Abstract
Postnatal development and survival of spiral ganglion (SG) neurons depend upon both neural activity and neurotrophic support. Our previous studies showed that electrical stimulation from a cochlear implant only partly prevents SG degeneration after early deafness. Thus, neurotrophic agents that might be combined with an implant to improve neural survival are of interest. Recent studies reporting that BDNF promotes SG survival after deafness, have been conducted in rodents and limited to relatively short durations. Our study examined longer duration BDNF treatment in deafened cats that may better model the slow progression of SG degeneration in human cochleae and provides the first study of BDNF in the developing auditory system. Kittens were deafened neonatally, implanted at 4-5 weeks with intracochlear electrodes containing a drug-delivery cannula, and BDNF or artificial perilymph was infused for 10 weeks from a mini-osmotic pump.
In BDNF-treated cochleae SG cells grew to normal size and were significantly larger than cells on the contralateral side. However, their morphology was not completely normal and many neurons lacked or had thinned perikaryl myelin. Unbiased stereology was employed to estimate SG cell density, independent of cell size. BDNF was effective in promoting significantly improved survival of SG neurons in these developing animals. BDNF treatment also resulted in higher density and larger size of myelinated radial nerve fibers, sprouting of fibers into the scala tympani, and improvement in electrically-evoked auditory brainstem response thresholds. Although BDNF may have potential therapeutic value in the developing auditory system, many serious obstacles currently preclude clinical application.
Keywords: auditory deprivation, auditory nerve, BDNF, cochlear implant, cochlear spiral ganglion, electrical stimulation, neonatal deafness, primary afferents, neurotrophins
INTRODUCTION
The cochlear spiral ganglion (SG) contains the primary afferent bipolar neurons that relay auditory information from the hair cells of the organ of Corti to the central auditory system. The hair cells provide the sole presynaptic input to these neurons, and the inner hair cells in mammals are innervated by a single synapse from each of 10 to 20 SG neurons, called type I neurons (Spoendlin, 1969, 1975, 1981; Kiang et al., 1982; Liberman et al., 1990). The type I cells comprise ~90-95% of the SG neurons in cats. They provide the primary information pathway to the central auditory system (Kiang et al., 1982, 1984) and are the neurons relevant to cochlear implant function. When hair cells degenerate in sensorineural hearing loss, the deafferented SG neurons gradually lose their distal dendrites (radial nerve fibers), and subsequently their cell somata within the cochlear modiolus also gradually degenerate. The normal development and maintenance of the SG neurons are regulated by a complex interplay of at least two types of survival-promoting mechanisms. First, the hair cells and supporting cells of the organ of Corti produce neurotrophic factors. Specifically, brain-derived neurotrophic factor (BDNF) and neurotrophin-3 (NT-3) have been shown to promote normal development and survival of SG neurons both in vitro (Hegarty et al., 1997; Lefebre et al., 1994; Malgrange et al, 1996; Mou et al., 1997,1998; Vieira et al., 2007) and in vivo (Ernfors et al., 1996; Farinas et al., 2001; Fritzsch et al., 1999, 2004, 2005; Miller et al., 1997, 2007; Rubel and Fritzsch, 2002; Staecker et al., 1996, 1998; Stankovic et al., 2004).
In addition, it is clear that depolarization elicited by elevated potassium promotes the survival of SG neurons in vitro (Hegarty et al., 1997; Hansen et al., 2001, 2003; Roehm and Hansen, 2005 ), although cultured neurons from neonatal animals may respond differently from mature neurons. Further, activity evoked by electrical stimulation from a cochlear implant (CI) in vivo also has been reported to elicit trophic effects on SG survival, both in deafened adult guinea pigs (Lousteau, 1987; Hartshorn et al., 1991; Miller and Altschuler, 1995; Miller et al., 1997; Mitchell et al., 1997; Miller et al., 2001; Kanzaki et al., 2002) and in cats deafened early in life (Leake et al., 1991, 1992, 1995, 1999; 2007, 2008). Our recent work in neonatally deafened cats (as a model of congenital deafness) indicates that intracochlear electrical stimulation, using temporally complex multichannel stimuli over several months, can partially prevent degeneration of SG neurons and promote substantial improvement in neural survival with SG densities of about 50% of normal maintained in implanted ears as compared to roughly 30% of normal on the contralateral side (Leake et al., 2007, 2008). Other studies, however, have found no evidence of trophic effects of electrical stimulation on overall SG survival in vivo in guinea pigs (Li et al., 1999), and Shepherd and co-workers report no difference in SG survival in early-deafened cats (Araki et al., 1998; Shepherd et al., 1994; Coco et al., 2006), although recently they reported a regional increase in SG survival along with increased SG cell size after electrical stimulation in partially deafened cats (Coco et al., 2007) or when BDNF is combined with stimulation (Shepherd et al., 2008). Together, findings suggest that differences among animal models and/or details of applied stimulation are critically important. However, even under optimum circumstances, it seems clear that electrical stimulation only partly prevents neural degeneration that occurs after deafness. Thus, there has been great interest recently in evaluating potential neurotrophic agents that may further promote neural survival in conjunction with a CI.
The best-characterized neurotrophic factors are members of the nerve growth factor (NGF) family of proteins called neurotrophins, including nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3), and neurotrophin-4/5, each of which binds to specific high-affinity receptors, the Trk family of receptors. Studies in SG cell culture preparation have provided strong evidence that SG survival is supported by both neurotrophins and membrane depolarization (Hansen et al., 2001, 2003; Hegarty et al., 1997; Zha et al., 2001; Wefstaedt et al., 2005) and suggest that multiple intracellular signaling mechanisms underlie this neural protection. Specifically, the survival-promoting effect of depolarization is mediated by L-type voltage gated Ca2+ channels and involves multiple distinct signaling pathways, including an autocrine neurotrophin mechanism, cAMP production and Ca2+/calmodulin-dependent protein kinase (CaMk)-mediated phosphorylation of the transcription factor CREB. Further, the neurotrophins BDNF and NT-3 are expressed by SG neurons and promote their survival by an autocrine mechanism that is additive with the effect of depolarization. We hypothesize, therefore, that the neural activity elicited by electrical stimulation in our earlier studies of neonatally deafened animals is effective in engaging these same mechanisms in vivo, and further, because exogenous neurotrophins would activate independent signaling pathways, intracochlear infusion of neurotrophins may elicit neurotrophic effects that would be additive to the survival-promoting effects of electrical stimulation.
Neurotrophins may be particularly relevant in neonatally-deafened animals because they regulate neuronal differentiation and survival during development (Korsching, 1993; Gao et al., 1995; Farinas et al., 2001; Fritzsch et al., 1999; Rubel and Fritsch, 2002) and are also involved in the development and maturation of the central auditory system (for review, see Rubel and Fritzsch, 2002; Fritzsch et al., 2005). Many previous studies have reported that exogenous neurotrophins can protect and promote the survival of SG neurons in adult animals after various insults including ototoxic drugs (Chikar et al., 2008; Ernfors et al., 1996; McGuinnes and Shepherd, 2005; Nakaizumi et al., 2004; Miller et al., 1997; Schindler et al., 1995; Shah et al., 1995; Shepherd et al., 2005, 2008; Staecker et al., 1996, 1998; Yamagata et al., 2004; Zheng et al., 1995; Zheng and Gao, 1996). Survival promoting effects on the SG also have been reported with other neurotrophic factors such as glial cell line-derived neurotrophic factor (GDNF) (Kanzaki et al., 2002; Maruyama et al., 2008; Ylikoski et al., 1998; Yagi et al., 2000) and fibroblast growth factor (FGF) (Glueckert et al., 2008). Recent studies have reported highly significant effects of BDNF in promoting SG survival (Agterberg et al., 2008, 2009; Glueckert et al., 2008; Miller et al., 2007; Shepherd et al., 2008; Song et al., 2009; Wise et al. 2005) and have explored alternative strategies for delivery of neurotrophins to the cochlea (Chikar et al., 2008; Endo et al., 2005; Pettingil et al., 2008; Nakaizumi et al., 2004; Warnecke et al., 2007; Richardson et al., 2008, 2009; Rejali, 2007). Several studies also have reported that BDNF elicits a significant increase in the size of SG cell somata, as compared to cells in the contralateral cochleae. In some cases the SG cells were reported to be even larger than normal (Agterberg et al., 2008; Glueckert et al., 2008; Shepherd et al., 2005, 2008). Most studies to date have used simple counts of SG cells or nuclei and measurements of the cross-sectional area of Rosenthal's canal to estimate neuronal density. Thus, the finding that SG cells are larger after BDNF treatment suggests a potential technical limitation of earlier studies because larger cell size may result in “double-counting” of cells (Mouton, 2002) and over-estimating neuronal survival. Although some studies have used model-based correction factors (e.g., Shepherd et al., 2005), no study to date has used unbiased stereology to assess SG survival independent of cell size. Also, prior studies have been limited to fairly short durations (usually 28 days) and to rodent species (guinea pigs, rats) in which a very rapid onset of deafness, resulting in rapid SG degeneration, was induced by administration of ototoxic drugs with loop diuretics (Versnel et al., 2007).
The present study was designed to extend previous research in several potentially important ways. First, we evaluated the effects of BDNF in a non-rodent species and in an animal model in which deafness was induced by daily systemic injections of an ototoxic drug, resulting in a slower rate of SG neuronal cell loss (Leake and Hradek, 1988) that may better model the slow SG degeneration seen in the human cochlea. In addition, BDNF infusion was extended to 10 weeks to explore whether neurotrophic effects could be maintained over a more prolonged period. Further, SG survival was evaluated using unbiased stereology to accurately assess SG cell survival independent of cell size. Finally, and most importantly, animals in this study were deafened as neonates and implanted at 4 weeks of age in order to assess the effects of BDNF during development. Given the role of neurotrophins in development and based upon the previous findings outlined above, we hypothesized that 10 weeks of intracochlear infusion of BNDF in would significantly ameliorate SG degeneration after early deafness. Our results indicate that BDNF treatment did promote a substantial increase in SG neural survival as compared to both untreated contralateral ears and control animals in which artificial perilymph was infused. In fact, BDNF in this animal model of congenital deafness maintained virtually all the SG neurons present at the time treatment was initiated. Further, BDNF promoted survival of a larger number of radial nerve fibers within the osseous spiral lamina and elicited sprouting of these fibers into the scala tympani. BDNF treatment also resulted in an increase in sensitivity to electrical stimulation with a significant reduction in thresholds for electrically evoked auditory brainstem responses (EABR) that occurred over the course of BDNF infusion, but not in control animals with artificial perilymph infusion.
METHODS
All procedures involving animals were approved by the Institutional Animal Care and Use Committee at the University of California, San Francisco and conformed to all NIH guidelines. The animals included in this study were bred in a closed colony maintained at the University.
Experimental groups and deafening histories
All animals in the experimental groups included in this study were deafened by daily injections of the ototoxic aminoglycoside antibiotic neomycin sulfate, 60 mg/kg of body weight (SQ, SID). Neomycin administration was initiated one day after birth and continued for 16 days, at which time auditory brainstem response (ABR) testing was performed as previously described (Leake et al., 1999, 2007). Acoustic stimuli (100 μs clicks, 20/s) were delivered through a canister headphone (STAX, model SMR-1/MK-2) coupled to the ear by a hollow ear bar inserted into the external ear canal. If a profound hearing loss was documented by absence of an ABR at 90 dB peak SPL, neomycin injections were discontinued. If residual hearing was observed, neomycin injections were continued in increments of 2 to 3 days, and ABR testing was repeated until the hearing loss was profound. The period of neomycin administration in individual experimental animals ranged from 16 to 21 days (Table 1). The first group of deafened control animals listed was examined at about 4 weeks of age, the age at which the other groups underwent unilateral cochlear implantation (left ear). Table 1 also summarizes the experimental histories of the 5 animals receiving BDNF and a group of control subjects in which the osmotic pumps delivered only artificial perilymph (AP), the vehicle for BDNF.
TABLE 1.
Deafening and BDNF Administration Histories
| Cat # |
Neomycin (days) |
Age at Implantation |
Type of Electrode |
Age at Study (days) |
Initial EABR Threshold |
Final EABR Threshold |
Threshold Shift |
|---|---|---|---|---|---|---|---|
| K264 | 20 | 24 | |||||
| K325 | 16 | 26 | |||||
| K334 | 21 | 35 | |||||
| K354 | 16 | 31 | |||||
| Mean age at study = 29 | |||||||
|
Neonatally Deafened + BDNF Infusion (10 weeks)
|
|||||||
| K206 | 19 | 26 | Basal, Short, 2 Contacts | 71 | n/a | n/a | n/a |
| K210 | 21 | 36 | Basal, Full, 4 Contacts | 107 | n/a | n/a | n/a |
| K214 | 16 | 33 | Apical, Full, 4 Contacts | 98 | 158μA | 79μA | −79μA (−6dB) |
| K217 | 16 | 26 | Basal, Short, 2 Contacts | 97 | 316μA | 199μA | −117μA (−4dB) |
| K310 | 18 | 33 | Basal, Short, 2 Contacts | 104 | 363μA | 158μA | −205μA (−7dB) |
| Mean = 31 | Mean age at study = 96 | ||||||
|
Neonatally Deafened + Artificial Perilymph Infusion (10 weeks)
|
|||||||
| K305 | 16 | 32 | Apical, Full, 6 Contacts | 97 | 286μA | 370μA | 84μA (3dB |
| K308 | 19 | 30 | Apical, Full, 6 Contacts | 95 | 164μA | 138μA | −26μA (0dB) |
| K312 | 15 | 39 | Basal, Short, 2 Contacts | 112 | 321μA | 313μA | 313μA (0dB) |
| K336 | 20 | 38 | Apical, Full, 6 Contacts | 118 | 163μA | 167μA | 4μA (1dB) |
| Mean age at study = 105 | |||||||
BDNF and Osmotic Pump Preparation
The cochlear implant electrode with a miniature cannula for intracochlear drug delivery used in this study has been described in detail previously (Rebscher et al., 2007). A shortened version of the electrode, which extended about 6 mm into the scala tympani and had 2-4 stimulating contacts was employed in all but one BDNF subject. One BDNF subject and two of the AP controls were implanted with a later-generation, longer electrode array (designed for future experiments combining drug-delivery with multichannel electrical stimulation). The port of the drug-delivery cannula in the short electrode was positioned within the scala tympani at the base of the left cochlea, approximately 3-4 mm inside the round window, which placed the tip of the drug delivery cannula at about 5-6 mm from the cochlear base. In the longer electrodes the drug-delivery port at the apical tip of the electrode was positioned about 360° from the round window or 12-13 mm from the base. The intracochlear electrode was secured in place by a dacron cuff that was fixed to the inferior aspect of the round window using tissue adhesive (Tissuemend II ™, Veterinary Products Laboratories, Phoenix, AZ). The drug delivery cannula within the cochlear implant (0.13 mm/0.005 inch ID) was connected to vinyl tubing (0.69 mm/0.027 inch ID; 1.143 mm /0.008 inch OD) which was connected to the regulator of the osmotic pump, which was implanted behind the right pinna.
Human recombinant BDNF for these studies was supplied by Amgen, Inc. of Thousand Oaks, CA. Osmotic pumps (Alzet, model #1002 or #2004; infusion rate 0.25 μl/hr) were filled and primed to ensure immediate infusion after implantation. Artificial perilymph (125 mM NaCl, 4.2 mM KCl, 1.3 mM CaCl2, 21 mM NaCHO3, pH 7.4, osmolarity 285-295 mOsm) was prepared from stock solutions. To provide normal autologous protein, blood was drawn from each subject, centrifuged, and the supernatant serum was added to the artificial perilymph (final concentration of 200 mg/dL). The solution was then passed through a sterile filter into several sterile microtubes, which were frozen and stored at −80° C until used to fill the osmotic pumps. Pumps were handled and filled in a UV sterile environment. BDNF (94μg/ml) was added to the sterile perilymph and the solution loaded into the pumps according to the manufacturer's instructions. Both the pump, which was then primed in a sterile saline bath, and the remaining BDNF/perilymph solution were placed in an incubation oven (37° C, 5 %CO2) for at least 24 hours until implantation. The extra solution was used to pre-fill the vinyl tubing and cannula before attaching the osmotic pump during the surgical implantation procedure. Because the animals were so small at the time of implantation (mean body weight, 490 g; range 395-590g), a small osmotic pump (Model #1002) that delivered 14 days of BDNF or AP was implanted initially. Two weeks later, animals underwent a brief surgical procedure to replace the initial pump with a larger model pump (#2004) containing a fresh 28-day supply of BDNF or AP, which was replaced one month later with a final 28-day pump. The pumps were examined upon removal from the animal and found to be fully depleted, with the exception of one animal, K210, in which about 60μl of BDNF remained in the final pump after 28 days. (This pump was filled initially with a volume about 220 μl, and after pumping at 0.25ul/hr for 28 days, a residual volume of less than 50μl should have been present; thus, this subject may not have received the full amount of BDNF over the last few days.) The pump, cannula and electrode components were intact in all subjects at the time of euthanasia, and there was no evidence that any leakage occurred.
Electrically evoked auditory brainstem response (EABR) thresholds
Auditory brainstem response thresholds to electrical pulses (EABR thresholds) were determined bi-weekly or monthly in most of the animals after implantation of the intracochlear electrode and osmotic pump. EABR measurements were made under isofluorane anesthesia, and the animals received no other stimulation from their implants during the BDNF treatment period. Electrical stimuli were charge-balanced biphasic square wave pulses (200 μsec/phase) generated by a real time processor and attenuated using a programmable attenuator (Tucker Davis RP2.1 and PA5). Pulses were delivered at 20 pps to a bipolar pair of electrodes on the CI positioned near the cochlear base via a custom-designed optically isolated and capacitively-coupled voltage-to-current converter that was calibrated prior to each recording session. Responses were recorded differentially from silver wires inserted through the skin (vertex – active; ipsilateral mastoid – reference; contralateral mastoid – ground), amplified (10,000x) and bandpass-filtered (0.3 – 10 kHz) using a battery-powered preamplifier (DAM-50), and digitized to 12-bit resolution at 20 kHz (National Instruments PCI-6071E). At each stimulus current level (1dB increments), responses to 1000 stimulus pulses were averaged and visually evaluated by two observers to estimate response threshold levels.
Assessment of biological activity of BDNF
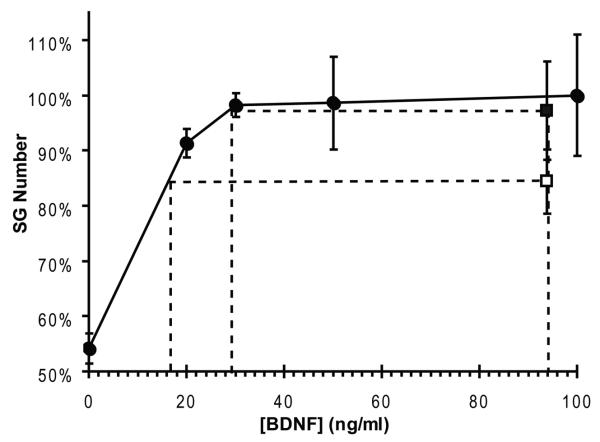
The biological activity of the residual BDNF remaining in the osmotic minipumps after 30 days of implantation in 2 animals was assessed for its ability to promote survival of cultured SG neurons by Dr. Steven Green at the University of Iowa. The BDNF was removed from the depleted pumps under sterile conditions, diluted to a nominal concentration of approx 100 ng/ml (1:1000 dilution based on the original concentration of 94μg/ml) and compared to fresh BDNF at known concentrations of 20, 30, 50, and 100 ng/ml. Dissociated neonatal rat spiral ganglion cell cultures, containing SG neurons and non-neuronal cells dissected from 5 day-old rat pups, were prepared by a modification of the method of Lefebvre et al. (1991) and maintained in N2-supplemented serum-free, high-glucose DMEM as described previously (Hegarty et al., 1997; Hansen et al., 2001). Briefly, after collection in ice-cold HBSS, ganglia were enzymatically and mechanically dissociated. Equal volumes of the dissociated cell suspension were plated in 96 well tissue culture plates treated with polyornithine and laminin. Cells were grown in 100 ml of culture media at 37°C in a 6.5% CO2 incubator. BDNF was added 2–3 hr after plating, and neuronal counts were performed after 48 hr in culture. The cells were fixed (4% paraformaldehyde, 20 min), and neurons were identified by immunofluorescence with mouse anti-high-molecular weight neurofilament (NF200) antibody (SIgma, St Louis, MO), followed by Alexa fluorescent dye-conjugated goat anti-mouse secondary antibody (Molecular Probes, Eugene OR) and Hoechst 33258 bisbenzamide to label the nuclei. The cells were visualized on a Zeiss Axiovert microscope equipped for fluorescent optics. All neurons were counted that showed NF200 immunoreactivity and had a visible nucleus that was not pyknotic. Each condition was replicated in triplicate. Figure 1 shows that the BDNF from one minipump, nominally at 100 ng/ml, was about as effective as 20 ng/ml fresh BDNF (about 20% of the original concentration), and the BDNF from the second minipump, nominally at 100 ng/ml, was as effective as 30 ng/ml fresh BDNF or approximately 30% of the original concentration (and not significantly different from the fresh BDNF control) after 30 days of implantation,suggesting that the BDNF still retained survival-promoting activity. Because the BDNF was diluted 1;1,000, the actual effective concentrations in the minipumps can be estimated as approximately 20 μg/ml and 30 μg/ml, respectively.
Figure 1.
The biological activity of BDNF remaining in the osmotic minipumps after 30 days of implantation in 2 animals was assessed for its ability to promote the survival of cultured SG neurons. BDNF from one minipump was about as effective as 20 ng/ml fresh BDNF (about 20% of the original concentration), and the BDNF from the second minipump was as effective as 30 ng/ml fresh BDNF or approximately 30% of the original concentration (and not significantly different from the fresh BDNF control) after 30 days of implantation. Data generously provided by Dr. Steven Green at the University of Iowa.
Histological preparation of cochlear specimens
Methods for preparation of cochlear specimens were identical to those described previously (Leake et al., 1999, 2007). Briefly, with the animal deeply anesthetized (sodium pentobarbital, I.V.), the stapes was removed, the round window opened and the cochleae were perfused with mixed aldehyde fixative (1.5% glutaraldehyde and 2.5% paraformaldehyde in 0.1M phosphate buffer, pH 7.4). An overdose of sodium pentobarbital then was administered, and transcardiac perfusion was performed with the same fixative solution. The temporal bones were placed in fixative overnight and then transferred to buffer for dissection. The otic capsule bone was thinned using diamond dental burrs. In implanted cochleae the bony capsule over the scala vestibuli of the basal turn was opened to visualize the individual electrode contacts; their positions were marked in the adjacent bone for later identification in the reconstructions, and the electrode was removed. The specimens were post-fixed in 1% phosphate buffered osmium tetroxide with 1.5% potassium ferricyanide added to enhance contrast, dehydrated and embedded in LX™ epoxy resin. Surface preparations containing both the organ of Corti and spiral ganglion were prepared and mounted in epoxy resin on glass slides. The basilar membrane was measured, and small segments (<1 mm) of the organ of Corti along with the adjacent spiral ganglion were excised in each 10% sector of the cochlea. These sectors of the cochlear spiral were remounted and oriented perpendicular to the basilar membrane for sectioning in the radial plane. For morphometric analysis sections were cut at a thickness of 5 μm, lightly stained with toluidine blue and examined with a Zeiss Axioskop II; in some cases for photomicroscopy to illustrate histological findings (e.g, to examine myelination of SG somata), additional semi-thin sections were cut at 1-2 μm. The organ of Corti was evaluated for the presence of hair cells, the condition of supporting cells, evidence of chronic infection/ inflammatory response, and trauma caused by surgical placement of the electrode. Morphometric analyses were carried out to assess spiral ganglion cell survival and cell size.
Histological and morphometric analyses
Unbiased stereology for assessment of spiral ganglion cell numerical density
Survival of the primary afferent auditory neurons was assessed by morphometric analysis of the density of SG cell somata in Rosenthal's canal as a function of cochlear location. Because previous reports (Agterberg et al., 2008, 2009; Glueckert et al., 2008; Shepherd et al., 2005, 2008) have shown that BDNF induces a significant increase in SG cell soma areas, we utilized a physical disector stereological method that accurately estimates the number of objects (nucleoli) in a given volume of tissue, independent of their size (Mouton, 2002). For this analysis, 4 sets of 6 serial sections (5 μm thickness; ~30 μm separation between serial sets) were collected from each of 9 regions, representing 10% sectors of the cochlea from 0 to 90% of the basilar membrane distance from the cochlear base (as determined in the surface preparation for each specimen). The disector method requires comparing adjacent serial sections and counting the number of “new” nucleoli of SG neurons that appear in the second section -- i.e. that are non-overlapping with those in the first section. The sections must be precisely aligned to make this determination, and we used Adobe Photoshop™ to facilitate this process. Two sections of a serial array were imaged at a resolution of 3.2 pixels/μm, 300 dpi and a screen magnification of ~3000X. Images were acquired using a Zeiss Axioskop 2, Axiocam MRc5 camera and a 40X/0.95 NA lens. In the native files a 330 by 250 μm field was equal to 2584 by 1936 pixels and the system acquired images at 150 dpi, resulting in a resolution of 7 pixels/μm. For the physical disector estimates of cell density, all cells with a visible nucleolus in the first serial section were selected using the Photoshop “wand” tool to select the cells, preserving cytoplasmic boundaries but excluding the nucleus and somatic myelin/satellite cell. Additional reference structures (e.g., vessels) were also selected. The selected structures were copied and pasted into a file with the second serial section, creating a template which was precisely aligned with the second section by matching the cells and reference structures. The “new” nucleoli appearing only in the second section were then counted. Four pairs of serial sections were analyzed for each cochlear sector; if necessary, additional pairs of sections were analyzed until approximately 50 cells were included in the data set for each sector. The magnetic lasso tool was also used to outline Rosenthal's canal in the serial pair. The images of cells and canal outlines were imported into the NIH Image J program (version 1.37v) and the canal areas (for numerical density) and cell areas (see below) were determined. All the image selection procedures for these and all other morphometric analyses reported in our study were performed blind.
SG cell area and density data for 4 normal adult cats were also collected using these methods in order to express data in experimental animals as a percentage of normal. For the group of deafened kittens studied at about 4 weeks of age, percentage of normal values were derived from comparisons to data for age-matched normal control subjects. For overall group comparisons to assess the effects of BDNF or AP on SG cell density, the mean number of nucleoli in a [100 μm]3 volume of Rosenthal's canal (see below) was calculated for each of the 9 cochlear sectors (0-90%).
Measurements of cross-sectional areas of spiral ganglion cells and Rosenthal's canal
The procedure for measuring the cross-sectional areas of SG cell somata was integrated with the estimation of SG cell numerical density as described above. The images used for the physical disector analysis in Photoshop (screen magnification of ~3000X) were imported as Tiff files into Image J. The “analyze particles" function was used to estimate cross-sectional areas of all SG cell somata containing a nucleolus, as previously selected for the disector analysis (i.e., profiles of the SG cell cytoplasmic boundary excluding Schwann cells and somatic myelin). Approximately 50 cells per cochlear sector were measured.
In 4 selected cochlear sectors (10-20, 30-40, 50-60, and 70-80% intervals of basilar membrane length) Rosenthal's canal was also measured in a more complete set of images (10 serial sections selected from the original 24 serial images used for the numerical density analysis). In Photoshop™ tiff images, the boundary of Rosenthal's canal was traced with the “magnetic lasso” tool, the 10 canal-outline images were converted to monochrome, the images were imported into Image J, and canal area was then measured using the “particle analysis” function.
Pixel density scans of SG somatic myelin and satellite cells
For this analysis additional 1 μm sections of the SG were cut orthogonal to the radial plane at a mid-cochlear location 50-60% (~13 mm) from the base. At least 5 unstained sections at 5 μm intervals were imaged with a resolution of 18 pixel per mm (Zeiss 100x Plan-Achromatic oil immersion objective, NA 0.2) and montages of the SG were assembled. SG cells with both a nucleolus and a visible satellite cell nucleus were selected, and the thickness and density of the osmiophillic myelin/satellite layer around the perimeter of the cell were analyzed at a point opposite the satellite cell nucleus, using a 30 pixel by 10 pixel scan in Image J and subtracting background staining of the SG cell cytoplasm. Cells that showed no dense perimeter were counted but excluded from analysis. Data were collected in 3 of the BDNF-treated cochleae, in the contralateral deaf cochleae of the same animals, in 3 deaf controls at 30 days of age and in 2 normal controls.
Transmission electron microscopy
In three animals (K210, K214, K217) a region in the BDNF-treated cochlea at a mid-cochlear location 50-60% from the base was selected for examination of the ultrastructure of the SG cells and sprouted radial nerve fibers within the scala tympani near the implanted electrode. Ultrathin sections were cut in the radial plane, sections were collected on grids, stained with lead citrate and uranyl acetate, and examined at 80 kV in a Jeol 100S transmission electron microscope.
Radial nerve fiber counts; measurements of cross-sectional areas
In order to evaluate radial nerve fiber survival and size, separate series of sections (5 μm thickness) were cut through the osseous spiral lamina tangentially, i.e., orthogonal to the radial plane. Three cochlear regions in the basal turn at about 3-6 mm, 7-9 mm and 11-13 mm (matched between left and right sides of each animal) were selected where the tissue blocks used for cell counts still had remaining tissue. Serial 5 μm sections were cut starting at the habenula and proceeding 135 μm toward the cochlear modiolus. Every third section was imaged (7.6 pixels per μm, 150 dpi), providing a total of 9 samples per location. In Photoshop™ the “rectangular marquee” tool (fixed size) was used to select a 100 μm length of the osseous spiral lamina in which to count and measure cross-sectional profiles of fibers. A reference structure was identified in all 9 sections of the series to ensure that counts sampled approximately the same location. The paint brush tool was used to mark each fiber for counts and the wand tool was used to select fibers for determining size. The selected fiber profiles were imported into Image J, counted and measured, and values averaged for the 9 sections at each location.
All photomicrographs were prepared using a Zeiss Axio-Cam (MRc5) digital camera and AxioVisionAc (v. 4.2) software to capture images. Brightness and contrast were adjusted in Adobe Photoshop (v. 8, 10; San Jose ,CA) to enhance cellular detail and to match images within a figure. Section blemishes were removed digitally from background areas using the paintbrush tool.
Statistical Analyses
The statistical comparison of the EABR threshold data for the BDNF and AP groups was made using the student's t-test (unpaired) to compare threshold shifts (initial less final values) measured in the two groups. Statistical comparisons of cochlear morphometric data (SG cell density, cell size, Rosenthal's canal area, radial nerve fiber density) between the two sides in each experimental group (BDNF group, AP group) were made using a two-way ANOVA pairwise multiple comparisons procedure (Tukey test) with treatment type and cochlear sector as factors. All groups had equal variance among the samples. In all but one instance the data were also normally distributed and thus did not violate the assumptions of the test. The cell size data for the deaf cochleae contralateral to the BDNF-treated ears failed the normality test; therefore, for this comparison a nonparametric version of two–way ANOVA (Friedman's test) was used.
Comparisons of the cross-sectional areas of radial nerve fibers among the BDNF, deaf and AP groups were made using a one using one way ANOVA pairwise multiple comparisons procedure (Tukey test). All statistical analyses were performed using Sigma Stat™ (version 2.03) or MatLab™ software (Friedman's test).
RESULTS
Experimental groups
Table 1 presents the individual histories for the experimental groups included in the study. A control group of 4 neonatally deafened animals was studied at a mean of 29 days of age, about the same time that other animals were implanted and began receiving BDNF or artificial perilymph (AP) infusion. Table 1 also shows the ages at implantation and at the time of study for 5 animals that received intracochlear infusion of BDNF, and a control group of 4 neonatally deafened subjects that were treated identically to the BDNF group, but in which the osmotic pumps were filled with AP. Mean age at implantation was 31 days for the BDNF group and 35 days for the AP group. One animal (K206) was studied after only 6 weeks of BDNF infusion at about 10 weeks of age; the other 4 subjects in the BDNF group and the 4 AP subjects were studied after 10 weeks of treatment at about 100 days of age.
EABR thresholds
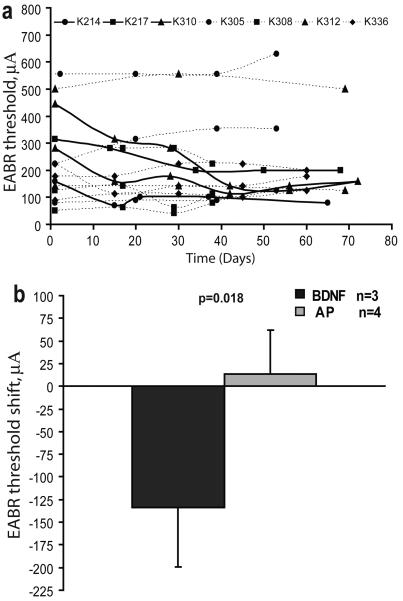
Evoked potential thresholds elicited by electrical stimulation delivered by bipolar pairs of intracochlear electrodes were tracked longitudinally at 2 week to 4 week intervals over the course of BDNF delivery in 3 subjects and in the 4 control subjects that received AP perilymph infusion. (Data could not be obtained due to health concerns in one BDNF subject and a broken electrode wire in another.) Mean initial and final thresholds and the resulting shifts are presented in Table 1. Considerable inter-subject variability was observed (Fig. 2a), and no systematic difference was observed between the BDNF and AP groups in the absolute mean threshold values (initial or final). However, comparison of the initial and final EABR thresholds in the BDNF-treated animals revealed a decrease (i.e., improved sensitivity) over time, with a mean shift of 134 μA or 6 dB (Fig. 2b). In contrast, the AP group showed no significant change in threshold. This difference between the BDNF group and the AP group in the EABR threshold shift recorded was statistically significant (Student's t-test, unpaired; P= 0.018).
Figure 2.
a. Electrically evoked auditory brainstem response (EABR) threshold data for bipolar pairs of electrodes recorded longitudinally for 10 weeks in neonatally deafened animals receiving intracochlear BDNF (solid bold lines) or artificial perilymph (lighter dotted lines). b. Mean data show a substantial improvement in threshold (difference between threshold at the time BDNF infusion was started and final threshold) of 134 μA (6 dB) in the BDNF-treated animals. In contrast, control animals studied receiving artificial perilymph showed a slight elevation in threshold.
Effects of BDNF on spiral ganglion cell size
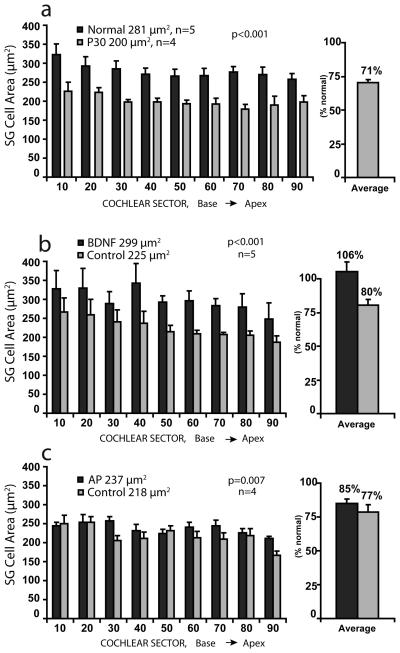
Evaluation of the organ of Corti showed that no hair cells were present in any of the deafened animals included in the study. In the control group of neonatally deafened animals studied at about 30 days of age, a significant reduction in the cross-sectional areas of SG cell perikarya had already occurred and the mean area averaged over all cochlear sectors was 71% of normal (Fig 3a). Both data sets show that SG cells are largest in the cochlear base and progressively smaller toward the apex.
Figure 3.
a. Cross-sectional areas of the somata of spiral ganglion (SG) neurons are shown for 9 cochlear sectors representing 10% intervals from base to apex in normal controls and in neonatally deafened animals examined at 30 days of age (age when other animals were implanted). b. Cell size data are shown for neonatally deafened animals examined after 10 weeks of intracochlear BDNF infusion. Significantly larger cell size was documented in the BDNF-treated cochleae (black bars) as compared to the contralateral side (gray bars) in the same animals. After BDNF treatment, the ganglion cells measured about 300 μm2, which was equivalent to normal adult size and 33% larger than cells on the other side, which measured about 225 μm2. c. Data for control deafened animals after 10 weeks of infusion of artificial perilymph (AP). A modest, but significant difference in cell size is seen with larger cells in the AP cochleae than on the contralateral side. Comparison of the two data sets shows that cells in the BDNF-treated ears are markedly larger than those in all 3 other groups (cochleae contralateral to the BDNF-treated ears; AP cochleae, cochleae contralateral to the AP ears).
In the group of 5 animals examined after 10 weeks of cochlear implantation and infusion of BDNF, the mean cross-sectional areas of cell somata of SG neurons in the BDNF-treated cochleae were consistently larger than cells on the contralateral side in all cochlear sectors (Fig. 3b). The overall trend toward decreasing cell size from base to apex was apparent in both the BDNF and control cochleae, as in the controls. Averaged over all cochlear regions, the mean cell area following BDNF infusion was about 300 μm2, as compared to about 225 μm2 for cells in the control cochlea (calculated cell diameters of 19.5 and 17μm, respectively), and this difference was highly statistically significant (two-way non-parametric ANOVA; p<0.001). Note that although the data for cells in the BDNF cochleae were normally distributed, the data for the contralateral deaf cochlea did not pass the test for normality, and a nonparametric version of two–way ANOVA, Friedman's test, was used for this comparison. When compared to data for normal adult cats, mean cell areas in the BDNF ears were not significantly different from normal (average, 106% of normal), whereas cells on the deafened control side averaged only 80% of normal adult size, comparable to cell size in animals studied at 30 days of age.
Data from the control group of 4 animals studied after 10 weeks of intracochlear infusion of artificial perilymph are presented in Figure 3c. There was a small but significant difference between the mean SG soma area measured in the AP cochleae at about 235 μm2 and cell size on the contralateral side which was about 220 μm2 (p=0.007; two way ANOVA). However, neither of these values was significantly different cell size in the deaf cochleae contralateral to BDNF-treated cochleae (225 μm2). Moreover, the SG cell areas in the BDNF-treated cochleae were significantly larger than cells in both the AP-treated and contralateral deaf cochleae (p<0.001).
Effects of BDNF on survival of spiral ganglion neurons
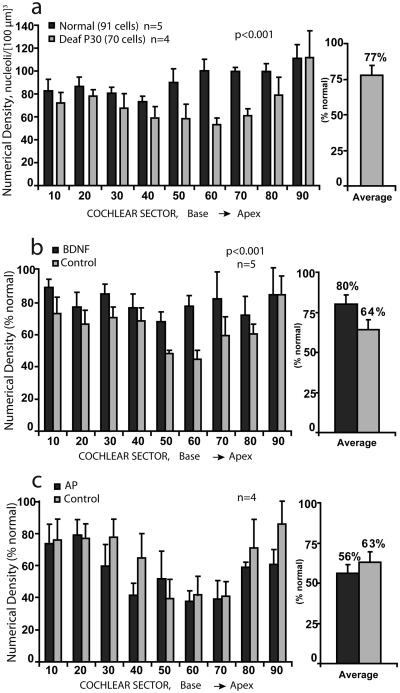
Counts of nucleoli of SG neurons obtained using the physical disector method were used to estimate the cell density in a [100 μm]3 volume of Rosenthal's canal for cochlear sectors from base to apex in all animals included in this study (Table 1). In the control group of neonatally deafened animals studied at about the age of implantation for the other groups, significant neural degeneration already had occurred and the mean SG numerical density (averaged over all cochlear sectors) was about 77% of normal (Fig. 4a). Figure 4b presents data for the 5 animals examined after 10 weeks of intracochlear BDNF infusion. The numerical density of SG cells in the BDNF-treated cochleae was consistently higher than that in the contralateral cochleae in all cochlear sectors, with the largest difference seen in middle cochlear sectors where cell loss was greatest. Averaged over the entire cochlea and expressed as percentage of normal, the data indicate that the mean cell density after BDNF infusion was about 80% of normal, as compared to about 64% in the contralateral deafened cochleae, and this difference was highly significant (Tukey Test; p< 0.001). Data from the 4 animals studied after 10 weeks of AP infusion are shown in Figure 4c. There was no significant difference between the mean SG cell density after AP infusion (56% of normal) as compared to the contralateral untreated side (63%).
Figure 4.
a. The numerical density of SG neurons is shown for 10% cochlear sectors from base to apex, with data expressed as percentage of normal for normal adult cats and for a control group of neonatally deafened animals studied at 30 days of age (age when other groups were implanted). SG survival is significantly reduced in the 30 day old deafened group (adult mean, 91 nucleoli [100 μm]3 ; 30-day deaf mean, 70 nucleoli [100 μm]3 ) b. SG numerical density data for neonatally deafened animals studied after 10 weeks of unilateral intracochlear BDNF infusion are shown The BDNF-treated cochleae (black bars) show higher density for all cochlear sectors than the contralateral cochleae (gray bars), although significant neural degeneration is evident on both sides. Averaged over all cochlear sectors, SG cell survival is about 80% of normal after BDNF treatment as compared to about 64% on the other side. c. Data for neonatally deafened animals that received AP infusion show no significant difference between the two sides.
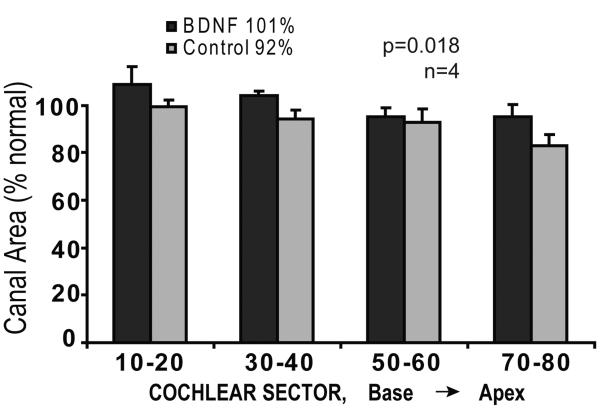
A factor that could affect SG neuronal density is any alteration in the size of Rosenthal's canal. Figure 5 shows the data obtained from direct measurements of the cross-sectional area of Rosenthal's canal in a subset of 4 cochlear intervals in the BDNF-treated cochlea and matched data from the contralateral cochleae. These data demonstrated that the canal area in the BDNF-treated side was equivalent to that of the normal adult cochlea, whereas the canal on the contralateral untreated ears was slightly smaller in all cochlear sectors (p=0.18) and averaged about 92% of normal overall. Note that the larger canal size in the BDNF cochleae would tend to bias our data toward a lower cell density in the BDNF-infused ears in comparison to the contralateral side, and thus only increases confidence in the finding of higher cell density after BDNF treatment. In the AP group there was no significant difference in Rosenthal's canal area between sides.
Figure 5.
Mean cross-sectional area of Rosenthal's canal in neonatally deafened animals after 10 weeks of BDNF infusion is shown for 4 cochlear sectors from base to apex. Canal area was slightly but significantly larger in BDNF-treated cochleae than on the contralateral side.
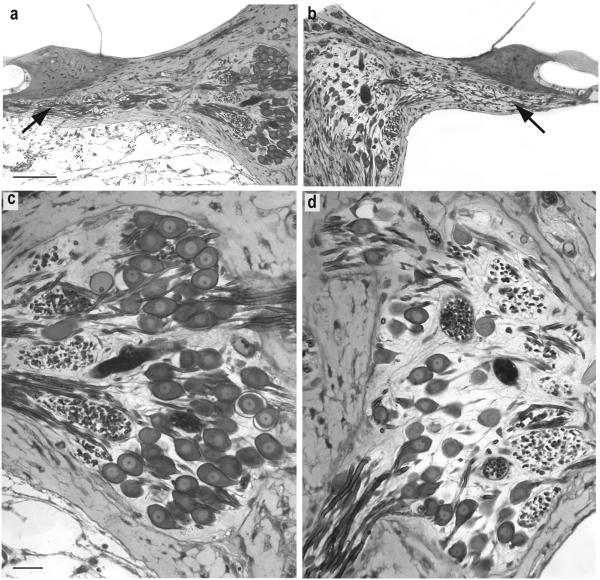
Representative histological sections illustrating the effects of BDNF infusion are presented in Figure 6. In these light microscopic images taken from a mid-cochlear sector (40-50% from the base), the higher density of cell somata of SG cells within Rosenthal's canal of the BDNF-treated cochlea (Fig. 6a,c) as compared to the same region in the contralateral ear (Fig. 6b,d) is evident. In addition, the images illustrate a higher density of radial nerve fibers in the osseous spiral lamina with BDNF treatment than in the opposite cochlea (arrows). In the higher magnification images, the larger cross-sectional areas of SG cell somata in the BDNF cochleae (Fig. 6c) also are apparent compared to cells on the contralateral side (Fig. 6d). Even the nucleoli appear larger on the BDNF side.
Figure 6.
Light microscopic images of histological sections from a neonatally deafened animal (K217) illustrating the marked neurotrophic effects of 10 weeks of unilateral intraocochlear BDNF infusion. The 40-50% cochlear sector of the left BDNF-treated cochlea (a,c) and the paired region from the contralateral deafened control cochleae (b,d) show a higher density of SG cells in Rosenthal's canal and a greater number radial nerve fibers within the osseous spiral lamina (arrows) after BDNF infusion. The fibrous tissue reaction to the implanted electrode is evident in the scala tympani of the left ear. The higher magnification images in c and d illustrate the quality of preservation and staining of the tissue after osmium post-fixation and the resolution of images used for morphometric analyses in the study. The higher density and size of SG perikarya in the BDNF-treated cochlea as compared to neurons on the opposite side are evident. (Scale bar in a = 50 μm; Scale bar in c = 25 μm).
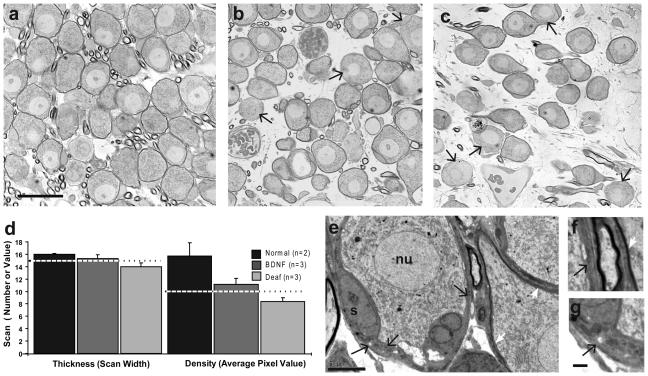
Although the SG neurons in the BDNF-treated cochleae were maintained at 80% of normal density and had cross-sectional areas equivalent to normal controls, it was apparent in examination of light microscopic images (Figs. 6a, 7b) that some of the surviving neurons did not have normal morphology. In normal cats the type I neurons (which comprise about 93% of the SG population and are considered the primary target for stimulation by the CI) are large bipolar neurons with myelinated cell somata (Fig. 7a). In the BDNF cochleae, however, the somatic myelin of some type I SG neurons appeared thinned (fewer myelin lamellae) or even completely absent (Fig. 7b). To assess this pathology quantitatively, pixel density scans were used to measure the thickness and density of the myelin/satellite cell layer around SG cell somata in unstained sections (Fig. 7 a,b,c) in the region of maximum BDNF effect and in normal controls. In about 10% of the neurons in BDNF-treated ears and 15% of cells on the contralateral side, the normally very dense myelin/satellite cell layer around the perimeter of the neuron showed no measurable contrast from the cell cytoplasm. Such cells were not observed in normal cochleae. Pixel density scan data for the remaining cells in both BDNF-treated and contralateral deaf ears (Fig. 7d) indicated reduced density as compared to normal (p< 0.05) at the location measured. This pathology was further examined in transmission electron microscopy in the three BDNF-treated ears by evaluating the perikaryl myelin and associated satellite cells in a random sample of 25-40 cells in the region of maximum BDNF effect. Substantial variability was noted among type I SG neuronal profiles within the same cochlear region (Fig. 7f) after 20 weeks of BDNF infusion. Specifically, many SG neurons had relatively normal myelin, but some cells were completely lacking myelin and others had only a few myelin lamellae. In both of these latter conditions the SG neurons often were surrounded by what appeared to be hypertrophied satellite cells.
Figure 7.
Light micrographs of the SG neurons in 1 μm unstained sections (cut orthogonal to the radial plane) in a normal cat (a), in a cochlea studied after 10 weeks of BDNF treatment (b) and in the contralateral deaf ear of the same subject (c). Images like these from the 50-60% cochlear sector (~13 mm from the cochlear base) were used to perform pixel density scan measurements of SG somatic myelin and satellite cells. Data indicate a slight reduction in width and significant reduction in density in both BDNF-treated and contralateral deaf cochleae (d). The dashed line shows data for deafened animals studied at 30 days of age (n=3). Observations in TEM images (e,f,g) parallel the quantitative data, showing that some type I SG cells after BDNF treatment had perikarya that were unmyelinated or very thinly myelinated. In image e one SG neuron on the left is unmyelinated, surrounded by hypertrophied satellite cells (black arrows) that are often associated with such pathological neurons and another neuron on the right has fairly normal myelin (white arrows). Images in f and g show higher magnifications of two regions of the satellite cell indicated by the black arrows in e and adjacent myelinated cell (white arrow in f). nu, SG cell nucleus; s, satellite cell. (Scale bar in a applies to a-c and = 25 μm; scale bar in e = 5 μm; scale bar in g applies to f,g = 1μm)
Radial nerve fiber number, size and distribution
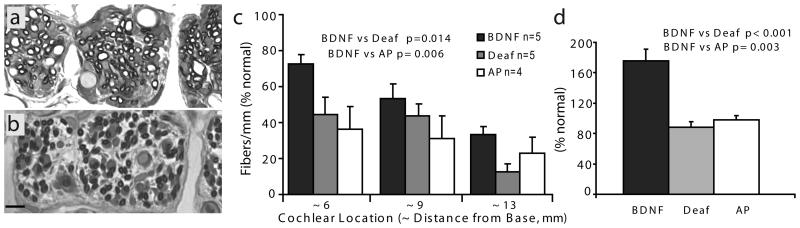
The SG dendrites or radial nerve fibers were assessed in tangential cross-sections of the osseous spiral lamina at 3 cochlear sectors within the basal half of the cochlear spiral (Fig. 8a,b). The number of cross-sectional profiles of radial nerve fibers was consistently higher in the BDNF-treated cochleae in all cochlear sectors as compared to the contralateral deafened ears (Fig. 8c). Note that the highest density and also the greatest difference in density between sides were observed in the most basal cochlear sector (approximately 3-6 mm from the base), which is the location closest to the tip of the BDNF delivery cannula. In this location an average of about 70% of the normal number of fibers were present in BDNF-treated cochleae as compared to about 45% in the same region of the contralateral ears and less than 40% in the cochleae examined after AP infusion. In the other two regions examined (about 7-9 mm and 11-13 mm from the base), fiber density in the BDNF cochleae decreased to approximately 50% and 30% of normal, respectively, but was still higher than on the contralateral side and in the AP ears. In addition to the higher density of radial nerve fibers, measurements of the cross-sectional areas of the dendritic profiles in the images demonstrated that fibers were much larger in the BDNF-treated cochleae than on the contralateral side (Fig. 8d). In fact, cross-sectional fiber areas after BNDF treatment averaged 175% of normal and were almost double the size of fibers in both the contralateral deafened cochleae (88% of normal) and in the AP-infused cochleae (98% of normal).
Figure 8.
Histological sections were cut tangential to the conventional radial plane to evaluate survival of the peripheral processes of the SG neurons in BDNF-treated (a) and contralateral control (b) cochleae in a 0.1 mm sector of the osseous spiral lamina (width of each micrograph = 0.1 mm). c. Counts of the radial nerve fiber profiles at three different cochlear locations within the basal turn demonstrated a significant improvement in survival after BDNF treatment (black bars) as compared to the contralateral deafened cochleae (lightly shaded bars) or after artificial perilymph infusion (white bars). d. Cross-sectional areas of the radial nerve fibers were also evaluated at the same 3 cochlear locations where fiber counts were performed. Averaged over the 3 locations, fibers in the BDNF-treated cochleae were markedly larger than normal and also much larger than fibers in the contralateral cochleae and in AP cochleae. Scale bar in b applies to a and b = 10 μm)
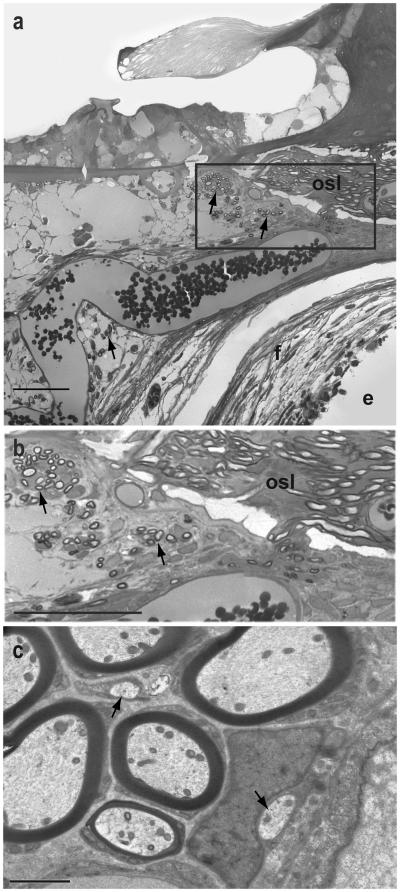
Another noteworthy finding after BDNF infusion was that the radial nerve fibers sprouted through the lower aspect of the osseous spiral lamina and grew into the scala tympani where they were observed in ectopic locations under the osseous spiral lamina and basilar membrane and within the fibrous tissue encapsulating the cochlear implant electrode (Fig. 9a,b). Such ectopic sprouted neuronal processes were noted in the BDNF-treated cochleae of all 5 animals examined, but were never observed in deafened control or AP-infused ears. The fibers were largely limited to the cochlear sector adjacent to the cochlear implant electrode, but within this region the number and distribution of fibers was quite variable among the individual cochleae. Transmission electron microscopy of these profiles in two BDNF-treated cochleae demonstrated the presence of both large myelinated and smaller unmyelinated profiles (Fig. 9c) and adjacent Schwann cells.
Figure 9.
a. Light microscopic image of the organ of Corti (30-40% cochlear sector) in a neonatally deafened animal (K310) after 10 weeks of BDNF infusion, illustrating the ectopic sprouting of radial nerve fibers from the osseous spiral lamina (osl) into the scala tympani (arrows) within the fibrotic tissue (f) encapsulating the cochlear implant electrode (e). b. Area outlined in a is shown at higher magnification. Fibers are seen exiting the osseous spiral lamina and forming small bundles (arrows). c. Transmission electron micrograph image of sprouted peripheral fibers in the scala tympani of another neonatally deafened animal (K214) after BDNF treatment illustrates several apparently large, well-myelinated axonal profiles (a) as well as 2 unmyelinated fibers (arrows). (Scale bars in a and b = 50 μm; scale bar in c = 2 μm).
DISCUSSION
EABR thresholds
A reduction in EABR thresholds (Shinohara et al., 2002; Shepherd et al., 2005) as well as larger amplitude of the suprathreshold EABR response (Agterberg et al., 2009) have been reported previously after 4 weeks of intracochlear infusion of BDNF; improved EABR thresholds have also been reported with intracochlear over-expression of BDNF by adenovirus (Chikar et al., 2008), as well as after intracochlear infusion of CTNF (Yamagata et al., 2004) or GDNF (Maruyama et al., 2008; Scheper et al., 2009) in adult-deafened guinea pigs. Our data extend those previous findings by demonstrating a significant decrease in EABR thresholds, which persisted over a longer period of BDNF infusion (10 weeks) and was elicited in developing animals that were deafened at birth and received BDNF starting at about 4 weeks of age. The specific mechanism(s) underlying the threshold reduction is unclear. One possibility is that the ectopic sprouting of the peripheral processes of SG neurons, the radial nerve fibers, down into the scala tympani directly above the stimulating CI electrodes could provide a closer electrode-to-neuron coupling and thus result in improved thresholds. However, fiber sprouting was not reported in the previously mentioned guinea pig studies, which also showed EABR threshold decreases. This discrepancy might be because sprouting may not have occurred after only 4 weeks of BDNF infusion. Alternatively, in many cases the histological methods utilized may not have allowed detection of sprouting fibers (i.e., tissues were not post-fixed in osmium tetroxide, making the small profiles very difficult to see). Radial nerve fiber sprouting into the scala tympani has been reported previously after 4 weeks combined infusion of BDNF and acidic fibroblast growth factor (aFGF) in guinea pigs (Glueckert et al., 2008) and in another study after 8 weeks of BDNF infusion (Staecker et al., 1996). Wise et al. (2005) reported sprouting onto the basilar membrane, as well as increased number and size of radial nerve fibers after combined BDNF and NT-3 infusion, but none of these earlier studies reporting sprouting documented EABR thresholds.
Aside from the sprouting seen in the BDNF-treated cochleae in our study, the larger size of the radial nerve fibers or some fundamental change in the SG neuronal membrane properties or channels also could account for the observed EABR threshold reduction. From the standpoint of clinical application of CIs, the maintenance of lower thresholds to electrical stimulation may be advantageous, as it potentially could result in improved dynamic range for applied electrical stimulation and also may also reduce power consumption and improve battery life for the speech processor.
Effects of Deafness and BDNF on SG cell soma size
Reduction in the size of SG neuron perikarya following profound deafness has been reported in many previous studies in various animal models (Agterberg et al., 2008, 2009; Araki et al., 1998; Elverland and Mair, 1980; Glueckert et al., 2008; Leake and Hradek, 1988; Leake et al., 1999; McGuinness and Shepherd, 2005; Shepherd et al., 2005; Wise et al., 2005). This finding presumably reflects the reduced metabolic requirements of the neurons due to the reduction in both spontaneous and driven spike activity after deafness (Hartmann et al., 1984; Liberman and Kiang, 1978; Shepherd and Javel, 1997). Further, when electrical stimulation from a CI is applied to (re-)activate these neurons, modest yet significant increases in SG soma area in the stimulated cochlea have been reported (Araki et al., 1998; Coco et al., 2007; Leake et al., 1999, 2007). However, the trophic effects on soma area associated with neurotrophin administration are much greater than increases in cell size associated with electrical stimulation. Several previous studies have shown that 4 weeks of intracochlear infusion of BDNF elicited an increase in SG perikaryal size, with cross-sectional areas that were significantly larger than those of normal controls (McGuinness and Shepherd, 2005; Shepherd et al., 2005, 2008; Agterberg et al., 2008; Glueckert et al., 2008). Although the mechanism(s) underlying this finding are unclear, it seems likely to be related to the relatively high concentrations of exogenous neurotrophin in the cochlea (as compared to the normal cochlea), as previously suggested by Shepherd et al. (2008).
Consistent with these previous studies, our data indicate that 10 weeks of intracochlear BDNF infusion promotes growth and maintenance of a significantly larger SG cell soma size as compared to the contralateral deafened cochleae in these developing animals. Cells in the BDNF-treated cochleae were full normal adult size but were not significantly larger than normal, although there was a tendency for cells to be slightly larger than normal (mean, 106% normal) especially in the basal cochlear sectors. In contrast, cells in the contralateral deafened control cochleae were only 80% of normal size. Expressed as percent increase over the control side, these data indicate a 33% larger SG somatic area is elicited by 10 weeks of BDNF treatment. Prior work has shown that SG cell somata in normal cats are full adult size and well-myelinated by one month of age (Romand and Romand, 1986, 1990) when BDNF treatment was initiated in deafened kittens in our study. In contrast, cells in the neonatally deafened group studied at about one month of age (the time of implantation of BDNF group) were significantly smaller than normal, with an average cell soma area of 71% of normal. Thus, in the BDNF group the SG perikarya apparently undergo delayed growth during the 10-weeks of BDNF treatment, which allows them to achieve and maintain normal adult size. In contrast, cells in the contralateral deafened ears and in ears studied after AP infusion retain smaller soma areas, comparable to those in deafened animals at one month of age.
Despite the finding that average SG cell areas were normal after 10 weeks of intracochlear BDNF infusion in the present study, we emphasize that the neurons did not all exhibit completely normal morphology. In the normal adult cat cochlea 93-95% of the ganglion cells are type I neurons with round nuclei and myelinated perikarya (Spoendlin, 1975, 1981; Kiang et al., 1982; Leake and Hradek, 1988). In contrast, many of the type I cells in the BDNF-treated cochleae lacked or had only very thin perikaryal myelin and often were surrounded by hypertrophied satellite cells. This finding is particularly interesting because the radial nerve fibers associated with these cells after BDNF infusion were present in larger numbers in the osseous spiral lamina and were well myelinated. Some of them even exhibited ectopic sprouting of myelinated neurites into the scala tympani. The functional significance of this reduction or loss of perikaryal myelin is unknown, but it must be considered indicative of significant pathology in some of the surviving SG neurons, raising a question about their ability to survive over the longer term. No ultrastructural studies were performed on deafened control or AP cochleae, but the pixel scan density measurements of the myelin/satellite cells suggest that pathology may be more severe in the deafened control ears.
Effects of BDNF on survival of spiral ganglion neurons
A highly significant effect of intracochlear infusion of exogenous BDNF in maintaining a higher numerical density of SG cells was observed in animals examined after 10 weeks of treatment. SG survival was systematically improved throughout the cochlea after BNDF treatment, but the maximum difference in neural density was seen in the middle cochlear sectors, ~40-70% from the base (represented frequency range of about 8 to 1.6 kHz), largely because this is where neuronal loss was greatest in the control ears. This pattern of SG neuronal loss is unique to animals that are deafened as neonates and is interesting because hair cell and supporting cell degeneration in these animals progress from base-to-apex (Leake et al., 1997), a pattern that is typical of aminoglycoside ototoxicity in adults (Leake and Hradek, 1988). These findings suggest that the initial SG neural degeneration in these young animals is not secondary to sensory cell degeneration, and that during the first 2-3 weeks postnatal when the onset of hearing normally occurs, neurons in the middle cochlear sectors are more sensitive to direct aminoglycoside ototoxicity than neurons in other regions. This likely reflects some regionally-varying aspect of cochlear maturation.
With BDNF infusion, the average SG numerical density was about 80% of normal as compared to 64% of normal on the contralateral side (P<0.001). When normalized to the survival in the control deaf cochleae, this represents an increase of about 25% in neuronal survival after BDNF treatment. In deafened animals studied at the age of implantation for the other groups, significant neural degeneration had already occurred, with a mean SG numerical density of 77% of normal. Thus, the finding of 80% SG numerical density after 10 weeks of BDNF infusion suggests that the treatment maintained virtually all the neurons present when treatment began. This effect, although highly significant, is somewhat more modest than previous findings in guinea pigs, where SG degeneration is more rapid. For example, one previous study reported that infusion of combined BDNF and aFGF (Glueckert et al., 2008) not only elicited enhanced survival of SG neurons, but that SG density in the basal turn was even higher than in normal hearing subjects, and the authors suggested that this finding could indicate that “…new SG neurons developed from endogenous progenitor/stem cells …” However, this study also documented highly significant increases in SG neuronal soma areas, with cells that were larger than normal after BDNF infusion. Thus, because simple counts of cells per unit area of Rosenthal's canal were used to determine neuronal density and survival, the effect of neurotrophins on SG survival may have been overestimated by overcounting due to the larger cell size. It is important to note that this technical problem is common to many previous studies evaluating the effects of BDNF and other neurotrophins on SG survival in rodents and may have resulted in some degree of overestimation of neurotrophic effects in the existing literature. We suggest that future studies ideally should apply unbiased stereological methods to ensure accurate assessment of neuronal survival independent of cell size, especially in view of the goal of assessing potential clinical interventions.
Another factor that could influence accurate assessment of neuronal survival is the size of Rosenthal's canal. Differences in the cross-sectional area of Rosenthal's canal have been reported previously in guinea pigs after electrical stimulation delivered by a cochlear implant (Li et al., 1999). This issue could be especially important in the present study because of the young age at which the animals were implanted, and possible alterations elicited by BDNF during a period when growth and maturation are still occurring. In the present study, however, Rosenthal's canal exhibited normal adult size in the BDNF-treated ears, and the small difference between the BDNF and contralateral cochleae (i.e., smaller contralateral canal size) would have biased our results toward higher density in the contralateral ears, resulting in less of a difference between the two sides. Thus, the data on canal size increase our confidence in the difference in SG density after BDNF infusion reported here.
Radial nerve fiber, size and distribution
Our data on survival of radial nerve fibers within the osseous spiral lamina demonstrate that greater numbers of fibers were present throughout the basal cochlear turn following BDNF infusion as compared to both the contralateral deaf ears and AP-treated cochleae. It is interesting to note that both the highest fiber density and the greatest difference between BDNF and deafened ears were observed in the most basal cochlear sector, about 3-5 mm from the base, where about 70% of the normal number of fibers were maintained. One possible explanation for this finding might be the fact that the port of the BDNF cannula is positioned near this location, so that fibers in this region may have been exposed to a higher concentration of the neurotrophic factor than in other cochlear regions. In the other two regions examined (about 7-9 mm and 11-13 mm from the base), survival decreased to approximately 50% and less than 40% survival, respectively, suggesting a possible gradient in neurotrophic effects. However, our findings that the BDNF neurotrophic effects of larger SG cell areas and improved SG survival were relatively uniform across all cochlear sectors argue against this possibility. Alternatively, the pattern of nerve survival may simply reflect the regional survival of SG neurons, which is also poorer in the middle cochlear sectors that would be equivalent to the 11-13 mm sector where the fiber survival is poorest.
The finding of an increase in the number of radial nerve fibers after BDNF treatment are consistent with the results of Wise et al. (2005) after combined infusion of BDNF and NT-3 infusion in adult guinea pigs. They also reported that radial nerve fiber counts in the BDNF treated cochleae were higher than in deaf ears, but not equivalent to normal ears. Similar data were reported by Gleuckert et al. (2008) after combined BDNF and aFGF treatment. Importantly, these authors also used immunohistochemical techniques (parvalbumin immunostaining) to show that the fiber profiles in the osseous spiral lamina and the sprouting fibers seen in ectopic locations were afferent peripheral processes of SG neurons.
Our data also indicate that the increased population of radial nerve fibers surviving in the BDNF-treated cochleae had substantially larger cross-sectional areas, measuring almost twice that of the contralateral deafened ears. In fact, after exogenous BNDF treatment, fiber areas were even significantly larger than normal (175% of normal) on average in the sampled areas of the basal cochlear turn. This may reflect the growth necessary for fibers to sprout extensively down into the scala tympani. Interestingly, our fiber size data indicate a much greater increase in cross-sectional areas than seen by Wise et al. (2005), perhaps because we extended neurotrophin delivery for 10 weeks, as compared to 4 weeks in the guinea pig study.
Clinical relevance
On average, adult human cochlear implant (CI) recipients using the latest technology enjoy speech recognition scores of about 80% correct on high-context sentences, and the goals for the most fortunate include better speech reception in noisy environments and improved music perception (Zeng, 2008). CI electrodes also are now being used in combination with hearing aids in individuals with significant residual hearing (Turner et al., 2008). The success of this “electro-acoustic” hearing, and the progressive hearing loss after surgery in some individuals, have re-focused attention on reducing trauma during CI implantation, maintaining residual hearing (Fraysse et al., 2006; James et al., 2006), and on the importance of the condition of the cochlea and auditory nerve for CI function. To this end, intra-scalar delivery of drugs (anti-inflammatory agents or neurotrophins) has been proposed in human CI subjects to promote maintenance of residual hearing or improved SG survival, and CI electrodes modified for drug delivery already have been developed (Hochmair et al., 2006; Paasche et al., 2003; Shepherd and Xu, 2002; Staecker et al., 2010). However, animal studies examining the long-term effects of potential neurotrophic agents and exploring alternative less invasive strategies for promoting SG survival have been relatively limited to date.
The issue of maintaining auditory nerve survival seems particularly important in the thousands of very young deaf children, including congenitally deaf infants, now are receiving CIs (Dettman et al., 2007), because they will depend on electrical hearing for many decades. It is encouraging that many children with CIs eventually are mainstreamed into public education settings, but it is important to note that many other pediatric CI recipients lag far behind in language development (Svirsky et al., 2000; Geers, 2004; Nicholas and Geers, 2007). The emphasis on implantation at very young ages is based on evidence for a critical period for language acquisition (Eggermont and Bock, 1986; Rubens, 1986; Rubens and Rapin, 1980) and on research showing that implantation before the age of two years results in significant advantages in speech perception (Svirsky et al., 2000; Svirsky et al., 2004; Geers, 2004; Nicholas and Geers, 2007). Studies in animals have emphasized that auditory deprivation during development is especially harmful in causing degeneration and/or reorganization in both the peripheral and central auditory system (Eggermont and Bock, 1986; Harris and Rubel, 2006; Kitzes, 1996; Kitzes et al., 1995; Moore and Kitzes, 1985; Moore and Kowalchuk, 1988; Niparko and Finger, 1997; Nordeen et al., 1983; Russell and Moore, 1995). With pediatric implants, it is generally assumed that restoring auditory input during this critical period will be more effective in preventing the degenerative consequences of deafness and that the immature auditory system will be better able to adapt to electrical stimulation.
However, we suggest that it is critically important to better understand the specific factors and mechanisms underlying SG degeneration following early onset deafness. To our knowledge the present study is the first report on the effects of exogenous neurotrophins in deafened, developing animals. Our findings in cats deafened prior to hearing onset suggest that intracochlear exogenous BDNF can promote improved survival of the cochlear SG neurons in an animal model of congenital deafness. Alam and coworkers (2007) have reported that there are two phases in the degeneration of SG neurons after early deafness in rats, an initial phase when apoptosis is correlated with reduced neurotrophic signaling (reduced CREB phosphorylation) and a later period after postnatal day 60 when activity in the proapoptotic JNK-Jun signaling pathway is tightly correlated with a slower phase of SG apoptosis. We hypothesize the initial rapid degeneration and later slower SG cell degeneration in our early-deafened cats is correlated with these mechanisms (Leake, 2007). If so, exogenous neurotrophins should be maximally effective during the early phase until about postnatal day 60, consistent with the results of the current study. Moreover, electrical stimulation should be effective in reducing degeneration during the later, slower phase of cell loss. This hypothesis will be addressed in future studies combining BDNF infusion with prolonged electrical stimulation from a CI.
Combined with many previous studies showing SG protection with BDNF in adult animals, our results suggest that BDNF may offer promise as a potential therapeutic agent to promote SG survival in the developing auditory system. However, the use of osmotic pumps to deliver neurotrophic factors clearly is not a good option for clinical application (Gillespie and Shepherd, 2005; Leake et al., 2008; Shepherd et al., 2008; Staecker et al., 2010). Cell-based therapies, perhaps in conjunction with gene transfer or some form of encapsulation to prevent migration and dispersal, may offer a better alternative, but these methods are still in early development and concerns about potential side-effects and risks have not yet been adequately addressed. Many important questions must be addressed with regard to selection of suitable neurotrophic factors, dosage, duration of treatment, long term effects, and development of appropriate drug delivery systems, prior to clinical application of neurotrophic factors within the inner ear (for reviews see: Gillespie and Shepherd, 2005; Staecker et al., 2010).
ACKNOWLEDGEMENTS
This work was supported by the U.S. National Institutes of Health, the National Institute on Deafness and Other Communication Disorders, Contract #HHS-N-263-2007-00054-C, The Epstein Fund and Hearing Research Inc. Human recombinant BDNF used for these studies was generously provided by Amgen Inc., Thousand Oaks, CA.
Grant sponsor: This research was supported by Contract #HHS-N-263-2007-00054-C from the National Institute on Deafness and Other Communication Disorders of the National Institutes of Health and the Epstein Fund.
LITERATURE CITED
- Agterberg MJ, Versnel H, van Dijk LM, de Groot JC, Klis SF. Enhanced survival of spiral ganglion cells after cessation of treatment with brain-derived neurotrophic factor in deafened guinea pigs. J Assoc Res Otolaryngol. 2009;10(3):355–67. doi: 10.1007/s10162-009-0170-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agterberg MJ, Versnel H, de Groot JC, Smoorenburg GF, Albers FW, Klis SF. Morphological changes in spiral ganglion cells after intracochlear application of brain-derived neurotrophic factor in deafened guinea pigs. Hear Res. 2008;244(1-2):25–34. doi: 10.1016/j.heares.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Alam SA, Robinson BK, Huang J, Green SH. Prosurvival and proapoptotic intracellular signaling in rat spiral ganglion neurons in vivo after the loss of hair cells. J Comp Neurol. doi: 10.1002/cne.21430. In Press. [DOI] [PubMed] [Google Scholar]
- Araki S, Atsushi K, Seldon HL, Shepherd RK, Funasaka S, Clark GM. Effects of chronic electrical stimulation on spiral ganglion neuron survival and size in deafened kittens. Laryngoscope. 1998;108:687–695. doi: 10.1097/00005537-199805000-00012. [DOI] [PubMed] [Google Scholar]
- Chikar JA, Colesa DJ, Swiderski DL, Di Polo A, Raphael Y, Pfingst BE. Over-expression of BDNF by adenovirus with concurrent electrical stimulation improves cochlear implant thresholds and survival of auditory neurons. Hear Res. 2008;245(1-2):24–34. doi: 10.1016/j.heares.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coco A, Epp SB, Fallon JB, Xu J, Millard RE, Shepherd RK. Does cochlear implantation and electrical stimulation affect residual hair cells and spiral ganglion neurons? Hear. Res. 2007;225:60–70. doi: 10.1016/j.heares.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettman SJ, Pinder D, Briggs RJ, Dowell RC, Leigh JR. Communication development in children who receive the cochlear implant younger than 12 months: risks versus benefits. Ear Hear. 2007;28(2 Suppl):11S–18S. doi: 10.1097/AUD.0b013e31803153f8. [DOI] [PubMed] [Google Scholar]
- Endo T, Nakagawa T, Kita T, Iguchi F, Kim TS, Tamura T, Iwai K, Tabata Y, Ito J. Novel strategy for treatment of inner ears using a biodegradable gel. Laryngoscope. 2005;115(11):2016–20. doi: 10.1097/01.mlg.0000183020.32435.59. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ, Bock GR, editors. Critical periods in auditory development. Acta Otolaryngol. (Stockholm) 1986;(Suppl. 429):1–64. [PubMed] [Google Scholar]
- Ernfors P, Duan ML, El-Shamy WM, Banlon B. Protection of auditory neurons from aminoglycoside toxicity by neurotrophin-3. Nat Med. 1996;2:463–467. doi: 10.1038/nm0496-463. [DOI] [PubMed] [Google Scholar]
- Elverland HH, Mair IW. Hereditary deafness in the cat. An electron microscopic study of the spiral ganglion. Acta Otolaryngol. 1980;90:360–9. doi: 10.3109/00016488009131737. [DOI] [PubMed] [Google Scholar]
- Farinas I, Jones KR, Tessarollo L, Vigers AJ, Kirstein M, deCaprona DC, Voppola V, Backus C, Reichardt LF, Fritzsch B. Spatial shaping of cochlear innervation by temporally regulated neurotrophin expression. J. Neurosci. 2001;21(16):6170–6180. doi: 10.1523/JNEUROSCI.21-16-06170.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraysse B, Macias AR, Sterkers O, Burdo S, Ramsden R, Dequine O, Klenzner T, Lenarz T, Rodriguez MM, Von Wallenberg E, James C. Residual hearing conservation and electroacoustic stimulation with the nucleus 24 contour advance cochlear implant. Otol. Neurotol. 2006;27(5):624–633. doi: 10.1097/01.mao.0000226289.04048.0f. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Pirvola U, Ylikoski J. Making and breaking the innervation of the ear: neurotrophic support during ear development and its clinical implications. Cell Tissue Res. 1999;295:369–382. doi: 10.1007/s004410051244. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Tessarollo L, Coppola V, Reichardt LF. Neurotrophins in the ear: their roles in sensory neuron survival and guidance. Prog. Brain. Res. 2004;146:265–278. doi: 10.1016/S0079-6123(03)46017-2. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Pauley S, Matei V, Katz DM, Xiang M, Tessarollo L. Mutant mice reveal the molecular and cellular basis for specific sensory connections to inner ear epithelia and primary nuclei of the brain. Hear Res. 2005;206:52–63. doi: 10.1016/j.heares.2004.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W-Q, Zheng JL, Karihaloo M. Neurotrophin-4/5 (NT-4/5) and brain-derived neurotrophic factor act at later stages of cerebellar granule cell differentiatiation. J. Neurosci. 1995;15:2656–2667. doi: 10.1523/JNEUROSCI.15-04-02656.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geers AE. Speech, language and relating skills after early cochlear implantation. Arch. Otolaryngol. Head Neck Surg. 2004;130:634–638. doi: 10.1001/archotol.130.5.634. [DOI] [PubMed] [Google Scholar]
- Gillespie LN, Shepherd RK. Clilnical application of neurotrophic factors: the potential for primary auditory neuron protection. Eur J Neurosci. 2005;22:2123–2133. doi: 10.1111/j.1460-9568.2005.04430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glueckert R, Bitsche M, Miller JM, Zhu Y, Prieskorn DM, Altschuler RA, Schrott-Fischer A. Deafferentation-associated changes in afferent and efferent processes in the guinea pig cochlea and afferent regeneration with chronic intrascalar brain-derived neurotrophic factor and acidic fibroblast growth factor. J Comp Neurol. 2008;507(4):1602–21. doi: 10.1002/cne.21619. [DOI] [PubMed] [Google Scholar]
- Hansen MR, Zha X-M, Bok J, Green SH. Multiple distinct signal pathways, including an autocrine neurotrophic mechanism contribute to the survival-promoting effect of depolarization on spiral ganglion neurons in vitro. J. Neuroscience. 2001;21:2256–2267. doi: 10.1523/JNEUROSCI.21-07-02256.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen MR, Bok J, Devaiah AK, Zha XM, Green SH. Ca2+/calmodulin-dependent protein kinases II and IV both promote survival but differ in their effects on axon growth in spiral ganglion neurons. J Neurosci Res. 2003;72(2):169–184. doi: 10.1002/jnr.10551. [DOI] [PubMed] [Google Scholar]
- Harris JA, Rubel EW. Afferent regulation of neuron number in the cochlear nucleus: cellular and molecular analyses of a critical period. Hear. Res. 2006;216–217:127–137. doi: 10.1016/j.heares.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Hartmann R, Topp G, Klinke R. Electrical stimulation of the cat cochlea: Discharge pattern of single auditory fibers. Adv. Audiol. 1984;1:18–29. doi: 10.1016/0378-5955(84)90094-7. [DOI] [PubMed] [Google Scholar]
- Hartshorn DO, Miller JM, Altschuler RA. Protective effect of electrical stimulation in the deafened guinea pig cochlea. Otolaryngol. Head Neck Surg. 1991;104:311–319. doi: 10.1177/019459989110400305. [DOI] [PubMed] [Google Scholar]
- Hawkins JE, Jr, Stebbins WC, Johnsson LG, Moody DB, Muraki A. The patas monkey as a model for dihydrostreptomycin ototoxicity. Acta Otolaryngol. 1977;83:123–129. doi: 10.3109/00016487709128821. [DOI] [PubMed] [Google Scholar]
- Hegarty J, Kay AR, Green SH. Trophic support of cultured spiral ganglion neurons by depolarization exceeds and is additive with that by neurotrophins or cAMP and requires elevation of [CA2+]i within a set range. J Neurosci. 1997;17:1959–1970. doi: 10.1523/JNEUROSCI.17-06-01959.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochmair I, Nopp P, Jolly C, Schmidt M, Schößer H, Garnham C, Anderson I. MED-EL cochlear implants: state of the art and a glimpse into the future. Trends Amplificat. 2006;10(4):201–220. doi: 10.1177/1084713806296720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James CJ, Fraysse B, Deguine O, Lenarz T, Mawman D, Ramos A, Ramsden R, Sterkers O. Combined electroacoustic stimulation in conventional candidates for cochlear implantation. Audiol. Neurootol. 2006;11(Suppl. 1):57–62. doi: 10.1159/000095615. [DOI] [PubMed] [Google Scholar]
- Kanzaki S, Stover T, Kawamoto K, Priskorn DM, Altschuler RA, Miller JM, Raphael Y. GDNF and chronic electrical stimulation prevent VIII cranial nerve degeneration following denervation. J. Comp Neurol. 2002;454:450–360. doi: 10.1002/cne.10480. [DOI] [PubMed] [Google Scholar]
- Kiang NYS, Rho JM, Northrop CC, Liberman MC, Ryugo DK. Hair cell innervation by spiral ganglion cells in adult cats. Science. 1982;217:129–177. doi: 10.1126/science.7089553. [DOI] [PubMed] [Google Scholar]
- Kiang NYS, Liberman MC, Levine RA. Auditory nerve activity in cats exposed to ototoxic drugs and high-intensity sounds. Ann Otol Rhinol Laryngol. 1976;85:752–768. doi: 10.1177/000348947608500605. [DOI] [PubMed] [Google Scholar]
- Kiang NYS, Liberman MC, Gage JS, Northrop CC, Dodds LW, Oliver ME. Afferent innnervation of the mammalian cochlea. In: Bolis L, Deynes RD, Maddrel SHP, editors. Comparative Physiology of Sensory Systems. Cambridge University Press; New York: 1984. pp. 143–161. [Google Scholar]
- Kitzes L. Anatomical and physiological changes in the brainstem induced by neonatal ablation of the cochlea. In: Salvi RJ, Henderson D, editors. Auditory System Plasticity and Regeneration. Thieme Medical Publishers; New York: 1996. pp. 256–274. [Google Scholar]
- Kitzes LM, Kageyama GH, Semple MN, Kil J. Development of ectopic projections from the ventral cochlear nucleus to the superior olivary complex induced by neonatal ablation of the contralateral cochlea. J. Comp. Neurol. 1995;353(3):341–363. doi: 10.1002/cne.903530303. [DOI] [PubMed] [Google Scholar]
- Korsching S. The neurotrophic factor concept: A reexamination. J. Neurosci. 1993;13:2739–2748. doi: 10.1523/JNEUROSCI.13-07-02739.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leake PA, Hradek GT. Cochlear pathology of long term neomycin induced deafness in cats. Hearing Res. 1988;33:11–34. doi: 10.1016/0378-5955(88)90018-4. [DOI] [PubMed] [Google Scholar]
- Leake PA, Hradek GT, Snyder RL, Rebscher SJ. Chronic intracochlear electrical stimulation induces selective survival of spiral ganglion cells in neonatally deafened cats. Hearing Res. 1991;54:251–271. doi: 10.1016/0378-5955(91)90120-x. [DOI] [PubMed] [Google Scholar]
- Leake PA, Hradek GT, Snyder RL. Chronic electrical stimulation by a cochlear implant promotes survival of spiral ganglion neurons in neonatally deafened cats. J. Comp. Neurol. 1999;412:543–562. doi: 10.1002/(sici)1096-9861(19991004)412:4<543::aid-cne1>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Leake PA, Hradek GT, Vollmer M, Rebscher SJ. Neurotrophic Effects of GM1 Ganglioside and Electrical Stimulation on Cochlear Spiral Ganglion Neurons in Cats Deafened as Neonates. J. Comp. Neurol. 2007;501:837–853. doi: 10.1002/cne.21275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leake PA, Kuntz AL, Moore CM, Chambers PL. Cochlear pathology induced by aminoglycoside ototoxicity during postnatal maturation in cats. Hearing Res. 1997;113:117–132. doi: 10.1016/s0378-5955(97)00133-0. [DOI] [PubMed] [Google Scholar]
- Leake PA, Snyder RL, Hradek GT, Rebscher SJ. Chronic intracochlear electrical stimulation in neonatally deafened cats: Effects of intensity and stimulating electrode location. Hearing Res. 1992;64:99–117. doi: 10.1016/0378-5955(92)90172-j. [DOI] [PubMed] [Google Scholar]
- Leake PA, Snyder RL, Hradek GT, Rebscher SJ. Consequences of chronic extracochlear electrical stimulation in neonatally deafened cats. Hearing Res. 1995;82:65–80. doi: 10.1016/0378-5955(94)00167-o. [DOI] [PubMed] [Google Scholar]
- Leake PA, Stakhovskaya O, Hradek GT. Factors influencing neurotrophic effects of electrical stimulation in the deafened, developing auditory system. Hearing Res. 2008;242(1-2):86–99. doi: 10.1016/j.heares.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre PP, Van de Water TR, Weber T, Rogister B, Moonen G. Browth factor interactions in cultures of dissociated adult acoustic ganglia: neuronotrophic effects. Brain Res. 1991;567(2):306–312. doi: 10.1016/0006-8993(91)90809-a. [DOI] [PubMed] [Google Scholar]
- Lefebvre PP, Malgrange B, Staecker H, Moghadass M, Van de Water TR, Moonen G. Neurotrophins affect survival and neuritogenesis by adult injured auditory neurons in vitro. Neuroreport. 1994;5:865–868. doi: 10.1097/00001756-199404000-00003. [DOI] [PubMed] [Google Scholar]
- Li L, Parkins CW, Webster DB. Does electrical stimulation of deaf cochleae prevent spiral ganglion degeneration? Hear Res. 1999;133:27–39. doi: 10.1016/s0378-5955(99)00043-x. [DOI] [PubMed] [Google Scholar]
- Liberman MC, Kiang NY. Acoustic trauma in cats. Cochlear pathology and auditory-nerve activity. Acta Otolaryngol. 1978;358(Suppl.):1–63. [PubMed] [Google Scholar]
- Liberman MC, Dodds LW, Pierce S. Afferent and efferent innervation of the cat cochlea: quantitative analysis with light and electron microscopy. J Comp Neurol. 1990;301:443–460. doi: 10.1002/cne.903010309. [DOI] [PubMed] [Google Scholar]
- Lousteau RJ. Increased spiral ganglion cell survival in electrically stimulated, deafened guinea pig cochleae. Laryngoscope. 1987;97:836–842. [PubMed] [Google Scholar]
- Malgrange B, Lefebvre PP, Martin D, Staecker H, Van de Water TR, Moonen G. NT-3 has a tropic effect on process outgrowth by postnatal auditory neurones in vitro. Neuroreport. 1996;7:2495–2499. doi: 10.1097/00001756-199611040-00019. [DOI] [PubMed] [Google Scholar]
- Maruyama J, Miller JM, Ulfendahl M. Glial cell line-derived neurotrophic factor and antioxidants preserve the electrical responsiveness of the spiral ganglion neurons after experimentally induced deafness. Neurobiol Dis. 2008;29:14–21. doi: 10.1016/j.nbd.2007.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuinness SL, Shepherd RK. Exogenous BDNF rescues rat spiral ganglion neurons in vivo. Otol Neurotol. 2005;26:1064–1072. doi: 10.1097/01.mao.0000185063.20081.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AL. Effects of chronic stimulation on auditory nerve survival in ototoxically deafened animals. Hear Res. 2001;151:1–14. doi: 10.1016/s0378-5955(00)00226-4. [DOI] [PubMed] [Google Scholar]
- Miller JM, Altschuler RA. Effectiveness of different electrical stimulation conditions in preservation of spiral ganglion cells following deafness. Ann Otol Rhinol Laryngol. 1995;166:57–60. [PubMed] [Google Scholar]
- Miller JM, Chi DH, O'Keefe LJ, Kruszka P, Raphael Y, Altschuler RA. Neurotrophins can enhance spiral ganglion cell survival after inner hair cell loss. Int. J. Devl Neurosci. 1997;15:631–643. doi: 10.1016/s0736-5748(96)00117-7. [DOI] [PubMed] [Google Scholar]
- Miller JM, Le Prell CG, Prieskorn DM, Wys NL, Altschuler RA. Delayed neurotrophin treatment following deafness rescues spiral ganglion cells from death and promotes regrowth of auditory nerve peripheral processes: effects of brain-derived neurotrophic factor and fibroblast growth factor. J Neurosci Res. 2007;85(9):1959–69. doi: 10.1002/jnr.21320. [DOI] [PubMed] [Google Scholar]
- Mitchell A, Miller JM, Finger PA, Heller JW, Raphael Y, Altschuler RA. Effects of chronic high-rate electrical stimulation on the cochlea and eighth nerve in the deafened guinea pig. Hear Res. 1997;105:30–43. doi: 10.1016/s0378-5955(96)00202-x. [DOI] [PubMed] [Google Scholar]
- Moore DR, Kitzes LM. Projections from the cochlear nucleus to the inferior colliculus in normal and neonatally cochlea-ablated gerbils. J. Comp. Neurol. 1985;240:180–195. doi: 10.1002/cne.902400208. [DOI] [PubMed] [Google Scholar]
- Moore DR, Kowalchuk NE. Auditory brainstem of the ferret: effects of unilateral cochlear lesions in cochlear nucleus volume and projections to the inferior colliculus. J. Comp. Neurol. 1988;272:503–515. doi: 10.1002/cne.902720405. [DOI] [PubMed] [Google Scholar]
- Mou K, Hunsberger CL, Cleary JM, Davis RL. Synergistic effects of BDNF and NT-3 on postnatal spiral ganglion neurons. J Comp Neurol. 1997;386(4):529–39. [PubMed] [Google Scholar]
- Mou K, Adamson CL, Davis RL. Time-dependence and cell-type specificity of synergistic neurotrophin actions on spiral ganglion neurons. J Comp Neurol. 1998;402:129–139. [PubMed] [Google Scholar]
- Mouton PR. Principles and practices of unbiased stereology: An introduction for bioscientists. The Johns Hopkins University Press; Baltimore and London: 2002. [Google Scholar]
- Nakaizumi T, Kawamoto K, Minoda R, Raphael Y. Adenovirus-mediated expression of brain-derived neurotrophic factor protects spiral ganglion neurons from ototoxic damage. Audiol Neurootol. 2004;9(3):135–43. doi: 10.1159/000077264. [DOI] [PubMed] [Google Scholar]
- Nicholas JG, Geers AE. Will they catch up? The role of age at cochlear implantation in the spoken language development of children with severe to profound hearing loss. J. Speech Lang. Hear. Res. 2007;50(4):1048–1062. doi: 10.1044/1092-4388(2007/073). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niparko JK, Finger PA. Cochlear nucleus cell size changes in the Dalmatian: model of congenital deafness. Otolaryngol Head Neck Surg. 1997;117:229–235. doi: 10.1016/s0194-5998(97)70179-7. [DOI] [PubMed] [Google Scholar]
- Nordeen KW, Killackey HP, Kitzes LM. Ascending projections to the inferior colliculus following unilateral cochlear ablation in the neonatal gerbil, meriones unguiculatus. J. Comp. Neurol. 1983;214:144–153. doi: 10.1002/cne.902140204. [DOI] [PubMed] [Google Scholar]
- Paasche G, Gibson P, Averbeck T, Becker H, Lenarz T, Stover T. Technical report: modification of a cochlear implant electrode for drug delivery to the inner ear. Otol. Neurotol. 2003;24(2):222–227. doi: 10.1097/00129492-200303000-00016. [DOI] [PubMed] [Google Scholar]
- Pettingill LN, Minter RL, Shepherd RK. Schwann cells genetically modified to express neurotrophins promote spiral ganglion neuron survival in vitro. Neuroscience. 2008;27;152(3):821–8. doi: 10.1016/j.neuroscience.2007.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rejali D, Lee VA, Abrashkin KA, Humayun N, Swiderski DL, Raphael Y. Cochlear implants and ex vivo BDNF gene therapy protect spiral ganglion neurons. Hear Res. 2007 Jun;228(1-2):180–7. doi: 10.1016/j.heares.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson RT, Wise AK, Thompson BC, Flynn BO, Atkinson PJ, Fretwell NJ, Fallon JB, Wallace GG, Shepherd RK, Clark GM, O'Leary SJ. Polypyrrole-coated electrodes for the delivery of charge and neurotrophins to cochlear neurons. Biomaterials. 2009 May;30(13):2614–24. doi: 10.1016/j.biomaterials.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson RT, Wise AK, Andrew JK, O'Leary SJ. Novel drug delivery systems for inner ear protection and regeneration after hearing loss. Expert Opin Drug Deliv. 2008:1059–76. doi: 10.1517/17425247.5.10.1059. Review. [DOI] [PubMed] [Google Scholar]
- Rebscher SJ, Hetherington AM, Snyder RL, Leake PA, Bonham BH. Design and fabrication of multichannel cochlear implants for animal research. J. Neurosci. Methods. 2007;166(1):1–12. doi: 10.1016/j.jneumeth.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roehm PC, Hansen MR. Strategies to preserve or regenerate spiral ganglion neurons. CUrr. Opin. Otolaryngol. Head Neck Surg. 2005;13:295–300. doi: 10.1097/01.moo.0000180919.68812.b9. [DOI] [PubMed] [Google Scholar]
- Romand R, Romand M-R. Perinatal growth of spiral ganglion cells in the kitten. Hearing Res. 1986;21:161–165. doi: 10.1016/0378-5955(86)90036-5. [DOI] [PubMed] [Google Scholar]
- Romand R, Romand M-R. Development of spiral ganglion cells in mammalian cochlea. J. Elect Mic Tech. 1990;15:144–154. doi: 10.1002/jemt.1060150206. [DOI] [PubMed] [Google Scholar]
- Rubens RJ. Unsolved issues around critical periods with emphasis on clinical application. Acta Otolaryngol. 1986;429(Suppl.):61–64. doi: 10.3109/00016488609122732. [DOI] [PubMed] [Google Scholar]
- Rubens RJ, Rapin I. Plasticity of the developing auditory system. Ann. Otol. Rhinol. Laryngol. 1980;89:303–311. doi: 10.1177/000348948008900403. [DOI] [PubMed] [Google Scholar]
- Rubel EW, Fritzsch B. Auditory system development: Primary auditory neurons and their targets. Ann Rev Neurosci. 2002;25:51–101. doi: 10.1146/annurev.neuro.25.112701.142849. [DOI] [PubMed] [Google Scholar]
- Russell RA, Moore DR. Afferent reorganization within the superior olivary complex of the gerbil: Development and induction by neonatal unilateral cochlear removal. J. Comp. Neurol. 1995;352:607–625. doi: 10.1002/cne.903520409. [DOI] [PubMed] [Google Scholar]
- Scheper V, Paasche G, Miller JM, Warnecke A, Berkingali N, Lenarz T, Stover T. Effects of delayed treatment with combined GDNF and continuous electrical stimulation on spiral ganglion survival in deafened guinea pigs. J Neurosci Res 2009. 2009;87(6):1389–1399. doi: 10.1002/jnr.21964. [DOI] [PubMed] [Google Scholar]
- Shah SB, Gladstone HB, Williams H, Hradek GT, Schindler RA. An extended study: protective effects of nerve growth factor in neomycin-induced auditory neural degeneration. Am J Otol. 1995;16(3):310–314. [PubMed] [Google Scholar]
- Schindler RA, Gladstone HB, Scott N, Hradek GT, Williams H, Shah SB. Enhanced preservation of the auditory nerve following cochlear perfusion with nerve growth factor. Am J Otol. 1995;16:304–309. [PubMed] [Google Scholar]
- Shepherd RK, Javel E. Electrical stimulation of the auditory nerve. I. Correlation of physiological responses with cochlear status. Hear. Res. 1997;108:112–144. doi: 10.1016/s0378-5955(97)00046-4. [DOI] [PubMed] [Google Scholar]
- Shepherd RK, Coco A, Epp SB. Neurotrophins and electrical stimulation for protection and repair of spiral ganglion neurons following sensorineural hearing loss. Hear Res. 2008;242(1-2):100–9. doi: 10.1016/j.heares.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd RK, Coco A, Epp SB, Crook JM. Chronic depolarization enhances the trophic effects of Brain-Derived Neurotrophic Factor in rescuing auditory neurons following a sensorinerual hearing loss. J Comp Neurol. 2005;486:145–158. doi: 10.1002/cne.20564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd RK, Xu J. A multichannel scala tympani electrode array incorporating a drug delivery system for chronic intracochlear infusion. Hear Res. 2002;172(1–2):92–98. doi: 10.1016/s0378-5955(02)00517-8. [DOI] [PubMed] [Google Scholar]
- Shinohara T, Bredberg G, Ulfendahl M, Pyykko I, Olivius NP, Kaksonen R, Lindstrom B, Altschuler R, Miller JM. Neurotrophic factor intervention restores auditory function in deafened animals. Proc. Natl. Acad. Sci. USA. 2002;99:1657–1660. doi: 10.1073/pnas.032677999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song BN, Li YX, Han DM. Delayed electrical stimulation and BDNF application following induced deafness in rats. Acta Otolaryngol. 2009;129(2):142–54. doi: 10.1080/00016480802043949. [DOI] [PubMed] [Google Scholar]
- Spoendlin HH. Innervation patterns in the organ of Corti of the cat. Acta Otolaryngol. 1969;67:239–254. doi: 10.3109/00016486909125448. [DOI] [PubMed] [Google Scholar]
- Spoendlin HH. Retrograde degeneration of the cochlear nerve. Acta Otolaryngol. 1975;79:266–275. doi: 10.3109/00016487509124683. [DOI] [PubMed] [Google Scholar]
- Spoendlin HH. Differentiation of cochlear neurons. Acta Otolaryngol. 1981;91:451–456. doi: 10.3109/00016488109138527. [DOI] [PubMed] [Google Scholar]
- Stankovic K, Rio C, Xia A, Sugawara M, Aams JC, Liberman MC, Corfas G. Survival of adult spiral ganglion neurons requires erbB receptor signaling in the inner ear. J Neurosci. 2004;24:8651–8661. doi: 10.1523/JNEUROSCI.0733-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staecker H, Jolly C, Garnham C. Cochlear implantatin: an opportunity for drug development. Drug Disc Today. 2010;15:314–321. doi: 10.1016/j.drudis.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Staecker H, Kopke R, Malgrange B, Lefebvre P, Van de Water TR. NT3 and BDNF therapy prevents loss of auditory neurons following loss of hair cells. NeuroReport. 1996;7:889–894. doi: 10.1097/00001756-199603220-00011. [DOI] [PubMed] [Google Scholar]
- Staecker H, Gabaizadeh R, Rederoff H, Van de Water TR. Brain-derived neurotrophic factor gene therapy prevents spiral ganglion degeneration after hair cell loss. Otolaryngol. Head Neck Surg. 1998;119:7–13. doi: 10.1016/S0194-5998(98)70194-9. [DOI] [PubMed] [Google Scholar]
- Svirsky MA, Robbins AM, Kirk KI, Pisoni DB, Miyamoto RT. Language development in profoundly deaf children with cochlear implants. Psychol. Sci. 2000;11(2):153–158. doi: 10.1111/1467-9280.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svirsky MA, Teoh SW, Neuburger H. Development of language and speech perception in congenitally, profoundly deaf children as a function of age at cochlear implantation. Audiol. Neurotol. 2004;9(4):224–233. doi: 10.1159/000078392. [DOI] [PubMed] [Google Scholar]
- Turner CW, Reiss J, Gantz BJ. Combined acoustic and electric hearing: Preserving residual acoustic hearing. Hear Res. 2008;242:164–171. doi: 10.1016/j.heares.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira M, Christensen BL, Wheeler BC, Feng AS, Kollmar R. Survival and stimulation of neurite outgrowth in a serum-free culture of spiral ganglion neurons from adult mice. Hear Res. 2007;230:17–23. doi: 10.1016/j.heares.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Versnel H, Agterberg MJ, de Groot JC, Smoorenburg GF, Klis SF. Time course of cochlear electrophysiology and morphology after combined administration of kanamycin and furosemide. Hear Res. 2007;231(1-2):1–12. doi: 10.1016/j.heares.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Warnecke A, Wissel K, Hoffmann A, Hofmann N, Berkingali N, Gro G, Lenarz T, Stöver T. The biological effects of cell-delivered brain-derived neurotrophic factor on cultured spiral ganglion cells. Neuroreport. 2007;18(16):1683–6. doi: 10.1097/WNR.0b013e3282f0b5d7. [DOI] [PubMed] [Google Scholar]
- Wefstaedt P, Scheper V, Lenarz T, Stöver T. Brain-derived neurotrophic factor/glial cell line-derived neurotrophic factor survival effects on auditory neurons are not limited by dexamethasone. Neuroreport. 2005;16(18):2011–4. doi: 10.1097/00001756-200512190-00008. [DOI] [PubMed] [Google Scholar]
- Wise AK, Richardson R, Hardman J, Clark G, O'Leary S. Resprouting and survival of guinea pig cochlear neurons in response to the administration of the neurotrophins brain-derived neurotrophic factor and neurotrophin-3. J. Comp. Neurol. 2005;487:147–165. doi: 10.1002/cne.20563. [DOI] [PubMed] [Google Scholar]
- Yagi M, Kanzaki S, Kawamoto K, Shin B, Shah PP, Magal E, Sheng J, Raphael Y. Spiral ganglion neurons are protected from degeneration by GDNF gene therapy. JARO. 2000;1:315–325. doi: 10.1007/s101620010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata T, Miller JM, Ulfendahl M, Olivius NP, Altschuler RA, Pyykkö I, Bredberg G. Delayed neurotrophic treatment preserves nerve survival and electrophysiological responsiveness in neomycin-deafened guinea pigs. J Neurosci Res. 2004 Oct 1;78(1):75–86. doi: 10.1002/jnr.20239. [DOI] [PubMed] [Google Scholar]
- Ylikoski J, Privola U, Virkkala J, Suvanto P, Liang X-Q, Magal E, Altschuler RA, Miller JM, Saarma M. Guinea pig auditory neurons are protected by glial cell line-derived growth factor from degeneration after noise trauma. Hearing Res. 1998;124:17–26. doi: 10.1016/s0378-5955(98)00095-1. [DOI] [PubMed] [Google Scholar]
- Zeng F-G, Rebscher S, Harrison WV, Sun X, Feng H. Cochlear Implants:System Design, Integration and Evaluation. IEEE Rev Biomed Eng. 2008;1:115–142. doi: 10.1109/RBME.2008.2008250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zha XM, Bishop JF, Hansen MR, Victoria L, Abbas PJ, Mouradian MM, Green SH. BDNF synthesis in spiral ganglion neurons is constitutive and CREB-dependent. Hearing Res. 2001;156:53–68. doi: 10.1016/s0378-5955(01)00267-2. [DOI] [PubMed] [Google Scholar]
- Zheng JL, Gao W-Q. Differential damage to auditory neurons and hair cells by ototoxins and neuroprotection by specific neurotrophins in rat cochlear organotypic cultures. Eur. J. Neurosci. 1996;8:1897–1905. doi: 10.1111/j.1460-9568.1996.tb01333.x. [DOI] [PubMed] [Google Scholar]
- Zheng JL, Stewart RR, Gao W-Q. Neurotrophin-4/5 enhances survival of cultured spiral ganglion neurons and protects them from cisplain ototoxicity. J Neurosci. 1995;7:5079–5087. doi: 10.1523/JNEUROSCI.15-07-05079.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]