Abstract
Multiple etiologies of liver disease lead to liver fibrosis through integrated signaling networks that regulate the deposition of extracellular matrix. This cascade of responses drives the activation of hepatic stellate cell (HSC) into a myofibroblast like phenotype that is contractile, proliferative and fibrogenic. Collagen and other extracellular matrix (ECM) components are deposited as the liver generates a wound healing response to encapsulate injury. Sustained fibrogenesis leads to cirrhosis, characterized by a distortion of the liver parenchyma and vascular architecture. Uncovering the intricate mechanisms that underlie liver fibrogenesis forms the basis for efforts to develop targeted therapies to reverse the fibrotic response and improve the outcomes of patients with chronic liver disease.
Keywords: Stellate cell, fibrogenesis, tyrosine kinase, growth factors, immune cells, adipokines
Introduction
Liver fibrosis is a reversible wound-healing response to either acute or chronic cellular injury that reflects a balance between liver repair and scar formation. During acute injury, the changes in liver architecture are transient and reversible. With chronic injury, there is progressive substitution of the liver parenchyma by scar tissue. Despite ongoing injury, the liver has a remarkable regenerative capacity, and, as a result, patients often progress slowly to cirrhosis over decades.
A key discovery in understanding fibrosis has been that the hepatic stellate cell (HSC) is the primary effector cell, orchestrating the deposition of ECM in normal and fibrotic liver. HSCs are resident perisinusoidal cells in the subendothelial space between hepatocytes and sinusoidal endothelial cells. They are strategically positioned to intimately interact with hepatocytes, endothelial cells, and nerve endings through their numerous processes extending across the space of Disse [1–3]. Additionally, the HSC plays a pivotal role in activating the immune response through secretion of cytokines and chemokines and interacting with immune cells. HSC also contributes to angiogenesis and the regulation of oxidant stress [4].
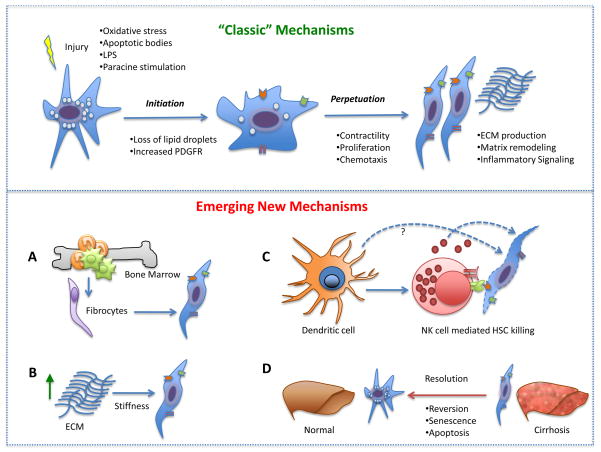
Activation of the HSC into a myofibroblast like phenotype can be provoked by a range of chronic injuries to the liver, amongst which are viral hepatitis, toxins, [non-] alcoholic steatohepatitis and autoimmune disorders [5]. Activation consists of two major phases, initiation [also called a “preinflammatory stage”] and perpetuation, followed by a resolution phase if the injury subsides [6]. Initiation refers to early changes in gene expression and phenotype that render the cells responsive to other cytokines and stimuli shortly after injury occurs (Figure 1). The initial paracrine stimulation, including exposure to lipid peroxides and products of damaged hepatocytes and signals from Kupffer and endothelial cells, drive early activation, as well as changes in surrounding extracellular matrix. Once the cell is primed for activation, perpetuation results from the effects of these stimuli on maintaining an activated phenotype and generating fibrosis. Sustained activation involves at least seven discrete changes in cell behavior: proliferation, chemotaxis, fibrogenesis, contractility, matrix degradation, retinoid loss, and WBC chemoattractant/cytokine release. The net effect of these changes is to increase accumulation of extracellular matrix. During this phase there is a release of proinflammatory, profibrogenic and promitogenic stimuli that act in an autocrine and paracrine manner. Resolution of fibrosis refers to pathways that cause either HSC apoptosis, senescence, or quiescence [7] (Figure 1).
Figure 1. Stellate cell activation through “classic” mechanisms (upper panel) and emerging new mechanisms (lower panel).
The HSC is the central effector in hepatic fibrosis and undergoes activation through a two-phase process. Initial liver injury results in hepatocyte cell apoptosis with generation of apoptotic bodies, reactive oxygen species, and paracrine stimulation of HSCs. Additionally, LPS from the gut can simulate HSCs. These initial stimuli allow the cell to become sensitized to additional activation by upregulating various receptors and is termed the initiation phase. Subsequently, it is able to secrete autocrine and paracrine growth factors, chemokines, and ECM. Maintenance of HSC activation is termed the perpetuation phase, and involves changes in HSC behavior, including proliferation, chemotaxis, fibrogenesis, and contractility. (A) Among other cell types that may contribute to ECM production, fibrocytes derived from the bone marrow are believed to transdifferentiate into myofibroblasts. (B) Mechanical stiffness of the ECM can be sensed by and activate HSCs. (C) The contribution of dendritic cells to fibrosis is complex and not yet well understood, however, they can activate NK cells. HSCs become sensitized to NK cell mediate apoptosis after cellular activation causes downregulation of their inhibitory MHC class I molecules. (D) New evidence suggests fibrosis can regress through reversion, senescence or apoptosis of HSCs.
Fibrosis progression and regression require specific signaling pathways; thus, understanding how they interact and evolve with injury can contribute to efforts to reverse fibrosis. Complex, well-orchestrated intracellular events lead to HSC survival and growth during progressive fibrosis, while permitting their regulated clearance during resolution of fibrosis. This review will focus on key pathways and gene regulation that leads to activation of HSCs and emerging mechanisms of fibrogenesis that have provided new targets for therapy.
Key pathways of HSC activation
Growth factor signaling
HSCs are an important source of growth factors in the liver, but also respond to these factors, emphasizing the importance of tightly regulated autocrine control of growth factor activity within the pericellular milieu.
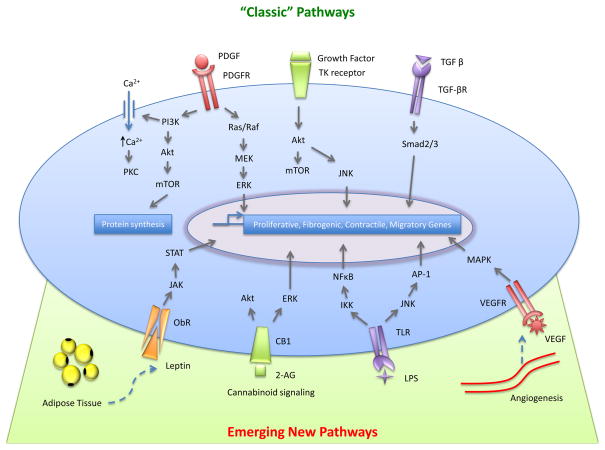
PDGF signaling is among the best characterized pathways of HSC activation[8]. PDGF binds its receptors, the receptor subunits dimerize with subsequent phosphorylation of the tyrosine residues in the intracellular domain. This leads to Ras-MAPK pathway activation, signaling through the PI3K-AKT/PKB pathway and mobilization of intracellular calcium ions to activate PKC family members [9] (Figure 2). All these events ultimately lead to cellular proliferation. Rapid induction of β-PDGF receptor, which is a hallmark of early HSC activation, is followed by development of a contractile, fibrogenic phenotype that correlates with the degree of fibrosis and inflammation [10, 11]. PDGF is the most potent mitogen towards HSCs, and its antagonism therefore has potential as an anti-fibrotic strategy [12]. In fact, pharmacological inhibition of the PDGFR-β chain in pre-clinical disease models has already shown promise as an anti-fibrotic [13, 14]. Sorafenib, a multiple receptor tyrosine kinase inhibitor targeting the PDGF receptor and the Raf/ERK signaling pathway, is effective in advanced HCC patients, and additionally displays anti-fibrotic activity in animal models of fibrosis [14].
Figure 2. Current and emerging signaling pathways regulating HSC activation.
PDGF signals partially through ERK activation and also through AKT via mTOR mediated protein synthesis regulation. PDGF activation also allows for influx of Ca2+ ions, which contributes to gene regulation. In addition to PDGF, several other growth factors can activate tyrosine receptors, which lead to Akt activation and then either mTOR or JNK activation. TGF-β recruits Smad2/3, leading to their phosphorylation and stimulation of fibrogenic gene expression. Emerging pathways of importance to fibrosis include contributions from adiopkines (leptin), neuroendocrine signals (2-AG), TLR signaling, and angiogenic signals (VEGF), among others.
Other signaling pathways converge on Akt activation, including c-Jun N-terminal kinase (JNK) phosphorylation and activation (Figure 2). JNK inhibition or genetic deletion in mice displays a reduction in liver fibrosis, as well as decreased PDGF and TGFβ signaling, implicating JNK inhibition as a potential target for drug development [15].
Transforming growth factor α (TGFα) and epidermal growth factor (EGF) are two potent epithelial growth factors that are secreted by, and also stimulate proliferation of HSCs [16, 17]. Release of these growth factors is also important for paracrine stimulation of hepatocyte proliferation during liver regeneration [16, 18]. Receptors for VEGF are also induced during HSC activation which contribute to enhanced mitogenesis in response to VEGF. The critical role of VEGF in angiogenesis, combined with its mitogenicity towards HSCs, establish HSCs as one of the cellular determinants of hepatic angiogenesis. Other HSC mitogenic pathways include those downstreatm of thrombin [19, 20], keratinocyte growth factor [21], and bFGF [22] (Figure 2).
Following the response to growth factors, the HSC remodels the ECM into one rich in fibril-forming collagens, particularly types I and III. The ECM components in turn act in a positive feedback loop by releasing additional matrix-bound growth factors resulting from increased protease activity, as well as increasing liver stiffness, both of which propagate HSC migration and contraction [23].
Fibrogenic Signaling Pathways
HSCs generate fibrosis not only by increasing cell number, but also by increasing matrix production per cell. In the normal liver, the basement membrane-like matrix of the space of Disse is comprised primarily of collagens IV and VI, which is progressively replaced by collagens I and III and cellular fibronectin during fibrogenesis [24].
TGFβ1 is derived from both paracrine and autocrine sources, and is the most potent fibrogenic cytokine in liver [25, 26]. TGFβ1 is stored as an inactivated protein bound to a latency-associated peptide. Once activated, TGFβ1 signals via its cognate receptors to Smad proteins, which lead to induction of collagen production [25] (figure 2). Quiescent HSCs are induced by TGFβ1 to transdifferentiate into myofibroblasts that secrete extracellular matrix. Whereas hepatocyte apoptosis and necrosis stimulate HSC fibrogenesis, TGFβ1 may sustain hepatocyte mass and regulate growth during regeneration [26]. Therefore antagonizing TGFβ1 therapeutically, will be challenging, since some of its action, for example its anti-inflammatory and growth regulatory roles, are important to sustain normal liver homeostasis [27].
Leptin, a key adipokine, has been implicated fibrogenesis through a number of pathways (detailed in adipokines, below). Downstream effects of leptin signaling during liver injury include increased release of TGF-β1 from Kupffer cells. In contrast, leptin deficiency may reduce fibrogenesis by decreasing the activity of norepinephrine. Reduced activity of norepinephrine, in turn, leads to decreased activity of natural killer cells, and thereby attenuates the release of additional profibrogenic cytokines and reduced ECM production [28].
Connective tissue growth factor (CTGF/CCN2) is a growth factor-modulator protein secreted from HSCs in the setting of hyperglycemia, hyperinsulinemia and ethanol-induced liver injury [29, 30]. It is a major fibrogenic signal whose activity can be both TGFβ-dependent when produced in hepatocytes, and TGFβ-independent when derived from HSCs [31]. CTGF may be a useful serum biomarker for fibrosis severity, as it is significantly correlated with fibrosis in patients with HCV in one study [32].
Chemokine Pathways
Chemokines are a class of small chemotactic molecules with cytokine-like functions, which are well known to orchestrate inflammatory responses within different organs [33]. Multiple effector cells in liver fibrosis are potential targets and sources of chemokines, each expressing a different combination of receptors and ligands. For example, HSCs express several receptors including CXCR3, CCR5, and CCR7, and secrete numerous chemokines, including CCL2, CCL3, CCL5, CXCL1, CXCL8, CXCL9 and CXCL10 [34]. Specific functions of these chemokines are being actively explored both to understand the pathophysiology of fibrosis, as well as to unearth new therapeutic targets.
In general, chemokines promote the migration of fibrogenic cells to the site of injury, thereby enhancing fibrogenesis through increased cell number and amplified inflammation. Notably, CCR5, whose ligand, RANTES, is induced by NFκB signaling, and stimulates HSC migration and proliferation [35]. In mouse models of fibrosis (CCl4 and bile duct ligation), CCR1- and CCR5-deficient mice displayed substantially reduced hepatic fibrosis and macrophage infiltration [36]. Genetic deletion of platelet-derived CXCL4 in mice also significantly reduces inflammation and reduces fibrosis [37]. In contrast, CXCL9, through its cognate receptor CXCR3, is anti-fibrotic [38]. It is therefore important to define the role of each chemokine independently and in combination, to better understand their overall impact on fibrosis progression and regression.
Adipokine Pathways
Adipokines are polypeptides secreted by adipose tissue in a regulated manner. While some of these molecules are expressed only by adipocytes, resident and infiltrating macrophages and components of the vascular stroma also contribute to the expression of others. Adipokines also contribute to the hepatic manifestations of obesity and are increasingly recognized as key mediators of fibrogenesis, especially in the setting of NAFLD (Non-Alcoholic Fatty Liver Disease) [39].
Leptin, a circulating adipogenic hormone, promotes stellate cell fibrogenesis and enhances TIMP-1 expression, which is associated with increased leptin signaling [40, 41]. It exerts its action through the leptin receptor (OB-R), which leads to stimulation of Janus kinase (Jak)-signal transduction and activates the Jak-signal transduction and activator of transcription (STAT) transcription signaling pathway [42] (Figure 2). Leptin has a pro-fibrotic action partially through suppression of peroxisome proliferator-activated receptor-γ (PPARγ), an anti-fibrogenic nuclear receptor that can reverse HSC activation and maintain HSC quiescence [43]. Leptin levels in circulating blood are proportionate to adipose mass, and enhanced leptin has been clearly tied to HSC fibrogenesis. Sources are likely to be both endocrine and autocrine, and its activity is amplified due to enhanced signaling through the leptin receptor, the expression of which is up-regulated during HSC activation [44]. Concurrently, down-regulation of adiponectin in obesity, a counter-regulatory hormone, might amplify the fibrogenic activity of leptin [45]. Adiponectin expressed is reduced in hepatic fibrosis, and mice lacking adiponectin have enhanced fibrosis after toxic liver injury [46].
Neuroendocrine Pathways
The fibrogenic function of HSCs is also influenced by neurochemical and neurotrophic factors that may be exploitable for targeted therapy. Upon chronic liver injury, the local neuroendocrine system is up-regulated, and activated HSCs express specific receptors, most prominently those regulating cannabinoid signaling [47]. Activated stellate cells are additionally a key source of the endogenous cannabinoid, 2-AG, which drives increased CB1 receptor signalling [48] (Figure 2). Stimulation of the CB1 receptor is profibrogenic, whereas the CB2 receptor is anti-fibrotic and hepatoprotective. CB1 receptor may also mediate steatosis, since daily cannabis use is a risk factor for steatosis and fibrosis in HCV. In fact, a peripheral CB1R antagonist that does not enter the CNS (where both receptors are also highly expressed), leads to improvement in fatty liver in an animal model [49]. Efforts are being expanded based on pre-clinical models to either antagonize CB1 or agonize CB2 for therapeutic use [50, 51].
Other neurotrophic or hormonal mediators also contribute to fibrosis. Opioid signaling increases proliferation and collagen production in HSCs [52]. Serotonin has a pro-fibrotic effect that synergizes with PDGF signaling [53]. Thyroid hormones enhance activation of HSC through increased p75NTR and activation of Rho, thereby accelerating development of liver fibrosis [54]. These cellular pathways merit further exploration as new drug targets since there are already agonists or antagonsists for them in clinical development.
Immune Interactions
The inflammatory response plays an important role in driving fibrogenesis, since persistent inflammation almost always precedes fibrosis. Activated HSCs secrete inflammatory chemokines [see above], interact directly with immune cells through expression of adhesion molecules [55], and modulate the immune system through antigen presentation [56]. Therefore, a positive feedback loop exists in which inflammatory and fibrogenic cells stimulate each other in amplifying fibrosis. Other cell types regulating progression and resolution of fibrosis include natural killer cells (NK)[57], T-cells [58], dendritic cells [59], macrophages [60], Kupffer cells [61], as well as HSCs [56] and endothelial cells [62].
Immune activation also provokes fibrosis through signaling in response to bacterial lipopolysaccharide (LPS), a ligand for the TLR4 receptor, which is expressed on both macrophages and HSCs [63, 64]. In fact, signaling of HSCs in response to either LPS or endogenous TLR4 ligands (high-mobility group box 1 (HMGB1), biglycan and heparan sulfate) may be even more important to the fibrogenic response than macrophage activation (Figure 2). Downstream of TLR4, BAMBI, a transmembrane suppressor of TGFβ1, when suppressed, leads to activation of TGFβ1 [65].
In another interaction, Kupffer cell activation leads to increased NF-κB activity and subsequent secretion of pro-inflammatory cytokines including tumor necrosis factor- α (TNF-α) and monocyte chemoattractant protein (MCP)-1, which provoke the activation of HSCs [61]. HSCs in turn respond to this stimulation by secreting macrophage colony-stimulating factor (M-CSF)[66], MCP-1 [67], IL-6 [68], CCL21 [4], RANTES, and CCR5 [35] leading to an amplified acute phase response with further activation of macrophages. TNF-α also induces neutrophil infiltration and stimulates mitochondrial oxidant production in hepatocytes, which are sensitized to undergo apoptosis. In mice deficient for both TNF receptors (types 1 and 2) fed with an MCD diet (an established model for fibrosing steatohepatitis), there is less Kupffer cell activation and reduced collagen deposition compared to wild-type mice [69], implicating TNF-α directly in Kupffer cell activation.
Oxidant stress [70] and apoptotic parenchymal cells [71] are also strong inducers of the immune system (see Oxidant stress below). Apoptotic cells resulting from liver injury may be phagocytosed by HSCs, which in turn stimulates an increase in NADPH oxidase (NOX) and cell survival [72, 73]. NOX induction also provokes oxidant stress, which contributes to HSC activation. Additionally, apoptotic hepatocyte DNA can interact with Toll like receptor 9 (TLR9) expressed on HSCs. Activation of TLR9 can repress HSC migration and increase collagen production [74]. Although blocking apoptosis of hepatocytes has been an attractive anti-fibrotic pathway, clinical trials using apoptosis inhibitors have been discontinued over fears that they may promote the risk of cancer.
Natural killer (NK) cells have an anti-fibrotic activity by inhibiting and/or killing activated HSCs (Figure 1). In liver injury, NK cells induce apoptosis of HSCs through production of interferon γ [59]. Quiescent HSCs are relatively resistant to apoptotic signals but become sensitive after activation and down-regulation of their class I MHC expression [75]. Moreover, immuno-suppressed individuals with decreased levels of NK cells in the liver have increased fibrosis, possibly due to less NK cell-mediated HSC apoptosis [76]. One mechanism for NK cell-mediated HSC death requires down-regulation of iKIR, an inhibitory receptor on NK cells, and down-regulation of MHC class I on HSCs for efficient killing. In mice and human co-culture models, knockdown of iKIR with siRNAs in lymphocytes stimulates NK cells and promotes their antifibrogenic activity [59].
Lastly, HSCs interact directly with various immune cells through their expression of adhesion molecules, including intercellular adhesion molecule 1 (ICAM-1) [55, 59] and vascular cell adhesion molecule 1 (VCAM-1)[77]. Expression of both adhesion molecules is increased in HSCs during injury, which is mediated by TNFα, and peaks with maximal cell infiltration. Thus, adhesion molecule induction on HSCs facilitates the recruitment of inflammatory cells to the injured liver.
Angiogenesis
Angiogenesis is a key component of the wound healing response to hepatic fibrosis and is essential for liver regeneration. Conversely, it is also implicated as a promoter of liver carcinogenesis; therefore, a fine balance of pro- and anti-angiogenic factors maintains a healthy liver [78].
The contractile phenotype of HSCs is thought to contribute to angiogenesis and portal hypertension in the cirrhotic liver [79]. HSCs are uniquely positioned in the perisinusoidal space where their long processes have a potential regulatory effect on pericapillary resistance and may help regulate intrahepatic blood flow via their contractile phenotype [80]. In fact, the contractile force generated by HSCs in response to vasoactive substances is sufficient to contract against sinusoidal pressure based on in vitro and in vivo studies [81]. Some vasoactive factors, especially endothelin-1 (ET-1) [82], constrict HSCs, whereas others, including nitric oxide and carbon monoxide (CO), promote relaxation. Interestingly, physiological concentrations of CO acts as an antioxidant, and are anti-inflammatory and anti-apoptotic. This cytoprotective effect is seen in response to cellular stress, in addition to its vasodilatory effect [83].
During progressive liver injury, there are areas of vascular disorganization that create a hypoxic milieu, which is a major stimulus to angiogenesis. Hypoxia-inducible factor-1alpha (HIF-1α) is an oxygen-sensitive transcription factor and the primary mediator of the hypoxia-induced angiogenic response. VEGF and PDGF are cytokines that drive both angiogenic and fibrogenic responses, each of which is induced in hypoxic states, in part by HIF-α [78]. HSCs are activated in response to hypoxia and play a key role in angiogenesis through interactions with endothelial cells via PGDF and VEGF signaling (Figure 2).
Endothelial cells, in addition to contributing to angiogenesis, exert paracrine effects on HSCs through nitric oxide (NO) synthase-derived NO production. Activated HSCs are more sensitive to NO-induced apoptosis, which may be a mechanism to counter-balance HSC expansion in injured livers [84].
NADPH oxidase/oxidant stress
Reactive oxygen species (ROS) are generated through lipid peroxidation, and both initiate and then perpetuate fibrosis [85]. ROS can originate from hepatocytes, macrophages, HSCs, cholangiocytes and inflammatory cells, and are enhanced by ethanol, polyunsaturated fatty acids, and iron. In alcoholics, there is strong induction of cytochrome P450 2E1 leading increased ROS and peri-central (zone 3) injury. NADPH oxidase (NOX) mediates liver injury and fibrosis through generation of oxidant stress and activation of HSCs, Kupffer cells and macrophages. NOX also contributes to angiotensin signaling [86]. Curtailing oxidative stress as a therapeutic option in patients with liver fibrosis is still under investigation. In a phase II clinical trial of the mitochondria-targeted anti-oxidant mitoquinone there was significantly decreased plasma ALT and AST in patients with chronic HCV infection, suggesting that the drug may decrease necroinflammation in the liver in these patients. A possible mechanisms includes induction of the antioxidant transcription factor Nrf2 [87, 88].
Ethanol metabolism leads to free fatty acid accumulation, which is an important stimulus to fibrogenesis. Free fatty acids which promote oxidant stress, are indirect activators of HSCs in culture [89]. In ethanol-fed mice, resveratrol improved mortality, transaminase levels and liver lesions [90]. However, cell culture experiments revealed activation of HSCs when treated with resveratrol under free fatty acid-rich conditions, contradicting the beneficial effects seen in obese patients [91].
Gene regulation during HSC activation
Regulated changes in gene expression are required for HSC activation, which reflect the convergence of several intracellular signaling cascades on regulatory regions of key molecular signals. Mechanisms of gene regulation include not only direct modulation of transcription factor activity, but also epigenetic regulation through promoter methylation, mRNA stabilization, and microRNA interactions. Additionally, post-translational regulation of transcription factors with modifications including phosphorylation, SUMOylation, prenylation, acetylation, and glucosylation, add another layer of complexity to gene regulation [92]. Collectively, these regulatory changes control the fibrogenic and proliferative state of the HSC.
Transcription Factors
Although countless transient changes in gene transcription occur after activation of HSCs, the prominent transcriptional targets include: type I collagen [alpha 1 and alpha 2 chains], α-SMA, TGFβ1, TGFβ receptors, MMP-2, and TIMPs 1 & 2 [93–95]. Among the transcription factors that activate these downstream targets are Ets-1, Mef2, CREB, Egr-1, Vitamin D receptor, Foxf1, JunD, and C/EBPβ [96]. LIM homeobox gene 2 (Lhx2) knockout mice embryos develop a spontaneous and progressive hepatic fibrosis, so is considered to be an anti-fibrotic transcription factor [97]. Forkhead box gene, group O (FoxO1) inhibits proliferation of HSCs, and is inactivated by phosphorylation downstream of PDGF and insulin signaling [97, 98].
HSC express several basic helix–loop–helix (bHLH) transcription factors, which bind to a DNA hexanucleotide sequence known as the E box. Some of these factors (MyoD, SREBP-1c, c-myb and c-Myc) regulate tissue development and differentiation. HSCs also express Id proteins, which operate as dominant-negative bHLH transcriptional regulators that inhibit differentiation and promote cell proliferation; surprisingly, they display an anti-fibrotic effect when over-expressed in a thioacetamide-induced mouse model of fibrosis [92]. C/EBPα is an anti-fibrotic transcription factor that declines in expression with HSC activation. Forced over-expression of C/EBPα in HSCs not only inhibits their proliferation but also decreases other fibrogenic features of the cell [99].
Nuclear Receptors
HSCs express a diverse group of nuclear receptors, including the retinoid responsive RXR and RAR, the farnesoid X receptor (FXR), the pregnane X receptor (PXR), and peroxisome proliferators-activated nuclear receptors (PPARs) [92]. RXR and FXR dimerize after sensing bile acids to suppress collagen gene expression through the action of SHP. PXR is highly expressed on HSCs and is activated by steroids, drugs, xenobiotics and antibiotics. PXR then dimerizes with RXR to induce cytochrome p450 enzymes. PPARγ nuclear receptor down-regulates HSC activation and reduces collagen gene expression [92].
Epigenetic Regulation
Epigenetics refers to changes in DNA methylation and associated histone modifications that influence the chromatin states and impact gene expression patterns, without affecting the sequence of the target DNA. Epigenetic regulation is a relatively new area of gene regulation, but its importance in HSC activation is already clear. HSC activation leads to global hypomethylation, reflective of the overall increase in protein synthesis needed to transdifferentiate a quiescent cell to an activated state [100]. However, induction of the molecules CBF1 and MeCP2 lead to IκB repression through promoter methylation, which is necessary for de-repressing NFκB activity and promoting HSC survival [94]. Therefore, although many genes need to be activated by de-methylation in fibrogenesis, there exist dominant pathways that must still be silenced in order to yield a fully activated HSC.
microRNA
MicroRNAs (miRNAs) represent a family of small non-coding RNAs controlling translation and transcription of many genes [101]. This new level of regulation has provoked the reassessment of previously well-understood pathways of disease to now consider the contribution of miRNAs. Although miRNA research has quickly expanded in the field of cancer, only recently have miRNAs been evaluated for their role in fibrogenesis.
Based on gene array analysis in mouse models of fibrosis, the miR-29-family of miRNAs is down-regulated in fibrotic livers. This down-regulation is also reported in patients with more advanced hepatic fibrosis. Mechanistically, miR-29 in HSCs is down-regulated via TGF-β, LPS, and NF-κB. Additionally, over-expressing miR-29b in HSCs lead to a decrease in collagen production [102]. There is still a lot to be learned about miRNAs in the field of fibrosis, but with each discovery comes the exciting possibility of a new drug target for therapy.
Summary
In the past decade, dramatic advances have advanced our understanding of the cellular and molecular mechanisms underlying liver fibrogenesis. The identification of activated HSC as the major fibrogenic cell type in the injured liver, as well as revealing the intricacies of HSC initiation and perpetuation have unearthed important targets for drug development, including PDGFR, TGF-β, EGF, and VEGF. These signals conspire to generate scar through enhanced HSC proliferation, contractility, fibrogenesis, matrix degradation, and pro-inflammatory signaling.
This review has also described the prominent new mechanisms of liver fibrosis and highlighted signaling pathways that are being actively studied. There is growing appreciation for the importance of immune interactions, chemokines, adipokines, neuroendocrine factors, and oxidant stress towards fibrogenesis. Steady advances in understanding how to exploit these various pathways towards fibrosis regression is generating realistic hopes for effective anti-fibrotic therapies.
Acknowledgments
UL is supported by the Brookdale Department of Molecule, Cell and Developmental Biology Training Grant. SLF is supported by NIH DK56621 AA 107067
Footnotes
Conflict of Interest
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wake K. Structure of the sinusoidal wall in the liver. In: Wisse E, Knook DL, Wake K, editors. Cells of the hepatic sinusoid. Leiden: The Kupffer Cell Foundation; 1995. pp. 241–246. [Google Scholar]
- 2.Bioulac-Sage P, Lafon ME, Saric J, et al. Nerves and perisinusoidal cells in human liver. J Hepatol. 1990;10:105–12. doi: 10.1016/0168-8278(90)90080-b. [DOI] [PubMed] [Google Scholar]
- 3.Ueno T, Sata M, Sakata R, et al. Hepatic stellate cells and intralobular innervation in human liver cirrhosis. Hum Pathol. 1997;28:953–9. doi: 10.1016/s0046-8177(97)90011-3. [DOI] [PubMed] [Google Scholar]
- 4.Bonacchi A, Petrai I, Defranco RM, et al. The chemokine CCL21 modulates lymphocyte recruitment and fibrosis in chronic hepatitis C. Gastroenterology. 2003;125:1060–76. doi: 10.1016/s0016-5085(03)01194-6. [DOI] [PubMed] [Google Scholar]
- 5.Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friedman SL. Mechanisms of hepatic fibrosis and therapeutic implications. Nature Clinical Practice in Gastroenterology & Hepatology. 2004;1:98–105. doi: 10.1038/ncpgasthep0055. [DOI] [PubMed] [Google Scholar]
- 7.Krizhanovsky V, Yon M, Dickins RA, et al. Senescence of activated stellate cells limits liver fibrosis. Cell. 2008;134:657–67. doi: 10.1016/j.cell.2008.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong L, Yamasaki G, Johnson RJ, et al. Induction of beta-platelet-derived growth factor receptor in rat hepatic lipocytes during cellular activation in vivo and in culture. J Clin Invest. 1994;94:1563–9. doi: 10.1172/JCI117497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelly JD, Haldeman BA, Grant FJ, et al. Platelet-derived growth factor (PDGF) stimulates PDGF receptor subunit dimerization and intersubunit trans-phosphorylation. J Biol Chem. 1991;266:8987–92. [PubMed] [Google Scholar]
- 10.Failli P, Ruocco C, De FR, et al. The mitogenic effect of platelet-derived growth factor in human hepatic stellate cells requires calcium influx. Am J Physiol. 1995:C1133–9. doi: 10.1152/ajpcell.1995.269.5.C1133. [DOI] [PubMed] [Google Scholar]
- 11.Borkham-Kamphorst E, Kovalenko E, van Roeyen CR, et al. Platelet-derived growth factor isoform expression in carbon tetrachloride-induced chronic liver injury. Lab Invest. 2008;88:1090–100. doi: 10.1038/labinvest.2008.71. [DOI] [PubMed] [Google Scholar]
- 12.Borkham-Kamphorst E, Stoll D, Gressner AM, et al. Antisense strategy against PDGF B-chain proves effective in preventing experimental liver fibrogenesis. Biochem Biophys Res Commun. 2004;321:413–23. doi: 10.1016/j.bbrc.2004.06.153. [DOI] [PubMed] [Google Scholar]
- 13.Ogawa S, Ochi T, Shimada H, et al. Anti-PDGF-B monoclonal antibody reduces liver fibrosis development. Hepatol Res. 2010;40:1128–41. doi: 10.1111/j.1872-034X.2010.00718.x. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Gao J, Zhang D, et al. New insights into the anti-fibrotic effects of sorafenib on hepatic stellate cells and liver fibrosis. J Hepatol. 2010 doi: 10.1016/j.jhep.2010.02.027. [DOI] [PubMed] [Google Scholar]
- 15.Kluwe J, Pradere JP, Gwak GY, et al. Modulation of hepatic fibrosis by c-Jun-N-terminal kinase inhibition. Gastroenterology. 2010;138:347–59. doi: 10.1053/j.gastro.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meyer DH, Bachem MG, Gressner AM. Modulation of hepatic lipocyte proteoglycan synthesis and proliferation by Kupffer cell-derived transforming growth factors type beta 1 and type alpha. Biochem Biophys Res Commun. 1990;171:1122–9. doi: 10.1016/0006-291x(90)90801-s. [DOI] [PubMed] [Google Scholar]
- 17.Win KM, Charlotte F, Mallat A, et al. Mitogenic effect of transforming growth factor-beta 1 on human Ito cells in culture: evidence for mediation by endogenous platelet-derived growth factor. Hepatology. 1993;18:137–45. [PubMed] [Google Scholar]
- 18.Mullhaupt B, Feren A, Fodor E, et al. Liver expression of epidermal growth factor RNA. Rapid increases in immediate-early phase of liver regeneration. J Biol Chem. 1994;269:19667–70. [PubMed] [Google Scholar]
- 19.Marra F, Grandaliano G, Valente AJ, et al. Thrombin stimulates proliferation of liver fat-storing cells and expression of monocyte chemotactic protein-1: potential role in liver injury. Hepatology. 1995;22:780–7. [PubMed] [Google Scholar]
- 20.Marra F, DeFranco R, Grappone C, et al. Expression of the thrombin receptor in human liver: up-regulation during acute and chronic injury. Hepatology. 1998;27:462–71. doi: 10.1002/hep.510270221. [DOI] [PubMed] [Google Scholar]
- 21.Steiling H, Muhlbauer M, Bataille F, et al. Activated hepatic stellate cells express keratinocyte growth factor in chronic liver disease. Am J Pathol. 2004;165:1233–41. doi: 10.1016/S0002-9440(10)63383-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu C, Wang F, Jin C, et al. Role of fibroblast growth factor type 1 and 2 in carbon tetrachloride-induced hepatic injury and fibrogenesis. Am J Pathol. 2003;163:1653–62. doi: 10.1016/S0002-9440(10)63522-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schuppan D, Ruehl M, Somasundaram R, et al. Matrix as modulator of stellate cell and hepatic fibrogenesis. Seminars in Liver Disease. 2001;21:351–372. doi: 10.1055/s-2001-17556. [DOI] [PubMed] [Google Scholar]
- 24.Brown B, Lindberg K, Reing J, et al. The basement membrane component of biologic scaffolds derived from extracellular matrix. Tissue Eng. 2006;12:519–26. doi: 10.1089/ten.2006.12.519. [DOI] [PubMed] [Google Scholar]
- 25.Inagaki Y, Okazaki I. Emerging insights into Transforming growth factor beta Smad signal in hepatic fibrogenesis. Gut. 2007;56:284–92. doi: 10.1136/gut.2005.088690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Breitkopf K, Godoy P, Ciuclan L, et al. TGF-beta/Smad signaling in the injured liver. Z Gastroenterol. 2006;44:57–66. doi: 10.1055/s-2005-858989. [DOI] [PubMed] [Google Scholar]
- 27.Yang L, Pang Y, Moses HL. TGF-beta and immune cells: an important regulatory axis in the tumor microenvironment and progression. Trends Immunol. 31:220–7. doi: 10.1016/j.it.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Z, Oben JA, Yang S, et al. Norepinephrine regulates hepatic innate immune system in leptin-deficient mice with nonalcoholic steatohepatitis. Hepatology. 2004;40:434–41. doi: 10.1002/hep.20320. [DOI] [PubMed] [Google Scholar]
- 29.Chen L, Charrier AL, Leask A, et al. Ethanol-stimulated differentiated functions of human or mouse hepatic stellate cells are mediated by connective tissue growth factor. J Hepatol. 2010 doi: 10.1016/j.jhep.2010.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hora C, Negro F, Leandro G, et al. Connective tissue growth factor, steatosis and fibrosis in patients with chronic hepatitis C. Liver Int. 2008;28:370–6. doi: 10.1111/j.1478-3231.2007.01608.x. [DOI] [PubMed] [Google Scholar]
- 31.Gressner OA, Lahme B, Demirci I, et al. Differential effects of TGF-beta on connective tissue growth factor [CTGF/CCN2] expression in hepatic stellate cells and hepatocytes. J Hepatol. 2007;47:699–710. doi: 10.1016/j.jhep.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 32.Kovalenko E, Tacke F, Gressner OA, et al. Validation of connective tissue growth factor [CTGF/CCN2] and its gene polymorphisms as noninvasive biomarkers for the assessment of liver fibrosis. J Viral Hepat. 2009 doi: 10.1111/j.1365-2893.2009.01110.x. [DOI] [PubMed] [Google Scholar]
- 33.Wasmuth HE, Tacke F, Trautwein C. Chemokines in liver inflammation and fibrosis. Semin Liver Dis. 30:215–25. doi: 10.1055/s-0030-1255351. [DOI] [PubMed] [Google Scholar]
- 34.Sahin H, Trautwein C, Wasmuth HE. Functional role of chemokines in liver disease models. Nat Rev Gastroenterol Hepatol. 2010;7:682–90. doi: 10.1038/nrgastro.2010.168. [DOI] [PubMed] [Google Scholar]
- 35.Schwabe RF, Bataller R, Brenner DA. Human hepatic stellate cells express CCR5 and RANTES to induce proliferation and migration. Am J Physiol Gastrointest Liver Physiol. 2003;285:G949–58. doi: 10.1152/ajpgi.00215.2003. [DOI] [PubMed] [Google Scholar]
- 36.Seki E, De Minicis S, Gwak GY, et al. CCR1 and CCR5 promote hepatic fibrosis in mice. J Clin Invest. 2009;119:1858–70. doi: 10.1172/JCI37444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zaldivar MM, Pauels K, von Hundelshausen P, et al. CXC chemokine ligand 4 [Cxcl4] is a platelet-derived mediator of experimental liver fibrosis. Hepatology. 2010;51:1345–53. doi: 10.1002/hep.23435. [DOI] [PubMed] [Google Scholar]
- 38.Wasmuth HE, Lammert F, Zaldivar MM, et al. Anti-fibrotic effects of CXCL9 and its receptor CXCR3 in livers of mice and humans. Gastroenterology. 2009;137:309–19. 319, e1–3. doi: 10.1053/j.gastro.2009.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marra F, Bertolani C. Adipokines in liver diseases. Hepatology. 2009;50:957–969. doi: 10.1002/hep.23046. [DOI] [PubMed] [Google Scholar]
- 40.Ikejima K, Okumura K, Kon K, et al. Role of adipocytokines in hepatic fibrogenesis. J Gastroenterol Hepatol. 2007;22 (Suppl 1):S87–92. doi: 10.1111/j.1440-1746.2007.04961.x. [DOI] [PubMed] [Google Scholar]
- 41.Leclercq IA, Farrell GC, Schriemer R, et al. Leptin is essential for the hepatic fibrogenic response to chronic liver injury. J Hepatol. 2002;37:206–213. doi: 10.1016/s0168-8278(02)00102-2. [DOI] [PubMed] [Google Scholar]
- 42.Hoteit MA, Anania FA. Treatment of fibrosis in nonalcoholic fatty liver disease. Curr Gastroenterol Rep. 2007;9:47–53. doi: 10.1007/s11894-008-0020-0. [DOI] [PubMed] [Google Scholar]
- 43.Zhou Y, Jia X, Wang G, et al. PI-3 K/AKT and ERK signaling pathways mediate leptin-induced inhibition of PPARgamma gene expression in primary rat hepatic stellate cells. Mol Cell Biochem. 2009;325:131–9. doi: 10.1007/s11010-009-0027-3. [DOI] [PubMed] [Google Scholar]
- 44.Choi SS, Syn WK, Karaca GF, et al. Leptin promotes the myofibroblastic phenotype in hepatic stellate cells by activating the hedgehog pathway. J Biol Chem. 2010 doi: 10.1074/jbc.M110.168542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wedemeyer I, Bechmann LP, Odenthal M, et al. Adiponectin inhibits steatotic CD95/Fas up-regulation by hepatocytes: therapeutic implications for hepatitis C. J Hepatol. 2009;50:140–9. doi: 10.1016/j.jhep.2008.08.023. [DOI] [PubMed] [Google Scholar]
- 46.Yamaguchi K, Yang L, McCall S, et al. Inhibiting triglyceride synthesis improves hepatic steatosis but exacerbates liver damage and fibrosis in obese mice with nonalcoholic steatohepatitis. Hepatology. 2007;45:1366–74. doi: 10.1002/hep.21655. [DOI] [PubMed] [Google Scholar]
- 47.Mukhopadhyay B, Liu J, Osei-Hyiaman D, et al. Transcriptional regulation of cannabinoid receptor-1 expression in the liver by retinoic acid acting via retinoic acid receptor-gamma. J Biol Chem. 2010;285:19002–11. doi: 10.1074/jbc.M109.068460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jeong WI, Osei-Hyiaman D, Park O, et al. Paracrine Activation of Hepatic CB[1] Receptors by Stellate Cell-Derived Endocannabinoids Mediates Alcoholic Fatty Liver. Cell Metab. 2008;7:227–35. doi: 10.1016/j.cmet.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 49.Tam J, Vemuri VK, Liu J, et al. Peripheral CB1 cannabinoid receptor blockade improves cardiometabolic risk in mouse models of obesity. J Clin Invest. 2010;120:2953–66. doi: 10.1172/JCI42551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Teixeira-Clerc F, Julien B, Grenard P, et al. CB1 cannabinoid receptor antagonism: a new strategy for the treatment of liver fibrosis. Nat Med. 2006;12:671–6. doi: 10.1038/nm1421. [DOI] [PubMed] [Google Scholar]
- 51.Teixeira-Clerc F, Belot MP, Manin S, et al. Beneficial paracrine effects of cannabinoid receptor 2 on liver injury and regeneration. Hepatology. 2010;52:1046–59. doi: 10.1002/hep.23779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.De Minicis S, Candelaresi C, Marzioni M, et al. Role of endogenous opioids in modulating HSC activity in vitro and liver fibrosis in vivo. Gut. 2008;57:352–64. doi: 10.1136/gut.2007.120303. [DOI] [PubMed] [Google Scholar]
- 53.Ruddell RG, Oakley F, Hussain Z, et al. A role for serotonin [5-HT] in hepatic stellate cell function and liver fibrosis. Am J Pathol. 2006;169:861–76. doi: 10.2353/ajpath.2006.050767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zvibel I, Atias D, Phillips A, et al. Thyroid hormones induce activation of rat hepatic stellate cells through increased expression of p75 neurotrophin receptor and direct activation of Rho. Lab Invest. 2010;90:674–84. doi: 10.1038/labinvest.2010.48. [DOI] [PubMed] [Google Scholar]
- 55.Hellerbrand, Wang SC, Tsukamoto H, et al. Expression of intracellular adhesion molecule 1 by activated hepatic stellate cells. Hepatology. 1996;24:670–6. doi: 10.1002/hep.510240333. [DOI] [PubMed] [Google Scholar]
- 56.Bomble M, Tacke F, Rink L, et al. Analysis of antigen-presenting functionality of cultured rat hepatic stellate cells and transdifferentiated myofibroblasts. Biochem Biophys Res Commun. 2010;396:342–7. doi: 10.1016/j.bbrc.2010.04.094. [DOI] [PubMed] [Google Scholar]
- 57.Gao B, Radaeva S, Park O. Liver natural killer and natural killer T cells: immunobiology and emerging roles in liver diseases. J Leukoc Biol. 2009;86:513–528. doi: 10.1189/jlb.0309135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Szabo G, Mandrekar P, Dolganiuc A. Innate immune response and hepatic inflammation. Semin Liver Dis. 2007;27:339–50. doi: 10.1055/s-2007-991511. [DOI] [PubMed] [Google Scholar]
- 59.Muhanna N, Tair LA, Doron S, et al. Amelioration of hepatic fibrosis by NK cell activation. Gut. 2011;60:90–8. doi: 10.1136/gut.2010.211136. [DOI] [PubMed] [Google Scholar]
- 60.Wynn TA, Barron L. Macrophages: master regulators of inflammation and fibrosis. Semin Liver Dis. 2010;30:245–57. doi: 10.1055/s-0030-1255354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu C, Tao Q, Sun M, et al. Kupffer cells are associated with apoptosis, inflammation and fibrotic effects in hepatic fibrosis in rats. Lab Invest. 2010;90:1805–16. doi: 10.1038/labinvest.2010.123. [DOI] [PubMed] [Google Scholar]
- 62.Knolle PA, Gerken G. Local control of the immune response in the liver. Immunol Rev. 2000;174:21–34. doi: 10.1034/j.1600-0528.2002.017408.x. [DOI] [PubMed] [Google Scholar]
- 63.Guo J, Loke J, Zheng F, et al. Functional linkage of cirrhosis-predictive single nucleotide polymorphisms of Toll-like receptor 4 to hepatic stellate cell responses. Hepatology. 2009;49:960–8. doi: 10.1002/hep.22697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pradere JP, Troeger JS, Dapito DH, et al. Toll-like receptor 4 and hepatic fibrogenesis. Semin Liver Dis. 2010;30:232–44. doi: 10.1055/s-0030-1255353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Seki E, De Minicis S, Osterreicher CH, et al. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med. 2007;13:1324–32. doi: 10.1038/nm1663. [DOI] [PubMed] [Google Scholar]
- 66.Pinzani M, Abboud HE, Gesualdo L, et al. Regulation of macrophage colony-stimulating factor in liver fat-storing cells by peptide growth factors. Am J Physiol. 1992;262:C876–81. doi: 10.1152/ajpcell.1992.262.4.C876. [DOI] [PubMed] [Google Scholar]
- 67.Czaja MJ, Geerts A, Xu J, et al. Monocyte chemoattractant protein 1 [MCP-1] expression occurs in toxic rat liver injury and human liver disease. J Leukoc Biol. 1994;55:120–6. doi: 10.1002/jlb.55.1.120. [DOI] [PubMed] [Google Scholar]
- 68.Tiggelman AM, Boers W, Linthorst C, et al. Interleukin-6 production by human liver [myo]fibroblasts in culture. Evidence for a regulatory role of LPS, IL-1 beta and TNF alpha. J Hepatol. 1995;23:295–306. [PubMed] [Google Scholar]
- 69.Li Z, Yang S, Lin H, et al. Probiotics and antibodies to TNF inhibit inflammatory activity and improve nonalcoholic fatty liver disease. Hepatology. 2003;37:343–50. doi: 10.1053/jhep.2003.50048. [DOI] [PubMed] [Google Scholar]
- 70.Guimaraes EL, Empsen C, Geerts A, et al. Advanced glycation end products induce production of reactive oxygen species via the activation of NADPH oxidase in murine hepatic stellate cells. J Hepatol. 2010;52:389–97. doi: 10.1016/j.jhep.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 71.Jaeschke H. Inflammation in response to hepatocellular apoptosis. Hepatology. 2002;35:964–6. doi: 10.1053/jhep.2002.0350964. [DOI] [PubMed] [Google Scholar]
- 72.Zhan SS, Jiang JX, Wu J, et al. Phagocytosis of apoptotic bodies by hepatic stellate cells induces NADPH oxidase and is associated with liver fibrosis in vivo. Hepatology. 2006;43:435–43. doi: 10.1002/hep.21093. [DOI] [PubMed] [Google Scholar]
- 73.Jiang JX, Mikami K, Venugopal S, et al. Apoptotic body engulfment by hepatic stellate cells promotes their survival by the JAK/STAT and Akt/NF-kappaB-dependent pathways. J Hepatol. 2009;51:139–48. doi: 10.1016/j.jhep.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Watanabe A, Hashmi A, Gomes DA, et al. Apoptotic hepatocyte DNA inhibits hepatic stellate cell chemotaxis via toll-like receptor 9. Hepatology. 2007;46:1509–18. doi: 10.1002/hep.21867. [DOI] [PubMed] [Google Scholar]
- 75.Melhem A, Muhanna N, Bishara A, et al. Anti-fibrotic activity of NK cells in experimental liver injury through killing of activated HSC. J Hepatol. 2006;45:60–71. doi: 10.1016/j.jhep.2005.12.025. [DOI] [PubMed] [Google Scholar]
- 76.Mehal W, Imaeda A. Cell death and fibrogenesis. Semin Liver Dis. 2010;30:226–31. doi: 10.1055/s-0030-1255352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Knittel T, Dinter C, Kobold D, et al. Expression and regulation of cell adhesion molecules by hepatic stellate cells [HSC] of rat liver: involvement of HSC in recruitment of inflammatory cells during hepatic tissue repair. Am J Pathol. 1999;154:153–67. doi: 10.1016/s0002-9440(10)65262-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rosmorduc O, Housset C. Hypoxia: a link between fibrogenesis, angiogenesis, and carcinogenesis in liver disease. Semin Liver Dis. 2010;30:258–70. doi: 10.1055/s-0030-1255355. [DOI] [PubMed] [Google Scholar]
- 79.Kinnman N, Housset C. Peribiliary myofibroblasts in biliary type liver fibrosis. Front Biosci. 2002;7:D496–503. doi: 10.2741/A790. [DOI] [PubMed] [Google Scholar]
- 80.Sims D. Recent Advances in pericyte biology and implications for health and disease. Can J Cardiol. 1991;7:431–443. [PubMed] [Google Scholar]
- 81.Thimgan MS, Yee HF., Jr Quantitation of rat hepatic stellate cell contraction: stellate cells’ contribution to sinusoidal resistance. Am J Physiol. 1999;277:G137–43. doi: 10.1152/ajpgi.1999.277.1.G137. [DOI] [PubMed] [Google Scholar]
- 82.Shao R, Yan W, Rockey DC. Regulation of endothelin-1 synthesis by endothelin-converting enzyme-1 during wound healing. J Biol Chem. 1999;274:3228–34. doi: 10.1074/jbc.274.5.3228. [DOI] [PubMed] [Google Scholar]
- 83.Zhang X, Shan P, Otterbein LE, et al. Carbon monoxide inhibition of apoptosis during ischemia-reperfusion lung injury is dependent on the p38 mitogen-activated protein kinase pathway and involves caspase 3. J Biol Chem. 2003;278:1248–58. doi: 10.1074/jbc.M208419200. [DOI] [PubMed] [Google Scholar]
- 84.Theodorakis NG, Wang YN, Wu JM, et al. Role of endothelial nitric oxide synthase in the development of portal hypertension in the carbon tetrachloride induced liver fibrosis model. Am J Physiol Gastrointest Liver Physiol. 2009 doi: 10.1152/ajpgi.00229.2009. [DOI] [PubMed] [Google Scholar]
- 85.MacDonald GA, Bridle KR, Ward PJ, et al. Lipid peroxidation in hepatic steatosis in humans is associated with hepatic fibrosis and occurs predominately in acinar zone 3. J Gastroenterol Hepatol. 2001;16:599–606. doi: 10.1046/j.1440-1746.2001.02445.x. [DOI] [PubMed] [Google Scholar]
- 86.De Minicis S, Brenner DA. NOX in liver fibrosis. Arch Biochem Biophys. 2007;462:266–72. doi: 10.1016/j.abb.2007.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rao VA, Klein SR, Bonar SJ, et al. The antioxidant transcription factor Nrf2 negatively regulates autophagy and growth arrest induced by the anticancer redox agent mitoquinone. J Biol Chem. 285:34447–59. doi: 10.1074/jbc.M110.133579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gane EJ, Weilert F, Orr DW, et al. The mitochondria-targeted anti-oxidant mitoquinone decreases liver damage in a phase II study of hepatitis C patients. Liver Int. 30:1019–26. doi: 10.1111/j.1478-3231.2010.02250.x. [DOI] [PubMed] [Google Scholar]
- 89.Wobser H, Dorn C, Weiss TS, et al. Lipid accumulation in hepatocytes induces fibrogenic activation of hepatic stellate cells. Cell Res. 2009;19:996–1005. doi: 10.1038/cr.2009.73. [DOI] [PubMed] [Google Scholar]
- 90.Kasdallah-Grissa A, Mornagui B, Aouani E, et al. Resveratrol, a red wine polyphenol, attenuates ethanol-induced oxidative stress in rat liver. Life Sci. 2007;80:1033–9. doi: 10.1016/j.lfs.2006.11.044. [DOI] [PubMed] [Google Scholar]
- 91.Bechmann LP, Zahn D, Gieseler RK, et al. Resveratrol amplifies profibrogenic effects of free fatty acids on human hepatic stellate cells. Hepatol Res. 2009;39:601–8. doi: 10.1111/j.1872-034X.2008.00485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mann J, Mann DA. Transcriptional regulation of hepatic stellate cells. Adv Drug Deliv Rev. 2009;61:497–512. doi: 10.1016/j.addr.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 93.Rippe RA, Brenner DA. From quiescence to activation: Gene regulation in hepatic stellate cells. Gastroenterology. 2004;127:1260–2. doi: 10.1053/j.gastro.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 94.Mann J, Oakley F, Akiboye F, et al. Regulation of myofibroblast transdifferentiation by DNA methylation and MeCP2: implications for wound healing and fibrogenesis. Cell Death Differ. 2007;14:275–85. doi: 10.1038/sj.cdd.4401979. [DOI] [PubMed] [Google Scholar]
- 95.Tsukamoto H, She H, Hazra S, et al. Anti-adipogenic regulation underlies hepatic stellate cell transdifferentiation. J Gastroenterol Hepatol. 2006;21 (Suppl 3):S102–5. doi: 10.1111/j.1440-1746.2006.04573.x. [DOI] [PubMed] [Google Scholar]
- 96.Friedman SL. Hepatic Stellate Cells – Protean, Multifunctional, and Enigmatic Cells of the Liver. Physiological Reviews. 2008;88:125–172. doi: 10.1152/physrev.00013.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wandzioch E, Kolterud A, Jacobsson M, et al. Lhx2−/− mice develop liver fibrosis. Proc Natl Acad Sci U S A. 2004;101:16549–54. doi: 10.1073/pnas.0404678101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Adachi M, Osawa Y, Uchinami H, et al. The Forkhead Transcription Factor FoxO1 Regulates Proliferation and Transdifferentiation of Hepatic Stellate Cells. Gastroenterology. 2007;132:1434–46. doi: 10.1053/j.gastro.2007.01.033. [DOI] [PubMed] [Google Scholar]
- 99.Huang GC, Zhang JS, Tang QQ. Involvement of C/EBP-alpha gene in in vitro activation of rat hepatic stellate cells. Biochem Biophys Res Commun. 2004;324:1309–18. doi: 10.1016/j.bbrc.2004.09.196. [DOI] [PubMed] [Google Scholar]
- 100.Ramani K, Yang H, Kuhlenkamp J, et al. Changes in the expression of methionine adenosyltransferase genes and S-adenosylmethionine homeostasis during hepatic stellate cell activation. Hepatology. 2010;51:986–95. doi: 10.1002/hep.23411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Garzon R, Marcucci G, Croce CM. Targeting microRNAs in cancer: rationale, strategies and challenges. Nat Rev Drug Discov. 2010;9:775–89. doi: 10.1038/nrd3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Roderburg C, Urban GW, Bettermann K, et al. Micro-RNA profiling reveals a role for miR-29 in human and murine liver fibrosis. Hepatology. 2010 doi: 10.1002/hep.23922. [DOI] [PubMed] [Google Scholar]