Abstract
Dystocia (difficult labor) is an important component of the management of nonhuman primates and results in significant fetal and maternal morbidity and increased use of veterinary resources. Dystocias can arise from abnormalities of the maternal pelvis or fetus or uncoordinated uterine activity. Although risk factors for stillbirths have been established in nonhuman primates, risk factors for dystocias have not. The objective of this study was to determine maternal and fetal risk factors for dystocia in macaques. Retrospective data were collected from 83 pigtailed macaques (Macaca nemestrina) diagnosed with dystocia. The diagnosis of dystocia was made based on clinical or pathologic evidence. Maternal records of age, reproductive history, experimental history, clinical records, and fetal birth weight and any applicable fetal necropsy reports were reviewed. The gestational age of the fetus, the infant's birth weight, total previous births by the dam, and the proportions of both viable delivery (inverse effect) and surgical pregnancy interventions (direct effect) in the dam's history generated a model that maximized the experimental variance for predicting dystocia in the current pregnancy and explained 24% of the dystocia deliveries. The number of total previous births and proportion of previous cesarean sections accounted for the greatest effect. This model can identify individual dams within a colony that are at risk for dystocias and allow for changes in breeding colony management, more intense monitoring of dams at risk, or allocation of additional resources.
Abbreviations: CN, clinically assisted nonviable birth; CV, clinically assisted viable birth; CSN, clinically necessary surgical nonviable birth; CSV, clinically necessary surgical viable birth; EV, experiment-related viable birth; EN, experiment-related nonviable birth; ESN, experiment-related surgical nonviable birth; ESV, experiment-related surgical viable birth; NN, natural or vaginal nonviable birth; NV, natural or vaginal viable birth
Dystocia (difficult labor) is an important consideration in the management of nonhuman primate breeding colonies.9 In addition to decreased production, these complications can result in significant fetal and maternal morbidity and mortality (that is, stillbirths). Dystocias increase the need and cost of veterinary care, increase the likelihood that females will be removed from the breeding group, and result in prolonged recovery time and longer postpartum intervals. Causes of dystocia are not well understood. In mammals, environmental (both physical and social), management/medical, and genetic factors have all been associated with these complications.23
Multiple mechanisms alone or in combination can result in a dystocia. These mechanisms can be generally classified into the ‘3 Ps’: power, passenger, and passage.9,30 Power abnormalities arise from uterine dysfunction either from insufficient or uncoordinated contractions or from inadequate maternal expulsive effort.9,10,30 The passenger (fetus) can cause a dystocia by inappropriate presentation, position, or development.9,10,30 The third mechanism—passage—involves an abnormality in the pelvis and can result from either bony or soft tissue abnormalities acting as an obstacle for passage.9,10,30
Dystocia may not be recognized in macaques for multiple reasons. Most nonhuman primates deliver during the night hours,18 when fewer colony personnel are available to observe the animal. Normal labor in nonhuman primates occurs over 5 to 7 h, and the placenta is usually delivered within 15 min after the infant.18 Subtle behavioral changes like restlessness, altered eating, frequent urination, and digital manipulation of the genitalia usually are the only signs of impending labor in macaques.18 Signs of dystocia in nonhuman primates can be equally subtle. In some circumstances, evidence such as an infant facial edema and bruising are the only signs. Other subtle signs include prolonged labor, maternal weakness, and overdue parturition.10,24,26 In other cases, such as breech presentation, dystocias may be obvious and can be seen by protruding fetal extremities.10,26 In addition to malpresentation, other causes of dystocia that have been documented in nonhuman primates include macrosomic fetuses, births of multiple fetuses, uterine masses, nutritional deficiencies, and traumatic fractures of bones.5,10 Squirrel monkeys, for instance, are a model for dystocia due to macrosomic fetuses, in that fetal body weight at the time of delivery can be as much as 17% of the dam's body weight.5,10 In humans, dystocias have been associated with a genetic predisposition, maternal height, maternal weight and weight gain during pregnancy, maternal age, and stress.21
Risk factors for stillbirths, which can be an outcome of dystocia, have been established for many species of nonhuman primates. In chimpanzees, increased parity and age have been associated with increased stillbirths.25 In baboons, maternal age, weight, and parity have all been associated with fetal loss.27 In rhesus macaques, maternal age has been associated with increased fetal loss.11 Interestingly, one study reported no correlation between maternal age and parity and stillbirths in Macaca fascicularis.13
Previous work on predicting abnormal births has focused mainly on determining when a monkey is anticipated to deliver and the use of pelviometry to establish physical risks. A Bishop score, similar to that used in humans, was developed for rhesus macaques in 1988.14 This system uses cervical position, cervical length, softness, dilation, and fetal head position to quantitatively determine readiness to give birth.14 Pelviometry in both squirrel monkeys and cynomologus macaques has been described.2,10 Pelviometry involves obtaining radiographic views of the pelvis and measurements of the pelvic inlet, midpelvis, and the pelvic outlet.2 From these measurements, risks of stillbirth can be predicted.2,10 Only a small difference, a 10% reduction in pelvic outlet diameter, results in a 20% area reduction in the pelvic canal, which can lead to a negative outcome.2 Identifying risk factors associated with dystocias may be an accurate way of predicting dystocias, but these factors have not yet been established for macaques.
Our hypothesis was that factors of maternal age, parity, gestational days, the dam's reproductive history (including stillbirths and experimental manipulations), and the infant's birth weight are risk factors for dystocia in pigtailed macaques (Macaca nemestrina). We speculated that these factors also would be risk factors for retained placentas and stillbirths. In addition, we explored the relationship of retained placentas and dystocia based on the hypothesis that uterine atony can result from dystocia and in turn lead to retained placenta. We explored the relationship of retained placenta on fetal death, a consequence of dystocia. Finally, we explored the direct relationship between dystocia and stillbirths.
Materials and Methods
Data collection and management.
Retrospective birth data were obtained from the Washington National Primate Research Center's Animal Records System. All animals were assigned to the breeding colony and any procedures performed on the animals were approved by the University of Washington's Institutional Animal Care and Use Committee. Data were collected from all births that occurred in the breeding colony of pigtailed macaques from October 1989 through December 2009. This colony is a closed colony of Indonesia-origin Macaca nemestrina. During this time frame, the colony progressed to become SPF for tuberculosis, SRV2, and STLV. Semiannual evaluations of the colony included physical exams, CBCs, blood chemistry screening, and serology testing for SRV2, STLV, and Macacine herpesvirus 1 (when available). Tuberculosis screening included intradermal tuberculin skin testing with mammalian old tuberculin. Only macaques that were negative for tuberculosis were allowed to remain in the colony, and no animals in this study were positive for tuberculosis. Screening for SRV2 and STLV started in the late 1980s. Of the study population, 43 were SRV2 antibody-positive, and 2 were virus-positive. All animals within the colony are STLV negative. Macacine herpesvirus (formerly Cercopithecine herpesvirus 1) is endemic within this colony, and all dams on this study that were screened were positive. No animals that were SIV- or SHIV-infected were included in this study. Dams within the colony usually start breeding at 3 y of age and are allowed to breed until they are no longer productive. In this study, age at first delivery ranged from 3 to 15 y old.
Data for analysis were restricted to outcome variables of naturally or vaginal nonviable deliveries (NN), clinically assisted vaginal nonviable deliveries (CN), clinically necessary cesarean delivery of a nonviable infant (CSN), and clinically necessary cesarean delivery of a viable infant (CSV). All births that resulted while a dam was involved in experimental protocols (coded in the records system as either experiment–viable [EV], experiment–nonviable [EN], experiment–cesarean section–viable [ESV], and experiment–cesarean–nonviable [ESN]) were not included in the dystocia and stillbirth data set from which retrospective data analysis was performed.
In addition, maternal clinical records, infant clinical records, and results of maternal and infant necropsies (when applicable) were reviewed for each delivery. These records included predictor variables of maternal origin, previous reproductive history including experimental reproductive history, maternal age at delivery, previous clinical illnesses, preventative care, and any complications observed with pregnancy, delivery, or after delivery. Previous reproductive history was recorded in 2 ways. First, the total number of previous deliveries was recorded. Each type of delivery (NV, NN, CV, CN, CSN, CSV, EV, EN, ESV, and ESN) was totaled and evaluated as both a whole number and the proportion of births. Next, the different types of births were placed into groups: previous viable births (NV, CV, CSV, ESV, and EV); nonviable births (NN, EN, CN, CSN, and ESN); previous births during experimental protocols (EV, EN, ESN, and ESV); and previous births that resulted in surgical intervention (CSV, CSN, ESV, and ESN). Infant records included sex, gestational age, weight, and sire.
Maternal clinical records and infant necropsy reports were reviewed for the diagnosis and cause of dystocia. Only cases where the diagnosis of dystocia was made by the clinical staff or pathologist and coded into the animal's record were included in our data set. Causes of clinical diagnosis of dystocia included malpresentation (that is, breech), placenta previa (placenta over the internal cervical os), uterine rupture, macrosomic fetus (excessive birth weight), placenta abruptio, and prolonged labor. Pathologic diagnosis of dystocia was most often a diagnosis of exclusion. When available, both the fetus and placenta were examined grossly; representative tissue sections were collected for histopathologic evaluation to rule out bacterial, viral, and fungal etiologies. When gross necropsy findings were inconclusive, bacterial culture was performed on fetal tissues and fluids. The lack of an infectious cause for fetal death coupled with ancillary findings such as tissue congestion and edema suggested dystocia. Macrosomia and fetal distress were considered diagnostic for dystocia in the absence of infection. Macrosomia was the most frequent gross diagnosis and often was corroborated by cerebral congestion and hemorrhage. Fetal distress, evidenced by deep aspiration of amniotic fluid, was diagnosed histologically through the presence of large numbers of keratinocytes within peripheral alveoli. In addition, all nonviable births were verified to be stillbirth by the pathologist at necropsy by confirming that the animal did not take a breath. Lack of a breath was confirmed by failure of the lungs to expand, sinking of the lungs in formalin, and histopathology.
An additional set of dams that delivered natural viable (NV) infants was included as a normal, baseline group to test for an experimental response. If any of the control dams had a previous negative outcome (CN, NN, CSN) or were diagnosed with dystocia, they were excluded from the normal control group. In addition, if a maternal record included previous negative outcomes that were produced by experimental manipulations (experiment cesarean nonviable [ESN] or experimental nonviable [EN]), the dams were excluded from the normal baseline group. Animals with previous history of viable outcomes during experimental protocols (experimental cesarean section viable [ESV] or experimental natural viable [EV]) were included.
Husbandry.
Macaques were housed in 1 of 2 conditions. The housing conditions at the Washington Regional (now National) Primate Research Center were described previously.16 Briefly, the random-mating colony was housed in indoor rooms (2.1 × 3.1 × 3 m high) in social harem groups of approximately 12 female and one male macaque. Animals housed in the indoor timed-mating colony were housed singly in cages according to guidelines in the Animal Welfare Act3 and Guide for the Care and Use of Laboratory Animals.19 All animals were housed under artificial light with a photoperiod of 14 h lights on, 10 h lights off. Every animal was fed a standard primate diet (chow) that was supplemented with fresh produce and grains.
Pregnancy diagnosis.
Each breeding female's menstrual cycle was observed on a daily basis as described.4 If a female macaque was observed to have missed a menstrual period, physical exams were performed to confirm pregnancy. Gestational day was determined by either fetal femoral length radiographically or at the time of fetal death. In 1990, ultrasonography was introduced to determine fetal gestation (fetal biparietal measurements) and was compared with colony biometric data.7,28 Ultrasonography also was used to monitor pregnancies as needed and to diagnose dystocias and their etiologies (that is, placenta previa, malpresentation, uterine rupture, and placenta abruption).
Statistics.
All statistical analyses were performed with Systat (version 7.0; Systat Software, Chicago, IL). Skewness and kurtosis were examined for normality of the data, and Fmax tests were used to test for homogeneity of variance. A Pearson correlation matrix was used to determine the correlation among continuous variables. All predictor variables were considered continuous variables, whereas pregnancy outcomes (for example, presence or absence of dystocia) were binomial; hypothesis tests of relationships among these variables were conducted by using general linear model techniques (logistic regression). Null hypothesis probabilities, proportion of variance (R2), and Cohen f effect sizes are reported for the logistic regression analyses. Relationships among outcome variables were explored by using biserial correlation coefficients. Null hypothesis P values of less than or equal to 0.05 were considered statistically significant. Effect sizes of 0.02 were considered small; effect sizes of 0.15 were considered medium; and effects of 0.35 were large effects.6
Results
The data set comprised 197 pigtailed macaques, each having between 0 to 14 previous pregnancy outcomes, for a total record of 602 previous deliveries. Of these examined pregnancies, 83 were dystocias, 11 were associated with retained placentas, and 49 were stillborn. The dam's age at which a dystotic delivery occurred averaged 10 y (range, 4 to 22 y). From the dystocias diagnosed clinically, 35 were due to malpresentation, 13 to prolonged labor, and 2 to macrosomic fetuses, with one case each of uterine rupture, placenta previa, and placental abruption.
Risk factors of dystocia.
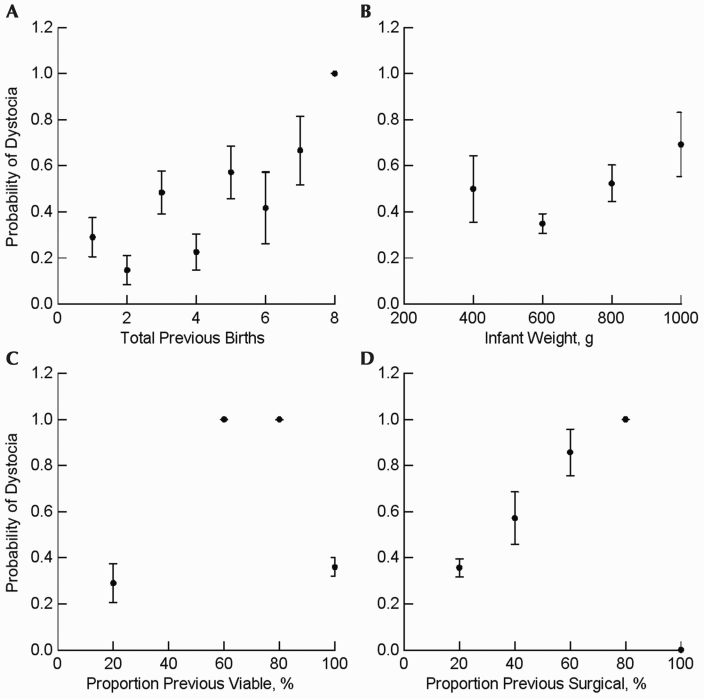
All data met parametric assumptions. The combination of gestational age, total previous births, the infant's birth weight (Figure 1 B), and the proportion of both viable deliveries (inverse effect; Figure 1 C) and surgical pregnancy interventions (direct effect) in the dam's history provided the best model for predicting the occurrence of dystocia in the current pregnancy and accounted for 24% of the variance in delivery outcomes (P < 0.000001, R2 = 0.24, f = 0.32; Figure 1). The predictor variables with the highest individual significance were total previous births (individual P = 0.000029; Figure 1 A) and proportion of surgical pregnancy interventions (individual P = 0.007; Figure 1 D). The highest probability of dystocia occurred in dams with few total births and previous surgical interventions (Figure 2). Parameters of previous nonviable births or experimental interventions were not significant risk factors of dystocia, nor was a history of retained placenta.
Figure 1.
Relationships between significant variables (mean ± 1 SD) and the probability of a dystocia outcome. (A) The probability of a dystocia outcome increases (P < 0.000001) with the amount of previous births. (B) Intermediate birth weights were least likely (P = 0.037) to be associated with dystocia. (C) Proportion of previous viable births was significantly (P = 0.016) associated with the probability of a dystocia outcome. (D) Higher percentage of previous births delivered by cesarean sections was associated (P = 0.007) with dystocia.
Figure 2.

Relationships among total previous births, proportion of previous surgical births, and the probability of resulting in a dystocia (Prob Dystocia).
No combinations of predictor variables were associated with the occurrence of retained placenta. In predicting stillbirth, only the number of nonviable outcomes in previous pregnancies was statistically significant (P = 0.024, R2 = 0.041), but the effect size (f = 0.043) was so small as to be not clinically relevant.
Other negative outcomes and dystocia.
We explored relationships among the outcome measures by using correlations: the occurrence of retained placenta and dystocia were unrelated (r = 0.058, P = 1.00); retained placenta and fetal death were unrelated (r = −0.038, P = 1.00); and dystocia and the occurrence of fetal death were unrelated (r = 0.009, P = 1.00).
Discussion
According to our model, the strongest predictors of dystocia in pigtailed macaques are the proportion of viable births, which was protective, and the proportion of previous cesarean sections, which was deleterious. In other words, a breeding female macaque with 3 previous births, all of which were delivered by cesarean section, would be at higher risk for dystocia than would be a female macaque with 6 previous viable births, 3 of which were delivered by cesarean section. Gestational age, total previous births, and the infant's birth weight also contributed to the risk of dystocia, and these 5 variables explained 24% of the variability in delivery outcomes. Each individual factor alone did not account for a significantly large amount of the variance; however, the interaction of all of the parameters tested accounted for 24% of the variance.
In our study, birth weights were significantly associated with a dystocia outcome. High birth weight has been previously described as a potential risk factor for dystocias in humans and nonhuman primates.1,10,20 Causes of increased infant birth weight and macrosomia in macaques include gestational diabetes, hydrocephalus, and fetal hydrops.10 High birth weight in human infants is associated with cephalopelvic disproportion, shoulder dystocia, gestational diabetes, higher cesarean rate, and birth injury.9 Although lower birth weights appeared to be associated with slightly higher probabilities of dystocia (Figure 1), the increase was not statistically significant. Attempts have been made in humans to determine fetal weight by ultrasonography.8,29 However, according to our present model, high fetal birth weight was only one factor associated with dystocia and, although significant, it was not responsible for the majority of the dystocias.
Previous cesarean sections are a significant determinant of dystocia. In humans, the safety of vaginal births after cesarean section is still being discussed. Prior to the 1970s, the standard of care was “once a cesarean, always a cesarean.” 9 However, trial of labor has become more acceptable.9 The primary medical concern involves the risk of uterine rupture, which can occur with either a classical uterine incision of the fundus or the low-transverse incision.9,22 Uterine rupture in macaques has been reported, and the leading causes are weakness of the uterine muscle from previous surgeries, obstructed labor, multiple births, and cephalopelvic disproportion.10 At our facility, cesarean sections have been performed by uterine incision at the fundus for the past 20 y. Interestingly, only one case of uterine rupture was the cause of dystocia in our data set (data not shown), and the dam involved had no previous cesarean sections. In addition, previous cesarean sections did not result in an increase risk of stillbirth in our study. Another interesting difference between humans and pigtailed macaques is that the proportion of previous cesarean sections to total deliveries, and not the number of cesarean sections, is significant. This situation may be related to the proportion of scar tissue present in the uterus decreasing the uterus’ ability to stretch and contract. Another theory is that tissue recovery time may be prolonged and may require a prolonged interbirth interval between surgical insults.
Dystocia in macaques can be a serious problem and lead to both infant and maternal morbidity. Anticipation of a problem may allow for better planning and more appropriate use of resources. Previous dystocia work included Bishop scores and pelviometry; however, both are problematic. Bishop scores are not performed frequently for several reasons. For example, labor in macaques can progress at different rates.4,10 Delivery progress is influenced by circadian cycles in macaques more so than in humans.17 In addition, macaque fetuses can change orientation from breech to cephalic presentation just prior to birth.17,18 Although Bishop scores can be used to determine whether induction of labor is appropriate, they do not allow for prediction of problems during delivery.14,18 Pelviometry is predictive of stillbirth,2 but the bony pelvis is only one of multiple components that determine the success of labor.
Applying our current model as a predictor of dystocia in pigtailed macaques could decrease the incidence of dystocia. For more than 20 y, the University of Washington's IACUC has limited the number of cesareans performed on a macaque to 3, including both experimentally and clinically necessary surgeries. This decision was an ethical judgment rather than a medically based decision. Although specifying the number of cesarean surgeries permitted may be necessary, risk factors for dystocias are more complex than a specific number of prior cesarean surgeries. It may be more prudent to apply the complex interaction of the proportion of live births compared with the proportion of surgical interventions to determine an individual animal's overall risk and to devote resources as necessary. If a dam is at high risk for developing dystocia, it may be necessary to remove her from the breeding colony or to place her on a study that would ensure cesarean section. Recently, our facility has implemented the use of closed-circuit videocameras to monitor high-risk obstetrical patients closely. This practice allows the care staff to observe the dam remotely and to obtain detailed descriptions of behavior, day or night, without disturbing the animal. In our experience, this system already has been instrumental in the early detection of and quick medical response to preterm labor of a macaque carrying twins.
Our model has demonstrated factors that are significant in the development of dystocia in pigtailed macaques. Potential factors that were not explored include maternal weight and stress. In humans, maternal obesity and a short stature have been shown to be significant risk factors.21 Animal heights are not recorded routinely in our records and, therefore, could not be included in the analysis. Although weights are collected routinely and entered into the maternal records, body condition scores and skin-fold measurements are better methods for determining obesity.12 Body condition scores have only been recorded for the past 5 y at our facility, so our records did not include sufficient data points for analysis. Maternal stress has been related to preterm labor, dysfunctional labor, and dystocia in humans.21 One study in macaques demonstrated that the presence of a sire and a stable social group increased the likelihood of a viable birth.15 The authors speculated that the presence of a sire decreased aggression within the group and that a stable social group decreased the level of stress in the pregnant dams.15 The role of stress resulting in dystocia in macaques has not been evaluated yet.
In summary, the current study is the first to identify risk factors for the development of dystocias in pigtailed macaques. The most significant risk factor was the proportion, not the number, of previous maternal cesarean sections. These findings may help in the management of nonhuman primate breeding colonies and may change dedication of resources. More work needs to be performed to determine why the proportion of previous cesarean sections, and not history of cesarean delivery, is significant. In addition, the role of stress and the outcome of dystocia still need to be evaluated.
Acknowledgments
We thank the clinical staff for maintaining detailed clinical records. This work was supported by grant R25 RR024512, which was funded by the National Center for Research Resources.
References
- 1.Adesina OA, Olayemi O. 2003. Fetal macrosomia at the University College Hospital, Ibadan: a 3-year review. J Obstet Gynaecol 23:30–33 [DOI] [PubMed] [Google Scholar]
- 2.Aksel S, Abee CR. 1983. A pelvimetry method for predicting perinatal mortality in pregnant squirrel monkeys (Saimiri sciuresus). Lab Anim Sci 33:165–167 [PubMed] [Google Scholar]
- 3. Animal Welfare Act as Amended. 2007. 7 USC §2131–2156.
- 4.Blakley GB, Beamer TW, Dukelow WR. 1981. Characteristics of the menstrual cycle in nonhuman primates. IV. Timed mating in Macaca nemestrina. Lab Anim 15:351–353 [DOI] [PubMed] [Google Scholar]
- 5.Brady AG. 2000. Research techniques for the squirrel monkey (Samiri sp.). ILAR J 41:10–18 [DOI] [PubMed] [Google Scholar]
- 6.Cohen J. 1988. Statistical power analysis for the behavioral sciences. Hillsdale (NJ): L Erlbaum Associates [Google Scholar]
- 7.Conrad S, Ha JC, Lohr C, Sackett G. 1995. Ultrasonic assessment of fetal growth in the pigtailed macaque (Macaca nemestrina). Am J Primatol 36:15–35 [DOI] [PubMed] [Google Scholar]
- 8.Coomarasamy A, Connock M, Thorton J, Khan KS. 2005. Accuracy of ultrasound biometry in the prediction of macrosomia: a systematic quantitative review. BJOG 112:1461–1466 [DOI] [PubMed] [Google Scholar]
- 9.Cunningham FG, Keveno KJ, Bloom SL, Hauth JC, Gilstrapp LC, 3rd, Wenstrom KD. 2005. Williams' obstetrics, 22nd ed New York (NY): McGraw-Hill [Google Scholar]
- 10.Ford EW, Roberts JA, Southers HL. 1998. Urogenital system, p 311–362 Bennett TB, Abee CR, Henrickson R. Nonhuman primates in biomedical research, diseases. San Diego (CA): Academic Press [Google Scholar]
- 11.Gagliardi C, Liukkonen JR, Phillippi-Falkenstein KM, Harrison RM, Kubisch HM. 2007. Age as a determinant of reproductive success among captive female rhesus macaques (Macaca mulatta). Reproduction 133:819–826 [DOI] [PubMed] [Google Scholar]
- 12.Garcia C, Huffman M, Shimizu K. 2010. Seasonal and reproductive variation in body condition in captive female Japanese macaques (Macaca fuscata). Am J Primatol 72:277–286 [DOI] [PubMed] [Google Scholar]
- 13.Gardin JF, Jerome CP, Jayo MJ, Weaver DS. 1989. Maternal factors affecting reproduction in a breeding colony of cynomolgus macaques (Macaca fascicularis). Lab Anim Sci 39:205–212 [PubMed] [Google Scholar]
- 14.Golub MS, Donald JM, Anderson JH, Ford EW. 1988. A labor-readiness index (Bishop score) for rhesus monkeys. Lab Anim Sci 38:435–438 [PubMed] [Google Scholar]
- 15.Ha JC, Robinette RL, Sackett GP. 1999. Social housing and pregnancy outcome in captive pigtailed macaques. Am J Primatol 47:153–163 [DOI] [PubMed] [Google Scholar]
- 16.Ha JC, Robinette RL, Sackett GP. 2000. Demographic analysis of the Washington Regional Primate Research Center pig-tailed macaque colony, 1967–1996. Am J Primatol 52:187–198 [DOI] [PubMed] [Google Scholar]
- 17.Hendrie TA, Peterson PE, Short JJ, Tarantal AF, Rothgarn E, Hendrie MI, Hendrickx AG. 1996. Frequency of prenatal loss in a macaque breeding colony. Am J Primatol 40:41–53 [DOI] [PubMed] [Google Scholar]
- 18.Hendrickx AG, Dukelow WR. 1995. Breeding, p 335–374 Bennett BT, Abee CR, Henrickson R. Nonhuman primates in biomedical research. Biology and management. San Diego (CA): Academic Press [Google Scholar]
- 19.Institute for Laboratory Animal Research 1996. Guide for the care and use of laboratory animals. Washington (DC): National Academies Press; [PubMed] [Google Scholar]
- 20.Ju H, Chadha Y, Donovan T, O'Rourke P. 2009. Fetal macrosomia and pregnancy outcomes. Aust N Z J Obstet Gynaecol 49:504–509 [DOI] [PubMed] [Google Scholar]
- 21.Lowe NK. 2007. A Review of factors associated with dystocia and cesarean section in nulliparous women. J Midwifery Womens Health 52:216–228 [DOI] [PubMed] [Google Scholar]
- 22.Lydon-Rochelle M, Holt VL, Easterling TR, Martin DP. 2001. Risk of uterine rupture during labor among women with a prior cesarean delivery. N Engl J Med 345:3–8 [DOI] [PubMed] [Google Scholar]
- 23.Olsen HG, Hayes BJ, Kent MP, Nome T, Svendsen M, Lien S. 2010. A genome wide association study for QTL affecting direct and maternal effects of stillbirth and dystocia in cattle. Anim Genet 41:273–280 [DOI] [PubMed] [Google Scholar]
- 24.Pilkington MD. 1987. Dystocia in a De Brazza's monkey. Vet Rec 120:603. [DOI] [PubMed] [Google Scholar]
- 25.Roof KA, Hopkins WD, Izard MK, Hook M, Schapiro SJ. 2005. Maternal age, parity, and reproductive outcome in captive chimpanzees (Pan troglodytes). Am J Primatol 67:199–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rushton E, McGrew WC. 1980. Breech birth of a chimpanzee (Pan troglodytes). A case report and literature review. J Med Primatol 9:189–193 [DOI] [PubMed] [Google Scholar]
- 27.Schlabritz-Loutsevitch NE, Moore CM, Lopez-Alvarenga JC, Dunn BG, Dudley D, Hubbard GB. 2008. The baboon model (Papio hamadryas) of fetal loss: maternal weight, age, reproductive history and pregnancy outcome. J Med Primatol 37:337–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shimizu K. 1988. Ultrasonic assessment of pregnancy and fetal development in 3 species of macaque monkeys. J Med Primatol 17:247–256 [PubMed] [Google Scholar]
- 29.Sood AK, Yancey M, Richards D. 1995. Prediction of fetal macrosomia using humeral soft tissue thickness. Obstet Gynecol 85:937–940 [DOI] [PubMed] [Google Scholar]
- 30.Uzelac PS, Strehlow SL. 2007. Complications in labor and delivery. DeCherney AH, Laren N, Goodwin TM, Lauger N. Current diagnosis and treatment obstetrics and gynecology. New York (NY): McGraw-Hill [Google Scholar]