Abstract
Thyroid nodules are common. Thyroid cancer is rarer. No guidelines exist for management of thyroid nodules in the Indian context and these recommendations are intended for this purpose. The consensus committee reviewed important articles, including previously published consensus statements. Management points were scored according to the level of evidence. These guidelines cover the clinical evaluation and include the interpretation of imaging and fine needle aspiration cytology of thyroid nodules. The guidelines also cover the management of special situations like thyroid incidentalomas, cystic thyroid lesion and nodules detected during pregnancy. The consensus guidelines represent a summary of current medical evidence for thyroid nodule management and the committee has attempted to optimize the guidelines for the clinical practice setting in India.
Keywords: Fine needle aspiration cytology, goiter, thyroid cancer, thyroid nodule
INTRODUCTION
The term “thyroid nodule” refers to a distinct lesion within the thyroid gland that is palpably or radiologically distinct from the surrounding thyroid parenchyma. Thyroid nodules are common, seen in about 8.5% of the population.[1] They are more common among women. In India the prevalence of a palpable thyroid nodule in the community is about 12.2%, according to a recent study.[2] However, thyroid cancer is quite rare, and the incidence is 8.7 per 100000 people per year, though this seems to be increasing over the years.[3] Hence, whenever a patient presents with a thyroid swelling, the task of the clinician is to distinguish the benign nodule from the malignant one. This is a difficult task, and no test is perfect in this regard. However, a reasonable amount of success can be achieved by a good clinical evaluation and investigations.
CONSENSUS METHODS
The consensus committee reviewed three types of manuscripts: (1) Original Articles pertaining to the management of thyroid nodules published in the last 3 years (2) Consensus Statements published by various international societies and (3) Selected reviews and commentaries to assess expert opinion. Based on these three sources, the committee identified important clinical questions that needed to be answered. The answers to these questions were scored by the authors according to the modified United States Preventive Services Task Force (see http://consensus.nih.gov) used by the American Thyroid Association (henceforth referred to as the USPSTF-ATA) for grading its panelists’ recommendations for managing thyroid nodules.[4] The grading is summarized below. It is important to note that only selected management points (and not all matter in the text) were subjected to consensus strength grading and the majority opinion was taken as the final score. The committee is aware that these guidelines will be used by thyroid specialists and non-specialists, and has tailored the recommendations to suit the management of thyroid nodules, which are very commonly encountered by doctors in the country [Table 1].
Table 1.
Rating of panelists’ evidence used for this consensus

Question 1. Which thyroid nodules need evaluation?
All thyroid nodules >1 cm need evaluation (recommendation strength: A). This includes both palpable and non-palpable nodules, the latter being radiologically distinct “incidentalomas” which are discovered by chance on imaging. Nodules that are below 1 cm need to be evaluated based on individual risk (see below).
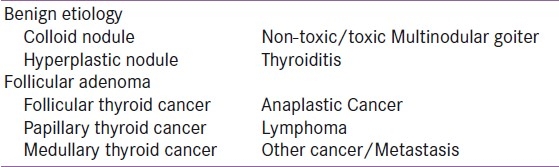
The etiologies of thyroid nodules are important as these have an important link with nodule evaluation. Local or systemic features to suggest any of the ominous ones among these etiologies [Table 2] should provoke nodule evaluation, irrespective of the size. The etiology of thyroid nodules is diverse [Table 2]. Benign causes include the colloid nodule and the classical multinodular goiter. Occasionally, Hashimoto's thyroiditis and Graves’ Disease may present with nodularity. Malignant causes include thyroid cancer, lymphoma as well as metastasis to the thyroid gland.
Table 2.
Important causes of thyroid nodules
Question 2. What “clinical” features increase the risk of a thyroid nodule harboring malignancy?
Here, the term “clinical” evaluation refers to history and physical examination, a very important factor in the Indian setting, where state-of-the-art facilities may not be uniformly available (recommendation strength: C). The focus of clinical evaluation is to differentiate thyroid cancers from benign swellings. The following features are suspicious and should suggest a malignant thyroid swelling: family history of thyroid cancer or thyroid cancer syndrome (for e.g., multiple endocrine neoplasia), rapid nodule growth, a very firm/hard nodule, clinical signs of fixity to surrounding structures, vocal cord paralysis/hoarseness of voice, regional lymph node enlargement or the presence of another lesion (for e.g., a lung mass on respiratory examination) that suggests a distant metastases. While the above-mentioned features are classical features, it must be remembered that many patients do not come with these typical features, and the presence of the following factors in addition to the thyroid nodule should evoke further investigations: a history of radiation, male gender, extremes of age (<20 or >70 years), history of neck irradiation, nodule >4 cm in size or the presence of any pressure symptoms.[5]
Question 3. What investigations are appropriate for thyroid nodule management?
A serum TSH, an ultrasound evaluation and an fine needle aspiration cytology (FNAC) is appropriate for almost all thyroid nodules (recommendation strength A). These tests and their interpretation are described below. The role of nuclear scintigraphy is also discussed.
The Serum TSH
A TSH value that is < 0.2 mU/L indicates hyperthyroidism and a TSH that is >4 mU/L indicates hypothyroidism, and both are situations where medical therapy may be considered (recommendation strength: B). If the serum TSH is low, then a nuclear scan may be performed to look for a hot nodule. If the “hot” nodule corresponds to the radiologically discrete nodule (see below) then no further evaluation may be required (recommendation strength: B). It is important to note that a high TSH (particularly a slightly high TSH) does not exclude malignancy and when associated with thyroid nodule, a full evaluation including ultrasound/FNAC still needs to be performed. (recommendation strength: B)
Ultrasonography
All cases of suspected or confirmed thyroid nodules must be evaluated by ultrasound (recommendation rating A). This includes incidentalomas discovered on CT, MRI or PET scanning. The ultrasonography (USG) is the most cost-effective imaging procedure, and is highly sensitive in assessing nodule size and number.[6] Firstly, the USG can discern if the palpable abnormality is indeed a thyroid nodule. In addition other features of the nodule can be described: size, regional lymphadenopathy, location and the percentage of nodule volume that is cystic (the last 2 nodule features can help plan an FNAB). Combining the USG with a Doppler can result in a better estimation of malignant potential: risk of malignancy is lower when a nodule has an exclusively perinodular vascular pattern and the risk of malignancy may be higher when there is a purely central vascular pattern. In short, the following USG patterns suggest malignancy: irregular shape, ill-defined borders, hypoechogenecity, solidity, heterogenous internal echoes, microcalcifications, absence of a halo, an anteroposterior to transverse diameter ratio (A/T) greater than 1, infiltration into regional structures and suspicious regional lymph nodes. Of note, while multinodularity does not exclude malignancy, the absence of a truly dominant nodule in this setting may make cancer an unlikely possibility.
Fine needle aspiration biopsy
Every patient with a palpable thyroid nodule should undergo an FNAB (recommendation strength A). Every patient with a thyroid nodule that is >1cm must undergo FNAB (recommendation strength B). Hyperthyroid patients with “cold” nodules on scintigraphy as well as hypothyroid subjects with discrete nodules on ultrasound need FNAB. The FNAB is unarguably the best single test, and uses a 23-27-G needle to aspirate samples for cytological assessment. With experience, adequate samples can be obtained in >95% cases. USG-guided FNAB can lower the occurrence of non-diagnostic smears.[7] Degenerating and cystic nodules are problematic to aspirate. In this setting, the accuracy is improved by using ultrasound-guided aspirations from the appropriate solid zones. Overall all, a USG-guided FNAB with an onsite confirmation of adequate cellularity of the smear by a trained cytopathologist is the investigation with the highest sensitivity and specificity. In suspicious nodules, taking multiple aspirates will help to improve the sensitivity.
Palpation versus ultrasonography-guided fine needle aspiration biopsy
This is important from the Indian perspective, where treatment strategies are often guided by ground realities such as the patient's economic status. Palpation-guided FNAB is less expensive, can be performed by the practitioner, and has a reasonable level of accuracy (recommendation strength B). However, USG-guided FNAB is a very superior test (recommendation strength A). Although USG-guided FNAB is always preferable, palpation-guided FNA may still be carried out when a thyroid nodule >1 cm in maximum diameter is confirmed via USG examination. The USG is important here, as physical examination is inaccurate in assessing nodule size, its origin from the thyroid gland itself (as opposed to origin from adjacent tissues) and the degree of cystic change. In certain situations, the USG-guided FNAB becomes mandatory, and palpation-guided FNAB should never be used- this includes the following scenarios (recommendation strength: F): non-palpable nodules, nodules with >25% cystic change, previously FNAB-inconclusive nodules as well as suspicious nodules. These issues regarding FNAB, in addition to issue of training, technique, interpretation and consent have been described in detail and will not be covered here.[8–10]
Choosing the nodule for fine needle aspiration biopsy in multinodular goiter
In cases of multinodular goiter, the following criteria may be used to decide on the appropriate nodule for FNAB in the order listed: (1) nodules >1 cm in largest diameter with microcalcifications, (2) nodules >1.5 cm which is predominantly solid or has coarse calcifications, (3) nodules >2 cm which are mixed solid cystic or have undergone “substantial” growth since last ultrasound, (4) the largest nodule, in which the last criteria is controversial. It is questionable whether the USG-guided FNAB should choose nodules based on the size, or whether it must be based on nodule characteristics. For the present the consensus committee does not recommend for or against the last criteria (recommendation strength: F). The consensus panel also declines to define “substantial” USG-detected growth of the nodule over time (recommendation strength: F). A recent consensus has outlined the aforementioned characteristics, while also categorically stating that FNAB is not essential in “diffusely enlarged glands with multiple nodules of similar US appearance without intervening normal parenchyma”.[11] This guideline also states that the first three aforementioned criteria be used in the decision for FNAB on solitary nodules.[11]
Radionuclide scanning
Nuclear scans use either one of the isotopes of iodine or technetium. These are handled differently by the follicular cells. Normal follicular cells take up both, but only radioiodine is organified and stored. Most benign and malignant neoplasms concentrate isotopes less avidly leading to a “cold” area on scanning. The rate of malignancy is about 10-15% in cold nodules, whereas malignancy is very unlikely in hot nodules. A hot nodule suggests hyperthyroidism-such nodules are usually not malignant. Nuclear scanning is a useful, but not an essential test in the routine evaluation of all thyroid nodules (recommendation strength I). This is in accordance with the well-accepted American Thyroid Association Guideline.[4]
Investigations to detect airway obstruction
Upper airways obstruction is rare in patients with goiter and is indicated by symptoms such as breathlessness and choking. There is a lack of correlation between the clinically assessed size of the goiter and the likelihood of upper airways obstruction. Plain radiography of the thoracic inlet and respiratory flow volume loops are specific means of identifying patients with functional tracheal compression who may need surgery (recommendation strength C). The better way to delineate deviation and compression of trachea is a spiral CT scan which could be done without contrast and is often required prior to operation.
Other tests
Anti-thyroid peroxidase antibodies are useful if Hashimoto's thyroiditis is suspected. However, thyroiditis can coexist with thyroid cancer and therefore a euthyroid patient with a nodule will still need evaluation despite antibodies being positive. The use of serum calcitonin measurements and serum thyroglobulin in the evaluation of thyroid nodules is controversial (recommendation strength F).[12]
Question 4. Is there a simple algorithm for thyroid nodule management?
It is often important to use a logical and step-wise approach in the management of thyroid nodules, and Figure 1 lists such a strategy. However, the consensus committee strongly recommends against following any simple algorithm for all subjects (recommendation strength: F) and opines that therapies be individualized.
Figure 1.
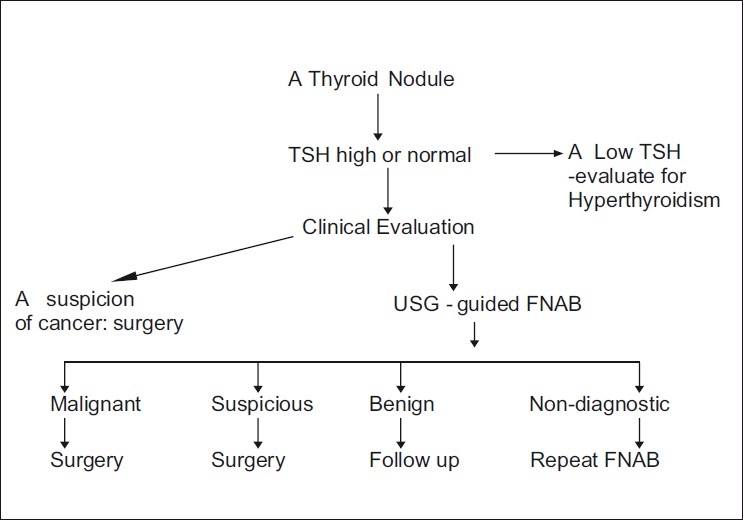
An algorithm for managing thyroid nodules. At any level, an intermediate result can justify the use of nuclear scintigraphy
Question 5. What are the indications for surgery?
Despite the many investigative options available to the clinician, the management of the nodule essentially depends on the FNAB result (recommendation strength: B). The possible reports from the cytopathologist are benign, malignant/suspicious, indeterminate and non-diagnostic.
The benign reports could either be colloid goiter, lymphocytic thyroiditis or a benign cyst. Subjects with a benign cytology are considered true negative if they are followed up for a period of at least 2 years – this will allow identification of those with changing symptoms for a repeat FNAB, and true negativity is confirmed if a diagnosis of thyroid neoplasia has not been made after a 2-year follow-up.
Surgery is required if malignant or suspicious cytology is reported. Rapid growth and increasing pressure effects (breathing difficulty) will signal the need for surgery. Some patients may undergo surgery for pressure effects like dysphagia, and other patients may opt for surgery because of cosmetic reasons due to the size of the goiter. It has been suggested that in subjects with a suspicious FNAB report, the rate of surgery may be reduced further by subjecting patients to radio nuclide scanning and performing surgery on those nodules that are cold or warm, and simply following up those with hot nodules without surgery as the risk of malignancy in these nodules is very low.
About 10-20% of cytological specimens may not have adequate material to enable an accurate interpretation and would be reported as non-diagnostic. A number of the non-diagnostic aspirates are due to cystic thyroid lesions. In cases where the report is persistently non-diagnostic, the USG results may be used to decide on the need for surgery (recommendation strength: C). In this category, if initial FNAC is non-diagnostic, it can be repeated under US guidance. If repeatedly non-diagnostic then based on clinical and US features and patient concern, a decision can be made for surgery or follow up. All the diagnostic procedures should be repeated in 6 months or earlier if the nodule enlarges.
A particular problem arises when the FNAB is indeterminate, i.e., when the FNAB is reported as follicular neoplasm and Hurthle neoplasms, as the differentiation between adenoma and carcinoma requires histological demonstration of vascular/capsular invasion. Surgery is generally advised.
In malignant/ suspicious cases, total thyroidectomy is the treatment of choice. The only exception could be a papillary microcarcinoma (<1 cm) carcinoma of the thyroid in the absence of local invasion. In this situation, the American Thyroid Association recommends hemithyroidectomy.[4] The choice of surgery (total or hemi- or subtotal thyroidectomy) in subjects with a benign cytology has often been a source of debate. In recent years, the consensus has veered toward total thyroidectomy, especially for bilateral benign multinodular goiters.[13] This is because of the chances of remnant hyperplasia or a histological surprise (a focus of malignancy in an otherwise classical multinodular goiter), may anyway necessitate completion thyroidectomy- and a second surgery.
Question 6. Is there an established role for non-surgical treatment ?
The use of levothyroxine suppression therapy to shrink non-malignant nodules is becoming less common, owing to its many disadvantages.[14] This treatment should be discouraged (recommendation strength: F) The advantages of this therapy are that it may slow nodule growth and prevent the appearance of new nodules. The disadvantages of suppressive levothyroxine therapy are modest efficacy, need for long term suppression, post-therapy regrowth, risk of atrial fibrillation/ cardiac arrhythmias and reduction in bone mineral density. I-131 therapy is an option in slightly enlarged, benign goiters, but benefits are limited by the very slow duration of action and need for contraception in female subjects. Ethanol injection into nodules (especially cystic ones) can reduce the goiter size by 45% at the end of 6 months, but has severe adverse effects: seepage of ethanol, hemorrhage into nodule, pain and even vocal cord paralysis. Laser therapy is still experimental, but it offers similar benefits as ethanol injection with lesser side effects. This is probably because of the higher degree of control and consequently lower risk of extra-nodular damage that this laser therapy entails.
Question 7. What is the optimal approach to a thyroid incidentaloma?
An impalpable thyroid nodule in a euthyroid patient detected incidentally by USG is termed a “thyroid incidentaloma”. The prevalence of non-palpable thyroid nodules discovered on sonography in Asian adults is about 13%.[15] In the same study, the malignancy rate among these incidentaolmas was 28.8%.[15] These nodules are detected when sonography is performed for non-thyroid-related neck problems. It is important to perform a TSH estimation to look for a toxic nodule. A general management approach is to observe those nodules that are less than or equal to 1 cm in size and perform an FNAB under ultrasound guidance for nodules greater than 1 cm.[4] Routine FNAB for all nodules < 1-1.4cm is less desirable.[16] However, it is important to remember that the prevalence of malignancy in subjects with a non-palpable nodule is the same as that in a palpable nodule: hence, close follow-up is quintessential (recommendation strength: B). If 6-monthly ultrasounds document an increase in size, an attempt at USG-guided FNAB may be appropriate (recommendation strength: B). If other additional risk factors for malignancy exist (like family history or past radiation or typical USG features), then further evaluation of even nodules <1 cm is justified. With the increasing use of positron emission tomography (PET) scanning, many incidentalomas are being detected.[17] In India, PET Scan is expensive and available only at selected centers, and hence the committee did not decide that this topic requires a detailed recommendation at this point of time. Further evaluation of these PET-detected lesions must be based on clinical and USG-based assessment of malignant potential.
Question 8. What is the ideal management for cystic thyroid lesions?
Most cystic lesions are degenerating benign adenomas, which may be seen on USG within the cyst wall or adjacent to the nodule. A cyst that is encountered in the course of performing FNAB should be drained completely since many such lesions are adequately treated by aspiration alone. The cyst fluid may be sent for cytological evaluation. However, this is unlikely to provide much diagnostic information as the fluid usually contains only degenerative debris. The color of the cyst fluid can offer a clue: an amber colored fluid is indicative of a benign lesion and a crystal clear fluid may be seen in a parathyroid cyst. It is important to note that both benign and malignant cysts may yield a bloody fluid. Cysts that recur after aspiration are best operated upon. Solid-cystic thyroid nodules have a higher incidence of malignancy than the purely cystic lesions. USG can differentiate between a solid and cystic lesion, but 80% of thyroid nodules are solid/solid-cystic. Hence if the ultrasound report is the sole criteria to decide about surgery then 80% of patients will proceed to surgery. A better option is to perform an FNAB from the solid portion of the solid-cystic lesion using USG guidance, and then decide on therapy in all cystic thyroid lesions.[18] In cases of persistent, uncharacterized cystic lesions, surgery is the best option (recommendation strength: C).
Question 9. How should thyroid nodules be evaluated in children?
Children with thyroid nodules may be assessed using the adult guidelines. A detailed recommendation on thyroid nodules in children is beyond the scope of these guidelines. However, a few generalizations can be made: (a) Thyroid nodules are uncommon in children (<1.5%) (b) The management must be more aggressive than in adults as the prevalence of malignancy in these nodules is higher. It has been reported that among children who undergo surgery for thyroid nodules, the incidence of thyroid cancer is about 26%.[19] (c) in addition to the classical adult causes of thyroid nodules, certain etiologies are more common in childhood thyroid nodules-ectopic thyroid, hemiagenesis, dyshormonogenesis and thyroglossal duct cyst. (d) Children with a history of dyshormonogensis are at higher risk of developing follicular thyroid cancer.[19,20] (e) Neck radiation in childhood increases the risk of future malignancies and finally (f) In general, children with thyroid nodules may be assessed using the adult guidelines (recommendation strength: C) i.e. TSH testing, USG and FNAB are the most important tests.[19]
Question 10. What is the management of thyroid nodules in pregnancy?
Pregnancy is associated with growth of pre-existing thyroid nodules as well as the growth of new nodules.[21] These are managed based on the stage of pregnancy at which it is detected and on the TSH level (recommendation strength: C). Detected in any trimester, no further evaluation is indicated for the nodule if the TSH is suppressed. Further testing, however, is necessary to rule out hyperthyroidism. Nodules detected during the third Trimester can safely be evaluated after completion of pregnancy as such delay is not expected to significantly affect the prognosis even if the nodule turns out to be malignant. When a nodule is detected in the first or second trimester and the TSH is normal or high, a FNAB is indicated. If it reports malignancy (DTC), the patient should be placed on suppressive therapy with levothyroxine (TSH to 0.1-1mU) and surgery considered in the second trimester (recommendation strength: B).[22] If the report is benign, no further evaluation is needed during pregnancy except levothyroxine to normalize the TSH. Evaluation after delivery may proceed in as indicated in the guidelines for the non-pregnant state. If FNAC is suspicious for malignancy, an US of the neck is recommended to look for suggestive sonographic features and for assessment of nodule size and for any suspicious lymph nodes. Even if the nodule appears benign by US, suppressive therapy with Thyroxine is recommended to maintain TSH in the range of 0.1 - 1.0 mU/L till completion of pregnancy.US may be repeated every Trimester for significant increase in dimensions. If there is no further enlargement, repeat FNAC need be performed only after delivery. If the nodule enlarges, a repeat FNAC/surgery may be considered. Alternatively, T4 suppression can be continued till pregnancy is completed and further evaluation planned as per guidelines. It has also been suggested that a distinction be made in suspicious lesions depending on whether they are of the “follicular neoplasm” type or the “papillary” type. It has been suggested from a small study that the former may be operated upon after delivery, while for the latter surgery may be considered in the 2nd trimester itself (recommendation strength: B).[22]
Conclusions and future perspectives
These guidelines have been made for the Indian setting, and hence the committee has not recommended on the use of immunocytological studies (particularly with galectin-3 immunostaining) and molecular cytogenetic studies (BRAF, RAS, RET/PTC and PAX8/PPARγ mutations) in predicting the risk of malignancy in thyroid nodules. However, the committee does predict their increasing use in future. Finally, a recent advancement in the management of thyroid nodules has been the use of recombinant TSH for amplifying the effect of I-131 in shrinking benign goiters. Recombinant TSH can increase the uptake of iodine by thyroid cells, and can thus augment the effect of I-131 on nodule size. Studies recently showed that recombinant TSH (rTSH) injections prior to I-131 therapy facilitated goiter shrinkage by an additional 50% as compared to I-131 alone.[23,24] However, pain and compressive symptoms were more frequent with rTSH therapy.[23] In another study on very large multinodular goiters, rTSH-based I-131 therapy was shown to improve tracheal compression and improve inspiratory capacity.[25] Although r-TSH is now available in India, this therapy is costly, controversial and experimental. The committee does not recommend its routine use (recommendation strength: F).
CONCLUSION
The management of thyroid nodules requires a combination of clinical evaluation followed by appropriate investigations- an individualized approach, rather than a broad algorithm is increasingly becoming relevant. These consensus guidelines represent a summary of current medical evidence for thyroid nodule management, optimized for the clinical practice setting in India.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Tunbridge WM, Evered DC, Hall R, Appleton D, Brewis M, Clark F, et al. The spectrum of thyroid disease in a community: The Whickham survey. Clin Endocrinol (Oxf) 1977;7:481–93. doi: 10.1111/j.1365-2265.1977.tb01340.x. [DOI] [PubMed] [Google Scholar]
- 2.Usha Menon V, Sundaram KR, Unnikrishnan AG, Jayakumar RV, Nair V, Kumar H. High prevalence of undetected thyroid disorders in an iodine sufficient adult south Indian population. J Indian Med Assoc. 2009;107:72–7. [PubMed] [Google Scholar]
- 3.Davies L, Welch HG. Increasing Incidence of Thyroid Cancer in the United States, 1973-2002. JAMA. 2006;295:2164–7. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 4.Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, et al. Revised American thyroid association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–214. doi: 10.1089/thy.2009.0110. American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. [DOI] [PubMed] [Google Scholar]
- 5.Tuttle RM, Lemar H, Burch HB. Clinical features associated with an increased risk of thyroid malignancy in patients with follicular neoplasia by fine-needle aspiration. Thyroid. 1998;8:377–83. doi: 10.1089/thy.1998.8.377. [DOI] [PubMed] [Google Scholar]
- 6.Koike E, Noguchi S, Yamashita H, Murakami T, Ohshima A, Kawamoto H, et al. Ultrasonographic characteristics of thyroid nodules: Prediction of malignancy. Arch Surg. 2001;136:334–7. doi: 10.1001/archsurg.136.3.334. [DOI] [PubMed] [Google Scholar]
- 7.Morgan JL, Serpell JW, Mb BS, Cheng MS, Mb F. Fine-needle aspiration cytology of thyroid nodules: How useful is it? ANZ J Surg. 2003;73:480. doi: 10.1046/j.1445-1433.2003.02670.x. [DOI] [PubMed] [Google Scholar]
- 8.Nayar R, Ivanovic M. The indeterminate thyroid fine-needle aspiration: Experience from an academic center using terminology similar to that proposed in the 2007 National Cancer Institute Thyroid Fine Needle Aspiration State of the Science Conference. Cancer Cytopathol. 2009;117:195–202. doi: 10.1002/cncy.20029. [DOI] [PubMed] [Google Scholar]
- 9.Baloch ZW, Cibas ES, Clark DP, Layfield LJ, Ljung BM, Pitman MB, et al. The National Cancer Institute Thyroid fine needle aspiration state of the science conference: A summation. Cytojournal. 2008;5:6. doi: 10.1186/1742-6413-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pitman MB, Abele J, Ali SZ, Duick D, Elsheikh TM, Jeffrey RB, et al. Techniques for thyroid FNA: A synopsis of the National Cancer Institute Thyroid Fine-Needle Aspiration State of the Science Conference. Diagn Cytopathol. 2008;36:407–24. doi: 10.1002/dc.20829. [DOI] [PubMed] [Google Scholar]
- 11.Frates MC, Benson CB, Charboneau JW, Cibas ES, Clark OH, Coleman BG, et al. Management of thyroid nodules detected at US: Society of radiologists in ultrasound consensus conference statement. Radiology. 2005;237:794–800. doi: 10.1148/radiol.2373050220. [DOI] [PubMed] [Google Scholar]
- 12.Castro MR, Gharib H. Continuing controversies in the management of thyroid nodules. Ann Intern Med. 2005;142:926–31. doi: 10.7326/0003-4819-142-11-200506070-00011. [DOI] [PubMed] [Google Scholar]
- 13.Delbridge L, Guinea AI, Reeve TS. Total thyroidectomy for bilateral benign multinodular goiter: Effect of changing practice. Arch Surg. 1999;134:1389–93. doi: 10.1001/archsurg.134.12.1389. [DOI] [PubMed] [Google Scholar]
- 14.Hegedus L. The thyroid nodule. N Engl J Med. 2004;351:1764–71. doi: 10.1056/NEJMcp031436. [DOI] [PubMed] [Google Scholar]
- 15.Kang HW, No JH, Chung JH, Min YK, Lee MS, Lee MK, et al. Prevalence, clinical and ultrasonographic characteristics of thyroid incidentalomas. Thyroid. 2004;14:29–33. doi: 10.1089/105072504322783812. [DOI] [PubMed] [Google Scholar]
- 16.McCartney CR, Stukenborg GJ. Decision analysis of discordant thyroid nodule biopsy guideline criteria. J Clin Endocrinol Metab. 2008;93:3037–44. doi: 10.1210/jc.2008-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang KW, Kim SK, Kang HS, Lee ES, Sim JS, Lee IG, et al. Prevalence and risk of cancer of focal thyroid incidentaloma identified by 18F-fluorodeoxyglucose positron emission tomography for metastasis evaluation and cancer screening in healthy subjects. J Clin Endocrinol Metab. 2003;88:4100–4. doi: 10.1210/jc.2003-030465. [DOI] [PubMed] [Google Scholar]
- 18.Bellantone R, Lombardi CP, Raffaelli M, Traini E, De Crea C, Rossi ED, et al. Management of cystic or predominantly cystic thyroid nodules: The role of ultrasound-guided fine-needle aspiration biopsy. Thyroid. 2004;14:43–7. doi: 10.1089/105072504322783830. [DOI] [PubMed] [Google Scholar]
- 19.Niedziela M. Pathogenesis, diagnosis and management of thyroid nodules in children. Endocr Related Cancer. 2006;13:427–53. doi: 10.1677/erc.1.00882. [DOI] [PubMed] [Google Scholar]
- 20.Cooper DS, Axelrod L, Degroot LJ, Vickery ALJ, Maloof F. Congenital goiter and the development of metastatic follicular carcinoma with evidence for a leak of nonhormonal iodide: Clinical, pathological, kinetic, and biochemical studies and a review of the literature. J Clin Endocrinol Metab. 1981;52:294–306. doi: 10.1210/jcem-52-2-294. [DOI] [PubMed] [Google Scholar]
- 21.Kung AW, Chau MT, Lao TT, Tam SC, Low LC. The effect of pregnancy on thyroid nodule formation. J Clin Endocrinol Metab. 2002;87:1010–4. doi: 10.1210/jcem.87.3.8285. [DOI] [PubMed] [Google Scholar]
- 22.Tan GH, Gharib H, Goellner JR, van Heerden JA, Bahn RS. Management of thyroid nodules in pregnancy. Arch Intern Med. 1996;156:2317–20. [PubMed] [Google Scholar]
- 23.Bonnema SJ, Nielsen VE, Boel-Jorgensen H, Grupe P, Andersen PB, Bastholt L, et al. Improvement of goiter volume reduction after 0.3 mg recombinant human thyrotropin-stimulated radioiodine therapy in patients with a very large goiter: A double-blinded, randomized trial. J Clin Endocrinol Metab. 2007;92:3424–8. doi: 10.1210/jc.2007-0501. [DOI] [PubMed] [Google Scholar]
- 24.Fast S, Hegedus L, Grupe P, Nielsen VE, Bluhme C, Bastholt L, et al. Recombinant human thyrotropin-stimulated radioiodine therapy of nodular goiter allows major reduction of the radiation burden with retained efficacy. J Clin Endocrinol Metab. 2010;95:3719–25. doi: 10.1210/jc.2010-0634. [DOI] [PubMed] [Google Scholar]
- 25.Bonnema SJ, Nielsen VE, Boel-Jorgensen H, Grupe P, Andersen PB, Bastholt L, et al. Recombinant human thyrotropin-stimulated radioiodine therapy of large nodular goiters facilitates tracheal decompression and improves inspiration. J Clin Endocrinol Metab. 2008;93:3981–4. doi: 10.1210/jc.2008-0485. [DOI] [PubMed] [Google Scholar]


