Abstract
Objective:
Pattern of sleep in hyperthyroid state / thyrotoxicosis has not been systematically studied. It is being characterized as poor without further elaboration. We analyzed the pattern of sleep in a large sample of individuals with thyrotoxicosis who came to our endocrine center in southern India.
Materials and Methods:
We identified individuals with the diagnosis of ‘thyrotoxicosis’ from our electronic medical record database, and evaluated clinical parameters and pattern of their sleep: difficulty in falling asleep (DFA), difficulty in maintaining sleep (DMS), excess daytime sleepiness). In the first phase, univariate analysis with logistic regression was performed. Multivariate logistic regression was performed in the next phase on variables with a P-value < 0.1: these were considered as potential categories/ variables.
Results:
In model response variable with DFA, multivariate logistic regression predicted that subjects with abnormal appetite (more 1.7 or less 2.2), change in bowel motion (loose 1.5 or constipation 2.8), in mood (easy loss of temper 3.4), change of voice -- hoarse 7.4 or moderately hoarse 3.1), tended to have higher chances of difficulty in falling asleep (DFA). Patients with tremor (yes = 5.4) had greater likelihood of difficulty in maintaining sleep (DMS).
Conclusions:
Individuals with hyperthyroidism/thyrotoxicosis principally had difficulty in falling asleep DFA, which was related to hyperkinetic features.
Keywords: Difficulty in falling asleep, hypothyroidism, logistic regression
INTRODUCTION
Thyrotoxicosis is the second most frequent functional thyroid disorder at our center.[1,2] Generally individuals with thyrotoxicosis present with hypermetabolic features, but pattern of sleep is not as well studied as in those with hypothyroidism.[1–4] Abnormalities in sleep regulation are often present in thyrotoxicosis[5] even in the neonatal period,[6] which can adversely affect the course of the disease.[7] Owing to the paucity of published information we performed a retrospective analysis of sleep patterns in subjects who presented with thyrotoxicosis to our Center.
MATERIALS AND METHODS
We have a live electronic medical database of subjects who present with endocrine disorders at our Center.[8] We identified individuals with the diagnosis of ‘thyrotoxicosis’ from our electronic medical record database, and evaluated clinical parameters and the pattern of their sleep (difficulty in falling asleep [DFA], difficulty in maintaining sleep [(DMS], excess daytime sleepiness [EDS]) as described previously[4] [Table 1]. Those who did not complain of sleep disturbances were taken as having normal sleep. The aim of this study was to identify factors affecting sleep in this group of subjects at the time of presentation A list continuous variables is presented in Table 1a and of categorical/dichotomous variables in Table 1b.
Table 1a.
Descriptive statistics for continuous variable
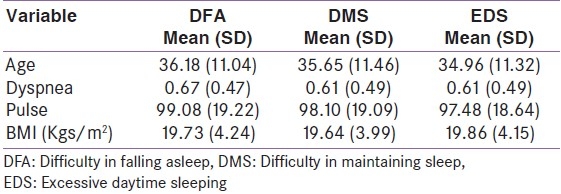
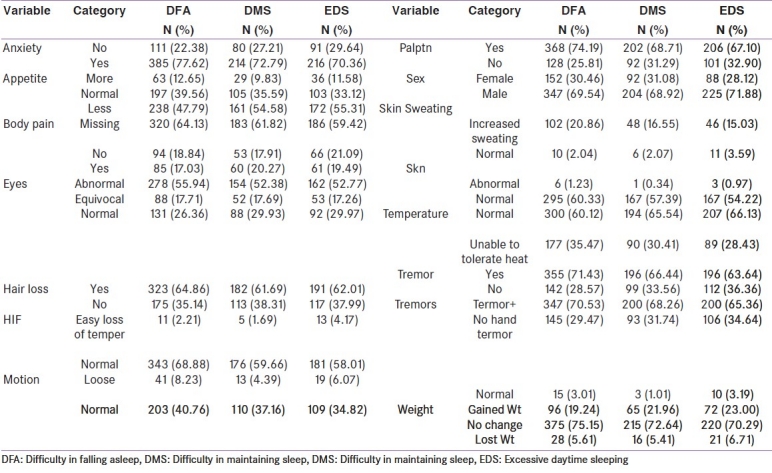
Table 1b.
Frequencies and percentages of categorical variables
Statistical analysis
Descriptive statistics are presented in Table 1a for continuous variables and in table 1b for categorical variables. The basic univariate analyses with logistic regression and results are shown in Table 2. From the univariate logistic regression analysis, the variables that had a P-value < 0.1 (90% statistical significance) were considered as potential variables in the multivariate logistic regression done as a second phase analysis. These results are presented in Table 3. The same exercise was adopted for all three models defined earlier namely DFA, DMS and EDS. To minimize the non-identifiably or co-linearity problems, stepwise logistic regression was performed.
Table 2.
Univariate logistic regression, odd ratios and P-values by each model: Patients diagnosed as Thyrotoxicosis disorder between 1992 and 2004 in EDC
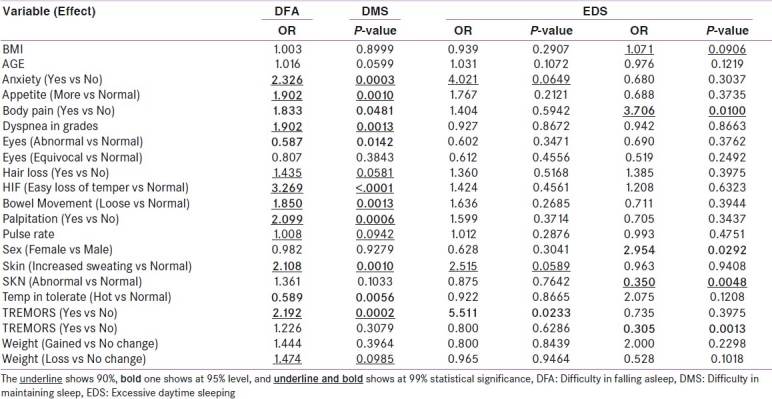
Table 3.
Multivariate logistic regression, odd ratios, P-values and 95% CI by each model: Patients diagnosed as Thyrotoxicosis disorder between 1992 and 2004 in EDC
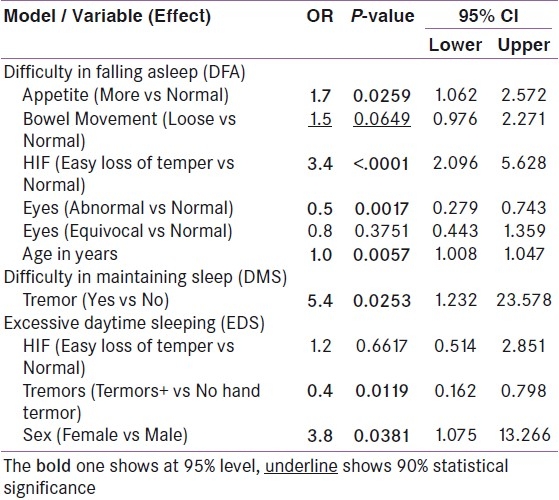
In the stepwise logistic regression, a hierarchical forward selection and a backward elimination approach, P-value criteria for data inclusion and exclusion were set at 0.05 and 0.09, respectively. The model automatically selected the variable if one of the categories was statistically significant at 95% level.
Odds ratios (OR), values and 95% confidence intervals of variables associated with the presence of DFA, DMS and EDS were estimated in a final logistic regression model that contained all variables that had entered or had been retained in the stepwise procedures [Table 2]. All analyses were done using SAS v9.2 software.
RESULTS
Out of twenty one variables considered [Tables 1a and b], seventeen were found to be statistically significant either partially or fully attaining at least 90% level of significance with DFA. But only three and ten variables, respectively, were found to be statistically significant at least for 90% significance level when the model response was performed for DMS and EDS, respectively. All models with the statistical significant variables were selected for multivariate logistic regression analysis.
In model response with variable DFA, multivariate logistic regression predicted that patients with abnormal appetite (more 1.7 or less 2.2), bowel movement abnormality (loose 1.5 or constipation 2.8), change in mood (easy loss of temper 3.4), changes in voice (hoarse 7.4 or moderately hoarse 3.1), had greater chances of difficulty in falling asleep (DFA) when compared to patients with normal appetite, normal bowel movements, normal affect or normal voice, respectively. Patients with tremor (yes = 5.4) are likely having the difficulty in maintaining sleep (DMS). Patients with hand tremors were as expected, less likely to have excessive daytime sleeping (EDS).
DISCUSSION
Sleep disorders have been described in a variety of endocrine disorders and span across entire age spectrum.[5,6] Earlier studies focused on sleep disturbances in thyrotoxicosis in relation to organic movement disorders[9,10] and periodic paralysis[11] but did not address sleep in general. A rare association of somnambulism was reported with hyperthyroidism.[3] Studies of patterns of sleep disturbances per se other than sleep apnea are lacking
In the current study, we found an association of hyperkinetic features (tremor, appetite change, bowel disturbances) with difficulty in falling asleep DFA and with difficulty in maintaining sleep DMS.
The sleep disturbances can potentially add to poor physical health of those with thyrotoxicosis,[12] which could be associated with increased percentage of sleep time spent in stages 3 and 4 (non-REM sleep).[13] A modification of sleep behavior has potential to improve overall wellness of patients with hyperthyroidism. Disturbances of sleep may directly or indirectly contribute to glucose dys-metabolism often reported in patients with hyperthyroidism. Thus understanding of patterns of sleep in hyperthyroid state begs attention. Our study is a step in that direction.
In conclusion, we have shown that thyrotoxicosis is associated principally with difficulty in falling asleep (DFA). Rather than merely affecting the quality of life in untreated thyrotoxicosis, there are potential adverse consequences for behavioral and cardiovascular performance as well.[14,15] It would be instructive to study the latter association in future in larger well well-designed prospective studies.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Sridhar GR. Pattern of thyroid diseases seen at an endocrine centre in Andhra Pradesh; 1991. In: Shah DH, Noronha OP, editors. Mumbai: Proc 4th Annual Conf Thyroid Assoc India. 1991. pp. 15–9. [Google Scholar]
- 2.Sridhar GR. Management of hyperthyroidism. J Assoc Physicians India. 2000;48:45–52. [Google Scholar]
- 3.Ajlouni KM, Ahmad AT, Al-Zahiri MM, Ammari FL, Jarah NS, Abulbara MA, et al. Sleepwalking associated with hyperthyroidism. Endocr Pract. 2005;11:5–10. doi: 10.4158/EP.11.1.5. [DOI] [PubMed] [Google Scholar]
- 4.Sridhar GR, Madhu K. Sleep in young untreated hypothyroid subjects. J Sleep Res. 1996;5:198–9. [PubMed] [Google Scholar]
- 5.Murakami Y, Kato Y. Sleep disorders in several pathologic states-endocrine diseases. Nippon Rinsho. 1998;56:457–60. [PubMed] [Google Scholar]
- 6.Watkins MG, Dejkhamron P, Huo J, Vazquez DM, Menon RK. Persistent neonatal thyrotoxicosis in a neonate secondary to a rare thyroid-stimulating hormone receptor activating mutation: Case report and literature review. Endocr Pract. 2008;14:479–83. doi: 10.4158/EP.14.4.479. [DOI] [PubMed] [Google Scholar]
- 7.LaDou J. Health effects of shift work. West J Med. 1982;137:525–30. [PMC free article] [PubMed] [Google Scholar]
- 8.Sridhar GR, Venkat YV. Information technology and endocrine sciences in the new millennium. Indian J Endocrinol Metab. 2000;4:70–80. [Google Scholar]
- 9.Amed MA, Martinez A, Yee A, Cahill D, Besag FM. Psychogenic and organic movement disorders children. Dev Med Child Neurol. 2008;50:300–4. doi: 10.1111/j.1469-8749.2008.02043.x. [DOI] [PubMed] [Google Scholar]
- 10.Loh LM, Hum AY, Teoh HL, Lim EC. Graves disease associated with spasmodic truncal flexion. Parkinsonism Relat Disord. 2005;11:117–9. doi: 10.1016/j.parkreldis.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 11.Correa-Luna LD, Reyes-Ortiz LM, Ramirez-Riveria J. Periodic paralysis: Rare presenting symptom of thyrotoxicosis. Bol Assoc Med PR. 2006;98:124–7. [PubMed] [Google Scholar]
- 12.Karges B, Krause G, Homoki J, Debatin K, deRoux N, Karges W. TSH receptor mutations U509A causes familial hyperthyroidism by release of interhelical constraints between transmembrane helices TMH3 and TMH5. J Endocrinol. 2005;186:377–85. doi: 10.1677/joe.1.06208. [DOI] [PubMed] [Google Scholar]
- 13.Ringkananont U, VanDurme J, Montanelli L, Ugrasbul F, Yu YM, Weiss RE, et al. Repulsive separation of the cytoplasmic ends of transmembrane helix 6 of the thyrotropin receptor as cause of hereditary nonautoimmune hyperthyroidism. Mol Endocrinol. 2006;20:893–903. doi: 10.1210/me.2005-0339. [DOI] [PubMed] [Google Scholar]
- 14.Joffe RT, Szuba MP, Stephen TH, Sokolov , Levitt AJ. The thyroid, sleep and depression. Biol Psychiatry. 1995;37:196–7. doi: 10.1016/0006-3223(94)00255-2. [DOI] [PubMed] [Google Scholar]
- 15.Sridhar GR, Lakshmi G. Sleep and obesity. J Gen Med. 2009;21:54–6. [Google Scholar]


