT, Carthy JM, McDonald PC, Wong BW, Esfandiarei M, Si X, Luo Z, Luo H, Rennie PS, McManus BM. Regulation of the versican promoter by the β-catenin-T-cell factor complex in vascular smooth muscle cells. J Biol Chem. 2005;280:13019–13028. doi: 10.1074/jbc.M411766200. [DOI] [PubMed] [Google Scholar]
- Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- Roelen BA, ten Dijke P. Controlling mesenchymal stem cell differentiation by TGFβ family members. J Orthop Sci. 2003;8:740–748. doi: 10.1007/s00776-003-0702-2. [DOI] [PubMed] [Google Scholar]
- Shi Y, Massagué J. Mechanisms of TGF-β signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- Siddappa R, Martens A, Doorn J, Leusink A, Olivo C, Licht R, van Rijn L, Gaspar C, Fodde R, Janssen F, van Blitterswijk C, de Boer J. cAMP/PKA pathway activation in human mesenchymal stem cells in vitro results in robust bone formation in vivo. Proc Natl Acad Sci U S A. 2008;105:7281–7286. doi: 10.1073/pnas.0711190105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlahos CJ, Matter WF, Hui KY, Brown RF. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) J Biol Chem. 1994;269:5241–5248. [PubMed] [Google Scholar]
- Winn SR, Randolph G, Uludag H, Wong SC, Hair GA, Hollinger JO. Establishing an immortalized human osteoprecursor cell line: OPC1. J Bone Miner Res. 1999;14:1721–1733. doi: 10.1359/jbmr.1999.14.10.1721. [DOI] [PubMed] [Google Scholar]
- Xia X, Batra N, Shi Q, Bonewald LF, Sprague E, Jiang JX. Prostaglandin promotion of osteocyte gap junction function through transcriptional regulation of connexin 43 by glycogen synthase kinase 3/β-catenin signaling. Mol Cell Biol. 2010;30:206–219. doi: 10.1128/MCB.01844-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YE. Non-Smad pathways in TGF-β signaling. Cell Res. 2009;19:128–139. doi: 10.1038/cr.2008.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Wang M, Tan X, Li TF, Zhang YE, Chen D. Smad3 prevents β-catenin degradation and facilitates β-catenin nuclear translocation in chondrocytes. J Biol Chem. 2010;285:8703–8710. doi: 10.1074/jbc.M109.093526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Zilberman Y, Wassermann K, Bain SD, Sadovsky Y, Gazit D. Estrogen modulates estrogen receptor α and β expression, osteogenic activity, and apoptosis in mesenchymal stem cells (MSCs) of osteoporotic mice. J Cell Biochem. 2001;36:144–155. doi: 10.1002/jcb.1096. [DOI] [PubMed] [Google Scholar]
- Zhou S, Turgeman G, Harris SE, Leitman DC, Komm BS, Bodine PVN, Gazit D. Estrogens activate bone morphogenic protein-2 gene transcription in mouse mesenchymal stem cells. Mol Endocrin. 2003;17:56–66. doi: 10.1210/me.2002-0210. [DOI] [PubMed] [Google Scholar]
- Zhou S, Eid K, Glowacki J. Cooperation between TGF-β and Wnt pathways during chondrocyte and adipocyte differentiation of human marrow stromal cells. J Bone Min Res. 2004;19:463–470. doi: 10.1359/JBMR.0301239. [DOI] [PubMed] [Google Scholar]
- Zhou S, Lechpammer S, Greenberger J, Glowacki J. Hypoxia Inhibition of adipocytogenesis in human bone marrow stromal cells requries TGFβ/Smad3 signaling. J Biol Chem. 2005;280:22688–22696. doi: 10.1074/jbc.M412953200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Greenberger JS, Epperly MW, Goff JP, Adler C, LeBoff MS, Glowacki J. Age-related intrinsic changes in human bone-marrow-derived mesenchymal stem cells and their differentiation to osteoblasts. Aging Cell. 2008;7:335–343. doi: 10.1111/j.1474-9726.2008.00377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, LeBoff MS, Glowacki J. Vitamin D metabolism and action in human bone marrow stromal cells. Endocrinology. 2010;151:14–22. doi: 10.1210/en.2009-0969. [DOI] [PMC free article] [PubMed] [Google Scholar]