Structured Abstract
Objective
To determine effects of 1) parenteral nutrition (PN) 2) exogenous Lymphotoxin β receptor (LTβR) stimulation in PN animals and 3) exogenous LTβR blockade in chow animals on NF-κB activation pathways and products: MAdCAM-1, chemokine (C-C motif) Ligand (CCL) 19, CCL20, CCL25, interleukin (IL)-4 and IL-10.
Summary Background Data
Lymphotoxin (LT) stimulates LTβR in Peyer's patches (PP) to activate NF-κB via the non-canonical pathway. The p100/RelB precursor yields p52/RelB producing MAdCAM-1, cytokines, and chemokines important in cell trafficking. TNFα, IL-1β, & bacterial products stimulate the inflammatory canonical NF-κB pathway producing p65/p50 and c-Rel/p50. PN decreases LTβR, MAdCAM-1 and chemokines in PP and lowers small intestinal (SI) IgA compared to chow.
Methods
Canonical (p50& p65) and non-canonical (p52 & Rel B) NF-κB proteins in PP were analyzed by TransAM NF-κB kit after 5 days of chow or PN, 2 days of LTβR stimulation or 3 days of LTβR blockade. MAdCAM-1, chemokines and cytokines in PP were measured by ELISA after LTβR stimulation or blockade.
Results
PN significantly reduced all NF-κB proteins in PP compared to chow. Exogenous LTβR stimulation during PN increased p50, p52, Rel B, MAdCAM-1, IL-4 and IL-10 in PP, but not p65, CCL19, CCL20 or CCL25 compared to PN. LTβR blockade reduced non-canonical products (p52 and Rel B), MAdCAM-1, CCL19, CCL20, CCL25, IL-4 and IL-10 but had no effect on the inflammatory pathway (p50 and p65) compared to chow.
Conclusion
Lack of enteral stimulation during PN decreases both canonical and non-canonical NF-κB pathways in PP. LTβR stimulation during PN feeding completely restores PP non-canonical NF-κB activity, MAdCAM-1, IL-4, IL-10, and partly the canonical pathway. LTβR blockade decreases the non-canonical NF-κB activity, MAdCAM-1, chemokines and cytokines without effect on the canonical NF-κB activity in PP.
Introduction
The mucosal associated lymphoid tissue (MALT) provides the specific immune protection of moist mucosal surfaces and constitutes the largest immune organ outside the liver and spleen. It produces IgA to protect against the huge load of bacteria and toxins within the gut lumen as well as the normally sterile pulmonary tissue. Naïve T&B cells destined for mucosal immunity express two integrins - L-selectin and α4β7 – directing them into Peyer's patches (PP) of the small intestine via interaction with mucosal addressin cellular adhesion molecule-1 (MAdCAM-1), a molecule expressed on the high endothelial venules of the Peyer's patches. 1,2 Regional chemokines stimulate migration of the cells into the Peyer's patches: CCL19 regulates T-cell entry, CCL-25 recruits antibody-secreting cells, and CCL-20 recruits dendritic cells.3 The T&B cells are sensitized in PP to luminal antigens absorbed by specialized M cells covering the PP.4 Our previous work shows that blockade of either the integrins- L-selectin and α4β7 - or the adhesion molecule- MAdCAM-1 - reduces cell entry into MALT, reduces secretory IgA and impair of mucosal immune protection.1,2 This work examines the specific pathways involved in this entry.
Lymphotoxin β receptor (LTβR) expressed in the endothelial surfaces of PPs control production of nuclear factor-kappa B (NF-κB) which is the key transcriptional regulator of these critical entry molecules in vitro. Normally, lymphotoxin α and β (LT α1β2) expressed on activated T&B cells stimulates the lymphotoxin β receptor (LTβR).5 This interaction activates NF-κB via the non-canonical pathway to produce MAdCAM-1, cytokines, and the specific chemokines critical to cell entry and function3. LTβR deficient (LTβR -/-) mice lack PP and lymph nodes and express low levels of MAdCAM-1, CCL-20, CCL25, and CXCL13. These mice also have lower levels of two Th-2 type IgA-stimulating cytokines, IL-4 & IL-10, and IgA itself. We and others showed that administration of a fusion protein which blocks LTβR interfering with this LTβR/ lymphotoxin interaction reduced the size and numbers of lymphocytes in PP. 5,6.
PN also interferes with this sequence. Our previous work3,7-9 demonstrated that PN feeding decreases the number of PP lymphocytes and the mRNA or/and protein levels of MAdCAM-1, CCL-20, CCL25, CXCL13 as well as LTβR itself compared to chow fed mice. The administration of a stimulatory (agonistic) LTβR antibody to PN mice prevented the reduction in PP lymphocytes and intestinal IgA.7 Also, LTβR blockade in chow fed animals significantly reduced MAdCAM-1 mRNA level and PP lymphocyte mass. 6 In this work we hypothesized that the PN effects occurs through down-regulation of NF-κB pathway(s). Two distinct NF-κB activation pathways have been described (Figure 1).10 The canonical (classical) NF-κB pathway is activated by proinflammatory cytokines, toll like receptor (TLR) ligands, or stresses such as γ-radiation, ultraviolet light and oxidative stress. These stimuli induce degradation of IκBα and the nuclear accumulation of mainly p50–RelA (p65) dimer, which regulate the expression of immunoregulatory and anti-apoptotic genes but not the cell entry proteins. The pathway which we hypothesize is relevant to the PN changes is the non-canonical (alternative) NF-κB pathway which is activated by receptors involved in lymphoid organogenesis and lymphocyte development. Receptors such as LTβR induce processing of p100 to p52 and nuclear accumulation of p52–RelB dimmers. This non-canonical NF-κB pathway appears to control processes directing PP T&B cell entry. In this work, we define in detail the mechanisms of PN-induced immune deficits and hypothesize that PN negatively affects LTβR signaling via the non-canonical NF-κB pathway. To test this hypothesis, we quantified the inflammatory canonical NF-κB pathway products - p50 & p65- as well as the non-canonical NF-κB pathway products - p52 and Rel B levels – together with levels of MAdCAM-1, CCL-19, CCL20, CCL25, IL-4 and IL-10 in the PP of animals receiving PN or chow feeding. To establish cause and effect, we measured these same parameters in PN fed animals administrated an agonistic LTβR mAb and chow fed animals receiving LTβR blockade.
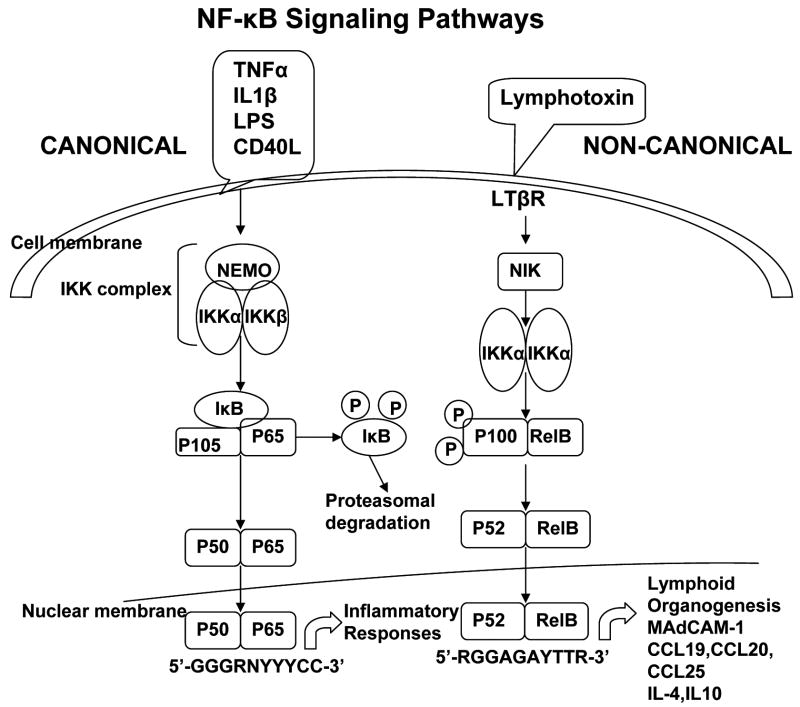
Figure 1. NF-κB Signaling Pathways.
Two distinct NF-κB activation pathways have been described. The canonical (classical) NF-κB pathway is activated by a large number of stimuli, including tumor necrosis factor (TNF)-α, interleukin(IL)-1β,lipopolysaccharide(LPS), and CD40 ligand(CD40L). These stimuli induce the degradation of IκBα and the nuclear accumulation of mainly p50–RELA (p65) dimer, which regulate the expression of immunoregulatory and anti-apoptotic genes. The non-canonical (alternative) NF-κB pathway is activated by receptors that are involved in lymphoid tissue organogenesis and lymphocyte development, such as Lymphotoxin β receptor(LTβR), which induces processing of p100 to p52 and the nuclear accumulation of p52–RELB dimmers,results in mucosal addressin cellular adhesion molecule-1 (MAdCAM-1), chemokine (C-C motif) ligand 19 (CCL19), CCL20, CCL25, interleukin (IL)-4, IL-10 responses.
Methods
Animals
All experimental protocols were approved by the Animal Care and Use Committee of the University of Wisconsin–Madison and Middleton Veterans Administration Hospital, Madison, WI. Six-week-old, male mice from Institute of Cancer Research (Harlan, Indianapolis, IN) were used for the experiments. The mice were kept in an environment with controlled temperature, humidity, and light cycle (12-hour light/12-hour dark). They were acclimatized for 1 week and fed a standard mouse chow (PMI Nutrition International, St. Louis, MO) ad libitum before protocol entry. During feeding protocol, mice had free access to water and were individually housed in metal metabolism cages with wire grid floors to eliminate coprophagia.
Experiment 1: Effects of PN on NF-κB activation pathways
Mice received catheters for IV infusion after intraperitoneal injection of a mixture of ketamine (100 mg/kg body weight) and acepromazine maleate (5 mg/kg body weight). A silicone rubber catheter (0.012″ I.D. by 0.025″ O.D.; Helix Medical, Inc., Carpinteria, CA) was inserted into the vena cava through the right jugular vein. The distal end of the catheter was tunneled subcutaneously and exited the midpoint of the tail. Mice were partially immobilized by tail restraint to protect the catheter during infusion. This technique of infusion in the mouse has been demonstrated to be an acceptable method of nutritional support, and this method does not produce physical or biochemical evidence of stress8.
Thirty-five mice were randomized to receive chow (Chow, n = 19) or parenteral nutrition (PN, n = 16). Mice received 0.9% saline at a rate of 4 mL/d with free access to water and chow for 2 days after surgery. PN mice initially received 4 mL/day of PN and advanced to a goal rate of 10 mL/day by the third day. The PN solution contains 6.0% amino acids, 34.9% dextrose (6002 kJ/L), electrolytes, and multivitamins, with a nonprotein calorie/nitrogen ratio of 535.8 kJ/g nitrogen. This feeding meets the calculated nutrient requirements of mice weighing 25 to 30g. 11
After 5 days of dietary treatments, animals were anesthetized and exsanguinated by cardiac puncture. The small intestine was removed, and the mesenteric fat and external vasculature were dissected away. After washing with 20 mL cold calcium and magnesium-free Hanks balanced salt solution (HBSS, BioWhittaker, Walkersville, MD), the PP were removed from the serosal side of the intestine. The PP were frozen in liquid N2 and stored at −80°C until the levels of Phosphorylated NF-κB proteins were measured.
Phosphorylated NF-κB proteins, p50, p65, p52 and Rel B in PP were analyzed by TransAM NF-κB Family kit (Active Motif,,Carlsbad, CA) after 5 days of chow or PN. TransAM™ NF-κB Kits are DNA-binding ELISAs that facilitate the study of transcription factor activation in mammalian tissue and cell extracts. The manufacturer's protocol was followed. Briefly, protein samples from whole-cell extracts were prepared by using Nuclear Extract Kit (Active Motif, Carlsbad, CA) on ice to disrupt and homogenize PP in 1 ml ice-cold Complete Lysis Buffer with an Omni's TissueMaster Homogenizer (Fisher Scientifc,Marietta, GA). Homogenization began at slow speeds until the PP were broken into smaller pieces and then the speed was increased to the maximum for 45-60 seconds. Care was taken to avoid the generation of excess heat or foam during homogenization. The homogenate was then transferred a to pre-chilled microcentrifuge tube, centrifuged at 10,000 × g for 10 minutes at 4 °C. The supernatant was then transferred to a new pre-chilled tube and centrifuged again. The supernatant fluid is the whole-cell lysate. The protein concentration was quantified by the dye-binding Bradford reagent (BioRad, Hercules, CA), and the samples were stored at −80°C until analyzed for NF-κB proteins.
After optimization, in each well, 5 μg of protein diluted in 20 μl Complete Lysis Buffer and 30 μl binding buffer were incubated at room temperature for 1 h in a 96-well plate coated with an oligonucleotide that contained the NF-κB consensus binding site under mild agitation. Then, the plate was washed. p65, p50, p52 or RelB antibodies were added to the wells. The plate was incubated for 1 h at room temperature, and then it was washed. Following this step, the secondary antibody was added to the wells. The plate was incubated for 1 h at room temperature, and then washed. Finally, the developing solution was added. After 5–10 min incubation at room temperature, the stop solution was added and the absorbance was read at 450 nm using a microplate reader (Molecular Devices Corporation, CA).
Experiment 2: Effects of LTβR Stimulation on NF- κB activities, MAdCAM-1, Chemokines and Cytokines
Mice received IV catheters as described in experiment 1. Two days after cannulation, 48 mice were randomized to Chow (n = 13), PN plus agonistic LTβR mAb (PN-LTβR, n = 17), or PN plus isotype control antibody (PN-Ig; n = 18). Chow and PN diets were identical to experiment 1. Using the previously optimized dose for the LTβR agonistic antibody obtained from dose response experiments show in our prior work 7, Chow mice received 200 μL of vehicle phosphate buffered saline (PBS), twice a day for 2 days. PN-LTβR and PN-Ig groups received the same volume containing 5 μg of hamster agonistic LTβR mAb (a generous gift from Jeffrey L. Browning, Biogen Idec, 12 Cambridge Center, Cambridge, MA) or 5 μg of hamster IgG1κ (Becton Dickinson Pharmingen, San Diego, CA), respectively, twice a day for 2 days.
After 2 days of treatment, mice were exsanguinated under anesthesia. The small intestine was excised from the duodenal bulb to ileocecal valve and the lumen flushed with 20 mL of HBSS. The PP were removed from the serosal side of the intestine then frozen in liquid N2 and stored at −80°C. Phosphorylated NF-κB proteins in PP were analyzed as Experiment 1.
Commercially available sandwich enzyme-linked immunosorbent assay (ELISA) kits were purchased for MAdCAM-1, CCL19, CCL20, CCL25 (R&D Systems, Minneapolis, MN), IL-4 and IL-10 (Becton Dickinson Pharmingen, San Diego, CA). Ninety-six-well plates were coated with capture antibody diluted to working dilution in phosphate-buffered saline (PBS) and incubated overnight at room temperature (RT) in a humid chamber. The following morning, plates were washed 3 times with PBS + 0.05% Tween 20 (PBS-T), and nonspecific binding was blocked by Reagent Diluent (R&D Systems, Minneapolis, MN) for 1 hour. Plates were washed 3 times before addition of samples. Samples (100 μL/well) were loaded at 80 μg/mL protein diluted with Reagent Diluent and incubated for 2 hours at RT. Plates were washed 3 times with PBS-T before addition of the detection antibody (100 μL/well) diluted 1:500 in Reagent Diluent and incubated at RT for 1 hour. Plates were washed 3 times before addition (100 μL/well) of the IgG-HRP conjugate at a dilution of 1:20,000 in Reagent Diluent. The conjugate was incubated at RT for 1 hour. Tetramethyl benzidine (TMB; BD Biosciences, San Diego, CA; 100 μL/well) substrate was added and incubated at RT for 15 minutes before the reaction was stopped with 50 μL/well of 2-N H2SO4. The absorbance was read at 450 nm using a microplate reader (Molecular Devices Corporation, CA).
Experiment 3: Effects of LTβR Blockade on NF- κB activities, MAdCAM-1, Chemokines and Cytokines
Twenty-seven mice received central venous catheters as described in Experiment 1. After recovering from surgery for 2 days, the animals were then randomly assigned to Chow- LTβR-Immunoglobulin (Chow- anti LTβR) group (n=10), Chow + control immunoglobulin (Ig) group (Chow-Ig n=9) and PN + control Ig group (PN-Ig, n=8). Using our previously optimized dose noted in our prior experiments 6, Chow- anti LTβR mice received 100 μg LTβR-Ig fusion protein IV (anti LTβR, 100 μg in 250 μL PBS; a generous gift from Yang-Xin Fu, Chicago University, Chicago, IL),,while Chow- Ig and PN-Ig groups received human IgG IV (100 μg in 250 μL PBS; Sigma, St. Louis, MO). The Chow- anti LTβR treated mice continued with chow ad libitum and 0.9% saline at 4 mL/d IV throughout the study. The control Ig-treated mice were randomly assigned to either chow or PN. The PN mice initially received 4 mL/d of PN and were advanced to a goal rate of 10 mL/d by the third day of feeding. After 3 days of these dietary treatments, the animals were anesthetized with a ketamine/acepromazine mixture and exsanguinated by cardiac puncture. The small intestine was excised from the pylorus to terminal ileum and the mesenteric fat and external vasculature were dissected away. Then, the intestinal lumen was flushed by 20 mL calcium- and magnesium-free Hanks' balanced salt solution (HBSS). The PP were removed from the intestine, frozen in liquid N2 and stored at −80°C until used in our assay. Phosphorylated NF-κB proteins in PP were analyzed as Experiment 1 while MAdCAM-1, CCL19, CCL20, CCL25, IL-4 and IL-10 were assayed as Experiment 2.
Statistical Analyses
All values were expressed as mean ± SD. Statistical analysis was performed with Statview software, by analysis of variance (ANOVA), followed by the Fisher's protected least significant difference post hoc test.
Results
Experiment 1: Effects of PN on Phosphorylated NF-κB in PP (Fig 2)
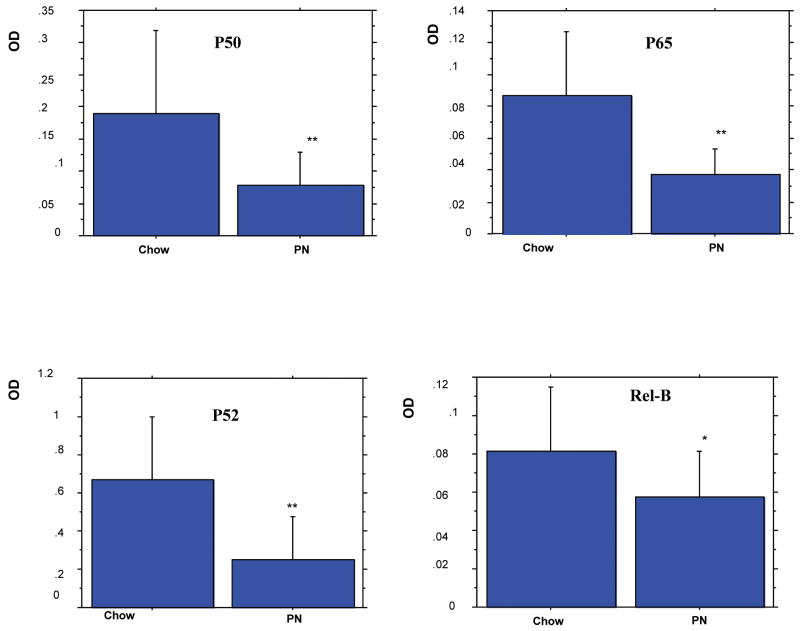
Figure 2.
Effects of parenteral nutrition (PN) on Nuclear Factor-κB (NF-κB) Activities in Peyer's patches (PP).
Data are expressed as mean OD ± SD. Expt Chow (n= 19) vs PN (n=16). PN significantly reduced all Phosphorylated NF-κB proteins in PP compared to Chow mice (* p<0.05, **p<0.01, PN vs Chow).
PN significantly reduced all Phosphorylated NF-κB proteins in PP compared to Chow mice (Fig.2): p50 (p<0.01), p65 (p<0.01), p52 (p<0.01) and Rel B (p<0.05).
Experiment 2: Effects of LTβR Stimulation on Phosphorylated NF-κB, MAdCAM-1, CCL19, CCL20, CCL25, IL-4 and IL-10 in PP (Fig 3&4)
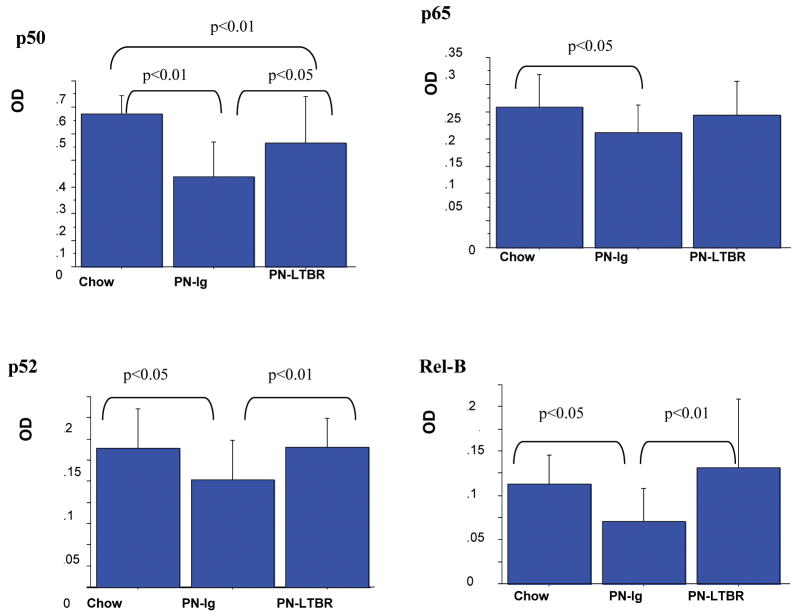
Figure 3.
Lymphotoxin β receptor (LTβR) Agonistic Antibody Stimulation of Nuclear Factor-κB (NF-κB) Activity in Peyer's patches (PP).
Data are expressed as mean OD ± SD. Stimulation of PN mice with LTβR agonistic antibody significantly increased inflammatory product p50 as well as the non-canonical p52 and Rel B in PP compared with PN controls. There were no significant differences in p65 or the non-canonical levels of p52 and Rel B between PN-LTβR and Chow mice. LTβR stimulation of PN animals failed to improve p50 to Chow levels.
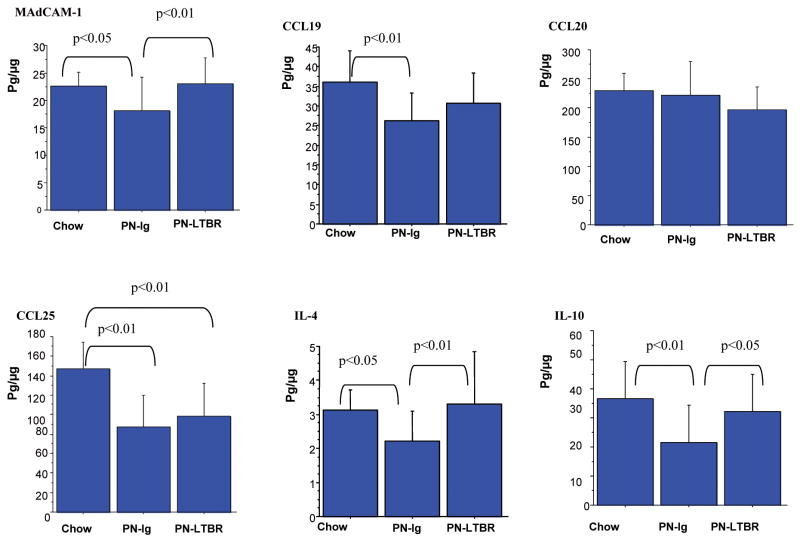
Figure 4.
Data were expressed as mean± SD pg/μg protein. PN significantly reduced MAdCAM-1, CCL19, CCL25, IL-4 and IL-10 compared to Chow mice. Compared to PN-Control mice, PN-LTβR significantly increased MAdCAM-1, IL-4 and IL-10 but not CCL20 and CCL25 in PP. There were no significant differences between PN-LTβR and Chow mice in levels of MAdCAM-1, CCL19, CCL20, IL-4 and IL-10.
Compared with PN-Ig (Fig.3), stimulation of PN mice with LTβR agonistic antibody significantly increased the NF-κB inflammatory pathway molecule p50 (p<0.01) and the NF-κB non-canonical pathway molecules p52 (p<0.01) and Rel B (p<0.05) in PP. There were no significant differences in levels of p65, p52 and Rel B between PN-LTβR and Chow mice, however, LTβR stimulation of PN animals failed to improve p50 to Chow levels (p<0.01). Compared to Chow feeding, PN-Ig (Fig.4) significantly lowered levels of MAdCAM-1 (p<0.05), CCL19 (p<0.01), CCL25 (p<0.01), IL-4 (p<0.05), and IL10 (p<0.01), but not CCL20. Compared to PN-Ig mice, PN-LTβR significantly increased MAdCAM-1 (p<0.01), IL-4(p<0.01) and IL-10(p<0.05) but had no effect on CCL19, CCL20 and CCL25 in PP There were no significant differences in levels of MAdCAM-1, CCL19, CCL20, IL-4 and IL-10 between PN-LTβR and Chow mice.
Experiment 3: Effects of LTβR Blockade on Phosphorylated NF-κB, MAdCAM-1, CCL19, CCL20, CCL25, IL-4 and IL-10 in PP (Fig 5&6)
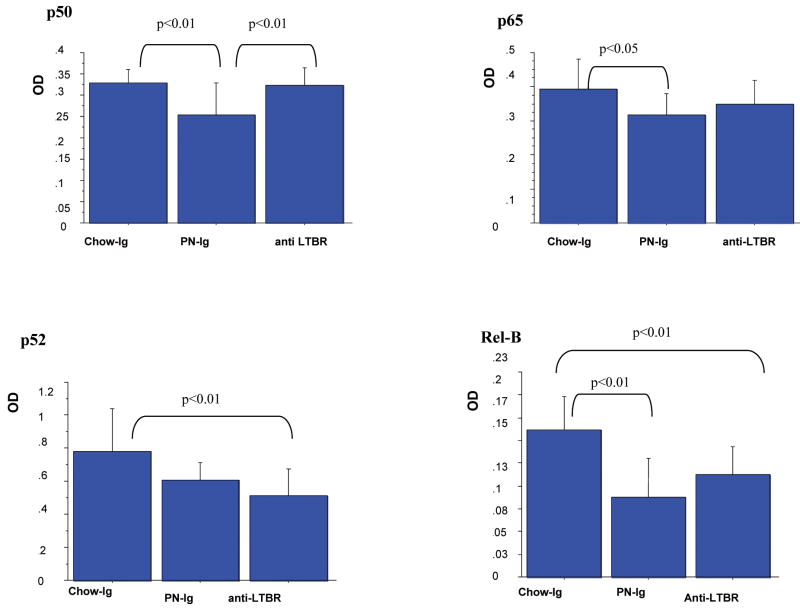
Figure 5.
Data are expressed as mean OD ± SD. Blockade of LTβR in Chow mice with LTβR-Ig fusion protein significantly reduced the non-canonical products of p52 (0.51±0.16 vs 0.78±0.26, p<0.01) and Rel B (0.11±0.03 vs 0.16±0.04, p<0.01) to PN levels but had no effect on the inflammatory pathway products of p50 and p65 compared with Chow. As in experiment 1, PN reduced all NF-κB pathway proteins.
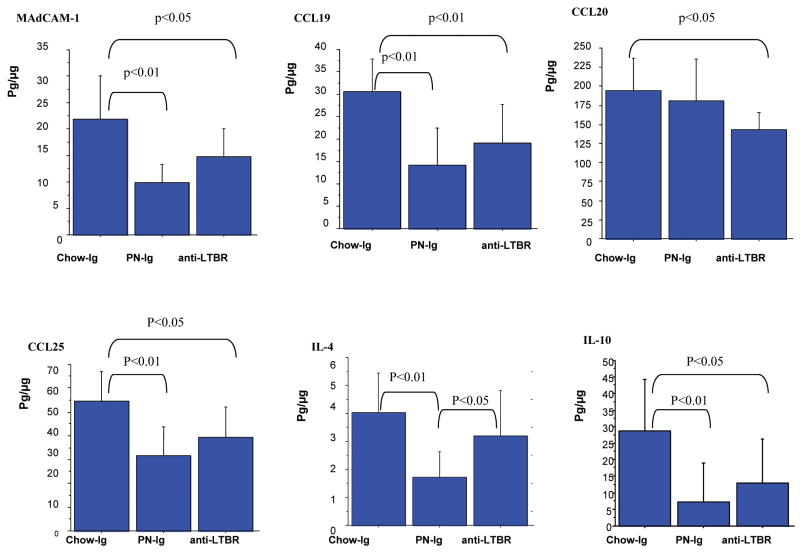
Figure 6.
Data were expressed as mean ± SD pg/μg protein. The blockade of LTβR with LTβR-Ig fusion protein significantly reduced levels of MAdCAM-1, CCL19, CCL20, CCL25, IL-4 and IL-10, compared with Chow mice. There were no significant differences between Chow- anti LTβR and PN-Ig in levels of MAdCAM-1, CCL19, CCL20, CCL25 and IL-10, except for IL-4.
Compared with Chow-Ig, blockade of LTβR in chow animals using the LTβR-Ig fusion protein significantly reduced the NF-κB non-canonical pathway molecules p52 (p<0.01) and Rel B (p<0.01) but had no effect on the NF-κB inflammatory pathway molecules p50 and p65, (Fig.5). The blockade of LTβR significantly reduced the non-canonical downstream products (Fig.6) of MAdCAM-1(p<0.05), CCL19 (p<0.01), CCL20 (p<0.05), CCL25 (p<0.05), IL-4(p<0.05) and IL-10 (p<0.05) compared to Chow-Ig.
There were no significant differences between Chow- anti LTβR and PN-Ig in levels of MAdCAM-1, CCL19, CCL20, CCL25 and IL-10. Only levels of IL-4 were higher in Chow- anti LTβR mice compared to PN (p<0.05).
Discussion
Intestinal lymphoid tissues induce adaptive immunity to pathogens while maintaining a symbiotic homeostasis with commensal bacteria in the gut lumen. Our prior work established that PN (with lack of enteral stimulation) reduces MAdCAM-1 within PP resulting in fewer CD4+ lymphocytes in the PP and lamina propria and lowers levels of 1) two critical Th2-type IgA stimulating cytokines, IL-4 & IL-10, 2) the chemokines CCL-20, CCL25, CXCL13 and 3) sIgA levels in the gut and lung 3,8,12,13. We confirmed that MAdCAM-1 recruits lymphocytes by showing that antibody blockade of MAdCAM-1 7 (as well as L-selectin or α4β71) reduced PP lymphocytes cell to PN levels. A unifying concept tying MAdCAM-1, cytokines, and the chemokines with lymphocyte migration is LTβR. After LT stimulation by activated T cells, LTβR regulates expression of PP MAdCAM-1 and tissue chemokine ligands necessary for lymphocyte trafficking as well as cytokine production presumably via one of the NFκB pathways 5,10. The current work confirms that NFκB is an integral step in the expression and production of tissue adhesion molecules and cytokines responsible for attracting T&B cells into the Peyer's patches. Stimulating (or blocking) LTβR resulted in an increase (or decrease) in MAdCAM-1 as well as several chemokines and cytokines which play critical roles in attachment, migration and stimulation of mucosal immune cells to maintain intact mucosal immunity. Surprisingly, inflammatory NFκB pathways were also controlled by LTβR stimulation to some extent.
Migration of immune cells to lymphoid organs and to effector sites is mediated by a multistep process whereby adhesion receptors, chemokines, and their receptors determine the specificity of immune cell homing.14-16 Both MAdCAM-1 and tissue specific chemokine ligands are expressed in response to LTβR. MAdCAM-1 is an important ligand for α4β7 integrin expressed on naive T&B cells destined for the mucosal immune system. In the adult mouse, a modified MAdCAM-1, especially interactive with α4β7, is constitutively expressed on high endothelial venules of PP. An unmodified MAdCAM-1, especially interactive with L-selectin, is expressed in mesenteric lymph nodes, postcapillary venules of the intestinal LP, the lactating mammary gland, and in sinus-lining cells in the spleen surrounding the periarteriolar lymphocyte sheath and follicle areas14,17,18. The difference in the modified and unmodified form is important since naïve T&B cells predominantly express α4β7 (with a lesser expression of L-selectin) while cells sensitized to antigen in the PP predominantly express L-selectin (with a lowered expression of α4β7). These specific modifications direct cell into and through the MALT from entry sites (the PP) to effector sites where IgA is produced and secreted for immune protection. Our studies with monoclonal antibody blockage of MAdCAM-1 revealed a role for this adhesion molecule in mediating lymphocyte migration into PP. In the presence of reduced MAdCAM-1 expression in the PP with PN (it is not reduced in effector sites18), antigen-specific IgA-secreting PC migration to the intestinal LP is compromised. In this study as in our previous work7, LTβR stimulation with agonistic antibody reverses the PN-induced MAdCAM-1 reduction in PP to levels equivalent to Chow fed animals while LTβR-Ig fusion protein in chow mice reduces MAdCAM-1 expression to levels equivalent to PN fed animals (Fig.5). These results occur in parallel with reduced levels of the non-canonical NF-κB activation pathway molecules, p52 and Rel B.
Our work supports our hypothesis that LTβR exerts its activity on these adhesion and migration products through the non-canonical NF-κB activation pathway. The non-canonical NF-κB pathway is activated when lymphotoxin on T and B cells binds to LTβR. This pathway only requires p52/RelB heterodimer. Gene transcription is slow and long- lasting with p52-dependent signals when compared to the inflammatory canonical NF-κB which is almost instantaneous.20 In this study, PN reduced p52 and Rel B production in parallel with MAdCAM-1, the chemokines CCL-25 and CCL-19 as well as IL-4 and IL-10. LTβR stimulation of PN mice with agonistic antibody reversed several of these including MAdCAM-1, IL-4 and IL-10 (but not the chemokines) while LTβR blockade with LTβR-Ig fusion protein in Chow mice reduced p52 and Rel B to similar levels as in PN fed animals together with MAdCAM-1, CCL-19, CCL-25, IL-4 and IL-10. The changes are somewhat circumstantial since we could not directly block or stimulate NF-κB activation to examine the response. To our knowledge non-toxic drugs that can be administered chronically in vivo to block or stimulate the non-canonical NF-κB activation pathway directly do not exist.
In addition to the critical role of MAdCAM-1 in cell entry, the other end products also exert important effects on mucosal immunity. IL-4, produced by CD4+ T cells 21, regulates antibody production, hematopoiesis and inflammation, as well as the development of effector T-cell responses. IL-4 can educate dendritic cells (DC) to differentially affect T cell effector activity and acts upon DC to instruct naive T cells to express the gut-associated homing receptor CCR9, which specifically binds CCL25 in small intestine.22 IL-10 is not specific to Th2 cells or regulatory T (TReg) cells but is much more broadly expressed by cells including Th1, Th2 and Th17 cell subsets, TReg cells, CD8+ T cells and B cells as well as cells in the innate immune system.23. While both IL-4 and IL-10 in PP are reduced by PN feeding, these reductions are corrected by LTβR stimulation with agonistic antibody during PN and reduced in chow fed mice after LTβR-Ig fusion protein to levels equivalent with PN fed animals. These cytokine changes are consistent with changes in the expression of p52 and Rel B, the non-canonical NF-κB proteins in our experimental system.
The only discordance between LTβR, the non-canonical NF-κB proteins and cell products related to the chemokine levels. While CCL-19 and CCL25 levels dropped with PN and LTβR blockade, LTβR simulation had no effect on CCL25 and minimal effect on CCL-19. This implies that CCL-19 and CCL25 expression may still need other simulation in addition to LTβR signaling to returns levels to normal. Neither PN nor stimulation affected CCL20 while LTβR blockade had some inhibitory effect upon this molecule. This may due to the cross compensation from different NF- κB pathways since recent research shows that TLR-5- and LTβR -dependent epithelial CCL20 expression involves the same NF-κB binding site but distinct NF- κB pathways and dynamics.24
The reduction in the canonical inflammatory pathway products, p50 and p65, with PN was unexpected. Our previous work experimental work established that PN provokes proinflammatory responses to stress and gut inflammation by increasing expression of ICAM-1 & p-selectin and increasing neutrophil accumulation in the gut endothelium 25,26, by increasing expression of pulmonary e-selectin 26, by promoting liver and pulmonary dysfunction after gut ischemia reperfusion 27, and by increasing ICAM-1, pulmonary mononuclear β2 integrin expression after a gut ischemic insult resulting in a higher mortality 28. This increased systemic inflammation is consistent with clinical data on metabolic responses and the development of multiple organ failure after PN 29,30. But these events reflect systemic, rather than local effects which may explain the discordance. Recently we noted that both injured patients and mice respond to trauma by increasing airway IgA in, presumably, a protective response. 31 In our animal model of controlled injury, the airway IgA response can be recreated by exogenous administration of tumor necrosis factor-α (TNFα) and eliminated by blocking TNFα through administration of an anti-TNFα monoclonal antibody 32. The increase in airway concentrations of TNFα is a local rather than a systemic response. Interestingly, the intestine also responds to injury by acutely increasing luminal levels of IgA just like the lung presumably in a similar manner. Since PN eliminates both the pulmonary and airway IgA response to injury 33, the reduction in the canonical inflammatory pathway products noted in our results may explain this PN-induced defect in the IgA response to injury.
In summary, we believe that our work confirms that defects in cell entry into the MALT induced by PN with lack of enteral feeding to support nutritional status is due to a LTβR-dependent depression in the non-canonical NF-κB activation pathway. This results in reduced expression in the levels of adhesion molecules, cytokines and, to some extent the chemokines, responsible for adhesion and attraction of naïve T&B cells into the mucosal immune system. In addition, PN with lack of enteral stimulation affects the inflammatory pathway as well. Clinically, development and administration of non-toxic agent to stimulate LTβR may provide an avenue to reverse the negative effects of PN on mucosal immunity.
Acknowledgments
We thank professor Yang-Xin Fu (Department of Pathology, University of Chicago, Chicago, IL) for his generous providing LTβR-Ig fusion protein IV, and Jeffrey L. Browning (Biogen Idec, 12 Cambridge Center, Cambridge, MA) for providing agonistic LTβR mAb. This research is funded by NIH Grant R01 GM53439. This material is also based upon work supported in part by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development Service. The contents of this article do not represent the views of the Dept. of Veterans Affairs or the United States Government.
Footnotes
No commerial sponsorship
Presented at the Surgical Infection Society meeting, April18-20, 2010 Las Vegas, NV
References
- 1.Reese SR, Genton L, Ikeda S, Le TC, Kudsk KA. L-selectin and α4β7 Integrin but not ICAM-1 Regulate Lymphocyte Distribution in Gut-Associated Lymphoid Tissue of Mice. Surgery. 2005;137(2):209–215. doi: 10.1016/j.surg.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 2.Ikeda S, Kudsk KA, Fukatsu K, Johnson C, Le T, Reese S, Zarzaur B. Enteral Feeding Preserves Mucosal Immunity Despite in vivo MAdCAM-1 Blockage of Lymphocyte Homing. Ann of Surg. 2003;237(5):677–685. doi: 10.1097/01.SLA.0000064364.40406.EA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hermsen JL, Gomez FE, Maeshima Y, Sano Y, Kang W, Kudsk KA. Decreased Enteral Stimulation Alters Mucosal Immune Chemokines. J Parenter Enteral Nutr. 2008;32(1):36–44. doi: 10.1177/014860710803200136. [DOI] [PubMed] [Google Scholar]
- 4.Brandtzaeg P, Kiyono H, Pabst R, Russell MW. Terminology: nomenclature of mucosa-associated lymphoid tissue. Mucosal Immunol. 2008;1:31–37. doi: 10.1038/mi.2007.9. [DOI] [PubMed] [Google Scholar]
- 5.Lo JC, Basak S, James SE, et al. Coordination between NF-κB family members p50 and p52 is essential for mediating LTβR signals in the development and organization of secondary lymphoid tissues. Blood. 2006;107:1048–1055. doi: 10.1182/blood-2005-06-2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kang W, Kudsk KA, Sano Y, et al. Effects of lymphotoxin beta receptor blockade on intestinal mucosal immunity. JPEN J Parenter Enteral Nutr. 2007;31(5):358–64. doi: 10.1177/0148607107031005358. discussion 364-5. [DOI] [PubMed] [Google Scholar]
- 7.Kang W, Gomez FE, Lan J, et al. Parenteral nutrition impairs gut-associated lymphoid tissue and mucosal immunity by reducing lymphotoxin Beta receptor expression. Ann Surg. 2006;244:392–399. doi: 10.1097/01.sla.0000234797.42935.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li J, Kudsk KA, Gocinski B, Dent D, Glezer J, Langkamp-Henken B. Effects of parenteral and enteral nutrition on gut-associated lymphoid tissue. J Trauma. 1995;39(1):44–52. doi: 10.1097/00005373-199507000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Zarzaur BL, Fukatsu K, Johnson CJ, Eng E, Kudsk KA. A temporal study of diet-induced changes in peyer patch MAdCAM-1 expression. Surgical Forum. 2001;52:194–196. [Google Scholar]
- 10.Pasparakis M. Regulation of tissue homeostasis by NF-kappaB signalling: implications for inflammatory diseases. Nat Rev Immunol. 2009 Nov;9(11):778–88. doi: 10.1038/nri2655. [DOI] [PubMed] [Google Scholar]
- 11.Sitren HS, Heller PA, Bailey LB, et al. Total parenteral nutrition in the mouse: development of a technique. J Parenter Enteral Nutr. 1983;7:582–586. doi: 10.1177/0148607183007006582. [DOI] [PubMed] [Google Scholar]
- 12.Gomez FE, Lan J, Kang W, Ueno C, Kudsk KA. Parenteral nutrition and fasting reduces MAdCAM-1 mRNA in Peyer's patches of mice. JPEN. 2007;31(1):47–52. doi: 10.1177/014860710703100147. [DOI] [PubMed] [Google Scholar]
- 13.Wu Y, Kudsk KA, DeWitt RC, Tolley EA, Li J. Route and type of nutrition influence IgA-mediated intestinal cytokines. Ann Surg. 1999;229(5):662–668. doi: 10.1097/00000658-199905000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schippers A, Leuker C, Pabst O, et al. Mucosal addressin cell-adhesion molecule-1 controls plasma-cell migration and function in the small intestine of mice. Gastroenterology. 2009;137(3):924–33. doi: 10.1053/j.gastro.2009.05.039. [DOI] [PubMed] [Google Scholar]
- 15.Gorfu G, Rivera-Nieves J, Ley K. Role of beta7 integrins in intestinal lymphocyte homing and retention. Curr Mol Med. 2009;9(7):836–50. doi: 10.2174/156652409789105525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeNucci CC, Pagan AJ, Mitchell JS, et al. Control of {alpha}4{beta}7 Integrin Expression and CD4 T Cell Homing by the {beta}1 Integrin Subunit. J Immunol. 2010;184:2458–2467. doi: 10.4049/jimmunol.0902407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 18.Poupon V, Cerf-Bensussan N. Adhesion molecules on mucosal lymphocytes. In: Ogra PL, Mestecky J, Lamm ME, Strober W, Bienenstock J, McGhee JR, editors. Mucosal Immunology. San Diego: Academic Press; 1999. pp. 523–40. [Google Scholar]
- 19.Fukatsu K, Zarzaur B, Johnson C, Wu Y, Wilcox H, Kudsk KA. Decreased MAdCAM-1 expression in Peyer's patches: A mechanism for impaired mucosal immunity during lack of enteral nutrition. Surg Forum. 2000:211–214. LI. [Google Scholar]
- 20.Hoffmann A, Baltimore D. Circuitry of nuclear factor kappaB signaling. Immunol Rev. 2006;210:171–186. doi: 10.1111/j.0105-2896.2006.00375.x. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki K, Fagarasan S. Diverse regulatory pathways for IgA synthesis in the gut. Mucosal Immunol. 2009;2(6):468–71. doi: 10.1038/mi.2009.107. [DOI] [PubMed] [Google Scholar]
- 22.Borte S, Pan-Hammarström Q, Liu C, et al. Interleukin-21 restores immunoglobulin production ex vivo in patients with common variable immunodeficiency and selective IgA deficiency. Blood. 2009;114(19):4089–98. doi: 10.1182/blood-2009-02-207423. [DOI] [PubMed] [Google Scholar]
- 23.Saraiva M, O'Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol. 2010;10(3):170–81. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- 24.Sirard JC, Didierlaurent A, Cayet D, et al. Toll-like receptor 5- and lymphotoxin beta receptor-dependent epithelial Ccl20 expression involves the same NF-kappaB binding site but distinct NF-kappaB pathways and dynamics. Biochim Biophys Acta. 2009;1789(5):386–94. doi: 10.1016/j.bbagrm.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 25.Fukatsu K, Lundberg AH, Hanna MK, Wu Y, Wilcox HG, Granger DN, Gaber AO, Kudsk KA. Route of nutrition influences intercellular adhesion molecule-1 expression and neutrophil accumulation in intestine. Arch Surg. 1999;134:1055–1060. doi: 10.1001/archsurg.134.10.1055. [DOI] [PubMed] [Google Scholar]
- 26.Fukatsu K, Lundberg AH, Hanna MK, Wu Y, Wilcox HG, Granger DN, Gaber AO, Kudsk KA. Increased expression of intestinal p-selectin and pulmonary e-selectin during IV-TPN. Arch Surg. 2000;135:1177–1182. doi: 10.1001/archsurg.135.10.1177. [DOI] [PubMed] [Google Scholar]
- 27.Fukatsu K, Zarzaur BL, Johnson CD, Lundberg AH, Wilcox HG, Kudsk KA. Enteral nutrition prevents remote organ injury and mortality following a gut ischemic insult. Ann Surg. 2001;233(5):660–668. doi: 10.1097/00000658-200105000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fukatsu K, Kudsk KA, Zarzaur BL, Sabek O, Wilcox HG, Johnson CD. Increased ICAM-1 and β2 integrin expression in parenterally fed mice after a gut ischemic insult. Shock. 2002;18(2):119–24. doi: 10.1097/00024382-200208000-00005. [DOI] [PubMed] [Google Scholar]
- 29.Moore FA, Moore EE. The evolving rationale for early enteral nutrition based on paradigms of multiple organ failure: a personal journey. Nutr Clin Pract. 2009 Jun-Jul;24(3):297–304. doi: 10.1177/0884533609336604. [DOI] [PubMed] [Google Scholar]
- 30.Fong YM, Marano MA, Barber A, He W, Moldawer LL, Bushman ED, Coyle SM, Shires GT, Lowry SF. Total parenteral nutrition and bowel rest modify the metabolic response to endotoxin in human. Ann Surg. 1989 Oct;210(4):449–56. doi: 10.1097/00000658-198910000-00005. discussion 456-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kudsk KA, Hermsen JL, Genton L, Faucher L, Gomez FE. Injury stimulates an innate respiratory IgA immune response in humans. J Trauma. 2008;64(2):316–325. doi: 10.1097/TA.0b013e3181627586. [DOI] [PubMed] [Google Scholar]
- 32.Hermsen JL, Sano Y, Gomez FE, Maeshima Y, Kang K, Kudsk KA. Parenteral Nutrition Inhibits a Tumor Necrosis Factor-α Mediated IgA Response to Injury. Surgical Infections (Larchmt) 2008;9(1):33–40. doi: 10.1089/sur.2007.029. [DOI] [PubMed] [Google Scholar]