Abstract
Testis xenografting is both a promising tool to study spermatogenesis and a means to preserve the genetic information and reproductive potential of prepubertal male animals. The present study was conducted to evaluate this technique using testis tissue from domestic ferrets, an important biomedical model and a model for the conservation of small carnivore species. Fresh testis tissue from 8-wk-old ferrets was implanted ectopically under the skin on the backs of castrated nude mice and subsequently evaluated for testosterone production and establishment of spermatogenesis at 10, 20, 25, and 30 wk after xenografting. A total of 40% of fresh ferret xenografts were harvested. Seminal vesicles were collected from the recipient mice and weighed as an assay for bioactive testosterone. The weights of seminal vesicles from the mice showed no significant difference from those of uncastrated, control nude mice, indicating that the xenografts were producing physiologically relevant amounts of testosterone. The ferret testis xenografts produced differentiating germ cells and sperm at the same time as did testis from age-matched control ferrets. These data demonstrate the ability of Mustelidae testicular tissue to establish spermatogenesis in nude mice after testis xenografting.
Abbreviations: SSC, spermatogonial stem cells
Spermatogenesis is a highly complex process that generates male gametes continuously once puberty is reached. This process begins with spermatogonial stem cells (SSC), which undergo a dynamic process of proliferation and differentiation with extensive support from the somatic Leydig and Sertoli cells.23 The complexity of the system has been an obstacle to recapitulating and studying spermatogenesis in vitro. In 1994, spermatogonial stem cell transplantation was introduced as the first functional assay of SSC, thereby greatly accelerating SSC research.4 Several years later, the technique of testis xenografting was reported as another promising tool which offered several complementary attributes to the study of spermatogenesis.9 Specifically, xenografting 1) retains the 3D architecture of the seminiferous tubules and interstitial cells, allowing cross-talk between these compartments; 2) allows testis tissue from a single donor to be engrafted into multiple recipient mice, which can then be treated differently, thereby removing variation between donors (the ‘donor effect’) as an experimental variable; and 3) removes the potential for Sertoli cell–germ cell incompatibilities that can limit the success of spermatogonial stem cell transplantation.5
In addition to facilitating research on spermatogenesis, xenografting technologies can be used practically as tools to preserve male genetic information, not only for wildlife conservation but also for human cancer patients.2,7,15,18 When a genetically valuable animal dies or a cancer patient needs chemotherapy that can destroy his SSC and therefore his future fertility, cryopreservation of sperm is the most common method used to conserve the donor's genetic information for future use. Because sperm are terminally differentiated cells incapable of self-replication and are only produced from sexually mature hosts, cryopreservation of sperm has some limitations. However, techniques using SSC can overcome these limitations: SSC can be harvested throughout an animal's lifetime after birth, and they can produce sperm indefinitely. Therefore, testis xenografting, which preserves SSC, could provide an important approach to preserve male genetic information.
Testis xenografting is performed by implanting 1- to 2-mm3 pieces of testis tissue in immunodeficient mice. Over time, the xenografts grow and produce sperm in the recipient. It has been shown that sperm produced from xenografts can produce offspring when used for intracytoplasmic sperm injection.11,19,24 Since the first testis xenografting was performed in the mouse, various donor species have been used, including pigs, goats, hamsters, monkeys, cattle, rabbits, cats, horses, and humans.9,14,16,17,20,21,24,25 In all cases thus far, a dramatic decline in success of xenografts has been noted when adult compared with prepubertal tissue has been used.1,3,20,22 Therefore, most studies have used neonatal or prepubertal donor tissue. We demonstrated that in cats, the precise age at which success drops is the onset of puberty, when meiotic cells first appear in the seminiferous tubules.10
Various donor species have shown production of sperm in xenografts, but the rate of progression of spermatogenesis and success of spermatogenesis varied tremendously among species. In particular, cats (Felis catus) showed a dramatic delay in producing sperm, regardless of the age of donor tissue.10,25 The progression of meiosis in the xenograft tubules was also abnormal.10 Testis xenografting in the dog has recently been reported, but success was somewhat lower than that reported for most agricultural species.1 Approximately 30% of carnivores are listed as endangered (http://www.iucnredlist.org/), and the survival of many species will depend on the success of captive management. Success is hampered by the high incidence of neonatal and juvenile mortality in many species, necessitating new approaches, such as those based on stem cells, to conserve the genetic information in those animals. Accordingly, we sought to investigate the efficacy of testis xenografting using donor tissue from a model for small carnivore species, namely domestic ferrets (Mustela putorius furo).
Materials and Methods
Animals.
Testes were obtained from domestic ferrets (Marshall BioResouces, North Rose, NY) undergoing routine surgical castration under general anesthesia; we used 8-wk-old ferrets (n = 4) as donors. Testes from 18-, 28-, and 31-wk-old ferrets were used as controls to match approximately the age of donor tissue when the xenografts were collected. All samples were stored and transported in sterile saline at 4 °C and used within 24 h after collection. The testes were washed in cold PBS, and visible blood was removed by blotting. One third of each testis was fixed overnight in Bouin solution for histologic assessment of the donor tissue at the time of xenografting. The remaining portion of each testis was processed for xenografting. The animals were housed inside a class 100 cabinet in sterilized caging on aspen chip bedding (changed weekly) at a temperature of 22 to 24 °C, humidity of 40% to 70%, 10 to 12 air exchanges hourly, and a 12:12-h light:dark cycle. Irradiated diet (ProLab IsoPro 3000, LabDiet, St Louis, MO) and autoclaved, acidified water were provided ad libitum. All experimental protocols were reviewed and approved by the Institutional Animal Care and Use Committee of Cornell University.
Xenografting procedure.
The tunica albuginea and rete testis were removed, and the testis parenchyma was cut into specimens measuring 1.5 to 2 mm3. These were kept on ice in DMEM containing 100 μg/mL streptomycin sulfate and 100 IU/mL penicillin until grafting. Male nude mice (age, 4 to 8 wk; Tac:Cr:(NCr)-Foxn1[nu], Taconic, Germantown, NY) were used as recipients (1 to 3 mice per donor). Anesthesia was induced and maintained with 1.5% to 3.5% isoflurane. Castration was performed by a midline abdominal approach, after which an incision was made on the dorsal midline, and xenografts were placed under the skin (3 to 4 grafts on each side, approximately 1 cm lateral of midline, and evenly spaced between the shoulders and the flanks). A 5-mm length of 6-0 silk (Ethicon, Somerville, NY) was used to mark the site of xenograft placement to facilitate retrieval, and to loosely tether the tissue to prevent movement. The dorsal incision was closed with skin staples (Braintree Scientific, Braintree, MA). At the end of surgery, carprofen (5 mg/kg) was used for analgesia.
Analysis of xenografts.
One or more larger fresh grafts were excised from multiple recipients at 10, 20, 25, and 30 wk after xenografting. The time points were selected based on extrapolation from similar studies in other carnivore species and on data regarding spermatogenesis in ferrets, showing that the stages and duration of ferret spermatogenesis are similar to those reported in other carnivores.6,12 The collected xenografts were fixed in Bouin solution overnight. The fixed xenografts were washed free of Bouin solution by using 70% ethanol and then dehydrated in ethanol prior to embedding in paraffin and sectioning at 4 μm. After mounting on slides, each section was deparaffinized with xylene; hydrated with 100% ethanol, 70% ethanol, and water; and stained with hematoxylin and eosin. The sections were examined by microscopy (model BX51, Olympus Canada, Ontario, Canada) and images were captured by using a digital camera system (model DP 70, Olympus Canada).
Testosterone bioassay
Seminal vesicles were removed by sharp dissection and weighed at the end of the study, at the time of removal of all remaining xenografts. A Student t test was performed to compare the weights of the seminal vesicles. All data were analyzed by using Origin 7.0 Software (OriginLab, Northampton, MA). Statistical significance was defined as a P value of less than 0.05.
Results
Histologic analysis of ferret xenografts.
A total of 37 of 86 (43.0%) ferret testis xenografts were recovered at 10, 20, 25, or 30 wk after placement. All xenografts collected at the 10 wk point showed primary spermatocytes as the most advanced stage of male germ cell development. Sperm were found in 14.3% of the grafts collected at 20 wk after xenografting (Table 1). These stages of germ cell development corresponded well with those in age-matched control testes (Figure 1). At the 10-wk time point (18 wk of age), primary spermatocytes similarly populated both control testis tissue and the most advanced grafts. At week 20 (28 wk of age), spermatids and a few spermatozoa were present in grafts and in their aged-matched control testis sections. At 25 wk after the procedure, the percentage of xenografts found to contain sperm was increased (30%, Table 1), but some grafts showed fluid-distended tubules (Figure 2). Although 7.7% of grafts still contained spermatozoa at 30 wk, more tubules were distended, and some were degenerating. Small tubular lumens with full spermatogenesis and no degeneration were observed in the corresponding control testes.
Table 1.
Xenograft recovery and parameters of spermatogenesis in 8-wk-old ferret testis xenografts
| Most advanced germ cell stages in xenografts (%) |
||||||
| Time after grafting (wk) | No. of grafts retrieved at time point | Spermatogonia | Spermatocyte | Round spermatid | Elongated spermatid | Sperm |
| 10 | 7 | 0 | 100 | 0 | 0 | 0 |
| 20 | 7 | 0 | 14.3 | 0 | 57.1 | 14.3 |
| 25 | 10 | 0 | 30.0 | 20.0 | 10.0 | 30.0 |
| 30 | 13 | 0 | 38.5 | 0 | 23.1 | 7.7 |
43% of all xenografts were recovered
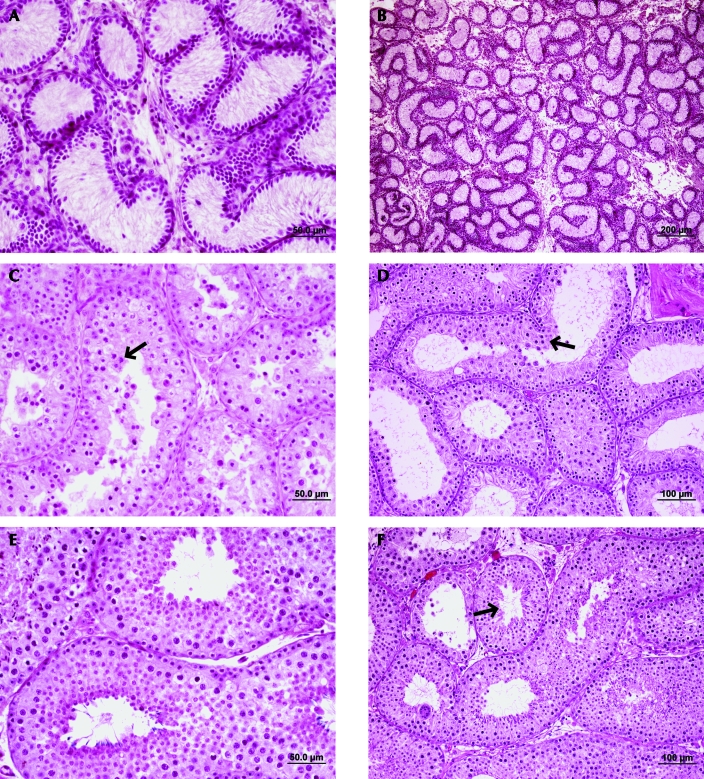
Figure 1.
Histology of ferret seminiferous tubules in age-matched control and testis xenograft sections. (A, B) No differentiating germ cells were observed in the testes collected from an 8-wk-old ferret. (C) In 18-wk-old mice, spermatocytes (arrowhead) were the most advanced germ cells in control testes. (D) In addition, this stage was the most advanced cell type observed in xenografts removed 10 wk after placement (arrowhead). (E) Full spermatogenesis was observed in the testis of a 28-wk-old ferret, as well as in (F) a xenograft collected 20 wk after placement. Hematoxylin and eosin stain.
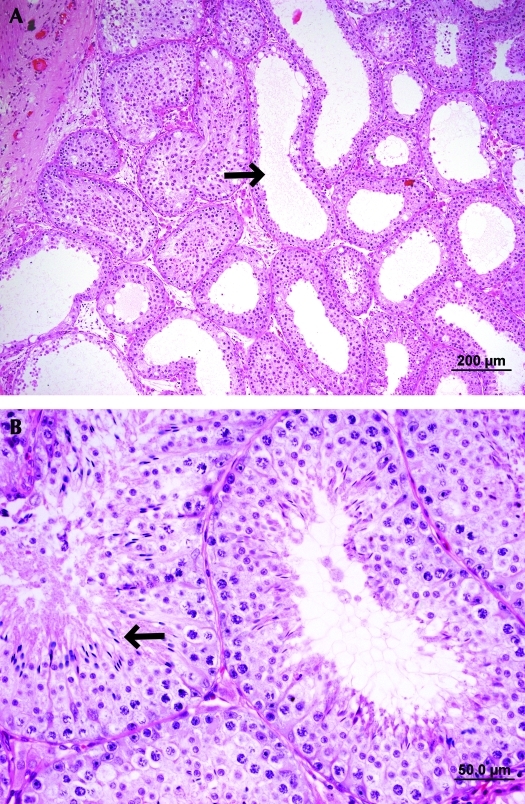
Figure 2.
Histologic sections of a ferret xenograft collected at 25 wk after grafting. (A) Some seminiferous tubules were fluid distended and showed empty lumens (arrowheads), whereas (B) other tubules supported full spermatogenesis (arrowhead). Hematoxylin and eosin stain.
Evaluation of xenograft testosterone production.
The seminal vesicle weight (mean ± 1 SD) of mice receiving xenografts was 237.7 ± 130.6 mg (n = 10), compared with 294.9 ± 16.3 mg in control mice; no significant differences were found. These data suggest that the ferret testis xenografts produced bioactive testosterone.
Discussion
In this study, we investigated the success and progression of spermatogenesis in ferret testis xenografts. Our data provide the first report of spermatogenesis in testis xenografts from domestic ferrets, which is an important model for biomedical studies as well as for wildlife conservation of other mustelids.
Ectopic testis xenografting has been performed by using numerous species as donors.1,3,9,14,17,18,21,24,25 However, there have been great discrepancies between species in terms of the timing of xenograft sperm production compared with that seen under normal physiologic conditions. Xenograft spermatogenesis in pigs and nonhuman primates showed a decreased time to sperm production compared with that when testis tissue was left in the donor.8,9 No difference was observed in the timing of sperm production of bovine xenografts, and sperm production was remarkably delayed in feline xenografts.13,16,25 In addition, we have noted delayed germ cell development in cat testis xenografts and documented that this delay remained regardless of donor age.10 In dogs, spermatogenesis in testis xenografts from immature and young donors was less efficient than that of intact testes of dogs of a similar age.1 In ferrets, an age of 8 wk corresponds to a time when most seminiferous tubules have spermatogonia as the most mature male germ cells and when spermatogenesis is just about to begin.6
In the present study, ferret xenografts showed lower total recovery rates (40%) compared with those we obtained for cats (64% to 82%). Although the recovery rate of ferret grafts was lower than those in other species, the progress of germ cell development in the ferret xenografts was well matched to that seen in intact control testes. Ferret xenografts demonstrated spermatocytes at 10 wk and sperm production at 20 wk after placement. The appearance of distended tubules is consistent with findings in mice, in which the tubules seem to degenerate after being productive, perhaps due to lack of an outflow tract.9 The production of high numbers of sperm, the apparently normal histologic appearance of the productive seminiferous tubules, and the apparently normal timing of sperm production in ferret testis xenografts compared with controls clearly distinguish ferret xenografts from both canine and feline testis xenografts. In this regard, our study demonstrates that the difficulties seen in feline and canine xenografts are not indicative that xenografts from all carnivores would fail to support spermatogenesis. Rather, differences in success lie at either the species, genus, or family level but are not uniform across the taxonomic order Carnivora. This finding has important practical implications for wildlife conservation and can help inform future lines of research. The factors underlying the differences in ability of testis xenografts to support spermatogenesis are not well characterized, with the exception of marmosets (Callithrix jacchus), which have an endocrinologic incompatibility with mice as recipients, in the form of a species difference in the luteinizing hormone receptor.26
Xenografts from ferrets actively produced testosterone, based on the weight of the seminal vesicles of the recipient mice. This tissue is very androgen-dependent, leading to its ability to be used as a bioassay for functional testosterone levels.17,19,22 This application proved that the interaction between the host endocrine system and Leydig cells was functionally normal.
This study is the first to report successful spermatogenesis in testicular xenografts from ferrets. Here we demonstrated that SSC development was similar to that seen in age-matched control ferret testis sections, which differs from the ability of testis xenografts to support spermatogenesis in other carnivore species. These results extend the use of ferrets for the study of spermatogenesis, not only as a biomedical model but also as a comparative model for differences among carnivore species. In addition, our results support further study of this approach for the purposes of conserving threatened or endangered Mustelid species.
Acknowledgments
We thank Marshall BioResources (North Rose, NY) for providing testis specimens from routine castrations. We also thank Dr Yeunhee Kim (Yale University, New Haven, CT) for sharing technical expertise, Mary Lou Norman for her help with histologic processing, and the Baker Institute laboratory animal care staff for their services. This work was supported in part by a grant from the Morris Animal Foundation (AJT) and the Baker Institute for Animal Health.
References
- 1.Abrishami M, Abbasi S, Honaramooz A. 2010. The effect of donor age on progression of spermatogenesis in canine testicular tissue after xenografting into immunodeficient mice. Theriogenology 73:512–522 [DOI] [PubMed] [Google Scholar]
- 2.Arregui L, Rathi R, Megee SO, Honaramooz A, Gomendio M, Roldan ER, Dobrinski I. 2008. Xenografting of sheep testis tissue and isolated cells as a model for preservation of genetic material from endangered ungulates. Reproduction 136:85–93 [DOI] [PubMed] [Google Scholar]
- 3.Arregui L, Rathi R, Zeng W, Honaramooz A, Gomendio M, Roldan ER, Dobrinski I. 2008. Xenografting of adult mammalian testis tissue. Anim Reprod Sci 106:65–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brinster RL, Zimmermann JW. 1994. Spermatogenesis following male germ-cell transplantation. Proc Natl Acad Sci USA 91:11298–11302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dobrinski I. 2005. Germ cell transplantation. Semin Reprod Med 23:257–265 [DOI] [PubMed] [Google Scholar]
- 6.Fox JG, Bell JA. 1998. Growth, reproduction, and breeding, p 526–531 Fox JG. Biology and diseases of the ferret. Baltimore (MD): Lippincott Williams and Wilkins [Google Scholar]
- 7.Goossens E, Geens M, De Block G, Tournaye H. 2008. Spermatogonial survival in long-term human prepubertal xenografts. Fertil Steril 90:2019–2022 [DOI] [PubMed] [Google Scholar]
- 8.Honaramooz A, Li MW, Penedo MCT, Meyers S, Dobrinski I. 2004. Accelerated maturation of primate testis by xenografting into mice. Biol Reprod 70:1500–1503 [DOI] [PubMed] [Google Scholar]
- 9.Honaramooz A, Snedaker A, Boiani M, Scholer H, Dobrinski I, Schlatt S. 2002. Sperm from neonatal mammalian testes grafted in mice. Nature 418:778–781 [DOI] [PubMed] [Google Scholar]
- 10.Kim Y, Selvaraj V, Pukazhenthi B, Travis AJ. 2007. Effect of donor age on success of spermatogenesis in feline testis xenografts. Reprod Fertil Dev 19:869–876 [DOI] [PubMed] [Google Scholar]
- 11.Nakai M, Kaneko H, Somfai T, Maedomari N, Ozawa M, Noguchi J, Ito J, Kashiwazaki N, Kikuchi K. 2010. Production of viable piglets for the first time using sperm derived from ectopic testicular xenografts. Reproduction 139:331–335 [DOI] [PubMed] [Google Scholar]
- 12.Nakai M, Van Cleeff JK, Bahr JM. 2004. Stages and duration of spermatogenesis in the domestic ferret (Mustela putorius furo). Tissue Cell 36:439–446 [DOI] [PubMed] [Google Scholar]
- 13.Oatley JM, de Avila DM, Reeves JJ, McLean DJ. 2004. Spermatogenesis and germ cell transgene expression in xenografted bovine testicular tissue. Biol Reprod 71:494–501 [DOI] [PubMed] [Google Scholar]
- 14.Oatley JM, Reeves JJ, McLean DJ. 2005. Establishment of spermatogenesis in neonatal bovine testicular tissue following ectopic xenografting varies with donor age. Biol Reprod 72:358–364 [DOI] [PubMed] [Google Scholar]
- 15.Pukazhenthi B, Comizzoli P, Travis AJ, Wildt DE. 2006. Applications of emerging technologies to the study and conservation of threatened and endangered species. Reprod Fertil Dev 18:77–90 [DOI] [PubMed] [Google Scholar]
- 16.Rathi R, Honaramooz A, Zeng W, Schlatt S, Dobrinski I. 2005. Germ cell fate and seminiferous tubule development in bovine testis xenografts. Reproduction 130:923–929 [DOI] [PubMed] [Google Scholar]
- 17.Rathi R, Honaramooz A, Zeng W, Turner R, Dobrinski I. 2006. Germ cell development in equine testis tissue xenografted into mice. Reproduction 131:1091–1098 [DOI] [PubMed] [Google Scholar]
- 18.Rodriguez-Sosa JR, Dobrinski I. 2009. Recent developments in testis tissue xenografting. Reproduction 138:187–194 [DOI] [PubMed] [Google Scholar]
- 19.Schlatt S, Honaramooz A, Boiani M, Scholer HR, Dobrinski I. 2003. Progeny from sperm obtained after ectopic grafting of neonatal mouse testes. Biol Reprod 68:2331–2335 [DOI] [PubMed] [Google Scholar]
- 20.Schlatt S, Honaramooz A, Ehmcke J, Goebell PJ, Rübben H, Dhir R, Dobrinski I, Patrizio P. 2006. Limited survival of adult human testicular tissue as ectopic xenograft. Hum Reprod 21:384–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schlatt S, Kim SS, Gosden R. 2002. Spermatogenesis and steroidogenesis in mouse, hamster, and monkey testicular tissue after cryopreservation and heterotopic grafting to castrated hosts. Reproduction 124:339–346 [DOI] [PubMed] [Google Scholar]
- 22.Schmidt JA, de Avila JM, McLean DJ. 2006. Grafting period and donor age affect the potential for spermatogenesis in bovine ectopic testis xenografts. Biol Reprod 75:160–166 [DOI] [PubMed] [Google Scholar]
- 23.Setchell BP. 1978. Spermatogenesis. p 184–194 Finn CA. The mammalian testis. Ithaca (NY): Cornell University Press [Google Scholar]
- 24.Shinohara T, Inoue K, Ogonuki N, Kanatsu-Shinohara M, Miki H, Nakata K, Kurome M, Nagashima H, Toyokuni S, Kogishi K, Honjo T, Ogura A. 2002. Birth of offspring following transplantation of cryopreserved immature testicular pieces and in-vitro microinsemination. Hum Reprod 17:3039–3045 [DOI] [PubMed] [Google Scholar]
- 25.Snedaker AK, Honaramooz A, Dobrinski I. 2004. A game of cat and mouse: xenografting of testis tissue from domestic kittens results in complete cat spermatogenesis in a mouse host. J Androl 25:926–930 [DOI] [PubMed] [Google Scholar]
- 26.Wistuba J, Mundry M, Luetjens CM, Schlatt S. 2004. Cografting of hamster (Phodopus sungorus) and marmoset (Callithrix jacchus) testicular tissues into nude mice does not overcome blockade of early spermatogenic differentiation in primate grafts. Biol Reprod 71:2087–2091 [DOI] [PubMed] [Google Scholar]