Abstract
Metabotropic glutamate receptors (mGluRs) modulate glutamatergic and GABAergic neurotransmission. mGluR8, a member of group III receptors, is generally located presynaptically where it regulates neurotransmitter release. Previously we reported higher measures of anxiety in 6- and 12-month-old mGluR8−/− male mice than age- and sex-matched wild-type mice and that acute pharmacological stimulation with the mGluR8 agonist (S)-3,4,-dicarboxyphenylglycine (DCPG) or the Positive Allosteric Modulator (PAM) AZ12216052 reduced measures of anxiety in wild-type mice. As in humans and animals, ageing is associated with enhanced measures of anxiety following non-social and social challenges, increased understanding of these measures and how to potentially modulate them is particularly important in the elderly. Here we determined whether the effects of AZ12216052 on measures of anxiety are mediated by mGluR8 using 24-month-old mGluR8−/− and wild-type male mice. AZ12216052 also reduced measures of anxiety in the elevated zero maze and the acoustic startle response in mGluR8−/− mice. The remaining anxiolytic effects of AZ12216052 in mGluR8−/− mice might involve mGluR4, as the mGluR4 PAM VU 0155041 also reduced measures of anxiety in wild-type mice. In contrast, mGluR8−/− mice show enhanced social interaction but AZ12216052 does not affect social interaction in wild-type mice. Thus, while mGluR8 is an attractive target to modulate measures of anxiety and social interaction, the effects of AZ12216052 on measures of anxiety likely also involve receptors other than mGluR8.
Keywords: metabotropic, group-III mGluR, allosteric modulator, zero maze, acoustic startle, social interaction
1. INTRODUCTION
G protein-coupled metabotropic glutamate receptors (mGluRs) modulate excitatory and inhibitory neurotransmission [1, 2]. Based on their sequence identity, pharmacological profile and signal transduction mechanisms, 8 distinct mGluR receptors (mGluR1-mGluR8) have been identified and classified into 3 groups. In transfected cells, mGluR8, like mGluR4, mGluR6, and mGluR7, a member of group-III receptors, is coupled to the inhibition of adenylyl cyclase [3, 4]. In neurons, group-III receptors are generally located presynaptically, where they regulate neurotransmitter release [5].
Because of their modulatory role, mGluRs are attractive targets for therapies aimed at treating anxiety disorders [6]. Consistent with a role for mGluR8 in the regulation of anxiety, increased measures of anxiety in the open field and the elevated plus maze, and an increased acoustic startle response were seen in 6- and 12-month-old mGluR8−/− male mice [7–9]. These effects seem age-dependent as no genotype differences were seen in the elevated plus maze or elevated zero maze in 3-month-old mice [10]. While potential effects of mGluR8 deficiency in utero, during postnatal development, or during early adulthood might have contributed to the observed behavioral phenotype, they do not seem required for the anxiety modulating effects. Acute pharmacological modulation using the mGluR8 agonist AZ12216052 in 2-month-old wild-type mice also reduced these measures of anxiety [11]. However, without testing the potential effects of this compound in mGluR8−/− mice, involvement of mGluR8-independent pathways cannot be excluded.
In addition to measures of anxiety assessed in the elevated zero maze and acoustic startle tests, mGluR8 might also play a role in measures of anxiety involving social challenges, such as those assessed in a social interaction test. In some animal models, these distinct measures show a similar pattern. Examples include streptozotocin (STZ)-induced diabetic animals [12], mice with a selective deletion in the Tac1 gene, encoding the neuropeptides substance P and neurokinin A [13], and mice deficient in the tumor suppressor gene Phosphatase and tensin homolog on chromosome ten (Pten) in limited differentiated neuronal populations in hippocampus and cortex [14]. However, in other animal models these distinct measures are clearly dissociated. For example, mice lacking fmr1, a mouse model of fragile X syndrome [15], or lacking the vasopressin V1a receptor [16], show reduced social interaction but decreased measures of anxiety in the elevated zero maze. Differences in brain areas affected in these models might contribute to these divergent results. For example, while hippocampal lesions reduce social interactions, they do not affect measures of anxiety in the elevated zero maze [17].
In humans and animals, ageing is associated with enhanced measures of anxiety following non-social and social challenges. Therefore, increased understanding of these measures and how to potentially modulate them is particularly important in the elderly. However, decreased social interaction in aged rodents does not seem to involve changes in Fos expression in brain areas involved in the anxiety circuitry and known to be activated by anxiogenic stimuli [18]. Thus also in the aged brain, no simple relationship between social interaction and measures of anxiety involving non-social challenges is apparent.
In this study, we used 24-month-old mGluR8−/− and wild-type mice to determine whether in aged mice the anxiety-reducing effects of AZ12216052 seen in the elevated zero maze and on the acoustic startle response are modulated by mGluR8. In addition, we tested the potential role of mGluR8 in measures of anxiety involving social challenges in mGluR8−/− and wild-type mice.
2. MATERIALS AND METHODS
2.1. Animals
Experimentally naïve two-year-old mGluR8−/− and C57BL/6J wild-type male mice were used for elevated zero maze, acoustic startle, and social interaction testing. Adult C57BL/6J wild-type male and female mice were used to assess the effects of AZ12216052 on social interaction. Mice were kept on 12:12 hr light-dark schedule (lights on at 6 AM) with chow (PicoLab Rodent Diet 20, #5053; PMI Nutrition International, St. Louis, MO) and water given ad libitum. The mice were group-housed until one day prior to the start of behavioral testing and subsequently singly housed. All the experiments reported here were conducted in accordance with NIH guidelines and approved by the Institutional Animal Care and Use Committee at OHSU.
2.2. Drugs
AZ12216052 was a gift from Astra Zeneca. The chemical structure of this compound and its primary site of action and selectivity for mGluR8 have been described [11]. AZ12216052 was dissolved in 30% DMSO in saline and administered at 10 mg/kg, 2 hours prior to testing. The volume of DMSO injected per mouse was 30 μl. This amount of DMSO did not cause any detectable sickness in the mice or any effect on activity in the home cage, but it did heighten basal anxiety-like measures. DMSO is often used to assess anti-nociceptive and anxiolytic effects of test compounds [19] and administered i.p. did not effect the antitumor agent paclitaxel-evoked paw withdrawal [20] or paw withdrawal latency to a thermal stimulus [21, 22]. Similarly, DMSO administered into the ventrolateral periaqueductal gray did not affect nociception [23].
VU 0155041 (Niswender et al., 2008, Tocris, Ellisville, Missouri) was dissolved in saline and and administered at 5, 10, or 20 mg/kg or dissolved in 30% DMSO in saline and administered at 5 mg/kg, 2 hours prior to testing.
2.3. Behavioral Analysis
2.3.1. Elevated Zero Maze
The elevated zero maze and acoustic startle tests were performed in the morning, between 9–11 am. The custom built elevated zero maze consisted of two enclosed areas and two open areas, identical in length to the open and closed arms of an elevated plus maze (35.5 cm; Kinder Scientific, Poway, CA). Mice were placed in the closed part of the maze and allowed free access for 10 min, as described [24]. They could spend their time either in a closed safe area or in a more anxiety-provoking open area. A video tracking system (Noldus Information Technology, Sterling, VA) set at 6 samples/second was used to calculate velocity, distance moved, and percent time spent in the open areas of the maze.
2.3.2. Acoustic Startle
Acoustic startle was tested in Hamilton-Kinder (Poway, CA) startle chambers using a paradigm sensitive to detect effects of AZ12216052 on the acoustic startle response [11] and to detect genotype difference in the acoustic startle response of 12-month-old wild-type and mGluR8−/− mice [9]. After a 5-min acclimation, the baseline response was measured. Thus the baseline was defined as the amplitude measures in the absence of any acoustic stimulus. Acoustic pulses were given, increasing from 80 dB to120 dB, using increments of 2 dB. Each stimulus was given once and lasted 30 ms. The inter-trial interval was varied and on average 21 sec. Startle amplitude, the maximum force in newtons (N) or peak voltage that occurred during a 500 msec window, was used as outcome measure. Movement independent of a startle response might result in enhanced pressure on the sensing plate and contribute to the mean startle response. Therefore, the peak startle amplitude rather than the mean startle response was analyzed. White noise was used for the acoustic stimuli. Wideback background noise (72 dB) was used during testing. The acoustic startle response, defined as the average startle amplitude in response to different intensities from 80 dB to 120 dB was assessed.
2.3.3. Social Interaction
In this task, the amount of time that a ‘test’ mouse spends exploring the ‘stimulus’ mouse is used to measure social investigation. Twenty-four hours prior to testing all test and stimulus mice were singly housed to encourage exploration during the subsequent testing. To avoid potential genotype differences in social interactions between test and stimulus mice, genotype-matched mice were used as pairs of stimulus and test mice. Testing began one hour after the onset of the active cycle. All testing occurred in the same room where the mice were housed to minimize stress during transportation that could influence social behaviors. To begin the trial, the ‘test’ mouse home cage was gently moved under a video camera and was topped with a thin sheet of clear plexiglass. Following a 10-minute acclimatization period, the stimulus mouse was carefully presented into the test mouse’s home cage and the subsequent behaviors were recorded for 5 minutes using Ethovision XT software (Noldus Information Technology, Wageningen, The Netherlands). The behavior was then manually scored from the video using Button Box software (Behavioral Research Solutions, LLC, Wisconsin, USA). The amount of time that the test mouse spent sniffing the head/neck, torso, and ano-genital region, and the amount of time spent following the stimulus mouse but not sniffing was scored as a continuous measurement. Since these measures are highly correlated, the total time exploring across all investigation types was used as the primary outcome measure. The total investigation type across each of 5 consecutive 1-minute time-bins was analyzed to determine if investigation increased or decreased over time. Also, the cumulative time investigating across all time bins was calculated. The former was analyzed using 2-way repeated-measures ANOVA with time-bin as the within-subjects factor and genotype (wild-type vs. mGluR8−/−) as the between-subjects factor. The total investigation time was analyzed using an independent samples Student’s t-Test.
2.3.4. Statistical analysis
Data are expressed as mean ± SEM. Differences among means were evaluated by ANOVA, followed by Student’s t-test or Tukey-Kramer posthoc tests, as indicated, using GraphPad Prism software (San Diego, CA). For the acoustic startle response, startle intensity was used as factor in the analysis. For all analyses, the null hypothesis was rejected at the 0.05 level.
3. RESULTS
3.1. Effects of AZ12216052 on measure of anxiety in the elevated zero maze and acoustic startle response of mGluR8−/− and wild-type mice
Previously, we reported that in 2-month-old mice AZ12216052 reduced measures of anxiety in the elevated zero maze and reduced the acoustic startle response [11]. To confirm whether these effects are modulated by mGluR8, we tested the effects of AZ12216052 on measures of anxiety of 24-month-old mGluR8−/− and wild-type mice in the elevated zero maze and acoustic startle response. Surprisingly, AZ12216052 (10 mg/kg) reduced measures of anxiety of mGluR8−/− and wild-type mice in the elevated zero maze (Fig. 1). These effects on measures of anxiety were not due to potential changes in activity levels. AZ12216052 did not affect the velocity or distance moved of the mice in either genotype. There was also an effect of genotype (F (1, 26) = 8.356, p < 0.01) with higher measures of anxiety in mGluR8−/− than wild-type mice.
Figure 1.
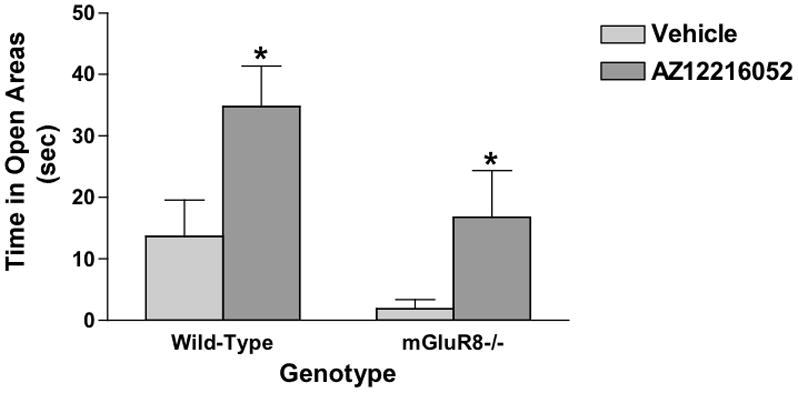
AZ12216052 reduces measure of anxiety of mGluR8−/− and wild-type mice in the elevated zero maze (F (1, 26) = 5.721, p < 0.05). *p < 0.05 versus vehicle-treated genotype-matched mice. AZ12216052 did not affect velocity of the mice in either genotype. n = 16 wild-type and n = 12 mGluR8−/− mice.
Acoustic startle was assessed using a paradigm sensitive to detect effects of AZ12216052 on the acoustic startle response [11] and of genotype differences in the acoustic startle response of 12-month-old wild-type and mGluR8−/− mice [9]. In addition to the elevated zero maze, AZ12216052 reduced the acoustic startle response of mGluR8−/− and wild-type mice without having effects on the baseline response (Fig. 2).
Figure 2.
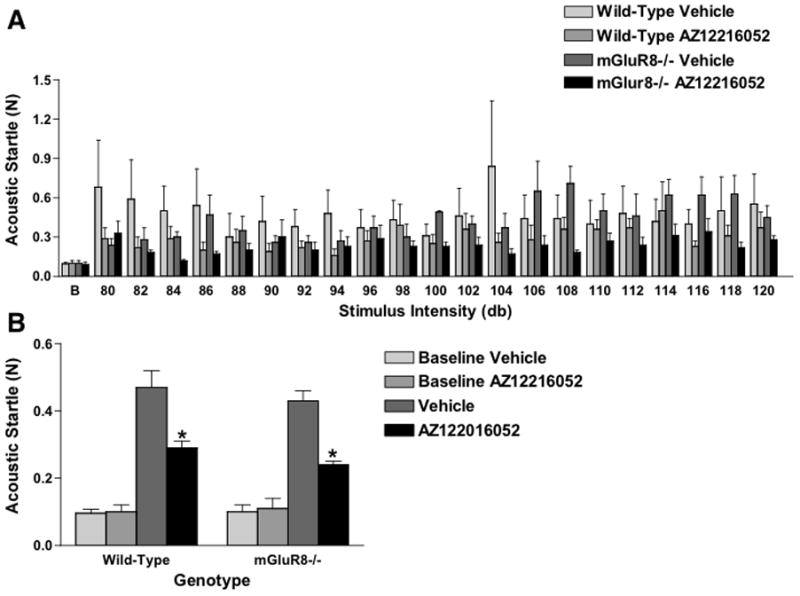
AZ12216052 reduces the acoustic startle response of mGluR8−/− and wild-type mice (F (1, 27) = 4.963, p < 0.05). A. Stimulus intensity startle magnitude responses. B is baseline. B. Acoustic startle responses collapsed across stimulus intensities. *p < 0.05, effects of AZ122016052 on acoustic startle response. There was no effect of AZ122016052 on the baseline startle response. n = 16 wild-type and n = 13 mGluR8−/− mice.
3.2. Effects of VU 0155041 on measure of anxiety of wild-type mice in the elevated zero maze
The remaining anxiolytic effects of AZ12216052 in mGluR8−/− mice might involve mGluR4-mediated signaling. Although up to 30 μM AZ12216052 had no effect in GTPγS binding assays using membranes prepared from mGluR4-expressing GHEK cells [11], in a more crude Fluorometric Imaging Plate Reader (FLIPR) assay, mGluR4 transfected cells did show a response to AZ12216052. Therefore, we assessed the potential effects of the mGluR4-selective PAM VU 0155041 on measures of anxiety of adult wild-type mice using the same experimental condition of a 2-hr interval between drug administration and behavioral testing that was used for the AZ12216052. When dissolved in saline, VU 0155041 reduced measures of anxiety at 5 mg/kg, but not at 10 or 20 mg/kg (Fig. 3). VU 0155041 did not affect the velocity or distance moved of the mice, thus its effects on measures of anxiety are not due to changes in activity levels. Since we found that DCPG reduced measures of anxiety in the elevated zero maze in the presence, but not in the absence, of 40% DMSO [11], we assessed the effects of VU 0155041 at 5 mg/kg in the presence of DMSO as compared to saline. The anxiety-reducing effects of VU 0155041 were similar under both conditions (DMSO: 12.75 ± 2.22; DMSO-VU 0155041: 19.13 ± 1.52, t (14) = 2.467, p < 0.05, n = 8 mice/treatment).
Figure 3.

VU 0155041 reduces measure of anxiety of wild-type mice in the elevated zero maze. Wild-type male mice were injected with saline (n = 14) or VU 0155041 at a dose of 5 (n = 8), 10 (n =16), or 20 (n = 8) mg/kg and behaviorally tested 2 hours later. At 5 mg/kg, VU 0155041 reduced measures of anxiety in the elevated zero maze (t (20) = 2.211, p < 0.05). *p < 0.05. VU 0155041 did not affect velocity of the mice.
3.3. mGluR8−/− mice show enhanced social interaction
To determine whether mGluR8 also plays a role in regulating anxiety under social conditions, we assessed social interaction in 24-month-old mGluR8−/− and wild-type mice. In contrast to measures of anxiety in the elevated zero maze, mGluR8−/− showed more social interaction than wild-type mice (Fig. 4). Since we found that AZ12216052 reduced measures of anxiety in the elevated zero maze and reduced the acoustic startle response [11], we next assessed potential effects of AZ12216052 on social interaction of adult male and female mice. AZ12216052 did not affect social interaction in male or female mice (Fig. 5).
Figure 4.
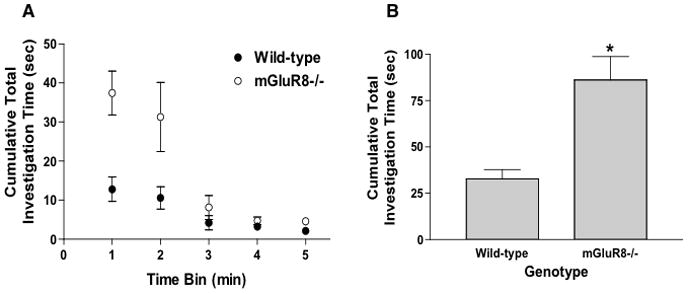
mGluR8−/− mice show enhanced social interaction. A. Time course of social interaction. There was an interaction between time bin and genotype (F (1, 59) = 9.349, p < 0.01). B. Social interaction data collapsed over the 5-min test session. t (10) = 4.750, p < 0.001. *p < 0.001. n = 8 wild-type and n = 8 wild-type stimulus mice; 4 mGluR8−/− test mice and n = 4 mGluR8−/− stimulus mice.
Figure 5.
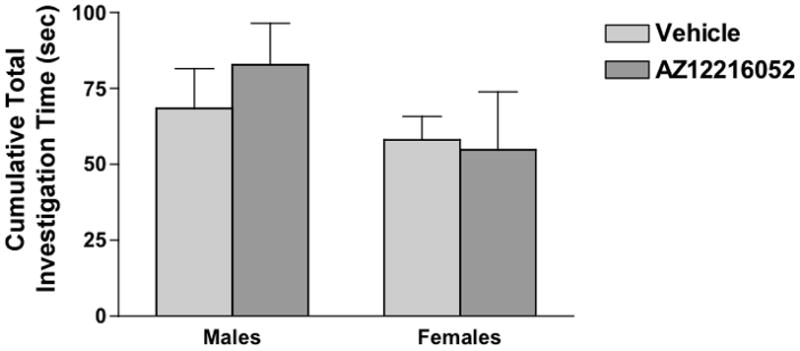
AZ12216052 does not affect social interaction of wild-type male or female mice. n = 12 male and n = 10 female wild-type test mice.
4. DISCUSSION
The data of the current study show that AZ12216052 also reduced measures of anxiety in the elevated zero maze and the acoustic startle response in mGluR8−/− mice. These effects might involve mGluR4, as the mGluR4 PAM VU 0155041 reduced measures of anxiety in wild-type mice. In contrast to measures of anxiety in the elevated zero maze and the acoustic startle response, mGluR8−/− mice show enhanced social interaction while AZ12216052 does not affect social interaction in wild-type mice. These data support opposing role of mGluR8 in measures of anxiety involving non-social and social challenges.
AZ12216052 reduced measures of anxiety in the elevated zero maze and the acoustic startle response. The magnitude of the pharmacological effect, as compared to vehicle injection, was similar in both genotypes for both outcome measures. These data support that effects of AZ12216052 on measures of anxiety involve receptors other than mGluR8.
Both vehicle-treated genotypes spent a relative low percentage of time in the anxiety-provoking areas of the elevated zero maze. This is unlikely required to detect enhanced measures of anxiety in mGluR8−/− mice, as Linden et al. [7] only found increased measures of anxiety in mGluR8−/− mice in the elevated plus maze under less anxiogenic conditions. Based on their results, they hypothesized that higher room illumination levels increased the anxiety of both wild-type and mGluR8−/− animals, masking the effect caused by the lack of the mGluR8 receptor. However, heightened anxiety levels might be required to detect effects of AZ12216052 on measures of anxiety, consistent with the notion that this compound is a positive allosteric modulator rather than a traditional agonist. Previously, we showed only anxiolytic effects of DCPG under conditions of elevated anxiety-like behavior [11].
In contrast to the genotype difference in measures of anxiety in the elevated zero maze, we did not see a genotype difference in the acoustic startle response in 24-month-old mice. It is possible that the genotype difference in the acoustic startle response observed at 6 and 12 months of age [8, 9] is age-dependent and not therefore not seen at 24 months of age. However, as the younger mice tested were not injected, we cannot exclude that potential effects of vehicle injections in the current study might have masked a genotype difference in 24-month-old mice.
Our data show that in addition to measures of anxiety in the elevated zero maze and acoustic startle tests, mGluR8 plays an opposite role in measures of anxiety involving social challenges. mGluR8−/− mice show enhanced social interaction but increased measures of anxiety in the elevated zero maze and acoustic startle response. Interestingly, the pattern of these changes is the opposite pattern seen in mice lacking the vasopressin V1a receptor [16]. Vasopressin has been shown to play a critical role in social interaction and pair bonding and regulation of anxiety under nonsocial challenges [16, 25, 26]. These data suggest that in mGluR8−/− mice enhanced vasopressin-mediated signaling might mediate the opposing effects on measures of anxiety involving social and non-social challenges. In contrast to measures of anxiety involving non-social challenges, acute pharmacological administration of AZ12216052 did not affect social interaction in wild-type mice. As the level of social interaction in 2-year-old mGluR8−/− mice was similar to that seen in adult wild-type mice but much higher than in 2-year-old wild-type mice, we cannot exclude that AZ12216052 might enhance social interaction under conditions of reduced levels of social interaction. This suggests that mGluR8 modulation might only be effective in reducing social anxiety levels when the levels are elevated. Alternatively, it is possible that AZ12216052 does not affect social interaction in wild-type mice because the receptor(s) it acts upon in addition to mGluR8 compensate for mGluR8-mediated signaling. Finally, it is possible that in contrast to measures of anxiety involving non-social challenges, effects of mGluR8 deficiency in utero, during postnatal development, or during adulthood are required for modulating social interaction.
In summary, AZ12216052 reduces measures of anxiety in the elevated zero maze and the acoustic startle response in mGluR8−/− and wild-type mice but does not affect social interaction in wild-type mice. mGluR8−/− mice show enhanced social interaction but increased measures of anxiety in the elevated zero maze and the acoustic startle response. While the PAM AZ12216052 shows great promise as pharmacotherapy for anxiety disorders, especially for the significant portion of patients with anxiety disorders showing benzodiazepine insensitivity [27–30], increased efforts are warranted to determine the mechanisms underlying the behavioral effects of mGluR8 and AZ12216052.
Acknowledgments
We thank Vijjay Chhahlani and Edwin Johnson at AstraZeneca (Wilmington, DE) for generously supplying the AZ12216052 compound. We thank Timothy Pfankuch and Ted Benice for assistance with the behavioral analysis. This work was supported by NIMH R01 MH77647.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pin JP, Duvoisin R. The metabotropic glutamate receptors: structure and functions. Neuropharmacology. 1995;34:1–26. doi: 10.1016/0028-3908(94)00129-g. [DOI] [PubMed] [Google Scholar]
- 2.Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:205–37. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- 3.Duvoisin RM, Zhang C, Ramonell K. A novel metabotropic glutamate receptor expressed in the retina and olfactory bulb. J Neurosci. 1995;15:3075–83. doi: 10.1523/JNEUROSCI.15-04-03075.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saugstad JA, Kinzie JM, Shinohara MM, Segerson TP, Westbrook GL. Cloning and expression of rat metabotropic glutamate receptor 8 reveals a distinct pharmacological profile. Mol Pharmacol. 1997;51:119–25. doi: 10.1124/mol.51.1.119. [DOI] [PubMed] [Google Scholar]
- 5.Cartmell J, Schoepp DD. Regulation of neurotransmitter release by metabotropic glutamate receptors. J Neurochem. 2000;75:889–907. doi: 10.1046/j.1471-4159.2000.0750889.x. [DOI] [PubMed] [Google Scholar]
- 6.Niswender C, Conn PJ. Metbotropic glutamate receptors: physiology, pharmacology, and disease. Pharmacol Toxicol. 2010;50:295–322. doi: 10.1146/annurev.pharmtox.011008.145533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Linden AM, Johnson BG, Peters SC, Shannon HE, Tian M, Wang Y, et al. Increased anxiety-related behavior in mice deficient for metabotropic glutamate 8 (mGlu8) receptor. Neuropharmacology. 2002;43:251–9. doi: 10.1016/s0028-3908(02)00079-5. [DOI] [PubMed] [Google Scholar]
- 8.Duvoisin R, Zhang C, Pfankuch T, O’Connor H, Gayet-Primo J, Quraishi S, et al. Increased measures of anxiety and weight gain in mice lacking the group III metabotropic glutamate receptor mGluR8. Eur J Neurosci. 2005:1–13. doi: 10.1111/j.1460-9568.2005.04210.x. [DOI] [PubMed] [Google Scholar]
- 9.Duvoisin R, Villasana L, Pfankuch T, Raber J. Sex-dependent cognitive phenotype of mice lacking mGluR8. Beh Brain Res. 2010;209:21–6. doi: 10.1016/j.bbr.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fendt M, Burki H, Imobersteg S, van der Putten H, McAllister K, Leslie J, et al. The effect of mGlu8 deficiency in animal models of psychiatric disease. Genes, Brain, Behav. 2010;9:33–44. doi: 10.1111/j.1601-183X.2009.00532.x. [DOI] [PubMed] [Google Scholar]
- 11.Duvoisin R, Pfankuch T, Wilson J, Grabell J, Chhajlani V, Brown D, et al. Acute pharmacological modulation of mGluR8 reduces measures of anxiety. Beh Brain Res. 2010;212:168–73. doi: 10.1016/j.bbr.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramanathan M, Jaiswal A, Bhattacharya S. Differential effects of diazepam on anxiety in streptozotocin induced diabetic and non-diabetic rats. Psychopharmacology. 1998;135:361–7. doi: 10.1007/s002130050523. [DOI] [PubMed] [Google Scholar]
- 13.Bilkei-Gorzo A, Racz I, Michel K, Zimmer A. Diminished anxiety- and depression-related behaviors in mice with selective deletion of the Tac1 gene. J Neurosci. 2002;22:10046–52. doi: 10.1523/JNEUROSCI.22-22-10046.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kwon C-H, Luikart B, Powell C, Zhou J, Matheny S, Zhang W, et al. Pten regulates neuronal arborization and social interaction in mice. Neuron. 2006;50:377–88. doi: 10.1016/j.neuron.2006.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Z, Smitch C. Dissociation of social and nonsocial anxiety in a mouse model of fragile X syndrome. Neurosci Lett. 2009;17:62–6. doi: 10.1016/j.neulet.2009.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Egashira N, Tanoue A, Matsuda T, Koushi E, Harada S, Takano Y, et al. Impaired social interaction and reduced anxiety-related behavior in vasopressin V1a receptor knockout mice. Beh Brain Res. 2007;178:123–7. doi: 10.1016/j.bbr.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 17.Deacon R, Bannerman D, Rawlins J. Anxiolytic effects of cytotoxic hippoampla lesions in rats. Behav Neurosci. 2002;116:494–7. doi: 10.1037//0735-7044.116.3.494. [DOI] [PubMed] [Google Scholar]
- 18.Salchner P, Luec G, Singewald N. Decreased social interaction in aged rats may not reflect changes in anxiety-related behaviour. Beh Brain Res. 2004;151:108. doi: 10.1016/j.bbr.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 19.Kathuria S, Gaetani S, Fegley D, Valino F, Duranti A, Tontine A, et al. Modulation of anxiety trough blockade of anandamide hydrolysis. Nat Med. 2002;9:76–81. doi: 10.1038/nm803. [DOI] [PubMed] [Google Scholar]
- 20.Rahn E, Zvonok A, Thakur G, Khalnolkar A, Makriyanic A, Hohman A. Selective activation of cannabinoid CB2 receptors suppresses neuropathic nociception induced by treatment with the chemotherapeutic agen paclitaxel in rats. J Pharmaco Exp Ther. 2008;327:584–91. doi: 10.1124/jpet.108.141994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malan T, Ibrahim M, Deng H, Lin Q, Mata H, Vanderah T, et al. CB2 cannabinoid receptor-mediated peripheral antinociception. Pain. 2001;93:239–45. doi: 10.1016/S0304-3959(01)00321-9. [DOI] [PubMed] [Google Scholar]
- 22.Ibrahim M, Porreca F, Lai J, Albrecht P, Rice F, Khodorova A, et al. CB2 cannboid receptor activation produces antinociception by stimulating peripheral release of endogenous opioids. Proc Nath Acad Sci USA. 2005;102:3093–8. doi: 10.1073/pnas.0409888102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fossum E, Lisowski M, Macey T, Ingram S, Morgan S. Microinjection of the vehicle dimethyl sulfoxide (DMSO) into the periaquaductal gray modulates morphine nociception. Brain Res. 2008;1204:53–8. doi: 10.1016/j.brainres.2008.02.022. [DOI] [PubMed] [Google Scholar]
- 24.Benice T, Rizk A, Pfankuch T, Kohama S, Raber J. Sex-differences in age-related cognitive decline in C57BL/6J mice associated with increased brain microtubule-associated protein 2 and synaptophysin immunoreactivity. Neuroscience. 2006;137:413–23. doi: 10.1016/j.neuroscience.2005.08.029. [DOI] [PubMed] [Google Scholar]
- 25.Dorsa DM, Petracca FM, Baskin DG, Cornett LE. Localization and characterization of vasopressin binding sites in the amygdala of the rat brain. J Neurosci. 1984;4:1764–70. doi: 10.1523/JNEUROSCI.04-07-01764.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rizk A, Curley J, Robertson J, Raber J. Anxiety and cognition in histamine H3 receptor −/− mice. Eur J Neurosci. 2004;19:1992–6. doi: 10.1111/j.1460-9568.2004.03251.x. [DOI] [PubMed] [Google Scholar]
- 27.Kaschka W, Feistel H, Ebert D. Reduced benzodiazepine receptor binding in panic disorder measured by iomazenil SPECT. J Psychiatr Res. 1995;29:427–34. doi: 10.1016/0022-3956(95)00019-2. [DOI] [PubMed] [Google Scholar]
- 28.Roy-Byrne P, Wingerson D, Radant A, Greenblatt D, Cowley D. Reduced benzodiazepine sensitivity in patients with panic disorder: comparison with patients with obsessive-compulsive disorder and normal subjects. Am J Psychiatr. 1996;153:1444–9. doi: 10.1176/ajp.153.11.1444. [DOI] [PubMed] [Google Scholar]
- 29.Schlegel S, Steinert H, Bockisch A, Hahn K, Schloesser R, Benkert O. Decreased benzodiazepine receptor binding in panic disorder measured by IOMAZENIL-SPECT. A preliminary report. Eur Arch Psychiatry Clin Neurosci. 1994;244:49–51. doi: 10.1007/BF02279812. [DOI] [PubMed] [Google Scholar]
- 30.Sundstrom I, Ashbrook D, Backstrom T. Reduced benzodiazepine sensitivity in patients with premenstrual syndrome: a pilot study. Psychoneuroendocrinology. 1997;22:25–38. doi: 10.1016/s0306-4530(96)00035-2. [DOI] [PubMed] [Google Scholar]


