Abstract
The specificity of relationships between anxiety and depressive symptoms, with each of the major atopic disorders of asthma, allergic rhinitis (AR), and atopic dermatitis (AD) was systematically investigated within a single study sample. Participants included 367 adolescents who participated in a community, longitudinal study investigating risk factors for the development of psychiatric and physical health problems. Mental health symptoms were assessed at 7, 9, 11, and 13 years of age. Lifetime history of atopic disorders was assessed by parent report at age 13. Analysis of variance was used to investigate the specificity of the associations between anxiety and depression, and each of the atopic disorders. Results indicated that anxiety was associated with a lifetime history of atopic disorders as a group. The association was significantly strengthened when controlling for depression and externalizing psychiatric symptoms. Among atopic disorders, “pure” anxiety was associated with asthma and AR, and having both asthma and AR strengthened the association compared to having either disorder alone. The association of “pure” anxiety with asthma and AR is consistent with existing data suggesting a relationship between anxiety and respiratory disorders. Having both asthma and AR appeared to confer an additive “dose effect” on the strength of the association. The lack of an association with depression suggests that other factors may contribute to the differential expression of anxiety and depression with atopic disorders. Findings demonstrate the importance of assessing the impact of co-morbid psychiatric symptoms and atopic disorders within individual studies to determine the specificity of underlying relationships between these conditions.
Keywords: anxiety, depression, asthma, allergic rhinitis, atopic dermatitis
1. Introduction
Considerable research has focused on the association between internalizing psychiatric symptoms of anxiety and depression, and atopic disorders, a familial group of IgE-mediated allergies that includes asthma, allergic rhinitis, and atopic dermatitis (Slattery, 2005). Although findings collectively suggest an association between these psychiatric and allergic disorders, the specificity of the relationship remains unclear including whether the association exists broadly among groups, or conversely, only among specific disorder subtypes. Knowledge of this specificity, i.e. unique relationships between anxiety, depression and each of the atopic disorders, is important in providing a framework for subsequent investigations aimed at elucidating underlying mechanisms contributing to the etiopathogenesis of both types of disorders, and relatedly, the development of novel treatments targeting these specific pathways. A recent meta-analysis by Chida and colleagues (2008) highlights the psychosomatic relevance of this research, with findings demonstrating a bidirectional relationship between mental health symptoms and atopic disorders (Chida et al., 2008).
Existing studies largely focus on the relationship of anxiety and/or depression with each of the major atopic disorders. The association between asthma and internalizing psychiatric symptoms has been investigated most extensively to date. Both clinical and community studies provide substantive evidence of a link between asthma and anxiety disorders, and panic disorder in particular (Goodwin & Pine, 2002; Hasler et al., 2005), in both adults and children (Goodwin, 2003; Katon et al., 2004). An association between depression and asthma has also been reported (Morrison et al., 2002; Nejtek et al., 2001; Opolski & Wilson, 2005; Van Lieshout et al., 2009; Zielinski & Brown, 2003), although results are less consistent, especially when considering other disease-related factors such as asthma severity (Mancuso et al., 2008; Morrison et al., 2002) and co-occurring anxiety (Brown, 2000; Goodwin et al., 2003b, 2005; Goodwin & Pine, 2002; Katon et al., 2007b; Ortega et al., 2002). Findings collectively suggest that asthma may be more consistently associated with anxiety alone or in combination with depression, and that the relationship with depression may be influenced to a greater degree by asthma-related factors such as symptom severity and illness chronicity (Janson-Bjerklie et al., 1992; Mancuso et al., 2008; Opolski & Wilson, 2005).
There are fewer studies of a potential association between allergic rhinitis (AR), and anxiety or depression. Clinical investigations of adults and youth suggest that AR is associated with increased state and trait anxiety symptoms, as well as generalized anxiety disorder (Addolorato et al., 1999; Hart et al., 1995). Other studies report increased rates of depression in subjects with AR, although as with asthma, the relationship appears to be influenced by a number of variables including seasonality and AR-associated sleep loss (Kovacs et al., 2003; Marshall et al., 2002; Nathan, 2007). Results of community studies are also mixed (Cuffel et al., 1999; Goodwin, 2002).
Investigations of psychosomatic correlates of atopic dermatitis (AD) have predominately focused on the assessment of psychological and personality characteristics associated with AD, with results commonly reporting difficulties with anger, hostility, and insecurity (Ginsburg et al., 1993; White et al., 1990). Studies investigating the relationship between internalizing symptoms and AD are inconclusive, and suggest that AD may be associated with anxiety, depression, or both anxiety and depression (Annesi-Maesano et al., 2006; Hashiro & Okumura, 1994; Hashizume et al., 2005; Linnet & Jemec, 1999; Wittkowski et al., 2004). Symptoms of chronic, trait anxiety appear to occur more consistently, compared to those of state anxiety (Annesi-Maesano et al., 2006; Ginsburg et al., 1993; Linnet & Jemec, 1999).
In summary, existing investigations focus on the relationship between anxiety and/or depression and specific atopic disorders, within divergent study samples. This is the first study, to our knowledge, to systematically investigate the specificity of the relationships between both anxiety and depression, and each of the major atopic disorders, within a single study sample. Use of this study design permits the assessment of specific and independent associations among symptoms of anxiety and depression, and each of the unique atopic disorders, that might otherwise be masked by the co-occurrence of related conditions. Results may shed new light on potential underlying mechanisms contributing to the frequent co-occurrence of these psychiatric and allergic states, and hence, open new windows to the development of targeted treatment interventions for these individuals.
Subjects are drawn from a community, longitudinal study of adolescents thus permitting assessment of trait-like mental health symptoms over time in relation to lifetime history of asthma, AR, and/or AD. The objectives of the present study are to 1) assess whether having any atopic disorder is associated with overall levels of anxiety vs. depressive symptoms and 2) assess the specificity of the relationship of each of the 3 atopic disorders (asthma, AR, and AD) with anxiety vs. depressive symptoms. We hypothesize that symptoms of anxiety will be more robustly associated with asthma, AR, and AD relative to symptoms of depression. Importantly, the investigation will also examine the potential impact of co-morbid psychiatric symptoms and atopic disorders on each of the possible associations, compared to relationships among “pure” anxiety, depression, asthma, AR, and AD.
2. Materials and methods
2.1. Subjects
Subjects included the 367 adolescents and mothers participating in a long-term longitudinal study (Wisconsin Study of Families and Work; WSFW) who had data on adolescent mental health problems and atopic disorders at the time of the Grade 7 assessment. The initial sample included 570 women recruited from pre-natal clinics. Eligibility requirements included being over age 18, in the second trimester of pregnancy, and because the original focus of the project was on issues of family and work, to be living with the father and either employed or homemakers (for details see (Hyde et al., 1995)). The study was approved by the Institutional Review Board of the University of Wisconsin-Madison. Informed consent was obtained from adults at each assessment; beginning at Grade 5, children provided informed assent.
The sample of 367 adolescents consisted of 175 boys and 192 girls; 11% represented ethnic minorities. At the time of recruitment (1990), 96% of the parents were married; family income ranged from less than $10,000 to over $200,000, (Mdn = $48,000). The 367 mothers ranged in age from 20 to 41 years; fathers ranged in age from 20 to 55 years. Of the mothers, 2% had less than a high school degree, 42% were high school graduates, and 56% were college graduates; of the fathers, 2% had less than a high school degree, 48% were high school graduates, and 50% were college graduates. The 367 families did not differ from the remainder of the original 570 families with respect to any of the above demographic characteristics with two minor exceptions: the participating mothers (M = 29.7, SD = 4.3) and fathers (M = 31.7, SD = 5.2) were 1 year older than non-participating mothers (M = 28.8, SD = 4.4) [t(568) = −2.22, p = 0.027] and fathers (M = 30.5, SD = 4.7) [t(548) = −2.71, p = 0.007]; and the participating fathers had a half-year more education (M = 15.2, SD = 2.5) than non-participating fathers (M = 14.8, SD = 2.6) [t(548) = −1.98, p = 0.049].
2.2. Measures
2.2.1. Children’s lifetime history of atopic disorders
At Grade 7, mothers were asked about the adolescents’ lifetime history of atopic disorders. For each of the three disorders (Asthma, Allergic Rhinitis, Atopic Dermatitis), mothers were asked whether or not (coded 1 vs. 0) their child ever had the condition. A summary measure of the three conditions was also constructed to define whether or not (coded 1 vs. 0) the adolescents had a Lifetime History of Any Atopic Disorder.
2.2.2. Adolescents’ Mental Health Symptoms at Grades 1, 3, 5, 7
Mothers, teachers, and children were interviewed during the spring of Grades 1, 3, 5 and 7 about the child’s mental health symptoms during the prior six months. Adults completed the Health and Behavior Questionnaire (Boyce et al., 2002; Essex et al., 2002); at Grade 1, children were administered the Berkeley Puppet Interview (Ablow et al., 1999; Measelle et al., 1998), which was modified to a flip-book format to be age-appropriate at the later assessments extending into adolescence. The mental health symptom items were derived primarily from the Ontario Child Health Study scales, well-established measures based on DSM-III criteria and with known reliability and validity (Boyle et al., 1993). The Generalized Anxiety Symptoms scale includes 11 items, e.g., Worries about doing better (all αs, across grades and reporters > 0.70, with exception of α = 0.61 for Grade 1 child report). The Depression Symptoms scale includes 12 items, e.g., Unhappy, sad, depressed (all αs > 0.77, with exception of α = 0.65 and 0.61 for Grade 1 mother and child report, respectively). The Externalizing Symptoms scale is the average of three subscales including oppositional defiant behaviors (n = 9 items, e.g., Defiant, talks back; all αs > 0.80, with exception of α = 0.42 and 0.63 for Grade 1 and 3 child report, respectively), conduct problems (n = 11 items, e.g., Destroys others’ belongings; all αs > 0.74, with exception of α = 0.60 for Grade 1 child report), and inattention/impulsivity (n = 15 items, e.g., Cannot concentrate; all αs > 0.80). For each of the three symptom scales, the scores of the mothers, teachers, and adolescents were combined using Principal Components Analysis (PCA), where the first component represented what the three reports shared in common (see (Kraemer et al., 2003) for the original conceptualization of this approach to combining data from multiple informants based on the WSFW data). Across all assessments, the first component of each PCA accounted for over 50% of the variance and the factor loadings of each informant were above 0.50.
2.2.3. Additional measures
In addition to the major measures defined above, several other variables were considered in analyses, including Child Sex (0=male; 1=female), Family Socioeconomic Status (assessed with a PCA composite of parental education level and family annual income at the time of recruitment), and whether or not (coded 1 vs. 0) the adolescent had a lifetime history of any other of nine Chronic Medical Conditions (e.g., diabetes, bowel disease, kidney disease, thyroid problems).
3. Results
3.1 Descriptive statistics
Table 1 presents descriptive statistics for the three atopic disorders and the psychiatric symptoms, and differences by child gender and family socioeconomic status (SES). Of the three atopic disorders, adolescents were most likely to have a lifetime history of allergic rhinitis, followed by asthma and atopic dermatitis, with prevalence rates similar to an epidemiological study of comparable aged youth (Shamssain & Shamsian, 2001). When considered together, approximately half of the adolescents had a lifetime history of at least one of the three atopic disorders: 109 adolescents had only one atopic disorder (asthma = 27, AR = 66, AD = 16); 45 had two atopic disorders (asthma + AR = 29, asthma + AD = 9, AR + AD = 7); and 16 had a lifetime history of all three atopic disorders.
Table 1.
Descriptive statistics for psychiatric symptoms and the three atopic disorders.
| N (%) | Anxiety Symptomsa M (SD) |
Depression Symptomsa M (SD) |
Externalizing Symptomsa M (SD) |
No Lifetime any Atopic Disorder N (%) |
Lifetime any Atopic Disorder N (%) |
Lifetime Asthmab N (%) |
Lifetime Allergic Rhinitisb N (%) |
Lifetime Atopic Dermititisb N (%) |
|---|---|---|---|---|---|---|---|---|
| Full Sample 367 (100.0) | .013 (.803) | .019 (.845) | .019 (.915) | 197 (53.7) | 170 (46.3) | 81 (22.1) | 118 (32.1) | 48 (13.2) |
| Female 192 (52.3) | .112 (.801) | .063 (.828) | −.216 (.775) | 115 (59.9) | 77 (40.1) | 42 (21.9) | 55 (28.6) | 19 (9.9) |
| Male 175 (47.7) | −.096 (.793) | −.029 (.864) | .276 (.988) | 82 (46.9) | 93 (53.1) | 39 (22.3) | 63 (36.0) | 29 (16.6) |
| M (SD) | Anxiety Symptoms r | Depression Symptoms r | Externalizing Symptoms r | No Lifetime any Atopic Disorder M (SD) | Lifetime any Atopic Disorder M (SD) | Lifetime Asthmaa M (SD) | Lifetime Allergic Rhinitisa M (SD) | Lifetime Atopic Dermititisa M (SD) |
| SESa .069 (.978) | −.268*** | −.278*** | −.300*** | .063 (.976) | .076 (.985) | .167 (.984) | .008 (.965) | .049 (.930) |
Standardized score
Counts for each of these atopic disorders are not mutually exclusive
p<0.001
Chi-square, t-tests, and correlational analyses were used to investigate differences in lifetime atopic disorders and mental health symptoms by child gender and family SES. Compared with girls, boys were significantly more likely to have a lifetime history of any atopic disorder (χ2 = 6.26, df = 1, p = 0.008), and they evidenced lower levels of anxiety symptoms [t(365) = −2.50, p = 0.013] and higher levels of externalizing symptoms [t(365) = 5.34, p < 0.001]. There were no SES differences in lifetime history of any atopic disorder [t(365) = −0.12, p = 0.903]. However, adolescents born into lower SES families evidenced higher levels of anxiety, depression, and externalizing symptoms. Given these associations, child sex and family SES were included as controls in all analyses.
3.2. Specificity of association of lifetime history of any atopic disorder with anxiety vs. depression symptoms from Grade 1 to Grade 7
Analysis of variance (ANOVA) was used to address the first major research question: whether the association of lifetime history of any atopic disorder was specific to overall levels of generalized anxiety symptoms vs. depressive symptoms from Grade 1 to Grade 7. Two initial ANOVAs were conducted, one with generalized anxiety symptoms as the dependent variable and one with depression symptoms as the dependent variable (Table 2, Model 1). The results showed that a lifetime history of any atopic disorder was associated with significantly higher overall levels of generalized anxiety symptoms, but there was no significant association with depression symptoms.
Table 2.
Results of ANOVA associations of lifetime atopic disorders (D/O) with generalized anxiety and depression symptoms.
| Symptoms (dependent variable) |
Model 1a |
Model 2b |
Model 3c |
Model 4d |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No Atopic D/O M (SE) |
Atopic D/O M (SE) |
F (1,363) |
No Atopic D/O M (SE) |
Atopic D/O M (SE) |
F (1,362) |
No Atopic D/O M (SE) |
Atopic D/O M (SE) |
F (1,362) |
No Atopic D/O M (SE) |
Atopic D/O M (SE) |
F (1,361) |
|
| Anxiety | −0.080 (.055) | .121 (.059) | 6.24* | −.066 (.037) | .104 (.040) | 9.46** | −.091 (.047) | .133 (.051) | 10.29** | −.069 (.037) | .107 (.040) | 10.24** |
| Depression | −.003 (.058) | .043 (.063) | 0.29 | .070 (.040) | −.041 (.043) | 3.47 | −.016 (.046) | .059 (.050) | 1.22 | .046 (.037) | −.013 (.039) | 1.20 |
Adjusted for child sex and SES
Adjusted for child sex, SES, and either depression or anxiety symptoms
Adjusted for child sex, SES, and externalizing symptoms
Adjusted for child sex, SES, either depression or anxiety symptoms, and externalizing symptoms
p<0.05;
p<0.01
Because we found, as expected, substantial co-occurrence of childhood anxiety and depression symptoms (r = 0.75; p < 0.001), as well as each of these with externalizing symptoms (anxiety, r = 0.48; p < 0.001; depression, r = 0.60; p < 0.001), a second set of ANOVAS was conducted to control for co-occurring symptoms (Table 2, Models 2 – 4). Results showed that the association of lifetime history of any atopic disorder with generalized anxiety symptoms was strengthened (i.e., F values increased, with stronger significance levels) when depression symptoms (Model 2), externalizing symptoms (Model 3), or both types of symptoms (Model 4) were controlled. However, the association of lifetime history of any atopic disorder with depression symptoms remained non-significant when anxiety and externalizing symptoms were controlled. Together, these results suggest that a lifetime history of any atopic disorder is associated more specifically with “pure” generalized anxiety symptoms rather than those co-occurring with either depression symptoms or, as we have previously shown (Infante et al., 2007), with externalizing symptoms. Thus, for the remaining analyses, externalizing symptoms and either depression (for analyses with anxiety as the dependent variable) or anxiety (for analyses with depression as the dependent variable) symptoms were controlled.
3.3. Specificity of type of atopic disorder with anxiety vs. depression symptoms
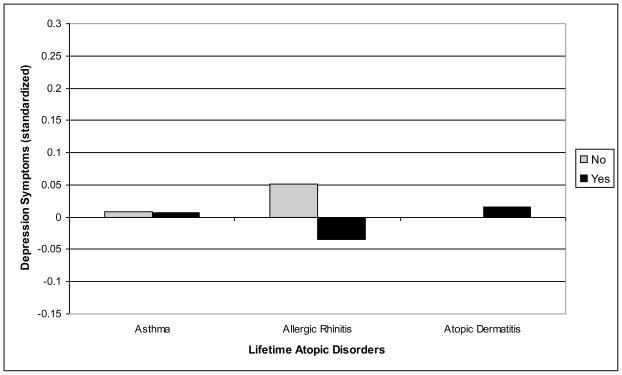
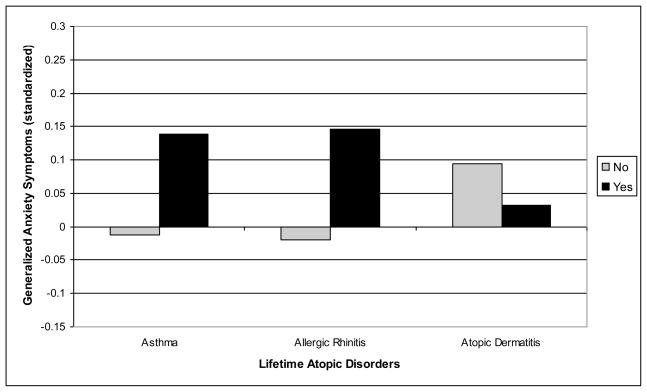
The second major research question focused on whether the association of atopic disorders with anxiety/depression symptoms is specific to any of the three atopic disorders considered. Two ANOVAS were conducted, one for anxiety symptoms and one for depression symptoms, including the three dichotomous variables defining a lifetime history of asthma, allergic rhinitis, and atopic dermatitis (Table 3). As expected based on the above results, there were no significant associations of depression symptoms with a lifetime history of asthma, allergic rhinitis, or atopic dermatitis. However, for anxiety symptoms, significant associations were found for asthma and allergic rhinitis, but not atopic dermatitis. These results, illustrated in Figures 1 and 2, suggest that there is specificity in the association of respiratory atopic disorders with generalized anxiety symptoms.
Table 3.
Results of ANOVA associations for each of the atopic disorders (lifetime) with generalized anxiety and depression symptoms.a
| Symptoms (dependent variable) | Asthma |
Allergic Rhinitis |
Atopic Dermatitis |
||||||
|---|---|---|---|---|---|---|---|---|---|
| No M (SE) | Yes M (SE) | F (1,357) | No M (SE) | Yes M (SE) | F (1,357) | No M (SE) | Yes M (SE) | F (1,357) | |
| Anxiety | −.013 (.049) | .139 (.061) | 4.53* | −.020 (0.49) | .146 (.054) | 7.49** | .094 (.038) | .032 (.075) | 0.53 |
| Depression | .009 (.048) | .007 (.060) | 0.001 | .051 (.048) | −.035 (.054) | 2.02 | <.001 (.037) | 0.16 (.074) | 0.04 |
Adjusting for child sex, SES, externalizing symptoms, and either depression or anxiety symptoms
p<0.05;
p<0.01
Fig. 1.
Specificity of lifetime atopic disorders and overall levels of depression symptoms from Grade 1 to 7.
Fig. 2.
Specificity of lifetime atopic disorders and overall levels of generalized anxiety symptoms from Grade 1 to 7.
3.4. Is there a dose effect?
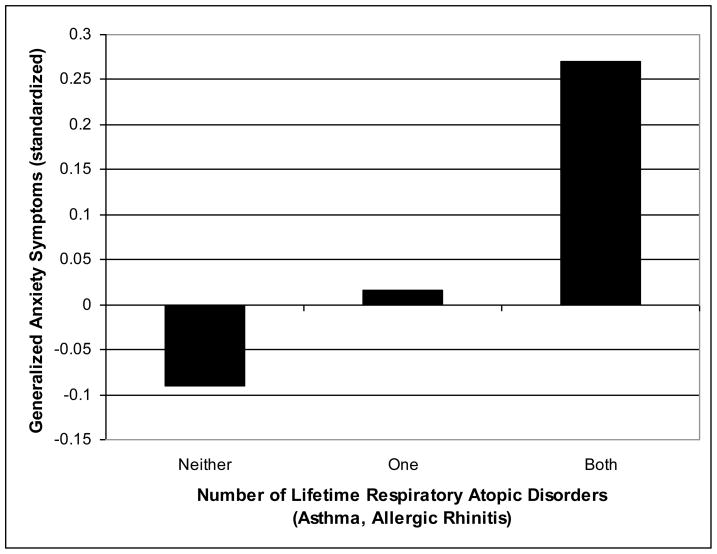
Because we found associations between generalized anxiety symptoms and both asthma and allergic rhinitis, we also investigated whether the association was stronger for adolescents with lifetime histories of both respiratory atopic disorders compared to those with only asthma or allergic rhinitis. To address this question, the above ANOVA for generalized anxiety was rerun with the two variables defining asthma and allergic rhinitis collapsed into a single variable defining adolescents with lifetime histories of neither (0), only one (1), or both (2) of these atopic disorders. The results, shown in Table 4 and illustrated in Figure 3, clarify that there is a dose effect. Further, a comparison of adolescents with no lifetime history of respiratory atopic disorders with the other two groups shows that adolescents with a lifetime history of either asthma or allergic rhinitis evidenced marginally (p = 0.085) higher levels of generalized anxiety symptoms, whereas adolescents with a lifetime history of both atopic disorders evidenced significantly (p < 0.001) higher levels of generalized anxiety symptoms from Grade 1 to Grade 7.
Table 4.
Results of ANOVA associations for asthma and/or allergic rhinitis with generalized anxiety symptoms.a
| Symptoms (dependent variable) | Neither Asthma nor Allergic Rhinitis M (SE) | Either Asthma or Allergic Rhinitis M (SE) | Both Asthma and Allergic Rhinitis M (SE) | F (2,358) |
|---|---|---|---|---|
| Anxiety | −.090 (.050) | .016 (.058) | .270 (.079) | 8.35*** |
Adjusting for child sex, SES, depression symptoms, externalizing symptoms, and atopic dermatitis
p<0.001
Fig. 3.
Dose effect of lifetime respiratory atopic disorders and overall levels of generalized anxiety symptoms from Grade 1 to 7.
Finally, to ensure that these associations were specific to atopic disorders, we included a variable distinguishing adolescents with and without a lifetime history of other chronic medical conditions. Results showed that the dose effect on generalized anxiety symptoms of number of respiratory atopic disorders remained significant [F(2,357) = 7.86, p < 0.001], and there was no additional effect of other chronic medical conditions [F(1,357) = 1.42, p = 0.235].
4. Discussion
This is the first study, to our knowledge, to address the central question of specificity of associations among symptoms of anxiety and depression, with the three major atopic disorders of asthma, AR, and AD using a single sample design. Moreover, analyses addressed the potential influences of co-morbid psychiatric symptoms and atopic conditions on these associations.
Results showed that symptoms of anxiety, but not depression, were broadly associated with a lifetime history of atopic disorders as a group. Moreover, a more robust association was found between atopic disorders and symptoms of “pure” anxiety vs. anxiety with co-morbid depression and/or externalizing symptoms. These findings are similar to those reported by our group in a previous clinical study of children and adolescents (Infante et al., 2007) in which only pure internalizing disorders were associated with atopic disorders compared to youth with co-morbid internalizing and externalizing disorders. Together, these findings suggest that externalizing psychiatric symptoms may partially obscure the identification of underlying associations of internalizing symptoms with atopic disorders, and importantly, that within internalizing symptoms, depression symptoms may partially obscure the more robust association of anxiety with atopic disorders. Further, among atopic disorders, pure anxiety symptoms were associated with asthma and AR, and not with AD, thus supporting existing studies suggesting a link between respiratory disorders and anxiety (Brenes, 2003; Goodwin & Buka, 2008; Nardi et al., 2009). Finally, the study found that having a lifetime history of both asthma and AR appeared to confer an additive “dose effect” in the association with pure anxiety. Depressive symptoms were not associated with any of the three atopic disorders in this community sample.
Results of our study agree with existing investigations reporting a more consistent association of asthma and AR with anxiety than depression (Brown, 2000; Goodwin et al., 2003b; Katon et al., 2007a; Ortega et al., 2002). Although asthma and AR are clinically distinct, contemporary research suggests that these disorders are more accurately conceptualized and treated as inflammation of one common airway (Nayak, 2003). If so, findings are further aligned with a well established body of evidence linking anxiety with respiratory abnormalities in children and adults (Nardi et al., 2009; Pine et al., 1998; Ryu et al., 2010). Our findings differ from existing studies reporting an association between depression, and asthma or AR, and suggest that other factors may contribute to this relationship, such as respiratory disease severity or chronicity (Janson-Bjerklie et al., 1992; Mancuso et al., 2008; Opolski & Wilson, 2005). Further, the lack of an association between anxiety or depression with AD as reported in other investigations may reflect other shared disease mechanisms not assessed in this study such as AD quality of life or AD disease severity (Hashiro & Okumura, 1997; Linnet & Jemec, 1999; Wittkowski et al., 2004).
Potential mechanisms underlying the association between anxiety, and asthma and AR remain unclear. Prevailing theories suggest that the association may reflect activation of an innate, fear-based neural network to perceived or experienced breathlessness or dyspnea (Ley, 1989; Nardi et al., 2009), or alternatively, activation of a suffocation alarm system due to alterations in central mechanisms of carbon dioxide sensitivity (Klein, 1993; Martinez et al., 2001). Findings of a more robust association of anxiety in adolescents with a lifetime history of both asthma and AR may reflect the effects of repeated respiratory inflammation and recurrent sensitization to conditioned dyspneic fear. Alternatively, having both asthma and AR may reflect increased respiratory disease severity contributing to an increased likelihood of co-occurring anxiety as, suggested by others. (Goodwin et al., 2003a; McQuaid et al., 2001; Wamboldt et al., 1998).
The association between anxiety and respiratory atopic disorders might also reflect broader issues related to a sense of well being as individuals attempt to adapt to ongoing illnesses. Although speculative, repeated experiences of respiratory distress might be expected to impact individuals differently compared to recurring AD, that is, repeated sensitization to experiences of breathlessness may sustain an innate and more robust link between respiratory distress and anxiety. Conversely, exacerbations of AD are commonly characterized by the subjective distress of intense pruritus and sleep loss (Bender et al., 2008) but do not typically pose a physiological threat to survival and homeostasis. Thus, co-occurrence of AD with depression and anxiety as reported in other studies may reflect other shared disease mechanisms such as quality of life (Linnet & Jemec, 1999; Wittkowski et al., 2004), disease severity (Hashiro & Okumura, 1997; Wittkowski et al., 2004), or other factors such as activation of pro-inflammatory cytokines through separate, non-atopic immune pathways (Anisman & Merali, 2003; Raison et al., 2006). Moreover, consistent with models of an “atopic march”, AD is more prevalent during infancy, and commonly resolves, compared to the later development of asthma and AR during childhood and adolescence (Cantani, 1999). Mental health symptoms in this study were assessed during late childhood and adolescence, and therefore, may not capture the impact of co-existing AD experienced during infancy/early childhood.
Our findings might also be explained by dysregulation of key neurobiological components of the stress response system. In particular, alterations of the hypothalamic-pituitary-adrenal (HPA) axis have been reported in children and adults with atopic disorders (Buske-Kirschbaum et al., 2002a, 2003; Van Lieshout et al., 2009), as well as anxiety and depression (Abelson et al., 2007; Van Lieshout et al., 2009; Young et al., 2004). HPA function is intimately linked to homeostatic regulation of the immune system, and under optimal function, constrains inflammatory responses activated by stress (Eskandari & Sternberg, 2002). In the case of asthma and AR, abnormalities in this system may result in unrestrained airway inflammation and activation of shared respiratory pathways. Support for this model is derived from a number of studies reporting an association between stressful life events and increased risk of asthma, allergies, anxiety, and depression (Arndt et al., 2008; Buske-Kirschbaum et al., 2002b; Kiecolt-Glaser et al., 2009; Liu et al., 2002; Roberts et al., 2009; Sandberg et al., 2000; Scott et al., 2008; Turyk et al., 2008; Wright, 2007).
Limitations of the present study include assessment of a single domain of anxiety symptoms (generalized anxiety), as patterns of association may differ for other types of anxiety such as panic disorder. The prevalence of panic disorder among children and young adolescents, however, is low, thereby decreasing the probability that our findings are affected by this underlying anxiety disorder, and further strengthening the likelihood that generalized anxiety symptoms, asthma, and AR may share common mechanisms of disease (Beesdo et al., 2009). Lifetime occurrence of atopic disorders is based upon parental report. While this method is commonly used to assess atopic disorders within clinical and epidemiologic studies (Goodwin, 2003), future investigations should attempt to integrate objective measures of atopic disorders such as standardized clinical exams (Oranje et al., 1997) and laboratory measures of atopy, e.g. skin testing (Timonen et al., 2002). Similarly, standardized measures of anxiety, depression, and co-occurring psychiatric symptoms should also be used, ideally integrating multi-informant report of symptoms as was done in this study to strengthen the validity of symptom assessment. Finally, although our study did control for potential confounding effects of co-morbidity among atopic disorder subtypes, anxiety, depression, and externalizing psychiatric symptoms, other chronic medical conditions, SES, and gender, we did not assess other potential variables such as family relationships, smoking, or family history of psychiatric or atopic disorders as investigated by others (Bender et al., 2000; Goodwin et al., 2004; Slattery et al., 2002).
In summary, this study significantly contributes to existing research by demonstrating the specificity of associations when substantially correlated measures of anxiety and depression are considered together with multiple, often co-morbid, atopic disorders. Results indicate that symptoms of anxiety were specifically associated with a lifetime history of asthma and AR, and that this association was partially obscured by co-existing AD, and depressive and externalizing symptoms. Previous studies may not fully reflect the true nature of the underlying relationships if effects of other co-morbid psychiatric symptoms and atopic disorders are not assessed. Clarification of these relationships is essential to the identification of potential underlying shared mechanisms, and the subsequent development of treatments targeting these specific pathways. Given the limitations of the present study, replication of the findings is important. Nevertheless, identification of these specific psychiatric – allergic disorder associations through systematic assessment of co-existing conditions provides an important basis for future studies investigating factors underlying these relationships.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abelson JL, Khan S, Liberzon I, Young EA. HPA axis activity in patients with panic disorder: review and synthesis of four studies. Depression and Anxiety. 2007;24:66–76. doi: 10.1002/da.20220. [DOI] [PubMed] [Google Scholar]
- Ablow JC, Measelle JR, Kraemer HC, Harrington R, Luby J, Smider N, Dierker L, Clark V, Dubicka B, Heffelfinger A, Essex MJ, Kupfer DJ. The MacArthur Three-City Outcome Study: evaluating multi-informant measures of young children’s symptomatology. Journal of the American Academy of Child and Adolescent Psychiatry. 1999;38:1580–1590. doi: 10.1097/00004583-199912000-00020. [DOI] [PubMed] [Google Scholar]
- Addolorato G, Ancona C, Capristo E, Graziosetto R, Di Rienzo L, Maurizi M, Gasbarrini G. State and trait anxiety in women affected by allergic and vasomotor rhinitis. Journal of Psychosomatic Research. 1999;46:283–289. doi: 10.1016/s0022-3999(98)00109-3. [DOI] [PubMed] [Google Scholar]
- Anisman H, Merali Z. Cytokines, stress and depressive illness: brain-immune interactions. Annals of Medicine. 2003;35:2–11. doi: 10.1080/07853890310004075. [DOI] [PubMed] [Google Scholar]
- Annesi-Maesano I, Beyer A, Marmouz F, Mathelier-Fusade P, Vervloet D, Bauchau V. Do patients with skin allergies have higher levels of anxiety than patients with allergic respiratory diseases? Results of a large-scale cross-sectional study in a French population. The British Journal of Dermatology. 2006;154:1128–1136. doi: 10.1111/j.1365-2133.2006.07186.x. [DOI] [PubMed] [Google Scholar]
- Arndt J, Smith N, Tausk F. Stress and atopic dermatitis. Current Allergy and Asthma Reports. 2008;8:312–317. doi: 10.1007/s11882-008-0050-6. [DOI] [PubMed] [Google Scholar]
- Beesdo K, Lau JY, Guyer AE, McClure-Tone EB, Monk CS, Nelson EE, Fromm SJ, Goldwin MA, Wittchen HU, Leibenluft E, Ernst M, Pine DS. Common and distinct amygdala-function perturbations in depressed vs anxious adolescents. Archives of General Psychiatry. 2009;66:275–285. doi: 10.1001/archgenpsychiatry.2008.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender BG, Annett RD, Ikle D, DuHamel TR, Rand C, Strunk RC. Relationship between disease and psychological adaptation in children in the Childhood Asthma Management Program and their families. CAMP Research Group. Archives of Pediatrics & Adolescent Medicine. 2000;154:706–713. doi: 10.1001/archpedi.154.7.706. [DOI] [PubMed] [Google Scholar]
- Bender BG, Ballard R, Canono B, Murphy JR, Leung DY. Disease severity, scratching, and sleep quality in patients with atopic dermatitis. Journal of the American Academy of Dermatology. 2008;58:415–420. doi: 10.1016/j.jaad.2007.10.010. [DOI] [PubMed] [Google Scholar]
- Boyce WT, Essex MJ, Woodward HR, Measelle JR, Ablow JC, Kupfer DJ. The confluence of mental, physical, social, and academic difficulties in middle childhood. I: exploring the “head waters” of early life morbidities. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41:580–587. doi: 10.1097/00004583-200205000-00016. [DOI] [PubMed] [Google Scholar]
- Boyle MH, Offord DR, Racine Y, Fleming JE, Szatmari P, Sanford M. Evaluation of the revised Ontario Child Health Study scales. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 1993;34:189–213. doi: 10.1111/j.1469-7610.1993.tb00979.x. [DOI] [PubMed] [Google Scholar]
- Brenes GA. Anxiety and chronic obstructive pulmonary disease: prevalence, impact, and treatment. Psychosomatic Medicine. 2003;65:963–970. doi: 10.1097/01.psy.0000097339.75789.81. [DOI] [PubMed] [Google Scholar]
- Brown ES. Psychiatric diagnoses in inner-city outpatients with moderate to severe asthma. International Journal of Psychiatry in Medicine. 2000;30:319–327. doi: 10.2190/7U7P-EJYL-5BKG-6106. [DOI] [PubMed] [Google Scholar]
- Buske-Kirschbaum A, Geiben A, Hollig H, Morschhauser E, Hellhammer D. Altered responsiveness of the hypothalamus-pituitary-adrenal axis and the sympathetic adrenomedullary system to stress in patients with atopic dermatitis. The Journal of Clinical Endocrinology and Metabolism. 2002a;87:4245–4251. doi: 10.1210/jc.2001-010872. [DOI] [PubMed] [Google Scholar]
- Buske-Kirschbaum A, Gierens A, Hollig H, Hellhammer DH. Stress-induced immunomodulation is altered in patients with atopic dermatitis. Journal of Neuroimmunology. 2002b;129:161–167. doi: 10.1016/s0165-5728(02)00168-6. [DOI] [PubMed] [Google Scholar]
- Buske-Kirschbaum A, von Auer K, Krieger S, Weis S, Rauh W, Hellhammer D. Blunted cortisol responses to psychosocial stress in asthmatic children: a general feature of atopic disease? Psychosomatic Medicine. 2003;65:806–810. doi: 10.1097/01.psy.0000095916.25975.4f. [DOI] [PubMed] [Google Scholar]
- Cantani A. The growing genetic links and the early onset of atopic diseases in children stress the unique role of the atopic march: a meta-analysis. Journal of Investigational Allergology & Clinical Immunology. 1999;9:314–320. [PubMed] [Google Scholar]
- Chida Y, Hamer M, Steptoe A. A bidirectional relationship between psychosocial factors and atopic disorders: a systematic review and meta-analysis. Psychosomatic Medicine. 2008;70:102–116. doi: 10.1097/PSY.0b013e31815c1b71. [DOI] [PubMed] [Google Scholar]
- Cuffel B, Wamboldt M, Borish L, Kennedy S, Crystal-Peters J. Economic consequences of comorbid depression, anxiety, and allergic rhinitis. Psychosomatics. 1999;40:491–496. doi: 10.1016/S0033-3182(99)71187-4. [DOI] [PubMed] [Google Scholar]
- Eskandari F, Sternberg EM. Neural-immune interactions in health and disease. Annals of the New York Academy of Sciences. 2002;966:20–27. doi: 10.1111/j.1749-6632.2002.tb04198.x. [DOI] [PubMed] [Google Scholar]
- Essex MJ, Boyce WT, Goldstein LH, Armstrong JM, Kraemer HC, Kupfer DJ. The confluence of mental, physical, social, and academic difficulties in middle childhood. II: developing the Macarthur health and Behavior Questionnaire. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41:588–603. doi: 10.1097/00004583-200205000-00017. [DOI] [PubMed] [Google Scholar]
- Ginsburg IH, Prystowsky JH, Kornfeld DS, Wolland H. Role of emotional factors in adults with atopic dermatitis. International Journal of Dermatology. 1993;32:656–660. doi: 10.1111/j.1365-4362.1993.tb04021.x. [DOI] [PubMed] [Google Scholar]
- Goodwin RD. Self-reported hay fever and panic attacks in the community. Annals of Allergy, Asthma & Immunology. 2002;88:556–559. doi: 10.1016/S1081-1206(10)61885-6. [DOI] [PubMed] [Google Scholar]
- Goodwin RD. Asthma and anxiety disorders. Advances in Psychosomatic Medicine. 2003;24:51–71. doi: 10.1159/000073780. [DOI] [PubMed] [Google Scholar]
- Goodwin RD, Buka SL. Childhood respiratory disease and the risk of anxiety disorder and major depression in adulthood. Archives of Pediatrics & Adolescent Medicine. 2008;162:774–780. doi: 10.1001/archpedi.162.8.774. [DOI] [PubMed] [Google Scholar]
- Goodwin RD, Jacobi F, Thefeld W. Mental disorders and asthma in the community. Archives of General Psychiatry. 2003a;60:1125–1130. doi: 10.1001/archpsyc.60.11.1125. [DOI] [PubMed] [Google Scholar]
- Goodwin RD, Lewinsohn PM, Seeley JR. Respiratory symptoms and mental disorders among youth: results from a prospective, longitudinal study. Psychosomatic Medicine. 2004;66:943–949. doi: 10.1097/01.psy.0000138123.70740.92. [DOI] [PubMed] [Google Scholar]
- Goodwin RD, Messineo K, Bregante A, Hoven CW, Kairam R. Prevalence of probable mental disorders among pediatric asthma patients in an inner-city clinic. The Journal of Asthma. 2005;42:643–647. doi: 10.1080/02770900500264770. [DOI] [PubMed] [Google Scholar]
- Goodwin RD, Olfson M, Shea S, Lantigua RA, Carrasquilo O, Gameroff MJ, Weissman MM. Asthma and mental disorders in primary care. General Hospital Psychiatry. 2003b;25:479–483. doi: 10.1016/s0163-8343(03)00071-9. [DOI] [PubMed] [Google Scholar]
- Goodwin RD, Pine DS. Respiratory disease and panic attacks among adults in the United States. Chest. 2002;122:645–650. doi: 10.1378/chest.122.2.645. [DOI] [PubMed] [Google Scholar]
- Hart EL, Lahey BB, Hynd GW, Loeber R. Association of chronic overanxious disorder with atopic rhinitis in boys: A four-year longitudinal study. Journal of Clinical Child Psychology. 1995;24:332–337. [Google Scholar]
- Hashiro M, Okumura M. Anxiety, depression, psychosomatic symptoms and autonomic nervous function in patients with chronic urticaria. Journal of Dermatological Science. 1994;8:129–135. doi: 10.1016/0923-1811(94)90007-8. [DOI] [PubMed] [Google Scholar]
- Hashiro M, Okumura M. Anxiety, depression and psychosomatic symptoms in patients with atopic dermatitis: comparison with normal controls and among groups of different degrees of severity. Journal of Dermatological Science. 1997;14:63–67. doi: 10.1016/s0923-1811(96)00553-1. [DOI] [PubMed] [Google Scholar]
- Hashizume H, Horibe T, Ohshima A, Ito T, Yagi H, Takigawa M. Anxiety accelerates T-helper 2-tilted immune responses in patients with atopic dermatitis. The British Journal of Dermatology. 2005;152:1161–1164. doi: 10.1111/j.1365-2133.2005.06449.x. [DOI] [PubMed] [Google Scholar]
- Hasler G, Gergen PJ, Kleinbaum DG, Ajdacic V, Gamma A, Eich D, Rossler W, Angst J. Asthma and panic in young adults: a 20-year prospective community study. American Journal of Respiratory and Critical Care Medicine. 2005;171:1224–1230. doi: 10.1164/rccm.200412-1669OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde JS, Klein MH, Essex MJ, Clark R. Maternity leave and women’s mental health. Psychology of Women Quarterly. 1995;19:257–285. [Google Scholar]
- Infante M, Slattery MJ, Klein MH, Essex MJ. Association of internalizing disorders and allergies in a child and adolescent psychiatry clinical sample. The Journal of Clinical Psychiatry. 2007;68:1419–1425. doi: 10.4088/jcp.v68n0915. [DOI] [PubMed] [Google Scholar]
- Janson-Bjerklie S, Ferketich S, Benner P, Becker G. Clinical markers of asthma severity and risk: importance of subjective as well as objective factors. Heart & Lung: The Journal of Critical Care. 1992;21:265–272. [PubMed] [Google Scholar]
- Katon W, Lin EH, Kroenke K. The association of depression and anxiety with medical symptom burden in patients with chronic medical illness. General Hospital Psychiatry. 2007a;29:147–155. doi: 10.1016/j.genhosppsych.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Katon W, Lozano P, Russo J, McCauley E, Richardson L, Bush T. The prevalence of DSM-IV anxiety and depressive disorders in youth with asthma compared with controls. The Journal of Adolescent Health. 2007b;41:455–463. doi: 10.1016/j.jadohealth.2007.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katon WJ, Richardson L, Lozano P, McCauley E. The relationship of asthma and anxiety disorders. Psychosomatic Medicine. 2004;66:349–355. doi: 10.1097/01.psy.0000126202.89941.ea. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Heffner KL, Glaser R, Malarkey WB, Porter K, Atkinson C, Laskowski B, Lemeshow S, Marshall GD. How stress and anxiety can alter immediate and late phase skin test responses in allergic rhinitis. Psychoneuroendocrinology. 2009;34:670–680. doi: 10.1016/j.psyneuen.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein DF. False suffocation alarms, spontaneous panics, and related conditions: An integrative hypothesis. Archives of General Psychiatry. 1993;50:306–317. doi: 10.1001/archpsyc.1993.01820160076009. [DOI] [PubMed] [Google Scholar]
- Kovacs M, Stauder A, Szedmak S. Severity of allergic complaints: the importance of depressed mood. Journal of Psychosomatic Research. 2003;54:549–557. doi: 10.1016/s0022-3999(02)00477-4. [DOI] [PubMed] [Google Scholar]
- Kraemer HC, Measelle JR, Ablow JC, Essex MJ, Boyce WT, Kupfer DJ. A new approach to integrating data from multiple informants in psychiatric assessment and research: mixing and matching contexts and perspectives. The American Journal of Psychiatry. 2003;160:1566–1577. doi: 10.1176/appi.ajp.160.9.1566. [DOI] [PubMed] [Google Scholar]
- Ley R. Dyspneic-fear and catastrophic cognitions in hyperventilatory panic attacks. Behaviour Research and Therapy. 1989;27:549–554. doi: 10.1016/0005-7967(89)90089-2. [DOI] [PubMed] [Google Scholar]
- Linnet J, Jemec GB. An assessment of anxiety and dermatology life quality in patients with atopic dermatitis. The British Journal of Dermatology. 1999;140:268–272. doi: 10.1046/j.1365-2133.1999.02661.x. [DOI] [PubMed] [Google Scholar]
- Liu LY, Coe CL, Swenson CA, Kelly EA, Kita H, Busse WW. School examinations enhance airway inflammation to antigen challenge. American Journal of Respiratory and Critical Care Medicine. 2002;165:1062–1067. doi: 10.1164/ajrccm.165.8.2109065. [DOI] [PubMed] [Google Scholar]
- Mancuso CA, Wenderoth S, Westermann H, Choi TN, Briggs WM, Charlson ME. Patient-reported and physician-reported depressive conditions in relation to asthma severity and control. Chest. 2008;133:1142–1148. doi: 10.1378/chest.07-2243. [DOI] [PubMed] [Google Scholar]
- Marshall PS, O’Hara C, Steinberg P. Effects of seasonal allergic rhinitis on fatigue levels and mood. Psychosomatic Medicine. 2002;64:684–691. doi: 10.1097/01.psy.0000021944.35402.44. [DOI] [PubMed] [Google Scholar]
- Martinez JM, Kent JM, Coplan JD, Browne ST, Papp LA, Sullivan GM, Kleber M, Perepletchikova F, Fyer AJ, Klein DF, Gorman JM. Respiratory variability in panic disorder. Depression and Anxiety. 2001;14:232–237. doi: 10.1002/da.1072. [DOI] [PubMed] [Google Scholar]
- McQuaid EL, Kopel SJ, Nassau JH. Behavioral adjustment in children with asthma: a meta-analysis. Journal of Developmental and Behavioral Pediatrics. 2001;22:430–439. doi: 10.1097/00004703-200112000-00011. [DOI] [PubMed] [Google Scholar]
- Measelle JR, Ablow JC, Cowan PA, Cowan CP. Assessing young children’s views of their academic, social, and emotional lives: an evaluation of the self-perception scales of the Berkeley Puppet Interview. Child Development. 1998;69:1556–1576. [PubMed] [Google Scholar]
- Morrison KM, Goli A, Van Wagoner J, Brown ES, Khan DA. Depressive Symptoms in Inner-City Children With Asthma. Primary Care Companion to the Journal of Clinical Psychiatry. 2002;4:174–177. doi: 10.4088/pcc.v04n0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardi AE, Freire RC, Zin WA. Panic disorder and control of breathing. Respiratory Physiology & Neurobiology. 2009;167:133–143. doi: 10.1016/j.resp.2008.07.011. [DOI] [PubMed] [Google Scholar]
- Nathan RA. The burden of allergic rhinitis. Allergy and Asthma Proceedings. 2007;28:3–9. doi: 10.2500/aap.2007.28.2934. [DOI] [PubMed] [Google Scholar]
- Nayak AS. The asthma and allergic rhinitis link. Allergy and Asthma Proceedings. 2003;24:395–402. [PubMed] [Google Scholar]
- Nejtek VA, Brown ES, Khan DA, Moore JJ, Van Wagner J, Perantie DC. Prevalence of mood disorders and relationship to asthma severity in patients at an inner-city asthma clinic. Annals of Allergy, Asthma & Immunology. 2001;87:129–133. doi: 10.1016/s1081-1206(10)62206-5. [DOI] [PubMed] [Google Scholar]
- Opolski M, Wilson I. Asthma and depression: a pragmatic review of the literature and recommendations for future research. Clinical Practice and Epidemiology in Mental Health. 2005;1:18. doi: 10.1186/1745-0179-1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oranje AP, Stalder JF, Taieb A, Tasset C, de Longueville M. Scoring of atopic dermatitis by SCORAD using a training atlas by investigators from different disciplines. ETAC Study Group. Early Treatment of the Atopic Child. Pediatric Allergy and Immunology. 1997;8:28–34. doi: 10.1111/j.1399-3038.1997.tb00139.x. [DOI] [PubMed] [Google Scholar]
- Ortega AN, Huertas SE, Canino G, Ramirez R, Rubio-Stipec M. Childhood asthma, chronic illness, and psychiatric disorders. The Journal of Nervous and Mental Disease. 2002;190:275–281. doi: 10.1097/00005053-200205000-00001. [DOI] [PubMed] [Google Scholar]
- Pine DS, Coplan JD, Papp LA, Klein RG, Martinez JM, Kovalenko P, Tancer N, Moreau D, Dummit ES, III, Shaffer D, Klein DF, Gorman JM. Ventilatory physiology of children and adolescents with anxiety disorders. Archives of General Psychiatry. 1998;55:123–129. doi: 10.1001/archpsyc.55.2.123. [DOI] [PubMed] [Google Scholar]
- Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends in Immunology. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts RE, Roberts CR, Chan W. One-year incidence of psychiatric disorders and associated risk factors among adolescents in the community. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2009;50:405–415. doi: 10.1111/j.1469-7610.2008.01969.x. [DOI] [PubMed] [Google Scholar]
- Ryu YJ, Chun EM, Lee JH, Chang JH. Prevalence of depression and anxiety in outpatients with chronic airway lung disease. The Korean Journal of Internal Medicine. 2010;25:51–57. doi: 10.3904/kjim.2010.25.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandberg S, Paton JY, Ahola S, McCann DC, McGuinness D, Hillary CR, Oja H. The role of acute and chronic stress in asthma attacks in children. Lancet. 2000;356:982–987. doi: 10.1016/S0140-6736(00)02715-X. [DOI] [PubMed] [Google Scholar]
- Scott KM, Von Korff M, Alonso J, Angermeyer MC, Benjet C, Bruffaerts R, de Girolamo G, Haro JM, Kessler RC, Kovess V, Ono Y, Ormel J, Posada-Villa J. Childhood adversity, early-onset depressive/anxiety disorders, and adult-onset asthma. Psychosomatic Medicine. 2008;70:1035–1043. doi: 10.1097/PSY.0b013e318187a2fb. [DOI] [PubMed] [Google Scholar]
- Shamssain MH, Shamsian N. Prevalence and severity of asthma, rhinitis, and atopic eczema in 13- to 14-year-old schoolchildren from the northeast of England. Annals of Allergy, Asthma & Immunology. 2001;86:428–432. doi: 10.1016/S1081-1206(10)62490-8. [DOI] [PubMed] [Google Scholar]
- Slattery MJ. Psychiatric comorbidity associated with atopic disorders in children and adolescents. Immunology and Allergy Clinics of North America. 2005;25:407–420. viii. doi: 10.1016/j.iac.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Slattery MJ, Klein DF, Mannuzza S, Moulton JL, 3rd, Pine DS, Klein RG. Relationship between separation anxiety disorder, parental panic disorder, and atopic disorders in children: a controlled high-risk study. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41:947–954. doi: 10.1097/00004583-200208000-00013. [DOI] [PubMed] [Google Scholar]
- Timonen M, Jokelainen J, Silvennoinen-Kassinen S, Herva A, Zitting P, Xu B, Peltola O, Rasanen P. Association between skin test diagnosed atopy and professionally diagnosed depression: a northern finland 1966 birth cohort study. Biological Psychiatry. 2002;52:349. doi: 10.1016/s0006-3223(01)01364-6. [DOI] [PubMed] [Google Scholar]
- Turyk ME, Hernandez E, Wright RJ, Freels S, Slezak J, Contraras A, Piorkowski J, Persky VW. Stressful life events and asthma in adolescents. Pediatric Allergy and Immunology. 2008;19:255–263. doi: 10.1111/j.1399-3038.2007.00603.x. [DOI] [PubMed] [Google Scholar]
- Van Lieshout RJ, Bienenstock J, MacQueen GM. A review of candidate pathways underlying the association between asthma and major depressive disorder. Psychosomatic Medicine. 2009;71:187–195. doi: 10.1097/PSY.0b013e3181907012. [DOI] [PubMed] [Google Scholar]
- Wamboldt MZ, Fritz G, Mansell A, McQuaid EL, Klein RB. Relationship of asthma severity and psychological problems in children. Journal of the American Academy of Child and Adolescent Psychiatry. 1998;37:943–950. doi: 10.1097/00004583-199809000-00014. [DOI] [PubMed] [Google Scholar]
- Wittkowski A, Richards HL, Griffiths CE, Main CJ. The impact of psychological and clinical factors on quality of life in individuals with atopic dermatitis. Journal of Psychosomatic Research. 2004;57:195–200. doi: 10.1016/S0022-3999(03)00572-5. [DOI] [PubMed] [Google Scholar]
- Wright RJ. Prenatal maternal stress and early caregiving experiences: implications for childhood asthma risk. Paediatric and Perinatal Epidemiology. 2007;21 (Suppl 3):8–14. doi: 10.1111/j.1365-3016.2007.00879.x. [DOI] [PubMed] [Google Scholar]
- Young EA, Abelson JL, Cameron OG. Effect of comorbid anxiety disorders on the hypothalamic-pituitary-adrenal axis response to a social stressor in major depression. Biological Psychiatry. 2004;56:113–120. doi: 10.1016/j.biopsych.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Zielinski TA, Brown ES. Depression in patients with asthma. Advances in Psychosomatic Medicine. 2003;24:42–50. doi: 10.1159/000073779. [DOI] [PubMed] [Google Scholar]