Abstract
Cellular responses to extrinsic and intrinsic insults have to be carefully regulated to properly coordinate cytoprotection, repair processes, cell proliferation and apoptosis. Stress signaling pathways, most prominently the Jun-N-terminal Kinase (JNK) pathway, are critical regulators of such cellular responses and have accordingly been implicated in the regulation of lifespan in various organisms. JNK signaling promotes cytoprotective gene expression, but also interacts with the Insulin signaling pathway to influence growth, metabolism, stress tolerance and regeneration. Here, we review recent studies in Drosophila that elucidate the tissue-specific and systemic consequences of JNK activation that ultimately impact lifespan of the organism.
Introduction
To maintain homeostasis, metazoans have to respond to extrinsic and intrinsic stressors by inducing defense mechanisms at the cellular, tissue and organismic level. Such mechanisms include cytoprotective gene expression, regenerative responses, apoptosis and metabolic adaptation. Signaling mechanisms that govern cellular and organismic responses to stress thus significantly impact stress tolerance, metabolic homeostasis and lifespan of the organism. To gain insight into the physiological processes maintaining homeostasis in adult animals, and into the causes for the age-related breakdown of these processes, it is thus critical to explore the interactions of stress-responsive signaling with regulatory processes that govern cytoprotection, metabolism, cell proliferation, and tissue regeneration.
The JNK signaling pathway: a conserved regulator of lifespan
Among the most versatile and ubiquitous stress sensors in metazoans is the Jun-N-terminal Kinase (JNK) signaling pathway. JNK is an evolutionarily conserved stress-activated protein kinase (SAPK) that is induced by a range of intrinsic and environmental insults (e.g. UV irradiation, reactive oxygen species, DNA damage, heat, bacterial antigens, and inflammatory cytokines; Figure 1). These stimuli selectively activate a member of the JNK Kinase Kinase family (at least 20 are known in mammals), which then phosphorylates and activates a dual-specificity Kinase of the MKK family that phosphorylates JNK on Serine/Threonine and Tyrosine residues (MKK4 and 7 in mammals) (Johnson and Nakamura, 2007; Weston and Davis, 2007). JNK itself has a number of nuclear and cytoplasmic targets, most prominently transcription factors, including the AP-1 family members Jun and Fos and the Forkhead Box O transcription factor FoxO (Johnson and Nakamura, 2007; Weston and Davis, 2007). Changes in the cellular transcriptome are thus a major part of the cellular response to JNK activation (Jasper et al., 2001; Johnson and Nakamura, 2007). In Drosophila, an important target gene of AP-1 is puckered (puc), which encodes a JNK-specific phosphatase that restricts JNK activity in a negative feedback loop (Martin-Blanco et al., 1998; McEwen and Peifer, 2005).
Figure 1. Physiologic consequences of JNK signaling in Drosophila.
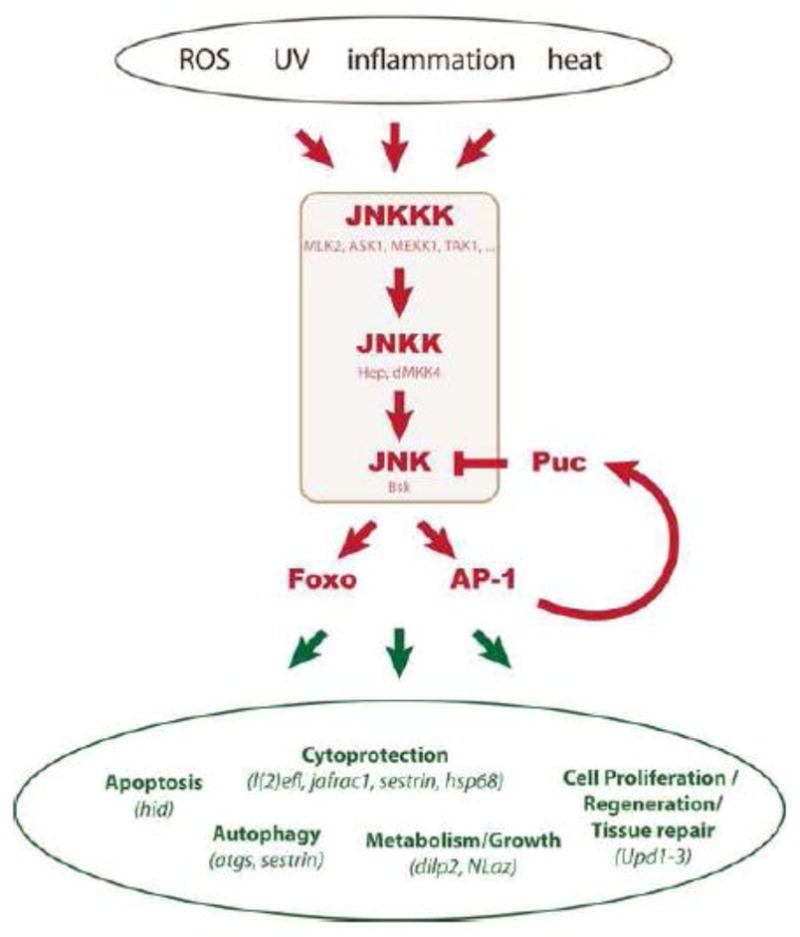
The Drosophila JNK signaling pathways consists of a single JNK (Basket), two JNKKs (Hemipterous and dMKK4) and a number of JNKKKs (including MLK: Mixed Lineage Protein Kinase 2/Slipper; ASK1: Apoptotic signal-regulating Kinase 1; TAK1: TGF- Activated Kinase 1; MEKK1: MEK Kinase1). The kinase cascade is activated by a variety of stressful insults, results in activation of the transcription factors AP-1 (Jun/Fos heterodimers) and Foxo, and causes a variety of tissue-specific and context-specific cellular responses. The JNK phosphatase Puckered (Puc) is a target gene of AP-1 and limits JNK activity in a negative feedback loop. Selected genes that are transcriptionally regulated in response to JNK activation, and that mediate specific physiologic consequences of JNK activation, are listed in green.
While the individual components of the JNK signaling pathway are represented by large gene families in vertebrates, the pathway is significantly less complex in flies, simplifying genetic analysis (Igaki, 2009; Johnson and Nakamura, 2007). Thus, only a single JNK (“Basket”) and two JNK Kinases (“Hemipterous”, Hep, a MKK7 homologue that mediates the majority of JNK signaling effects in flies, and the somewhat less studied dMKK4, which acts in parallel with Hep in the induction of apoptosis and immune responses, but might also mediate specific heavy metal responses (Boutros et al., 2002; Chen et al., 2002; Geuking et al., 2009)) are encoded in the Drosophila genome. The diverse and highly context-dependent consequences of JNK activation, however, are conserved between vertebrates and invertebrates. JNK signaling regulates a wide array of cellular functions, ranging from apoptosis over morphogenesis and cell migration to cytoprotection and metabolism in flies and mice (Igaki, 2009; Johnson and Nakamura, 2007; Sabio and Davis). These diverse effects of JNK activation are specified in a context-dependent manner by signal integration between JNK and other cellular signaling pathways (e.g. NFkappaB and EGFR signaling in the decision between apoptosis and survival (Janes et al., 2006; Karin and Gallagher, 2005; Lin, 2003; Pham et al., 2004)). Highlighting the importance of JNK signaling as a determinant of cellular responses to stress, its misregulation has been implicated in a wide range of pathologies, including neurodegenerative diseases, diabetes, and cancer (Hotamisligil, 2010; Karin and Gallagher, 2005; Sabio and Davis; Weston and Davis, 2002).
In flies, JNK is required during development for morphogenetic processes (embryonic dorsal closure and thorax closure in pupae), as well as for synaptic plasticity and for stress-induced apoptosis (Etter et al., 2005; Igaki, 2009; Luo et al., 2007). Interestingly, moderate activation of JNK signaling results in increased stress tolerance and extended lifespan (Libert et al., 2008; Wang et al., 2003, 2005). Flies heterozygous for puc, or in which JNKK/Hep is overexpressed in neuronal tissue, are resistant to the oxidative stress-inducing compound Paraquat, while JNKK/Hep mutant flies exhibit increased stress sensitivity and are deficient in the induction of a transcriptional stress response (Wang et al., 2003). Supporting the protective role for JNK signaling in flies, puc heterozygotes or Hep over-expressing animals are long-lived under normal conditions (Libert et al., 2008; Wang et al., 2003, Table 1). Similar consequences of JNK activation have been described in C.elegans. Worms with increased JNK activity due to inhibition of the JNK phosphatase VHP-1 (VH1 dual-specificity phosphatase) are protected against heavy metal toxicity, and over-expression of JNK increases lifespan under normal conditions (Mizuno et al., 2004; Oh et al., 2005).
Table 1. Lifespan studies with JNK signaling components and JNK target genes in Drosophila.
References in which demographic data for loss- or gain-of-function conditions for JNK signaling components have been reported are listed. Tissue-specific manipulations are identified and lifespan changes are listed as increased (+) or decreased (−) compared to control genotypes.
| Genotype | Control Genotype | Tissue | Lifespan | Reference |
|---|---|---|---|---|
| pucE69, ry506/+ | +/+ (OreR) | Ubiquitous | + | Wang et al., 2003 |
| pucA251.1/+ | +/+ (OreR) | + | ||
| pucE69, ry506/ry506 | ry506/ry506 | + |
Wang et al., 2003 Libert et al., 2008 |
|
| pucE69/+ |
hep1/y hep1/y;;pucE69/+ |
+ | Wang et al., 2003 | |
|
yw/+;;pucE69, ry506/+ yw/y;;pucE69/+ |
yw/+ or y;;pucE69, ry506/dfoxo25 yw/+ or y;;dfoxo25/+ yw/+ or y;;ry506/+ |
+ | Wang et al., 2005 | |
|
yw/+;;pucE69, ry506/+ yw/y;;pucE69/+ |
yw/+ or y;;pucE69, ry506/dfoxo21 yw/+ or y;;dfoxo21/+ yw/+ or y;;ry506/+ |
+ | ||
| w/Y;armGal4/+;UASl(2)efl/+ | w/Y;armGal4 | + | ||
| armGal4/UASHsp68 | armGal4/+ | + | Wang et al., 2003 | |
| NLazNW5/NW5 | +/+ (isogenic) | − | Hull-Thompson et al., 2009 | |
| DaGal4/UASNLaz | DaGal4/+ and UASNLaz/+ | + | ||
| w; Dilp2Gal4/UASHep | w; Dilp2Gal4/+ | IPCs | + | Wang et al., 2005 |
| elavC155Gal4/y; UAS Hep/+ | ElavGal4/y | Pan- neuronal | + | Wang et al., 2003 |
| w, elavC155Gal4/y;;UASl(2)efl/+ | w, elavC155Gal4/y | + | Wang et al., 2005 | |
| elavGal4/UASjafrac1 | elavGal4/+ and +/+ | + | Lee et al., 2009 | |
| elavGS/UASjafrac1 (+RU) | elavGS/UASjafrac1 (−RU) | + | ||
| esgGal4/UASHep | esgGal4/+ | Intestine (ISCs/EBs) | − | Biteau et al., 2008 |
| 5961GS/UASBskRNAi (+RU) | 5961GS/UAS BskRNAi (−RU) | + | Biteau et al., 2010 | |
| 5961GS/UASBskDN (+RU) | 5961GS/UAS BskDN (−RU) | + | ||
| esgGal4/UASBskRNAi;tubGal80ts (29ºC) | esgGal4/+; tubGal80ts (29ºC) | − | ||
| esgGal4/UASHep;tubGal80ts (29ºC) | esgGal4/+;tubGal80ts (29ºC) | − | ||
| esgGal4/UASjafrac1 | esgGal4/+ | + | ||
| esgGal4/UASHsp68 | esgGal4/+ | + | ||
| 5961GS/UASjafrac1+Hsp68 (+RU) | 5961GS/UASjafrac1+Hsp68 (−RU) | + |
Mechanisms of lifespan extension by JNK signaling
JNK thus emerges as an evolutionarily conserved determinant of metazoan lifespan. However, chronic JNK activation also causes degenerative diseases, diabetes and cancer in vertebrates, suggesting that the pleiotropic cellular consequences of JNK activation can both positively and negatively influence longevity. It is expected that tissue-specific functions of JNK signaling critically modulate the overall physiologic consequences of elevating its activity. At the same time, it is likely that the strength and duration of JNK activation determines whether lifespan is extended or compromised (Karpac and Jasper, 2009). In this respect, JNK activation might be considered a mediator of hormesis, in which low-level exposure to stressful insults can extend lifespan (Le Bourg, 2009). Due to its pleiotropic nature and the dose-dependency of cellular responses to JNK activation, the mechanism(s) by which JNK promotes longevity are expected to be manifold, and are only starting to be understood. In the following, we review studies in Drosophila that highlight several mechanisms by which JNK signaling influences lifespan:
Cytoprotection
Many age-related diseases are associated with oxidative damage, and protection against such damage by scavenging reactive oxygen species (ROS), as well as repair of damaged macromolecules by chaperones or DNA repair enzymes is expected to positively influence lifespan (Nathan and Ding, 2010; Sykiotis and Bohmann, 2010). A battery of such cytoprotective genes are induced in flies in response to exposure to the oxidative stress-inducing compound Paraquat. This induction is dependent on JNK activity, suggesting that the lifespan extension observed in JNK gain-of-function conditions is caused, at least in part, by promoting overall cellular stress resistance and damage repair (Wang et al., 2003). This idea is supported by the fact that selected cytoprotective JNK target genes also extend lifespan and/or promote overall stress tolerance of the organism when over-expressed in various tissues. Among the proteins encoded by these target genes are the heat-shock proteins Hsp68 and L(2)efl, the peroxiredoxin Jafrac1, and Sestrin, a potential antioxidant and a negative feedback regulator of Tor signaling that may limit ROS accumulation in aging tissues by promoting autophagy (Lee et al., 2010; Lee et al., 2009; Wang et al., 2003, 2005). The tissue-specific functions of these target genes are only partially understood. While neuronal expression of Jafrac1 is sufficient for lifespan extension, loss of Sestrin causes metabolic imbalances, muscle degeneration and cardiac dysfunction (Lee et al., 2010; Lee et al., 2009). Similarly, JNK signaling is required in differentiated cells of the intestinal epithelium to prevent excessive sensitivity of these cells to oxidative stress (Biteau et al., 2008). The cytoprotective effects of JNK and its target genes are thus likely to be widespread, affecting a majority of tissues in the adult fly.
JNK/IIS antagonism
In addition to regulating cellular defenses and repair processes, JNK also intersects with the Insulin/IGF signaling (IIS) pathway to influence lifespan. Reduced IIS signaling activity extends lifespan in flies, mice and worms by negatively regulating Foxo transcription factors (Karpac and Jasper, 2009; Kenyon, 2005; Tatar et al., 2003). JNK counteracts IIS activity, and JNK-mediated lifespan extension is accordingly dependent on the activity of Foxo in both flies and worms (Karpac and Jasper, 2009; Oh et al., 2005; Wang et al., 2005). The antagonism between JNK and IIS activity is evolutionarily conserved and is mediated by at least three separate mechanisms (Figure 2):
Figure 2. Endocrine interactions mediating JNK – IIS antagonism.
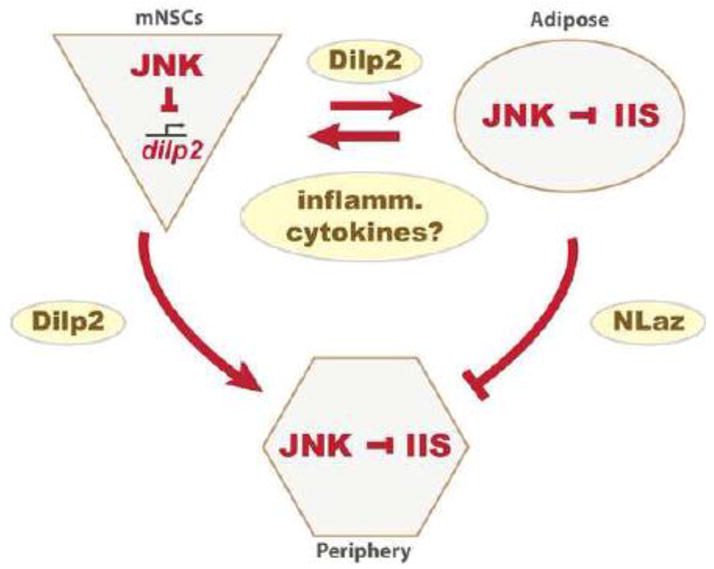
JNK negatively regulates insulin signal transduction cell-autonomously in adipose and peripheral tissues, represses the expression of dilp2 in mNSCs, and promotes expression of the lipocalin NLaz in adipose tissue. NLaz inhibits IIS activity in the periphery. Based on studies in vertebrates, inflammatory cytokines (including Upd1-3) are likely to further influence the interaction between JNK and IIS in flies.
Cell autonomously, JNK inhibits insulin signal transduction and promotes Foxo nuclear translocation, suppressing cell growth in larval fatbodies of flies, and regulating insulin sensitivity in vertebrates (Hotamisligil, 2006; Oh et al., 2005; Wang et al., 2005). The cell-autonomous regulation of Foxo activity by both IIS and JNK signaling also regulates cell survival in response to DNA damage, such as in UV-irradiated Drosophila pupal retinae (Luo et al., 2007). The molecular mechanism of this signaling interaction is complex: In vertebrates and worms, a direct phosphorylation of at least some Foxo isoforms by JNK has been identified (Essers et al., 2004; Oh et al., 2005), but the corresponding phosphorylation sites are not conserved in the Drosophila protein. Other points of signaling crosstalk in vertebrates include JNK-mediated inhibitory phosphorylation of insulin-receptor substrate (IRS), and JNK-mediated phosphorylation of 14-3-3 proteins, which inhibits the negative regulation of Foxo by 14-3-3 (Hotamisligil, 2010; Sunayama et al., 2005). Of the JNK phosphorylation sites described in vertebrate IRS, 14-3-3 and Foxo proteins, only the sites identified on 14-3-3 are conserved in the Drosophila homologue, suggesting that this specific interaction might be evolutionarily conserved. Accordingly, Drosophila 14-3-3 regulates stress tolerance and lifespan of the fly by inhibiting Foxo activity (Nielsen et al., 2008).
JNK regulates IIS signaling activity systemically by inhibiting the expression of the Drosophila insulin-like peptide dilp2 in the medial neurosecretory cells (mNSCs) of the fly brain (Karpac et al., 2009; Karpac and Jasper, 2009; Wang et al., 2005). Strikingly, JNK activity (as determined by puc expression) is observed in these cells under normal (unstressed) conditions, suggesting that JNK may be activated sporadically in these cells to govern metabolic homeostasis. When JNK activity is repressed in mNSCs, either by expression of a dominant-negative JNK/Bsk or by expressing a dsRNA against JNK/Bsk, flies lose the ability to adapt to oxidative stress, showing impaired peripheral stress responses and reduced developmental growth restriction in response to heat stress. These adaptive processes are critical for survival, as flies with reduced JNK activity in mNSCs show increased mortality when exposed to Paraquat. Conversely, activating JNK signaling in these cells is sufficient to extend lifespan (Karpac et al., 2009; Karpac and Jasper, 2009; Wang et al., 2005).
JNK activity in mNSCs thus serves as a stress sensor in flies that allows adapting metabolism and growth to changing environmental conditions. Interestingly, this function of JNK may be conserved in vertebrates, where it was reported that JNK can suppress the expression of insulin in pancreatic beta cells (Kaneto et al., 2002).
JNK activation further influences IIS activity by inducing the expression of secreted factors, including inflammatory cytokines and Lipocalins, from a variety of tissues, including adipose. Work in mammalian systems has shown that chronic activation of JNK in adipose tissue of obese mice induces the expression of tumor necrosis factor alpha (TNFa) and interleukin 6 (IL6), both of which can promote insulin resistance in the liver, causing diabetes (Hotamisligil, 2010; Sabio and Davis, 2010). This ultimately pathological consequence of the systemic antagonism between JNK signaling and IIS has its likely evolutionary source in the need for metabolic adaptation to stressful environmental conditions (Hull-Thompson et al., 2009; Karpac and Jasper, 2009). Supporting this view, JNK also interacts with the Lipocalin family member Neural Lazarillo (NLaz) to regulate metabolic homeostasis in adult flies, promoting starvation tolerance. Lipocalins are a family of mostly secreted proteins that bind small hydrophobic ligands (Åkerström et al., 2006; Flower, 1996). Various studies implicate Lipocalins in the regulation of systemic insulin action and of stress responses (Ganfornina et al., 2008; Muffat et al., 2008; van Dam and Hu, 2007; Yan et al., 2007; Yang et al., 2005). NLaz is a homologue of Retinol Binding Protein 4 (RBP4) and Apolipoprotein D, and represses IIS activity in peripheral tissues by an unknown mechanism, influencing overall metabolic homeostasis, stress tolerance and lifespan (Hull-Thompson et al., 2009). Strikingly, re-expression of NLaz in the fatbody of animals deficient in JNK signaling can revert the metabolic imbalances and starvation sensitivity of these flies, highlighting the importance of JNK-mediated expression of secreted IIS repressors (Hull-Thompson et al., 2009; Karpac and Jasper, 2009). The control of IIS signal transduction by Lipocalins is likely to be conserved in vertebrates, as RBP4 is induced in conditions in adipose-specific GLUT4-deficient mice and contributes to insulin resistance (Yang et al., 2005).
JNK and the control of cell survival
JNK is a well-established regulator of apoptosis, and induces cell death when its activity is sustained, i.e. when it is not restricted after an initial pulse (Janes et al., 2006; Pham et al., 2004). While it remains unclear if modulating the rate of apoptosis in the adult significantly influences lifespan of flies (Shen et al., 2009), the proper regulation of cell death and survival is expected to be critical for long-term tissue homeostasis, especially in high-turnover tissues such as the intestinal epithelium (Biteau et al., 2008; Buchon et al., 2009; Jiang et al., 2009). Interestingly, while the antagonistic interaction between JNK and IIS critically controls metabolic homeostasis, it also regulates decisions between cell survival and death in flies, indicating that the control of tissue homeostasis by this interaction may influence longevity, at least in part. In the developing Drosophila retina, JNK activates Foxo and AP-1 in response to UV-induced DNA damage, inducing expression of the pro-apoptotic protein head-involution defective (Hid). The regulation of hid by Foxo allows sensitive control of its transcription by the relative activities of the JNK, IIS, and EGFR signaling pathways in the retina, determining if damaged cells are repaired or undergo apoptosis according to nutrition and growth factor availability (Janes et al., 2006; Luo et al., 2007; Pham et al., 2004). The antagonism between JNK and RTK signaling activities is thus critical to maintain the balance between cell repair and death in the developing retina, promoting epithelial homeostasis after tissue damage (Luo et al., 2007). Interestingly, JNK also promotes paracrine signaling from apoptotic cells that influences epithelial homeostasis non-autonomously: when epithelial homeostasis is disrupted by mutations in polarity genes, JNK-mediated expression of growth factors in dying cells promotes compensatory proliferation of cells surrounding the damaged tissue. If apoptosis is impaired in this context, for example by oncogenic Ras signaling, JNK can promote excessive growth and proliferation, resulting in hyperplasia of the retina (Igaki, 2009). JNK activation thus significantly impacts the maintenance of epithelial homeostasis in developing tissues and has to be precisely controlled to ensure proper tissue maintenance and morphogenesis during development.
Epithelial homeostasis and Tissue regeneration/stem cells
While it remains unclear whether these functions of JNK in developing tissues are relevant to the regulation of lifespan, recent studies suggest that JNK also influences epithelial homeostasis in adult Drosophila tissues, notably in the intestinal epithelium. JNK activation promotes proliferation of intestinal stem cells (ISCs) in response to oxidative stress and infection, a mitogenic function of JNK that is not commonly observed in other cell types. JNK appears to act both autonomously in ISCs to promote proliferation (Biteau et al., 2008; Buchon et al., 2009), as well as non-autonomously, by inducing the IL6-like cytokines Unpaired 1–3 (Upd1-3) in stressed enterocytes (Jiang et al., 2009). Upds in turn activate the Jak/Stat signaling pathway in ISCs, promoting proliferation. JNK is thus part of a regenerative response of ISCs that ensures recovery of the intestinal epithelium after injury (Jiang et al., 2009). Interestingly, JNK becomes widely activated in the intestinal epithelium of aging flies, promoting excessive proliferation of ISCs (Biteau et al., 2008). This over-proliferation is accompanied by mis-differentiation of ISC daughter cells due to JNK-mediated ectopic activation of Notch signaling, causing dysplasia in this tissue. Unchecked or chronic JNK activation in these cells thus disrupts epithelial homeostasis and shortens lifespan (Biteau et al., 2008). Strong inhibition of JNK signaling activity in the ISC lineage, on the other hand, also shortens lifespan due to complete inhibition of ISC proliferation, while moderate decrease in JNK signaling activity (through weak expression of BskRNAi or BskDN) reduces age-relaed dysplasia and increases lifespan (Biteau et al., 2010; Table 1). Intriguingly, the underlying cause for the widespread ectopic activation of JNK signaling in old intestinal epithelia is likely to be age-related changes in the intestinal microflora that induce a chronic inflammatory state (Buchon et al., 2009). It is possible that JNK cooperates with another stress-activated protein kinase, p38 MAPK, in this response, as p38 is also required for ISC proliferation and mediates the EC response to bacterial antigens (Ha et al., 2009; Park et al., 2009).
Discussion and outlook
The lifespan extension induced by JNK signaling in flies thus seems to be caused by a large number of cellular and systemic changes that influence metabolic and tissue homeostasis, cell survival and cell damage repair, but is also counteracted by deleterious consequences of excessive and chronic JNK activation, such as disruption of normal intestinal regeneration, or the induction of chronic insulin resistance and diabetes observed in vertebrates. Strikingly, recent findings suggest even more complex roles for JNK signaling in the regulation of cellular and organismic physiology, highlighting the need for further investigation into how JNK influences lifespan:
JNK signaling plays an important role in innate immune responses, and has been found to promote the efficacy of the immune response (Boutros et al., 2002; Libert et al., 2008). The specific signaling interactions between JNK signaling and innate immune response pathways that regulate immune efficacy, however, remain unclear.
The recently established importance of the JNK target gene sestrin in the maintenance of tissue function in aging flies further suggests that JNK interacts with the Tor signal transduction pathway, a central regulator of protein synthesis, cell growth and autophagy, and a mediator of Dietary Restriction-induced lifespan extension (Lee et al., 2010; Zid et al., 2009). Sestrin inhibits Tor signaling (Lee et al., 2010). By inducing sestrin, JNK thus inhibits Tor signaling, promoting autophagy and influencing the response of the organism to nutritional cues. The tissue-specific requirement for sestrin downstream of JNK signaling in the regulation of lifespan has yet to be established, but an important function for JNK in the regulation of autophagy is emerging (Wu et al., 2009), indicating that the lifespan extension observed in JNK gain-of-function conditions may be mediated, at least in part, by controlling this critical cellular process. Supporting this view, lifespan extension by increasing autophagy in Drosophila has recently been reported (Eisenberg et al., 2009).
The control of inflammatory cytokines, in particular of Upds, by JNK in flies further suggest additional mechanisms by which JNK can influence IIS activity systemically and thus influence lifespan. Secreted Upds are ligand activators of the JAK/Stat signaling pathway, and are induced by JNK signaling in response to localized tissue damage (Jiang et al., 2009; Pastor-Pareja et al., 2008). The suppression of IIS activity by IL6 in vertebrates suggests that Upds might serve a similar role and might suppress IIS sensitivity in peripheral tissues in response to localized JNK activation.
JNK might further interact with other longevity-promoting stress response signaling pathways to control cellular stress responses. The Drosophila homologue of the Nrf2 (vertebrates) and SKN-1 (C.elegans) transcription factors, Cap-n-collar C (CncC), has recently been found to significantly influence oxidative stress tolerance in flies and to increase lifespan (Sykiotis and Bohmann, 2010). CncC promotes the expression of antioxidant and detoxifying proteins, and it is possible that JNK signaling interacts with this regulator to promote cytoprotection. This idea is supprted by the fact that SKN-1 is regulated directly by p38-mediated phosphorylation in worms. Interestingly, SKN-1 is also required for DR-induced lifespan extension in C.elegans, further highlighting the importance of characterizing the relationship of these factors in the regulation of Drosophila lifespan (Bishop and Guarente, 2007).
Another stress-responsive transcription factor, P53, potentially influences the cellular response to JNK activation. P53 has complex effects on Drosophila lifespan (Bauer and Helfand, 2009; Biteau and Jasper, 2009), but expression of a dominant-negative version of p53 in mNSCs represses dilp2 transcription, thus regulating IIS activity in a manner reminiscent of conditions in which JNK is activated (Bauer and Helfand, 2009).
While significant progress has thus been made in establishing potential mechanisms by which JNK signaling regulates lifespan in Drosophila, further studies are needed to understand the relative contribution of the described mechanisms to overall lifespan of the animal. In particular, it will be important to establish the significance of the antagonistic pleiotropy of JNK in different tissues and at different activation intensities. Since all described consequences of JNK activation in flies appear to be evolutionarily conserved, such studies are also expected to provide important insight into the etiology of age-related diseases specifically, and the aging process generally, in vertebrates.
Acknowledgments
H.J. is supported by the National Institute on Aging (NIH RO1 AG028127), the National Eye Institute (NIH RO1 EY018177), the Glenn Foundation, and the Ellison Medical Foundation (AG-SS-2224-08). J.K. is supported by an NIH postdoctoral fellowship (F32 DK083862).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- Johnson GL, Nakamura K. The c-jun kinase/stress-activated pathway: regulation, function and role in human disease. Biochim Biophys Acta. 2007;1773:1341–8. doi: 10.1016/j.bbamcr.2006.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston CR, Davis RJ. The JNK signal transduction pathway. Curr Opin Cell Biol. 2007;19:142–9. doi: 10.1016/j.ceb.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Jasper H, Benes V, Schwager C, Sauer S, Clauder-Munster S, Ansorge W, Bohmann D. The genomic response of the Drosophila embryo to JNK signaling. Dev Cell. 2001;1:579–86. doi: 10.1016/s1534-5807(01)00045-4. [DOI] [PubMed] [Google Scholar]
- Martin-Blanco E, Gampel A, Ring J, Virdee K, Kirov N, Tolkovsky AM, Martinez-Arias A. puckered encodes a phosphatase that mediates a feedback loop regulating JNK activity during dorsal closure in Drosophila. Genes Dev. 1998;12:557–70. doi: 10.1101/gad.12.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen DG, Peifer M. Puckered, a Drosophila MAPK phosphatase, ensures cell viability by antagonizing JNK-induced apoptosis. Development. 2005;132:3935–46. doi: 10.1242/dev.01949. [DOI] [PubMed] [Google Scholar]
- Igaki T. Correcting developmental errors by apoptosis: lessons from Drosophila JNK signaling. Apoptosis. 2009;14:1021–8. doi: 10.1007/s10495-009-0361-7. [DOI] [PubMed] [Google Scholar]
- Boutros M, Agaisse H, Perrimon N. Sequential activation of signaling pathways during innate immune responses in Drosophila. Dev Cell. 2002;3:711–22. doi: 10.1016/s1534-5807(02)00325-8. [DOI] [PubMed] [Google Scholar]
- Chen W, White MA, Cobb MH. Stimulus-specific requirements for MAP3 kinases in activating the JNK pathway. J Biol Chem. 2002;277:49105–10. doi: 10.1074/jbc.M204934200. [DOI] [PubMed] [Google Scholar]
- Geuking P, Narasimamurthy R, Lemaitre B, Basler K, Leulier F. A non-redundant role for Drosophila Mkk4 and hemipterous/Mkk7 in TAK1-mediated activation of JNK. PLoS ONE. 2009;4:e7709. doi: 10.1371/journal.pone.0007709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabio G, Davis RJ. cJun NH(2)-terminal kinase 1 (JNK1): roles in metabolic regulation of insulin resistance. Trends Biochem Sci. 2010 doi: 10.1016/j.tibs.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes KA, Gaudet S, Albeck JG, Nielsen UB, Lauffenburger DA, Sorger PK. The Response of Human Epithelial Cells to TNF Involves an Inducible Autocrine Cascade. Cell. 2006;124:1225–39. doi: 10.1016/j.cell.2006.01.041. [DOI] [PubMed] [Google Scholar]
- Karin M, Gallagher E. From JNK to pay dirt: jun kinases, their biochemistry, physiology and clinical importance. IUBMB Life. 2005;57:283–95. doi: 10.1080/15216540500097111. [DOI] [PubMed] [Google Scholar]
- Lin A. Activation of the JNK signaling pathway: breaking the brake on apoptosis. Bioessays. 2003;25:17–24. doi: 10.1002/bies.10204. [DOI] [PubMed] [Google Scholar]
- Pham CG, Bubici C, Zazzeroni F, Papa S, Jones J, Alvarez K, Jayawardena S, De Smaele E, Cong R, Beaumont C, Torti FM, Torti SV, Franzoso G. Ferritin heavy chain upregulation by NF-kappaB inhibits TNFalpha-induced apoptosis by suppressing reactive oxygen species. Cell. 2004;119:529–42. doi: 10.1016/j.cell.2004.10.017. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140:900–17. doi: 10.1016/j.cell.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston CR, Davis RJ. The JNK signal transduction pathway. Curr Opin Genet Dev. 2002;12:14–21. doi: 10.1016/s0959-437x(01)00258-1. [DOI] [PubMed] [Google Scholar]
- Etter PD, Narayanan R, Navratilova Z, Patel C, Bohmann D, Jasper H, Ramaswami M. Synaptic and genomic responses to JNK and AP-1 signaling in Drosophila neurons. BMC Neurosci. 2005;6:39. doi: 10.1186/1471-2202-6-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X, Puig O, Hyun J, Bohmann D, Jasper H. Foxo and Fos regulate the decision between cell death and survival in response to UV irradiation. Embo J. 2007;26:380–90. doi: 10.1038/sj.emboj.7601484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libert S, Chao Y, Zwiener J, Pletcher SD. Realized immune response is enhanced in long-lived puc and chico mutants but is unaffected by dietary restriction. Mol Immunol. 2008;45:810–7. doi: 10.1016/j.molimm.2007.06.353. [DOI] [PubMed] [Google Scholar]
- Wang MC, Bohmann D, Jasper H. JNK signaling confers tolerance to oxidative stress and extends lifespan in Drosophila. Dev Cell. 2003;5:811–6. doi: 10.1016/s1534-5807(03)00323-x. [DOI] [PubMed] [Google Scholar]
- Wang MC, Bohmann D, Jasper H. JNK extends life span and limits growth by antagonizing cellular and organism-wide responses to insulin signaling. Cell. 2005;121:115–25. doi: 10.1016/j.cell.2005.02.030. [DOI] [PubMed] [Google Scholar]
- Mizuno T, Hisamoto N, Terada T, Kondo T, Adachi M, Nishida E, Kim DH, Ausubel FM, Matsumoto K. The Caenorhabditis elegans MAPK phosphatase VHP-1 mediates a novel JNK-like signaling pathway in stress response. Embo J. 2004;23:2226–34. doi: 10.1038/sj.emboj.7600226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh SW, Mukhopadhyay A, Svrzikapa N, Jiang F, Davis RJ, Tissenbaum HA. JNK regulates lifespan in Caenorhabditis elegans by modulating nuclear translocation of forkhead transcription factor/DAF-16. Proc Natl Acad Sci U S A. 2005;102:4494–9. doi: 10.1073/pnas.0500749102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpac J, Jasper H. Insulin and JNK: optimizing metabolic homeostasis and lifespan. Trends Endocrinol Metab. 2009;20:100–6. doi: 10.1016/j.tem.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bourg E. Hormesis, aging and longevity. Biochim Biophys Acta. 2009;1790:1030–9. doi: 10.1016/j.bbagen.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Nathan C, Ding A. Nonresolving inflammation. Cell. 2010;140:871–82. doi: 10.1016/j.cell.2010.02.029. [DOI] [PubMed] [Google Scholar]
- Sykiotis GP, Bohmann D. Stress-activated cap'n'collar transcription factors in aging and human disease. Sci Signal. 2010;3:re3. doi: 10.1126/scisignal.3112re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Budanov AV, Park EJ, Birse R, Kim TE, Perkins GA, Ocorr K, Ellisman MH, Bodmer R, Bier E, Karin M. Sestrin as a feedback inhibitor of TOR that prevents age-related pathologies. Science. 2010;327:1223–8. doi: 10.1126/science.1182228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KS, Iijima-Ando K, Iijima K, Lee WJ, Lee JH, Yu K, Lee DS. JNK/FOXO-mediated neuronal expression of fly homologue of peroxiredoxin II reduces oxidative stress and extends life span. J Biol Chem. 2009;284:29454–61. doi: 10.1074/jbc.M109.028027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biteau B, Hochmuth CE, Jasper H. JNK activity in somatic stem cells causes loss of tissue homeostasis in the aging Drosophila gut. Cell Stem Cell. 2008;3:442–55. doi: 10.1016/j.stem.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon C. The plasticity of aging: insights from long-lived mutants. Cell. 2005;120:449–60. doi: 10.1016/j.cell.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Tatar M, Bartke A, Antebi A. The endocrine regulation of aging by insulin-like signals. Science. 2003;299:1346–51. doi: 10.1126/science.1081447. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–7. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- Essers MA, Weijzen S, de Vries-Smits AM, Saarloos I, de Ruiter ND, Bos JL, Burgering BM. FOXO transcription factor activation by oxidative stress mediated by the small GTPase Ral and JNK. Embo J. 2004;23:4802–12. doi: 10.1038/sj.emboj.7600476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunayama J, Tsuruta F, Masuyama N, Gotoh Y. JNK antagonizes Akt-mediated survival signals by phosphorylating 14-3-3. J Cell Biol. 2005;170:295–304. doi: 10.1083/jcb.200409117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen MD, Luo X, Biteau B, Syverson K, Jasper H. 14-3-3 Epsilon antagonizes FoxO to control growth, apoptosis and longevity in Drosophila. Aging Cell. 2008;7:688–99. doi: 10.1111/j.1474-9726.2008.00420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpac J, Hull-Thompson J, Falleur M, Jasper H. JNK signaling in insulin-producing cells is required for adaptive responses to stress in Drosophila. Aging Cell. 2009;8:288–295. doi: 10.1111/j.1474-9726.2009.00476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneto H, Xu G, Fujii N, Kim S, Bonner-Weir S, Weir GC. Involvement of c-Jun N-terminal kinase in oxidative stress-mediated suppression of insulin gene expression. J Biol Chem. 2002;277:30010–8. doi: 10.1074/jbc.M202066200. [DOI] [PubMed] [Google Scholar]
- Hull-Thompson J, Muffat J, Sanchez D, Walker DW, Benzer S, Ganfornina MD, Jasper H. Control of metabolic homeostasis by stress signaling is mediated by the lipocalin NLaz. PLoS Genet. 2009;5:e1000460. doi: 10.1371/journal.pgen.1000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Åkerström B, Borregaard N, Flower DR, Salier JP. Lipocalins Landes Bioscience. Austin, TX: 2006. [Google Scholar]
- Flower DR. The lipocalin protein family: structure and function. Biochem J. 1996;318 ( Pt 1):1–14. doi: 10.1042/bj3180001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganfornina MD, Do Carmo S, Lora JM, Torres-Schumann S, Vogel M, Allhorn M, Gonzalez C, Bastiani MJ, Rassart E, Sanchez D. Apolipoprotein D is involved in the mechanisms regulating protection from oxidative stress. Aging Cell. 2008 doi: 10.1111/j.1474-9726.2008.00395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muffat J, Walker DW, Benzer S. Human ApoD, an apolipoprotein up-regulated in neurodegenerative diseases, extends lifespan and increases stress resistance in Drosophila. Proc Natl Acad Sci U S A. 2008;105:7088–93. doi: 10.1073/pnas.0800896105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dam RM, Hu FB. Lipocalins and insulin resistance: etiological role of retinol-binding protein 4 and lipocalin-2? Clin Chem. 2007;53:5–7. doi: 10.1373/clinchem.2006.080432. [DOI] [PubMed] [Google Scholar]
- Yan QW, Yang Q, Mody N, Graham TE, Hsu CH, Xu Z, Houstis NE, Kahn BB, Rosen ED. The adipokine lipocalin 2 is regulated by obesity and promotes insulin resistance. Diabetes. 2007;56:2533–40. doi: 10.2337/db07-0007. [DOI] [PubMed] [Google Scholar]
- Yang Q, Graham TE, Mody N, Preitner F, Peroni OD, Zabolotny JM, Kotani K, Quadro L, Kahn BB. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. 2005;436:356–62. doi: 10.1038/nature03711. [DOI] [PubMed] [Google Scholar]
- Shen J, Curtis C, Tavare S, Tower J. A screen of apoptosis and senescence regulatory genes for life span effects when over-expressed in Drosophila. Aging (Albany NY) 2009;1:191–211. doi: 10.18632/aging.100018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biteau B, Karpac J, Supoyo S, Degennaro M, Lehmann R, Jasper H. Lifespan extension by preserving proliferative homeostasis in Drosophila. PLoS Genet. 2010;14:e1001159. doi: 10.1371/journal.pgen.1001159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchon N, Broderick NA, Chakrabarti S, Lemaitre B. Invasive and indigenous microbiota impact intestinal stem cell activity through multiple pathways in Drosophila. Genes Dev. 2009;23:2333–44. doi: 10.1101/gad.1827009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Patel PH, Kohlmaier A, Grenley MO, McEwen DG, Edgar BA. Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut. Cell. 2009;137:1343–55. doi: 10.1016/j.cell.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha EM, Lee KA, Seo YY, Kim SH, Lim JH, Oh BH, Kim J, Lee WJ. Coordination of multiple dual oxidase-regulatory pathways in responses to commensal and infectious microbes in drosophila gut. Nat Immunol. 2009;10:949–57. doi: 10.1038/ni.1765. [DOI] [PubMed] [Google Scholar]
- Park JS, Kim YS, Yoo MA. The role of p38b MAPK in age-related modulation of intestinal stem cell proliferation and differentiation in Drosophila. Aging (Albany NY) 2009;1:637–51. doi: 10.18632/aging.100054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zid BM, Rogers AN, Katewa SD, Vargas MA, Kolipinski MC, Lu TA, Benzer S, Kapahi P. 4E-BP extends lifespan upon dietary restriction by enhancing mitochondrial activity in Drosophila. Cell. 2009;139:149–60. doi: 10.1016/j.cell.2009.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Wang MC, Bohmann D. JNK protects Drosophila from oxidative stress by trancriptionally activating autophagy. Mech Dev. 2009;126:624–37. doi: 10.1016/j.mod.2009.06.1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg T, Knauer H, Schauer A, Buttner S, Ruckenstuhl C, Carmona-Gutierrez D, Ring J, Schroeder S, Magnes C, Antonacci L, Fussi H, Deszcz L, Hartl R, Schraml E, Criollo A, Megalou E, Weiskopf D, Laun P, Heeren G, Breitenbach M, Grubeck-Loebenstein B, Herker E, Fahrenkrog B, Frohlich KU, Sinner F, Tavernarakis N, Minois N, Kroemer G, Madeo F. Induction of autophagy by spermidine promotes longevity. Nat Cell Biol. 2009;11:1305–14. doi: 10.1038/ncb1975. [DOI] [PubMed] [Google Scholar]
- Pastor-Pareja JC, Wu M, Xu T. An innate immune response of blood cells to tumors and tissue damage in Drosophila. Dis Model Mech. 2008;1:144–54. doi: 10.1242/dmm.000950. discussion 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop NA, Guarente L. Two neurons mediate diet-restriction-induced longevity in C. elegans. Nature. 2007;447:545–9. doi: 10.1038/nature05904. [DOI] [PubMed] [Google Scholar]
- Bauer JH, Helfand SL. Sir2 and longevity: the p53 connection. Cell Cycle. 2009;8:1821. doi: 10.4161/cc.8.12.9010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biteau B, Jasper H. It’s all about balance: p53 and aging. Aging (Albany NY) 2009;1:884–6. doi: 10.18632/aging.100102. [DOI] [PMC free article] [PubMed] [Google Scholar]


