Abstract
Given that avoidance is a core feature of anxiety disorders, Wistar-Kyoto (WKY) rats may be a good model of anxiety vulnerability for their hypersensitivity to stress and trait behavioral inhibition. Here, we examined the influence of strain and shock intensity on avoidance acquisition and extinction. Accordingly, we trained WKY and Sprague-Dawley (SD) rats in lever-press avoidance using either 1.0-mA or 2.0-mA foot-shock. After extinction, neuronal activation was visualized by c-Fos for overall activity and parvalbumin immunoreactivity for gamma-aminobutyric acid (GABA) neuron in brain areas linked to anxiety (medial prefrontal cortex and amygdala). Consistent with earlier work, WKY rats acquired lever-press avoidance faster and to a greater extent than SD rats. However, the intensity of foot shock did not differentially affect acquisition. Although there were no differences during extinction in SD rats, avoidance responses of WKY rats trained with the higher foot shock perseverated during extinction compared to those WKY rats trained with lower foot shock intensity or SD rats. WKY rats trained with 2.0-mA shock exhibited less GABAergic activation in the basolateral amygdala after extinction. These findings suggest that inhibitory modulation in amygdala is important to ensure successful extinction learning. Deficits in avoidance extinction secondary to lower GABAergic activation in baslolateral amygdala may contribute to anxiety vulnerability in this animal model of inhibited temperament.
Keywords: Anxiety, avoid, extinction, lever-press, Wistar-Kyoto (WKY) rat, behavioral inhibition, c-Fos
Introduction
Anxiety disorders develop as an interaction of trait vulnerabilities (e.g., behavioral inhibition), early life experiences and environmental exposures [26,36,37,39,78,88]. The interplay of these influences determines coping and failures to effectively cope. Accordingly, avoidance from both behavioral and emotional perspectives is at the core of all anxiety disorders (e.g., posttraumatic stress disorder (PTSD), obsessive-compulsive disorder, social anxiety disorder as well as generalized anxiety disorder) [2]. Avoidance and the growth of avoidance often distinguish between those exposed to stressors who will eventually develop an anxiety disorder and those who will not [25,40,62,63]. Avoidance exhibited by those with an anxiety disorder may be differentiated in terms of sensitivity, pervasiveness, disruptiveness and persistence, that is, the conditions by which avoidance is acquired, the degree to which avoidance is employed, the degree that avoidance interferes with normal function, and its resistance to extinction, respectively. As such, the propensity for acquiring avoidance and its resistance to extinction may be the final common path toward anxiety disorders [13,43,49].
The inbred WKY rat models human trait behavioral inhibition, a vulnerability factor in developing anxiety disorders [58]. Like a behaviorally inhibited human, WKY rats display extreme withdrawal in the face of social and nonsocial challenges [11,24,50,66,84,92] and are hyperresponsive to stress stimulation in terms of neuroendocrine responses (exaggerated stress-induced corticosterone, plasma adrenocorticotropin hormone (ACTH), and norepinephrine levels) compared to outbred rat strains (Wistar, and SD rats) [3,23,29,31,67,75,93]. Although WKY rats have an inhibited temperament, they acquire an active avoidance response faster than SD rats. Moreover, WKY rats display perseveration of avoidance responding in the absence of foot shock but continued presence of an explicit safety signal [83].
Stress intensity is often cited as a contributing factor in the development of anxiety disorders [10,25,30,57,86]. Given the relationship between anxiety disorders and avoidance, one expects avoidance acquisition to accelerate or to reach greater asymptotic levels under conditions of greater stressor intensity. In rats, lever-press avoidance is more prevalent as stressor intensity increases [8,72]. In addition, acquisition of lever-press avoidance is enhanced and extinction is slower in rats trained with more intense shock [8,20]. Others have suggested that increasing intensity above a minimum level does not affect avoidance acquisition rate [22]. However, poorer lever-press avoidance acquisition has also been reported with increasing stressor intensity and attributed to competing responses; that is, intense stressors induce behavioral adjustments (i.e., freezing) incompatible with an arbitrary active avoidance response such as lever pressing [6,7]. Thus, it is unclear from previous research how stressor intensity would affect avoidance acquisition and extinction in a stress-sensitive rat strain, like the WKY rat.
The neural circuitry underlying avoidance extinction and its perseveration in anxiety states is largely a matter of speculation. Converging data in humans suggest that cingulate cortex, insular cortex, amygdala, thalamus and periaquaductal gray are involved in avoidance acquisition [59,82,87,89,90]. While critical areas involved in extinction of fear conditioning have been elucidated recently, those responsible for avoidance extinction are much less defined. We have recently shown that the medial septum is important for avoidance extinction but not acquisition [64]. However, the involvement of the medial prefrontal cortex (mPFC) and basolateral amygdala (BlA) [53,69], two key structures for fear extinction, remain unresolved in the extinction of active avoidance task. Distinct neural circuits in the basal amygdala (BA) are connected to the hippocampus and mPFC and different circuits are activated during fear conditioning or extinction, suggesting that distinct behavioral states can be triggered by activation of separate neuronal populations [34]. Projections from the mPFC to the BlA inhibit amygdala output under highly emotional situations [73,79,80]. Because mPFC projections preferentially innervates gamma-aminobutyric acid (GABA) interneurons in the BlA and these interneurons innervate projection neurons [80], stimulation of the mPFC predominantly suppresses the output of BA [1,5,61,80]. Thus, we speculate that the rat mPFC and the GABAergic inhibitory interneurons in the BlA may be important in the interaction of stressor intensity, vulnerability and extinction of avoidance.
The present study investigated the interaction of strain and shock intensity in avoidance acquisition and extinction. Given the stress-sensitivity of WKY rats, we predicted that more intense foot shock would be disruptive to lever pressing and acquisition. Training with intense foot shock was also expected to slow extinction in WKY rats. To begin to understand the neural circuitry underlying extinction of avoidance, neuronal activation in the mPFC and amygdala were quantified. We hypothesized that rats demonstrating retarded extinction would exhibit reduced activation of GABAergic interneurons in the BA.
Methods and Materials
Animals
Twenty-eight Sprague-Dawley (SD) and 28 Wistar-Kyoto (WKY) male rats (approximately 60 days of age at the start of the experiment) were obtained from Harlan Sprague-Dawley Laboratories (Indianapolis, IN). Four rats from each strain that did not receive behavioral training were used to obtain baseline c-Fos activity. Rats were housed in individual cages with free access to food and water in a room maintained on a 12:12 hour day/night cycle for at least two weeks prior to experimentation. Experiments occurred between 0700 and 1900 hours in the light portion of the cycle (individuals were tested in approximately the same time period of each session). All procedures received prior approved by the Institutional Animal Care and Use Committee in accordance with AAALAC standards.
Open-field Test
Naïve rats were tested in the open field. The apparatus consisted of a gray cylindrical arena, 82 cm in diameter with 30 cm high aluminum walls. The arena floor was divided into three concentric circles. The smallest inner circle had a diameter of 20 cm measured from the center of the arena. The second circle had a diameter of 50 cm and the arena wall defined the outer limit of the third circle. Each of the outer circles was divided by radial lines into equally sized areas of approximately 251 cm2. All lines were drawn in black paint. A 100-W bulb located directly above the center of the open field provided constant illumination.
The open-field test consisted of placing a rat in the center circle of the arena and video recording its behavior for 2 min. Videotapes were scored for the following behaviors: 1) latency to leave the center segment of the arena (i.e., time spent in the center) and 2) the number of segments entered by a rat with all four feet. The arena was wiped with a soap solution between the testing of each rat.
Lever-press Escape/Avoidance Training
The apparatus was described previously [83]. Training was conducted in 16 identical operant chambers (Coulbourn Instruments, Langhorn, PA). Each operant chamber was enclosed in a sound-attenuated box. Scrambled 1.0-mA or 2.0-mA foot-shock was delivered through the grid floor (Coulbourn Instruments, Langhorn, PA). The auditory warning signal was a 1000-Hz, 75-dB tone (10 dB above background noise). A 3-minute intertrial interval (ITI) was explicitly signaled with a 5-Hz blinking cue light located above the lever. Graphic State Notation software (v. 3.02, Coulbourn Instruments, Langhorn, PA) controlled the stimuli and recorded response times.
Each session began with a 60-s stimulus-free period. A trial commenced with the presentation of the auditory warning signal. During avoidance acquisition training, a lever-press during the first 60 s of the warning signal constituted an avoidance response, terminated the warning signal, and triggered the intertrial interval (ITI) with a blinking cue light. In the absence of a lever-press in the first 60 s, 0.5 s foot shocks were delivered with an inter-shock interval of 3 s. A maximum of 99 foot shocks could be delivered on each trial. A lever-press during the shock period constituted an escape response, terminated the shock and warning signal and triggered the intertrial interval with blinking cue light. During avoidance extinction training, both shock and the blinking cue light were deactivated. A lever-press made during the first 60 s of the warning signal constituted an avoidance response while the lever-press made during the rest of the warning signal constituted an escape response. Both responses terminated the warning signal and initiated a non-signaled safety period (e.g. without the blinking cue light). Each session consisted of 20 trials.
Sequence of Behavioral Procedures
Each animal was initially evaluated in the open field and then for startle reactivity (not reported here). For each strain, rats were stratified on total ambulation scores in the open field and then randomly assigned within each stratum to the 1.0-mA or 2.0-mA foot shock group for avoidance training. Avoidance training sessions (12 acquisition sessions followed by 9 extinction sessions) occurred 3 times per week (every 2–3 days). A rat that failed to emit a lever-press response by the end of the fourth training session was removed from the study (local IACUC requirement). One SD rat in the low intensity group was dropped from the study for this reason.
Immunocytochemistry
Two days after the last day of extinction training, 5 rats from each training group were selected for a brief re-exposure to extinction contingencies. Rats with response latencies on the 9th extinction session that were closest to their group’s mean latency were selected. The abbreviated extinction session was 30 min; otherwise, procedures were identical to the previous extinction sessions. Because the re-exposure session had a fixed time, rats were exposed to different numbers of trials depending on their behavior.
Ninety minutes after the end of the re-exposure session, rats were anaesthetized with sodium pentobarbital (150mg/kg) and transcardially perfused with 200 ml of 0.9% saline, followed by 200 ml of 10% buffered formalin. Brains were extracted, post-fixed in 10% formalin at 4°C overnight, and then stored at 4°C in a 30% (w/v) sucrose in 0.1 M phosphate buffer (PB) solution until the brains sank.
Coronal brain sections (25 micrometers) were prepared on a freezing microtome from the mPFC (4.20 mm ~ 2.53 mm anterior to bregma) and the amygdala (2.04 mm ~ 3.24 mm posterior to bregma) [68]. Every sixth section of the mPFC was stained for c-Fos protein, which has been used as a marker of neuronal activation [15]. Every fourth section of the amygdala was immunostained for both c-Fos and parvalbumin neurons. Immunocytochemistry procedures were similar to those described previously [56,65] Briefly, sections were incubated in rabbit anti-c-Fos IgG (#sc-52, 1:1000, Santa Cruz, CA) or mouse anti-parvalbumin IgG (#P3088, 1:1000, Sigma-Aldrich, MO). Sections for c-Fos staining were incubated overnight at room temperature while sections for parvalbumin staining were incubated for 24 hours at 4 °C, followed by another incubation with secondary antibodies (biotinylated donkey IgGs, 1:200, Jackson ImmunoResearch Laboratory, PA) for 2 hours at room temperature. Visualization was performed using the avidin-biotin method (Vector Laboratories, Burlington, CA) with nickel-enhanced diaminobenzidine for c-Fos and diaminobenzidine alone for parvalbumin.
c-Fos immunoreactive (ir) nuclei and/or parvalbumin(ir) perikarya were counted in the anterior cingulate, prelimbic and infralimbic regions of the prefrontal cortex (ACC, PLC, and ILC, respectively) and the basolateral, lateral, central and medial amygdala nuclei (BA, LaA, CeA, MeA, respectively). Estimates of the number of immunostained neurons and regional volume were obtained using standard stereology procedures [64,94, 96]. The optical fractionator method (Stereo Investigator v.7.0, MicroBrightField, Colchester, VT) was used to obtain the estimates on a microscope with an x-, y-, z-axis motorized stage (Bio Point 30, Ludl Electronic Products, Hawthorne, NY). The counting frame had a height of 10 μm with 300 μm × 200μm dimensions for the amygdala and 80 μm × 60 μm for medial prefrontal area. Cell counts and volume measures were assessed in the left hemisphere for a random half of the rats and in the right hemisphere for the other half. Counts were performed by a person blind to the treatment and strain of the animal.
Data analysis
All data are expressed as means ± the standard error of the mean. Statistical results are reported only where significant differences were found. For avoidance training, the number of avoidance responses and nonreinforced responses during the first minute of the ITI (ITRs) were analyzed. Mean values were obtained for each training session. Acquisition and extinction were separately analyzed.
Results
Open-field Test
WKY rats were inhibited compared to SD rats. WKY rats (9.6 ± 1.0 s) exhibited longer latencies to leave the center segment than SD rats (6.4±0.9 s), t(45) = 5.3, p < .05. Moreover, WKY rats (24.6 ± 2.3) segments) exhibited reduced activity compared to SD rats (58.3±2.4 segments), t(45) =101.1, p < .001.
Avoidance acquisition
Both strains acquired the avoidance response, exhibiting mean avoidance above 60% by the end of training (Figure 1). WKY rats acquired the avoidance response faster and to a higher asymptotic level than SD rats. However, shock intensity did not affect acquisition in either strain. Using a 2 × 2 × 12 (Strain × Intensity × Session) mixed-ANOVA, the main factors of Strain, F(1,43) = 13.4 and Session, F(11,473) = 48.2, and the Strain × Session interaction, F(11,473) = 2.3, were all significant (ps< 0.01). Although foot shock intensity did not affect avoidance responses, shock intensity did alter ITRs with higher intensity associated with greater numbers of ITRs in both SD and WKY rats (Figure 2). Moreover, WKY rats emitted more ITRs than SD rats during early but not late acquisition sessions. These differences were confirmed by a 2 × 2 × 12 (Strain × Intensity × Sessions) mixed ANOVA. The main effect of Intensity, F(1,43) = 7.8, and the interaction of Strain × Sessions, F(11,473) = 2.0, were significant (ps< .05).
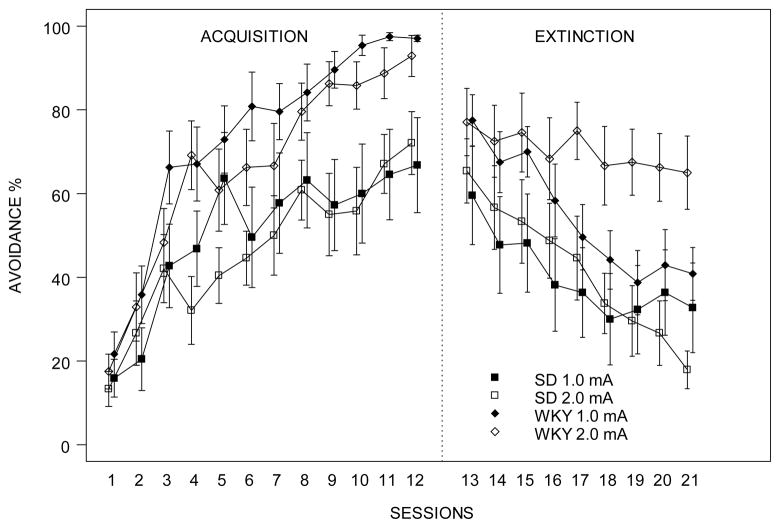
Figure 1.
Avoidance lever-press of SD and WKY rats by sessions.
Avoidance response in the phases of acquisition (12 sessions) and extinction (9 sessions) was expressed as avoidance ratio per session. Each session was composed of 20 trials. Avoidance lever-press increased during acquisition in both strains regardless of shock intensity, while WKY rats made more avoidance lever-presses than SD rats. However, during extinction, WKY rats extinguished slower as compared to SD rats in general. Higher shock intensity resulted in greater number of avoidance lever-press in WKY rats indicating resistance to extinguish. Each data point represents group mean ± S.E.M. (n=11–12/group).
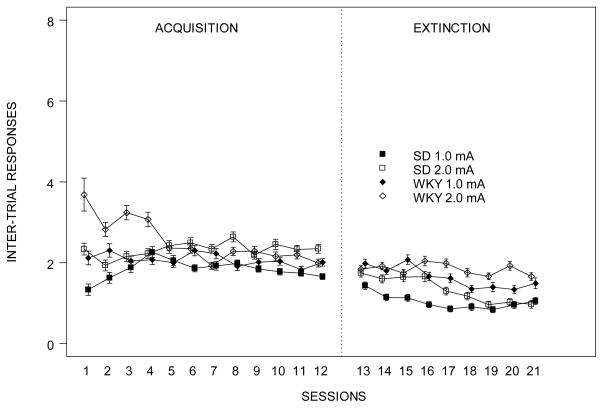
Figure 2.
Lever presses (ITRs) of SD and WKY rats during the first minute of safety period by session.
Lever presses during the first minute of ITI (ITRs) was expressed as the number of responses in acquisition and extinction. WKY rats made more lever-presses compared to SD rats during early acquisition sessions. Higher shock intensity resulted in greater number of lever-presses during extinction in both strains. Each data point represents group mean ± S.E.M. (n=11–12/group).
Extinction
WKY rats trained with 2.0-mA foot shock perseverated during the extinction phase (Figure 1). SD rats trained with 1.0-mA and 2.0-mA foot shock, and WKY rats trained with 1.0-mA foot shock reduced their avoidance responding in the absence of foot shock and the ITI signal. In contrast, WKY rats trained with 2.0-mA foot shock did not appreciably reduce their avoidance responding during the 9 extinction sessions with mean responding remaining above 60% for all extinction sessions. In a 2 × 2 × 9 (Strain × Intensity × Sessions) mixed ANOVA, main effects of Strain, F(1,43) = 7.6, and Sessions, F(8,344) = 21.7, and the Strain × Intensity × Sessions interaction, F(8, 344) = 3.7, were significant (ps< .01). WKY rats trained with 2.0-mA foot shock made greater avoidance responses compared to the other groups in the last session, confirmed by Strain × Intensity interaction, F(1,43)=6.27, p< .05.
Similar to the acquisition phase, a greater number of ITRs were emitted by rats trained with the 2.0-mA compared to 1.0-mA foot shock (Figure 2). WKY rats trained with 2.0-mA foot shock maintained their high rate of ITRs through extinction sessions in contrast to the other 3 groups, which showed a more pronounced reduction of ITRs. A 2 × 2 × 9 (Strain × Intensity × Sessions) mixed ANOVA yielded main effects of Strain, F(1,43) = 15.8, and of Session, F(8,344) = 11.9, and interactions of Intensity × Session, F(8,344) = 2.2 and Strain × Intensity × Session, F(8,344) = 3.5, all ps<0.01.
Immunocytochemistry
Neuronal activation patterns associated with an abbreviated extinction session were mapped using immunocytochemistry to the immediate early gene product c-Fos.
mPFC
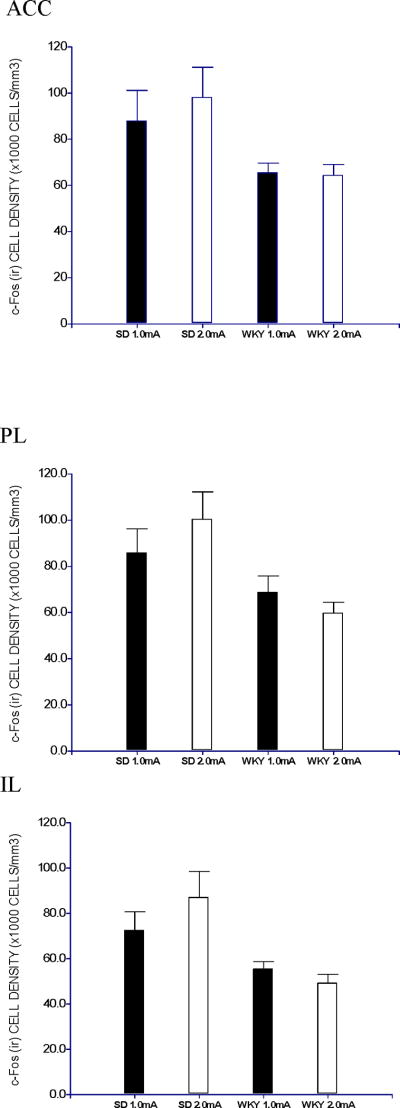
Fewer mPFC cells were activated in WKY rats compared to SD rats during the abbreviated extinction session. Less c-Fos (ir) cells were observed in ACC, PLC and ILC regions of WKY rats compared to SD rats (Figure 3, and Tables 1 and 2). No difference in c-Fos staining was observed between rats trained at 1.0- and 2.0-mA shock. Thus, despite the fact that WKY rats trained with 2.0-mA shock were much more resistant to extinction of avoidance responding compared to the other groups, shock intensity was not associated with differences in c-Fos (ir) of any mPFC subregions. A 2 × 2 ANOVA demonstrated main effects of Strain in each of the mPFC sub-regions, ACC, F(1,16) = 8.3, PLC, F(1,16) = 8.5, and ILC, F(1,16) = 12.5, ps< .05.
Figure 3.
Density of c-Fos (ir) cells in mPFC, namely anterior cingulate cortex (ACC), prelimbic (PL) and infralimbic (IL).
Density of c-Fos (ir) cells in the mPFC, namely anterior cingulate cortex (ACC), prelimbic (PL) and infralimbic (IL), was depicted. WKY rats exhibited lower density of c-Fos (ir) cells in all three sub-regions as compared to SD rats (§, ps<0.05), regardless of intensity of foot shock. Data is expressed as group mean ± S.E.M. (n=5/group).
Table 1.
Immunocytochemistry data in sub-regions of mPFC and amygdala
| Regions | Groups | ACC | PL | IL | BA | LA | CE | ME |
|---|---|---|---|---|---|---|---|---|
| c-Fos (ir) | SD-1.0-mA | 78.66 ± 13.22 b | 85.88 ± 10.33 b | 72.55 ± 8.08 b | 4.77 ± 0.3 | 3.85 ± 0.51 | 3.24 ± 0.261 | 10.00 ± 1.87 |
| SD-2.0-mA | 98.13 ± 13.01 b | 100.37 ± 11.83b | 86.98 ± 11.46 b | 4.90 ± 0.43 | 3.36 ± 0.33 | 3.55 ± 0.48 | 11.15 ± 1.96 | |
| WKY-1.0-mA | 65.44 ± 4.14 b | 68.75 ± 7.03 b | 55.38 ± 3.36 b | 4.42 ± 0.23 | 5.48 ± 1.04 | 2.29 ± 0.42 | 11.37 ± 2.51 | |
| WKY-2.0-mA | 64.32 ± 4.71 b | 59.73 ± 4.66 b | 49.20 ± 3.8 b | 3.76 ± 0.41 | 3.50 ± 0.29 | 2.89 ± 0.41 | 13.13 ± 1.90 | |
| PV (ir) | SD-1.0-mA | NA | NA | NA | 6.20 ± 0.12 b | 5.34 ± 0.39 | NA | NA |
| SD-2.0-mA | NA | NA | NA | 6.48 ± 0.55 b | 5.09 ± 0.35 | NA | NA | |
| WKY-1.0-mA | NA | NA | NA | 5.19 ± 0.39 b | 3.69 ± 0.87 | NA | NA | |
| WKY-2.0-mA | NA | NA | NA | 4.29 ± 0.54 b | 4.77 ± 0.49 | NA | NA | |
| activated PV (ir) ratio (%) | SD-1.0-mA | NA | NA | NA | 51.36 ± 2.79 a,c | 38.98 ± 3.69 | NA | NA |
| SD-2.0-mA | NA | NA | NA | 44.04 ± 2.43 a,c | 27.39 ± 3.61 | NA | NA | |
| WKY-1.0-mA | NA | NA | NA | 62.12 ± 1.31a,c | 67.57 ± 11.80 | NA | NA | |
| WKY-2.0-mA | NA | NA | NA | 38.23 ± 1.28a,c | 29.11 ± 5.90 | NA | NA | |
Density of c-Fos and PV (ir) cells in sub-regions of mPFC and amygdala and activated PV (ir) cell ratio in amygdalar sub-regions.
Immunocytochemistry data are presented as mean (x1000 cells/mm3) ± S.E.M. (x1000 cells/mm3) in mPFC and amygdala in WKY and SD rats trained with 2.0-mA or 1.0-mA foot shocks. Post hoc analysis was conducted using Tukey’s test for specific group comparison.
P < .05, significant difference between shock intensities. strains.groups within the same strain.
P < .05, significant difference between strains. within rats trained with the same intensity of foot shock.
P< .05, post hoc significance.
Table 2.
Statistical results of two-way ANOVA for immunocytochemistry data in mPFC and amygdalar sub-regions.
| staining | ROI | sub-region | TEST | MainFactors | F-values | p-values | Interactions | F-values | p-values | pos hoc Tukey’s |
|---|---|---|---|---|---|---|---|---|---|---|
| c-Fos | mPFC | CG | ANOVA | strain * | F(1,16)=8.26 | p=0.011029 | strain × intensity | F(1,16)=0.34 | p=0.57 | |
| intensity | F(1,16)=0.22 | p=0.65 | ||||||||
| IL | ANOVA | strain * | F(1,16)=13.57 | p=0.002 | strain × intensity | F(1,16)=1.91 | p=0.19 | |||
| intensity | F(1,16)=0.31 | p=0.59 | ||||||||
| PL | ANOVA | strain * | F(1,16)=10.5 | p=0.005 | strain × intensity | F(1,16)=1.74 | p=0.21 | |||
| intensity | F(1,16)=0.09 | p=0.76 | ||||||||
| Amygdala | BA | ANOVA | strain | F(1,16)=1.0 | p=0.33 | strain × intensity | F(1,16)=0.17 | p=0.69 | ||
| intensity | F(1,16)=0.07 | p=0.79 | ||||||||
| CE | ANOVA | strain | F(1,16)=4.02 | p=0.06 | strain × intensity | F(1,16)=0.19 | p=0.67 | |||
| intensity | F(1,16)=1.13 | p=0.30 | ||||||||
| LA | ANOVA | strain | F(1,16)=1.83 | p=0.19 | strain × intensity | F(1,16)=1.28 | p=0.28 | |||
| intensity | F(1,16)=3.72 | p=0.07 | ||||||||
| ME | ANOVA | strain | F(1,16)=0.34 | p=0.57 | strain × intensity | F(1,16)=0.07 | p=0.80 | |||
| intensity | F91,16)=0.23 | p=0.63 | ||||||||
| parvalbumin | Amygdala | BA | ANOVA | strain * | F(1,16)=4.65 | p=0.047 | strain × intensity | F(1,16)=0.18 | p=0.68 | |
| intensity | F(1,16)=0 | p=0.97 | ||||||||
| LA | ANOVA | strain | F(1,16)=2.38 | p=0.14 | strain × intensity | F(1,16)=1.2 | p=0.29 | |||
| intensity | F(1,16)=0.16 | p=0.69 | ||||||||
| Activated parvalbumin (ir) cells ratio |
Amygdala | BA | ANOVA | strain | F(1,16)=2.72 | p=0.12 | strain × intensity * | F(1,16)=9.29 | p=0.008 | WKY 2.0-mA vs. SD 1.0-mA and WKY 1.0-mA; WKY 1.0-mA vs. WKY 2.0-mA, SD 2.0-mA and SD 1.0-mA |
| intensity * | F(1,16)=38.98 | p=0.00 | ||||||||
| LA | ANOVA | strain * | F(1,16)=4.57 | p=0.048 | strain × intensity | F(1,16)=3.6 | p=0.076 | |||
| intensity * | F(1,16)=12.48 | p=0.003 | ||||||||
Amygdala
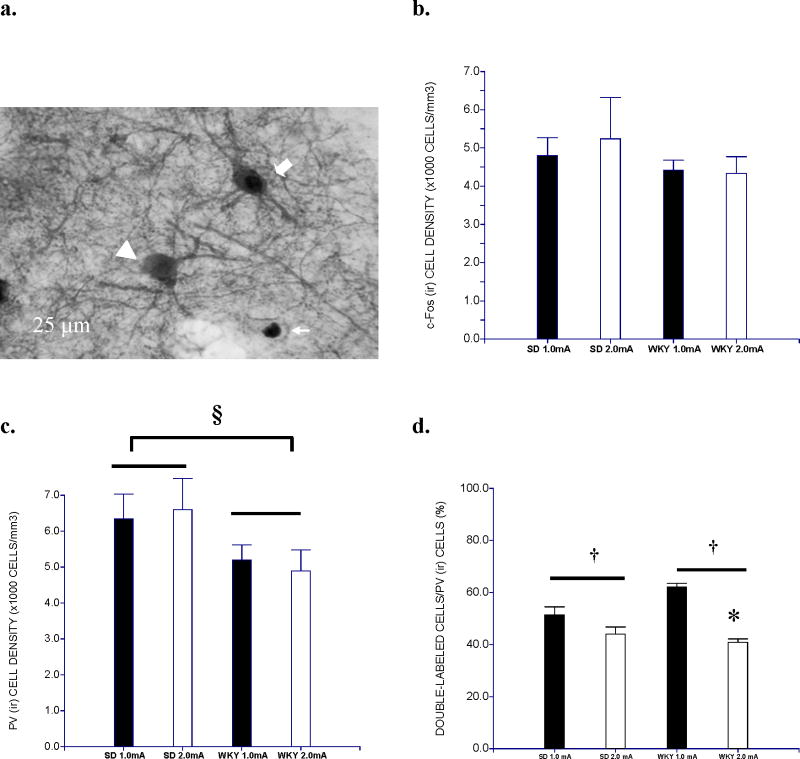
The abbreviated extinction session differentially activated parvalbumin neurons in the BA (Figure 4, and Tables 1 and 2). While similar c-Fos (ir) neurons were found as a function of strain and stressor intensity (Figure 4b), WKY rats had lower levels of parvalbumin (ir) cells compared to SD rats, confirmed by main effect of Strain, F(1,16) = 4.7, p< .05 (Figure 4c). Moreover, a smaller proportion of parvalbumin cells were activated (i.e., double labeled c-Fos (ir) and parvalbumin (ir)) in WKY rats trained with a 2.0-mA shock compared to all other groups (Figure 4d). Additionally, WKY rats in general had less activated parvalbumin cells than SD rats. These findings were supported by a main effect of Intensity, F(1,16) = 38.98, and an interaction of Strain × Intensity, F(1,16)=9.29, ps< .01. Post hoc analyses indicated that less GABAergic neurons were activated in WKY rats trained at 2.0-mA shock than those trained at 1.0-mA shock, p<.05.
Figure 4.
Double staining of c-Fos and PV in the basolateral amygdala (BA).
Density of c-Fos (ir) and PV (ir) cells in the BA was depicted. C-Fos (ir) (arrow), PV (ir) (arrow head) and c-Fos/PV (ir) (block arrow) cells were visualized by DAB-NiCl2, DAB and DAB/ DAB-NiCl2 staining (100x) (a). C-Fos (ir) cell density did not differ between strains or conditions (b). However, WKY rats exhibited lower density of PV (ir) cells as compared to SD rats (§, p<0.05) (c). The proportion of double-labeled cells in PV (ir) cells was lower in rats trained under 2.0-mA shock (†, p<0.01); WKY rats trained under 2.0-mA shock exhibited lower ratio compared to their counterpart trained under 1.0-mA shock (*, post hoc p<0.05) (d). Data is expressed as group mean ± S.E.M. (n=5/group).
LaA neurons were differentially activated after the abbreviated extinction session depending on shock intensity (Tables 1 and 2). Density of c-Fos (ir) tended to be less in rats trained with 2.0-mA than 1.0-mA shock, main effect of Stressor Intensity, F(1,16)=3.72, p=0.07. In contrast to the BA, the density of parvalbumin (ir) cells in the LaA did not differ between strains. Moreover, LaA parvalbumin (ir) cells were less activated during extinction if the rat was trained with 2.0-mA shock, main effect of Stressor Intensity, F(1,16)=12.48, p< .01. WKY rats had more activated parvalbumin (ir) cells, main effect of Strain, F(1,16)=4.57, p<.05. Moreover, WKY rats trained at 1.0-mA shock appear to have higher ratio of activated parvalbumin (ir) cells than WKY rats trained at 2.0-mA shock although the strain × intensity only reached p=0.08. Neurons in the CeA and MeA were less affected by shock intensity than BA and LaA neurons. In the CeA, a trend was observed for less c-Fos (ir) staining in WKY rats compared to SD rats, F(1,16)=4.02, p=0.062 (data not shown). c-Fos (ir) staining in MeA did not differ between shock intensity or strain. Differences in parvalbumin staining was not evaluated in the CeA or MeA because parvalbumin is scarcely located in GABAergic neurons of deep amygdalar nuclei as demonstrated previously [41].
Discussion
The present study was designed to examine the interaction of strain and stressor intensity in the acquisition and extinction of lever press avoidance. Consistent with previous work [18,19,21], SD rats exhibited similar lever press avoidance acquisition to foot shock modestly differing in intensity. Observation of vocalization and foot movements elicited training suggested that exposure to 2.0-mA foot shock represented a more intense event for both rat strains. Although acquisition did not differ, ITRs were more numerous in rats trained with more intense shock. Thus, these data support the contention that ITRs reflect an emotional aspect of avoidance responding [12,83]. Also consistent with the literature, inbred WKY rats acquired lever press avoidance faster than outbred SD rats [4,83]. Contrary to expectations, an interaction of strain and stressor intensity was not observed. Training with a more intense foot shock did not affect the avoidance performance of WKY rats.
As to extinction, WKY and SD rats trained with 1.0-mA foot shock extinguished at similar rates. These data are seemingly inconsistent with our prior work which showed substantial perseveration of avoidance in WKY rats [83]. We reasoned the discrepancy is due to a modification in our present extinction procedure. Previously, the extinction phase began with the simple removal of the shock. We speculated in our previous work that presentation of the blinking light during the ITI period insulated the WKY detecting a change in US contingency during the extinction phase. In the present study, the extinction phase began with the removal of the shock as well as the blinking light during the intertrial interval. In the present study, extinction rates for SD and WKY rats did not differ, supporting the contention continued presence of the ITI cue during the extinction phase retards extinction of the avoidance response to a greater degree in WKY rats compared to SD rats.
In contrast to acquisition, an interaction of strain and foot shock intensity was evident during extinction. Although similar rates of extinction were observed in SD rats training with the two intensities, WKY rats trained at 2.0-mA shock extinguished considerably slower than WKY rats trained at 1.0-mA. In fact, there was virtually no change in avoidance responding up to and including the 9th session. The lack of extinction was further supported by the persistence ITRs in WKY trained with the higher foot shock intensity. Resistance to extinction has been observed after extremely intense foot shocks in outbred rats[19,20]. Perseveration of avoidance responses after exposure to intense stressors supports the characterization of WKY rats as more sensitive to avoidance extinction independent of avoidance acquisition.
Although there is mounting evidence in humans and rats implicating the mPFC and amygdala in fear extinction [9,16,17,47,53,60,69] and neuropathology of anxiety [14,27], there is a dearth of information regarding the neuronal circuits involved in avoidance extinction and its perseveration. We have previously shown that the medial septum and by implication the hippocampus is important for avoidance extinction but not avoidance acquisition [64], supporting the idea of separate circuits involved in acquisition and extinction of avoidance similar to the fear conditioning [33]. One issue to resolve is the similarities (or differences) of the neural circuits involved in fear conditioning and avoidance. In the present study, we examined two key brain areas important in fear extinction to determine whether they were also involved in avoidance extinction. Neuronal activation of mPFC and amygdala subregions was visualized during an abbreviated extinction session. In response to the abbreviated extinction session, WKY rats exhibited lower c-Fos (ir) in prefrontal regions (ACC, ILC and PLC) than SD rats. The lower activation of prefrontal regions in WKY rats may reflect the reduced ‘warm up’ – the tendency for well-trained rats to exhibit escape early in a training session–observed in WKY rats compared to SD rats. Reduced warm up is reflected by the increased number of trials experienced by WKY rats compared to SD rats in the abbreviated, fixed time extinction session (SD rats trained with normal intensity foot shock = 5.2 ± .5 trials; SD trained with higher intensity = 5.2 ± .2 trials; WKY rats trained with normal intensity = 6.6 ± .6 trials; WKY rats trained with the higher intensity = 8.8 ± .6 trials). Thus, the lack of warm up exhibited by WKY rats may be reflected in the reduction of mPFC c-Fos (ir) activation. Reduced activation of the IL and PL regions of the rat PFC are reminiscent of findings in anxious patients displaying reduced activity in areas of the prefrontal cortex [32,38,42]. For anxious patients, such reduced activation patterns may represent a source of vulnerability to anxiety states or may develop concomitant with anxiety. Thus, our present data suggest that reduced mPFC activation may be associated with a lack of warm up and leading to enhanced avoidance acquisition and insulation from changes in contingencies.
While training with intense foot shock and the attendant perseveration of avoidance responding by WKY rats was not associated with changes in mPFC activation, different activational patterns were observed in amygdala sub-regions. WKY rats exhibited less parvalbumin labeling than SD rats in the BA but not in the LaA. Parvalbumin is a calcium binding protein that is found in 50~ 60% GABAergic BA and LaA neurons [41], and the reduced parvalbumin (ir) neurons in the BA is suggestive of less GABAergic tone in the BA. The MeA and CeA did not have parvalbumin staining to an appreciable extent in all rats consistent with earlier work [41]. At present, it is unclear whether the reduced parvalbumin (ir) in the BA of WKY rats is an inherent difference between strains or results from previous avoidance acquisition or extinction sessions.
c-Fos (ir) did not differ in the amygdala subregions either as a function of strain, shock intensity or the interaction. Although overall activation did not differ, the activation of parvalbumin neurons was less in WKY rats trained with the more intense stressor compared WKY rats trained with a lower shock intensity or SD rats trained at either intensity. Thus, the reduced GABAergic tone of WKY rats was compounded by reduced activation of GABAergic cells when training involved more intense shock. The reduced GABAergic activation likely results in greater activation of non-GABAergic neurons, explaining why overall activation did not differ between shock intensities. These data suggest that reduced GABAergic tone in the BA is a sensitive index of extinction perseveration in WKY rats. Both GABAA receptor antagonism and chronic stress can reduce inhibitory tone in the BA and result in enhanced fear conditioning response in rats [76,77]. The BA and LaA aspects of the amygdala have received substantial attention with regards to their role in acquisition and extinction of aversive memories [44,45,46,47,48,51,74,81,85,97]. Both anatomical and electrophysiological evidence indicate that BA parvalbumin (ir) neurons are associated with synchronization of the firing activity of local pyramidal neurons, which is essential for consolidation as well as recall of fear-related memory [52,61,76]. Thus, vulnerability to acquire avoidance may be traced to inherently less activation of the prefrontal cortex and less inhibitory tone in the BA, whereas impediments to the extinction of avoidance behavior may reflect inherently low GABAergic activation in the BA.
Clearly, the mapping of activation during avoidance acquisition and extinction is in its infancy. Activational patterns were assessed after several weeks of avoidance training, weeks of extinction training in the absence of shock and safety signal, and an abbreviated extinction training session. Thus, a further analysis on neuronal activation is necessary to differentiate effects of acquisition and extinction and to evaluate a possible interaction of acquisition and extinction of these strains. Nonetheless, the present study suggests that strain differences in acquisition and extinction are reflected in alterations in mPFC and amygdala activity.
To determine whether basal c-Fos expression differed between WKY and SD rats, c-Fos staining was assessed in four behaviorally naïve rats from each strain. In these rats, c-Fos expression was scarce but similar in both strains in mPFC and amygdalar regions (data not shown). Our results are consistent with previous observations in forebrain in home-cage rats [33,35,91], demonstrating c-Fos (ir) cell numbers were low.
Recent studies have increased our appreciation of vulnerability toward the risk of developing anxiety disorders [28,71] and illustrated the intimate relationship of avoidance and avoidant behavior in the development of anxiety disorders [63]. Thus, the role of vulnerability in avoidance sensitivity and its persistence is critical to an understanding of the development of anxiety disorders. WKY rats model vulnerability to anxiety disorders, as they display a rare combination of behavioral inhibition, stress sensitivity, and enhanced avoidance acquisition. Under conditions of more intense stressor exposure, avoidance perseveration is selectively apparent in WKY rats. Avoidance sensitivity is associated with less activation in the prefrontal cortex and less GABAergic neurons in BA of the amygdala. In addition, reduced activation of GABAergic neurons in the amygdala is associated with avoidance perseveration. Detailing the neurobiological processes subsuming avoidance sensitivity and its perseveration has the potential to advance our understanding of the dynamic interaction of vulnerability and the development of anxiety disorders, such as PTSD.
Acknowledgments
This research was supported by NIH grants and a Department of Veterans Affairs Merit Review research program and the Stress & Motivated Behavior Institute of the New Jersey Medical School. The described experimentation was conducted with approval by the VANJHCS Institutional Animal Care and Use and Research and Development Committees. The authors would like to thank the technical expertise of Toni Marie Dispenziere and Tracey Longo in the behavioral testing of the animals in these experiments.
References
- 1.Akirav I, Raizel H, Maroun M. Enhancement of conditioned fear extinction by infusion of the GABA(A) agonist muscimol into the rat prefrontal cortex and amygdala. Eur J Neurosci. 2006;23:758–764. doi: 10.1111/j.1460-9568.2006.04603.x. [DOI] [PubMed] [Google Scholar]
- 2.American Psychiatric Association. DSM IV-Diagnostic and Statistical Manual of Psychiatric Disorders. 4. American Psychiatric Association; Washington D.C: 1994. [Google Scholar]
- 3.Baum AE, Solberg LC, Churchill GA, Ahmadiyeh N, Takahashi JS, Redei EE. Test- and behavior-specific genetic factors affect WKY hypoactivity in tests of emotionality. Behavioural Brain Research. 2006;169:220–230. doi: 10.1016/j.bbr.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beck KD, Jiao X, Pang KC, Servatius RJ. Vulnerability factors in anxiety determined through differences in active-avoidance behavior. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:852–860. doi: 10.1016/j.pnpbp.2010.03.036. [DOI] [PubMed] [Google Scholar]
- 5.Berlau DJ, McGaugh JL. Enhancement of extinction memory consolidation: the role of the noradrenergic and GABAergic systems within the basolateral amygdala. Neurobiol Learn Mem. 2006;86:123–132. doi: 10.1016/j.nlm.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 6.Bolles RC, Warren JA. The acquisition of bar press avoidance as a function of shock intensity. Psychonomic Science. 1965;3:297–298. [Google Scholar]
- 7.Bolles RC. Species-specific defense reactions and avoidance learning. Psychological Review. 1970;77:32–48. [Google Scholar]
- 8.Boren JL, SIDMAN M, Herrnstein RJ. Avoidance, escape, and extinction as functions of shock intensity. J Comp Physiol Psychol. 1959;52:420–426. doi: 10.1037/h0042727. [DOI] [PubMed] [Google Scholar]
- 9.Bouton ME, Garcia-Gutierrez A, Zilski J, Moody EW. Extinction in multiple contexts does not necessarily make extinction less vulnerable to relapse. Behav Res Ther. 2006;44:983–994. doi: 10.1016/j.brat.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 10.Braunstein-Bercovitz H, mentman-Ashkenazi I, Lubow RE. Stress affects the selection of relevant from irrelevant stimuli. Emotion. 2001;1:182–192. doi: 10.1037/1528-3542.1.2.182. [DOI] [PubMed] [Google Scholar]
- 11.Braw Y, Malkesman O, Merenlender A, Bercovich A, Dagan M, Overstreet DH, Weller A. Withdrawal emotional-regulation in infant rats from genetic animal models of depression. Behavioural Brain Research. 2008;193:94–100. doi: 10.1016/j.bbr.2008.04.026. [DOI] [PubMed] [Google Scholar]
- 12.Brennan FX, Beck KD, Servatius RJ. Leverpress escape/avoidance conditioning in rats: safety signal length and avoidance performance. Integr Physiol Behav Sci. 2003;38:36–44. doi: 10.1007/BF02734259. [DOI] [PubMed] [Google Scholar]
- 13.Brush FR, Brush ES, Solomon RL. Traumatic avoidance learning: the effects of CS-US interval with a delayed-conditioning procedure. [References] Journal of Comparative and Physiological Psychology. 1955;48:285–293. doi: 10.1037/h0043496. [DOI] [PubMed] [Google Scholar]
- 14.Campbell-Sills L, Simmons AN, Lovero KL, Rochlin AA, Paulus MP, Stein MB. Functioning of neural systems supporting emotion regulation in anxiety-prone individuals. NeuroImage. 2011;54:689–696. doi: 10.1016/j.neuroimage.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chaudhuri A, Zangenehpour S, Rahbar-Dehgan F, Ye F. Molecular maps of neural activity and quiescence. Acta Neurobiol Exp (Wars ) 2000;60:403–410. doi: 10.55782/ane-2000-1359. [DOI] [PubMed] [Google Scholar]
- 16.Cheng DT, Knight DC, Smith CN, Helmstetter FJ. Human Amygdala Activity During the Expression of Fear Responses. Behavioral Neuroscience. 2006;120:1187–1195. doi: 10.1037/0735-7044.120.5.1187. [DOI] [PubMed] [Google Scholar]
- 17.Cheng DT, Knight DC, Smith CN, Stein EA, Helmstetter FJ. Functional MRI of Human Amygdala Activity During Pavlovian Fear Conditioning: Stimulus Processing Versus Response Expression. Behavioral Neuroscience. 2003;117:3–10. doi: 10.1037//0735-7044.117.1.3. [DOI] [PubMed] [Google Scholar]
- 18.D'Amato MR, Etkin M, Fazzaro J. Effects of shock type and intensity on anticipatory responses. J Comp Physiol Psychol. 1968;66:527–529. doi: 10.1037/h0026364. [DOI] [PubMed] [Google Scholar]
- 19.D'Amato MR, Fazzaro J. Discriminated lever-press avoidance learning as a function of type and intensity of shock. J Comp Physiol Psychol. 1966;61:313–315. doi: 10.1037/h0023146. [DOI] [PubMed] [Google Scholar]
- 20.D'Amato MR, Fazzaro J, Etkin M. Discriminated bar-press avoidance maintenance and extinction in rats as a function of shock intensity. J Comp Physiol Psychol. 1967;63:351–354. doi: 10.1037/h0024386. [DOI] [PubMed] [Google Scholar]
- 21.D'Amato MR, Keller D, Biederman G. Discriminated avoidance learning as a function of parameters of discontinuous shock. Journal of Experimental Psychology. 1965;70:543–548. doi: 10.1037/h0022689. [DOI] [PubMed] [Google Scholar]
- 22.Das Gracas De SD, ves De Moraes AB, Todorov JC. Shock intensity and signaled avoidance responding. J Exp Anal Behav. 1984;42:67–74. doi: 10.1901/jeab.1984.42-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fairbanks JM, Klein RG. Behavioral inhibition study of offspring of depressed and anxious adults. Biological Psychiatry. 1996;39:523–523. [Google Scholar]
- 24.Ferguson SA, Cada AM. Spatial learning/memory and social and nonsocial behaviors in the spontaneously hypertensive, Wistar-Kyoto and Sprague-Dawley rat strains. Pharmacol Biochem Behav. 2004;77:583–594. doi: 10.1016/j.pbb.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 25.Foa EB, Stein DJ, McFarlane AC. Symptomatology and psychopathology of mental health problems after disaster. J Clin Psychiatry. 2006;67 (Suppl 2):15–25. [PubMed] [Google Scholar]
- 26.Fox NA, Henderson HA, Marshall PJ, Nichols KE, Ghera MM. Behavioral inhibition: linking biology and behavior within a developmental framework. Annu Rev Psychol. 2005;56:235–262. doi: 10.1146/annurev.psych.55.090902.141532. [DOI] [PubMed] [Google Scholar]
- 27.Freitas-Ferrari MC, Hallak JEC, Trzesniak C, Filho AS, Machado-de-Sousa JP, Chagas MH, Nardi AE, Crippa JA. Neuroimaging in social anxiety disorder: A systematic review of the literature. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2010;34:565–580. doi: 10.1016/j.pnpbp.2010.02.028. [DOI] [PubMed] [Google Scholar]
- 28.Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, Pitman RK. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci. 2002;5:1242–1247. doi: 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gold PW, Chrousos GP. The endocrinology of melancholic and atypical depression: relation to neurocircuitry and somatic consequences. Proc Assoc Am Physicians. 1999;111:22–34. doi: 10.1046/j.1525-1381.1999.09423.x. [DOI] [PubMed] [Google Scholar]
- 30.Grillon C, Duncko R, Covington MF, Kopperman L, Kling MA. Acute Stress Potentiates Anxiety in Humans. Biol Psychiatry. 2007 doi: 10.1016/j.biopsych.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grillon C, Pine DS, Baas JM, Lawley M, Ellis V, Charney DS. Cortisol and DHEA-S are associated with startle potentiation during aversive conditioning in humans. Psychopharmacology (Berl) 2006;186:434–441. doi: 10.1007/s00213-005-0124-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guyer AE, Lau JYF, Clure-Tone EB, Parrish J, Shiffrin ND, Reynolds RC, Chen G, Blair RJR, Leibenluft E, Fox NA, Ernst M, Pine DS, Nelson EE. Amygdala and Ventrolateral Prefrontal Cortex Function During Anticipated Peer Evaluation in Pediatric Social Anxiety. Arch Gen Psychiatry. 2008;65:1303–1312. doi: 10.1001/archpsyc.65.11.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hale MW, Bouwknecht JA, Spiga F, Shekhar A, Lowry CA. Exposure to high- and low-light conditions in an open-field test of anxiety increases c-Fos expression in specific subdivisions of the rat basolateral amygdaloid complex. Brain Res Bull. 2006;71:174–182. doi: 10.1016/j.brainresbull.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 34.Herry C, Ciocchi S, Senn V, Demmou L, Muller C, Luthi A. Switching on and off fear by distinct neuronal circuits. Nature. 2008;454:600–606. doi: 10.1038/nature07166. [DOI] [PubMed] [Google Scholar]
- 35.Hess US, Lynch G, Gall CM. Changes in c-fos mRNA expression in rat brain during odor discrimination learning: differential involvement of hippocampal subfields CA1 and CA3. J Neurosci. 1995;15:4786–4795. doi: 10.1523/JNEUROSCI.15-07-04786.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hirshfeld DR, Rosenbaum JF, Biederman J, Bolduc EA, Faraone SV, Snidman N, Reznick JS, Kagan J. Stable behavioral inhibition and its association with anxiety disorder. J Am Acad Child Adolesc Psychiatry. 1992;31:103–111. doi: 10.1097/00004583-199201000-00016. [DOI] [PubMed] [Google Scholar]
- 37.Hirshfeld-Becker DR, Biederman J, Henin A, Faraone SV, Davis S, Harrington K, Rosenbaum JF. Behavioral inhibition in preschool children at risk is a specific predictor of middle childhood social anxiety: a five-year follow-up. J Dev Behav Pediatr. 2007;28:225–233. doi: 10.1097/01.DBP.0000268559.34463.d0. [DOI] [PubMed] [Google Scholar]
- 38.Hopper JW, Frewen PA, van der Kolk BA, Lanius RA. Neural correlates of reexperiencing, avoidance, and dissociation in PTSD: symptom dimensions and emotion dysregulation in responses to script-driven trauma imagery. J Trauma Stress. 2007;20:713–725. doi: 10.1002/jts.20284. [DOI] [PubMed] [Google Scholar]
- 39.Kagan J, Reznick JS, Snidman N. The physiology and psychology of behavioral inhibition in children. Child Dev. 1987;58:1459–1473. [PubMed] [Google Scholar]
- 40.Karamustafalioglu OK, Zohar J, Guveli M, Gal G, Bakim B, Fostick L, Karamustafalioglu N, Sasson Y. Natural Course of Posttraumatic Stress Disorder: A 20-Month Prospective Study of Turkish Earthquake Survivors. J Clin Psychiatry. 2006;67:882–889. doi: 10.4088/jcp.v67n0604. [DOI] [PubMed] [Google Scholar]
- 41.Kemppainen S, Pitkanen A. Distribution of parvalbumin, calretinin, and calbindin-D(28k) immunoreactivity in the rat amygdaloid complex and colocalization with gamma-aminobutyric acid. J Comp Neurol. 2000;426:441–467. doi: 10.1002/1096-9861(20001023)426:3<441::aid-cne8>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 42.Lanius RA, Williamson PC, Hopper J, Densmore M, Boksman K, Gupta MA, Neufeld RW, Gati JS, Menon RS. Recall of emotional states in posttraumatic stress disorder: an fMRI investigation. Biol Psychiatry. 2003;53:204–210. doi: 10.1016/s0006-3223(02)01466-x. [DOI] [PubMed] [Google Scholar]
- 43.Lau JY, Lissek S, Nelson EE, Lee Y, Roberson-Nay R, Poeth K, Jenness J, Ernst M, Grillon C, Pine DS. Fear conditioning in adolescents with anxiety disorders: results from a novel experimental paradigm. J Am Acad Child Adolesc Psychiatry. 2008;47:94–102. doi: 10.1097/chi.0b01e31815a5f01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Laurent V, Marchand AR, Westbrook RF. The basolateral amygdala is necessary for learning but not relearning extinction of context conditioned fear. Learn Mem. 2008;15:304–314. doi: 10.1101/lm.928208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Laurent V, Westbrook RF. Distinct contributions of the basolateral amygdala and the medial prefrontal cortex to learning and relearning extinction of context conditioned fear. Learn Mem. 2008;15:657–666. doi: 10.1101/lm.1080108. [DOI] [PubMed] [Google Scholar]
- 46.Li G, Nair SS, Quirk GJ. A biologically realistic network model of acquisition and extinction of conditioned fear associations in lateral amygdala neurons. J Neurophysiol. 2009;101:1629–1646. doi: 10.1152/jn.90765.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Likhtik E, Pelletier JG, Paz R, Pare D. Prefrontal control of the amygdala. J Neurosci. 2005;25:7429–7437. doi: 10.1523/JNEUROSCI.2314-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Likhtik E, Popa D, pergis-Schoute J, Fidacaro GA, Pare D. Amygdala intercalated neurons are required for expression of fear extinction. Nature. 2008;454:642–645. doi: 10.1038/nature07167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lovibond PF, Mitchell CJ, Minard E, Brady A, Menzies RG. Safety behaviours preserve threat beliefs: Protection from extinction of human fear conditioning by an avoidance response. Behav Res Ther. 2009;47:716–720. doi: 10.1016/j.brat.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 50.Malkesman O, Braw Y, Zagoory-Sharon O, Golan O, Lavi-Avnon Y, Schroeder M, Overstreet DH, Yadid G, Weller A. Reward and anxiety in genetic animal models of childhood depression. Behav Brain Res. 2005;164:1–10. doi: 10.1016/j.bbr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 51.Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Hermann H, Tang J, Hofmann C, Zieglgansberger W, Di MV, Lutz B. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- 52.McDonald AJ, Mascagni F, Mania I, Rainnie DG. Evidence for a perisomatic innervation of parvalbumin-containing interneurons by individual pyramidal cells in the basolateral amygdala. Brain Research. 2005;1035:32–40. doi: 10.1016/j.brainres.2004.11.052. [DOI] [PubMed] [Google Scholar]
- 53.Milad MR, Rauch SL, Pitman RK, Quirk GJ. Fear extinction in rats: Implications for human brain imaging and anxiety disorders. Biological Psychology. 2006;73:61–71. doi: 10.1016/j.biopsycho.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 56.Miller JP, McAuley JD, Pang KCH. Spontaneous fos expression in the suprachiasmatic nucleus of young and old mice. Neurobiology of Aging. 2005;26:1107–1115. doi: 10.1016/j.neurobiolaging.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 57.Mineka S, Zinbarg R. Conditioning and ethological models of anxiety disorders: stress-in-dynamic-context anxiety models. Nebr Symp Motiv. 1996;43:135–210. 135–210. [PubMed] [Google Scholar]
- 58.Mineka S, Zinbarg R. A Contemporary Learning Theory Perspective on the Etiology of Anxiety Disorders: It's Not What You Thought It Was. American Psychologist. 2006;61:10–26. doi: 10.1037/0003-066X.61.1.10. [DOI] [PubMed] [Google Scholar]
- 59.Mobbs D, Petrovic P, Marchant JL, Hassabis D, Weiskopf N, Seymour B, Dolan RJ, Frith CD. When fear is near: threat imminence elicits prefrontal-periaqueductal gray shifts in humans. Science. 2007;317:1079–1083. doi: 10.1126/science.1144298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Morgan MA, Romanski LM, LeDoux JE. Extinction of emotional learning: contribution of medial prefrontal cortex. Neurosci Lett. 1993;163:109–113. doi: 10.1016/0304-3940(93)90241-c. [DOI] [PubMed] [Google Scholar]
- 61.Muller JF, Mascagni F, McDonald AJ. Pyramidal cells of the rat basolateral amygdala: synaptology and innervation by parvalbumin-immunoreactive interneurons. J Comp Neurol. 2006;494:635–650. doi: 10.1002/cne.20832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.North CS, Pfefferbaum B, Tivis L, Kawasaki A, Reddy C, Spitznagel EL. The course of posttraumatic stress disorder in a follow-up study of survivors of the Oklahoma City bombing. Ann Clin Psychiatry. 2004;16:209–215. doi: 10.1080/10401230490522034. [DOI] [PubMed] [Google Scholar]
- 63.O'donnell ML, Elliott P, Lau W, Creamer M. PTSD symptom trajectories: From early to chronic response. Behav Res Ther. 2006 doi: 10.1016/j.brat.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 64.Pang KC, Jiao X, Sinha S, Beck KD, Servatius RJ. Damage of GABAergic neurons in the medial septum impairs spatial working memory and extinction of active avoidance: Effects on proactive interference. Hippocampus. 2010 doi: 10.1002/hipo.20799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pang KCH, Nocera R. Interactions Between 192-IgG Saporin and Intraseptal Cholinergic and GABAergic Drugs: Role of Cholinergic Medial Septal Neurons in Spatial Working Memory. Behavioral Neuroscience. 1999;113:265–275. doi: 10.1037//0735-7044.113.2.265. [DOI] [PubMed] [Google Scholar]
- 66.Pare WP. Enhanced retrieval of unpleasant memories influenced by shock controllability, shock sequence, and rat strain. Biol Psychiatry. 1996;39:808–813. doi: 10.1016/0006-3223(95)00220-0. [DOI] [PubMed] [Google Scholar]
- 67.Pare WP, Redei E. Depressive behavior and stress ulcer in Wistar Kyoto rats. J Physiol Paris. 1993;87:229–238. doi: 10.1016/0928-4257(93)90010-q. [DOI] [PubMed] [Google Scholar]
- 68.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4. Academic Press; San Diego, USA: 2004. [Google Scholar]
- 69.Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 71.Pitman RK, Gilbertson MW, Gurvits TV, May FS, Lasko NB, Metzger LJ, Shenton ME, Yehuda R, Orr SP. Clarifying the origin of biological abnormalities in PTSD through the study of identical twins discordant for combat exposure. Ann N Y Acad Sci. 2006;1071:242–254. doi: 10.1196/annals.1364.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Powell RW. The effect of shock intensity upon responding under a multiple-avoidance schedule. J Exp Anal Behav. 1970;14:321–329. doi: 10.1901/jeab.1970.14-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Quirk GJ, Armony JL, LeDoux JE. Fear conditioning enhances different temporal components of tone-evoked spike trains in auditory cortex and lateral amygdala. Neuron. 1997;19:613–624. doi: 10.1016/s0896-6273(00)80375-x. [DOI] [PubMed] [Google Scholar]
- 74.Quirk GJ, Likhtik E, Pelletier JG, Pare D. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J Neurosci. 2003;23:8800–8807. doi: 10.1523/JNEUROSCI.23-25-08800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Redei E, Pare WP, Aird F, Kluczynski J. Strain differences in hypothalamic-pituitary-adrenal activity and stress ulcer. Am J Physiol. 1994;266:R353–R360. doi: 10.1152/ajpregu.1994.266.2.R353. [DOI] [PubMed] [Google Scholar]
- 76.Reznikov LR, Reagan LP, Fadel JR. Activation of phenotypically distinct neuronal subpopulations in the anterior subdivision of the rat basolateral amygdala following acute and repeated stress. J Comp Neurol. 2008;508:458–472. doi: 10.1002/cne.21687. [DOI] [PubMed] [Google Scholar]
- 77.Rodriguez Manzanares PA, Isoardi NA, Carrer HF, Molina VA. Previous stress facilitates fear memory, attenuates GABAergic inhibition, and increases synaptic plasticity in the rat basolateral amygdala. J Neurosci. 2005;25:8725–8734. doi: 10.1523/JNEUROSCI.2260-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rosenbaum JF, Biederman J, Bolduc-Murphy EA, Faraone SV, Chaloff J, Hirshfeld DR, Kagan J. Behavioral inhibition in childhood: a risk factor for anxiety disorders. Harv Rev Psychiatry. 1993;1:2–16. doi: 10.3109/10673229309017052. [DOI] [PubMed] [Google Scholar]
- 79.Rosenkranz JA, Grace AA. Modulation of basolateral amygdala neuronal firing and afferent drive by dopamine receptor activation in vivo. J Neurosci. 1999;19:11027–11039. doi: 10.1523/JNEUROSCI.19-24-11027.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rosenkranz JA, Grace AA. Dopamine attenuates prefrontal cortical suppression of sensory inputs to the basolateral amygdala of rats. J Neurosci. 2001;21:4090–4103. doi: 10.1523/JNEUROSCI.21-11-04090.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Royer S, Pare D. Bidirectional synaptic plasticity in intercalated amygdala neurons and the extinction of conditioned fear responses. Neuroscience. 2002;115:455–462. doi: 10.1016/s0306-4522(02)00455-4. [DOI] [PubMed] [Google Scholar]
- 82.Samanez-Larkin GR, Hollon NG, Carstensen LL, Knutson B. Individual differences in insular sensitivity during loss anticipation predict avoidance learning. Psychol Sci. 2008;19:320–323. doi: 10.1111/j.1467-9280.2008.02087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Servatius RJ, Jiao X, Beck KD, Pang KC, Minor TR. Rapid avoidance acquisition in Wistar-Kyoto rats. Behav Brain Res. 2008;192:191–197. doi: 10.1016/j.bbr.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 84.Servatius RJ, Ottenweller JE, Beldowicz D, Guo W, Zhu G, Natelson BH. Persistently exaggerated startle responses in rats treated with pyridostigmine bromide. J Pharmacol Exp Ther. 1998;287:1020–1028. [PubMed] [Google Scholar]
- 85.Sevelinges Y, Desgranges B, Ferreira G. The basolateral amygdala is necessary for the encoding and the expression of odor memory. Learn Mem. 2009;16:235–242. doi: 10.1101/lm.1247609. [DOI] [PubMed] [Google Scholar]
- 86.Silver RC, Holman EA, McIntosh DN, Poulin M, Gil-Rivas V. Nationwide longitudinal study of psychological responses to September 11. JAMA. 2002;288:1235–1244. doi: 10.1001/jama.288.10.1235. [DOI] [PubMed] [Google Scholar]
- 87.Simmons A, Strigo I, Matthews SC, Paulus MP, Stein MB. Anticipation of aversive visual stimuli is associated with increased insula activation in anxiety-prone subjects. Biol Psychiatry. 2006;60:402–409. doi: 10.1016/j.biopsych.2006.04.038. [DOI] [PubMed] [Google Scholar]
- 88.Smoller JW, Rosenbaum JF, Biederman J, Kennedy J, Dai D, Racette SR, Laird NM, Kagan J, Snidman N, Hirshfeld-Becker D, Tsuang MT, Sklar PB, Slaugenhaupt SA. Association of a genetic marker at the corticotropin-releasing hormone locus with behavioral inhibition. Biol Psychiatry. 2003;54:1376–1381. doi: 10.1016/s0006-3223(03)00598-5. [DOI] [PubMed] [Google Scholar]
- 89.Straube T, Schmidt S, Weiss T, Mentzel HJ, Miltner WH. Dynamic activation of the anterior cingulate cortex during anticipatory anxiety. Neuroimage. 2009;44:975–981. doi: 10.1016/j.neuroimage.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 90.Suslow T, Kugel H, Rauch AV, Dannlowski U, Bauer J, Konrad C, Arolt V, Heindel W, Ohrmann P. Attachment avoidance modulates neural response to masked facial emotion. Hum Brain Mapp. 2009 doi: 10.1002/hbm.20778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Suwanprathes P, Ngu M, Ing A, Hunt G, Seow F. c-Fos immunoreactivity in the brain after esophageal acid stimulation. Am J Med. 2003;115(Suppl 3A):31S–38S. doi: 10.1016/s0002-9343(03)00190-6. [DOI] [PubMed] [Google Scholar]
- 92.Tejani-Butt S, Kluczynski J, Pare WP. Strain-dependent modification of behavior following antidepressant treatment. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2003;27:7–14. doi: 10.1016/s0278-5846(02)00308-1. [DOI] [PubMed] [Google Scholar]
- 93.van Peer JM, Roelofs K, Spinhoven P. Cortisol administration enhances the coupling of midfrontal delta and beta oscillations. International Journal of Psychophysiology. 2008;67:144–150. doi: 10.1016/j.ijpsycho.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 94.West MJ. New stereological methods for counting neurons. Neurobiol Aging. 1993;14:275–285. doi: 10.1016/0197-4580(93)90112-o. [DOI] [PubMed] [Google Scholar]
- 96.West MJ, Bach G, Soderman A, Jensen JL. Synaptic contact number and size in stratum radiatum CA1 of APP/PS1DeltaE9 transgenic mice. Neurobiol Aging. 2009;30:1756–1776. doi: 10.1016/j.neurobiolaging.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 97.Yang YL, Lu KT. Facilitation of conditioned fear extinction by d-cycloserine is mediated by mitogen-activated protein kinase and phosphatidylinositol 3-kinase cascades and requires de novo protein synthesis in basolateral nucleus of amygdala. Neuroscience. 2005;134:247–260. doi: 10.1016/j.neuroscience.2005.04.003. [DOI] [PubMed] [Google Scholar]