Abstract
Identifying patients prior to treatment who are more likely to benefit from chemotherapeutic agents or more likely to experience adverse events is the aim of personalized medicine. Pharmacogenomics offers a potential means of achieving this through the discovery of predictive germline genetic biomarkers. When applied particularly to the treatment of head and neck cancers, such information could offer significant benefit to patients as a means of potentially reducing morbidity associated with platinum-based chemotherapy. We developed a genome-wide cell-based approach to identify single nucleotide polymorphisms (SNPs) associated with platinum susceptibility and then evaluated these SNPs as predictors for response and toxicity in head and neck cancer patients treated with platinum-based therapy as part of a phase II clinical trial. Sixty head and neck cancer patients were evaluated. Of 45 genome-wide SNPs examined, we found that two SNPs, rs6870861 (p=0.004; false discovery rate [FDR] <0.05) and rs2551038 (p=0.005; FDR <0.05), were significantly associated with overall response to carboplatin-based induction chemotherapy when incorporated into a model along with total carboplatin exposure. Interestingly, these two SNPs are strongly associated with the baseline expression of ≥20 genes (all p≤10−4) and two of the genes (SLC22A5 and SLCO4C1) are important organic cation/anion transporters known to affect platinum uptake and clearance. Several other SNPs were nominally associated with carboplatin-related hematologic toxicities. These findings importantly demonstrate that a genome-wide cell-based model can identify novel germline genetic biomarkers of platinum susceptibility which are replicable in a clinical setting with treated cancer patients and appear clinically meaningful for potentially enabling future personalization of care in such patients.
Keywords: pharmacogenomics, platinating agents, head and neck cancers
Introduction
The utilization of biomarkers of treatment susceptibility in the field of oncology is an important and burgeoning field since it represents a means towards potential personalization of cancer care. Pharmacogenomics represents one aspect of this effort, and genetic predictors of response or toxicity have been proposed or are being sought for an increasing number of chemotherapy agents. Such genetic information could allow physicians to pre-identify those patients most likely to respond to chemotherapies (or those most likely to experience severe side-effects but not benefit at all), or to predict which patients might require alternative doses.
Our laboratory recently refined a genome-wide approach that permits the identification of multi-genic chemotherapy susceptibility determinants based upon germline, rather than tumor DNA 1. Using a model which employs well-genotyped human lymphoblastoid cell lines (LCLs) 2 established from healthy individuals in the International HapMap Project 3, our laboratory has treated over 500 LCLs with various chemotherapy agents to produce individual drug-specific “sensitivity phenotypes” for each cell line. Then, genome-wide association studies (GWAS) —which have become widely used in the field of genetics 4 —are performed on these lines to associate the chosen phenotype (chemotherapy-related sensitivity) with germline single nucleotide polymorphisms (SNPs) and/or genes that are preferentially shared by the individuals demonstrating the phenotype, as compared to individuals who do not. This powerful, statistical association analysis allows identification of individual SNPs and genes which may govern a chemotherapy drug’s susceptibility. Importantly, it is unbiased to well studied genes, and has permitted the discovery of many never-before implicated SNPs influencing chemotherapy susceptibility. The next step in the validation of these SNPs is to demonstrate their utility in clinical models. Specifically, it remains to be tested whether, in patient samples, these SNPs will correlate with toxicity susceptibility, tumor sensitivity, or both. This study represents the important translational step of testing these novel genetic variants in an informative clinical setting.
Head and neck cancer therapy represents an appropriate and meaningful clinical setting in which to apply pharmacogenomics because morbidity can be significant during treatment 5, and treatment outcomes can be influenced greatly by whether a patient can tolerate and responds to chemotherapy 6. Recently, in an attempt to explore the potential for decreased toxicity without loss of efficacy, the use of carboplatin as a replacement for cisplatin in the treatment of head and neck cancer has been of interest. Carboplatin has been explored both as an agent in the induction setting 7, 8, and has been used concurrently with radiation therapy 9, 10. However, carboplatin is not without its own, potentially severe, toxicities—chiefly hematologic—and its profile of susceptibility may not completely overlap that of cisplatin, making predictive genetic biomarkers potentially very important to consider in the treatment decision.
We hypothesized that the top SNPs derived from our genome-wide cell-based model of carboplatin sensitivity would associate and validate as genetic “platinum susceptibility” biomarkers in a phase II clinical trial of patients receiving carboplatin-based therapy for head and neck cancer. We report here the pharmacogenomic findings from the evaluation of these biomarkers in these head and neck cancer patients. The clinical results of the therapeutic trial have been reported separately 11.
Methods
Cell Lines
The International HapMap LCLs were purchased from the Coriell Institute for Medical Research (Camden, NJ). Specifically, LCLs derived from 30 CEU trios (Centre d'Etude du Polymorphisme Humain [CEPH] comprised of Utah residents with northern and western European ancestry; HAPMAPPT01), 59 unrelated YRI (Yoruba people from Ibadan, Nigeria; HAPMAPPT03), 45 unrelated CHB (Han Chinese in Beijing; HAPMAPPT02); and 45 unrelated JPT (Japanese in Tokyo; HAPMAPPT05) were used in an in vitro cell based model for this study. These cell lines were diluted three times a week at a concentration of 200,000–350,000 cells/mL in RPMI 1640 media (Mediatech, Herndon, VA) supplemented with 15% fetal bovine serum (HyClone, Logan, UT) and 1% L-glutamine (MediaTech, Herndon,VA). Cell lines were maintained at a 37°C, 5% CO2 and 95% humidity environment.
Establishing Carboplatin Sensitivity in LCLs
Cell growth inhibition after increasing concentrations of carboplatin treatment (0, 10, 20, 40, and 80 µM for 72 hours) were evaluated using the alamar blue assay from Sigma (St. Louis, MO) 12. The carboplatin concentration at which cell growth is inhibited by 50%, termed IC50, along with the percentage of cellular survival after 10, 20, 40 and 80 µM carboplatin treatment were all considered as phenotypes of cellular sensitivity to carboplatin.
Identification of Genetic Predictors for Carboplatin Sensitivity
The overall study design can be found in Figure 1. A genome-wide triangular approach which integrates genotype, transcriptional gene expression, and cellular sensitivity to drug phenotypes developed in our lab 13 was applied to identify genetic predictors of carboplatin sensitivity. Specifically, GWAS was first performed between more than 2 million SNP genotypes and the cellular phenotypes of interest (including percent survival after increasing concentrations of carboplatin treatment as well as carboplatin IC50) in 30 CEU trios as described previously 12. All SNPs whose genotypes were associated with at least one carboplatin sensitivity phenotype at p≤0.0001 were further evaluated for whether they were related to the baseline mRNA expression of any genes using the SCAN database (www.scandb.org), an online resource we developed to evaluate genome-wide genetic and gene expression relationships 14. Bonferroni correction for multiple testing was employed in this step. Lastly, linear regression analysis was performed between the identified expression levels and the original carboplatin sensitivity phenotypes. This produced a list of SNPs and related genes that survived the full rigorous, triangular association testing.
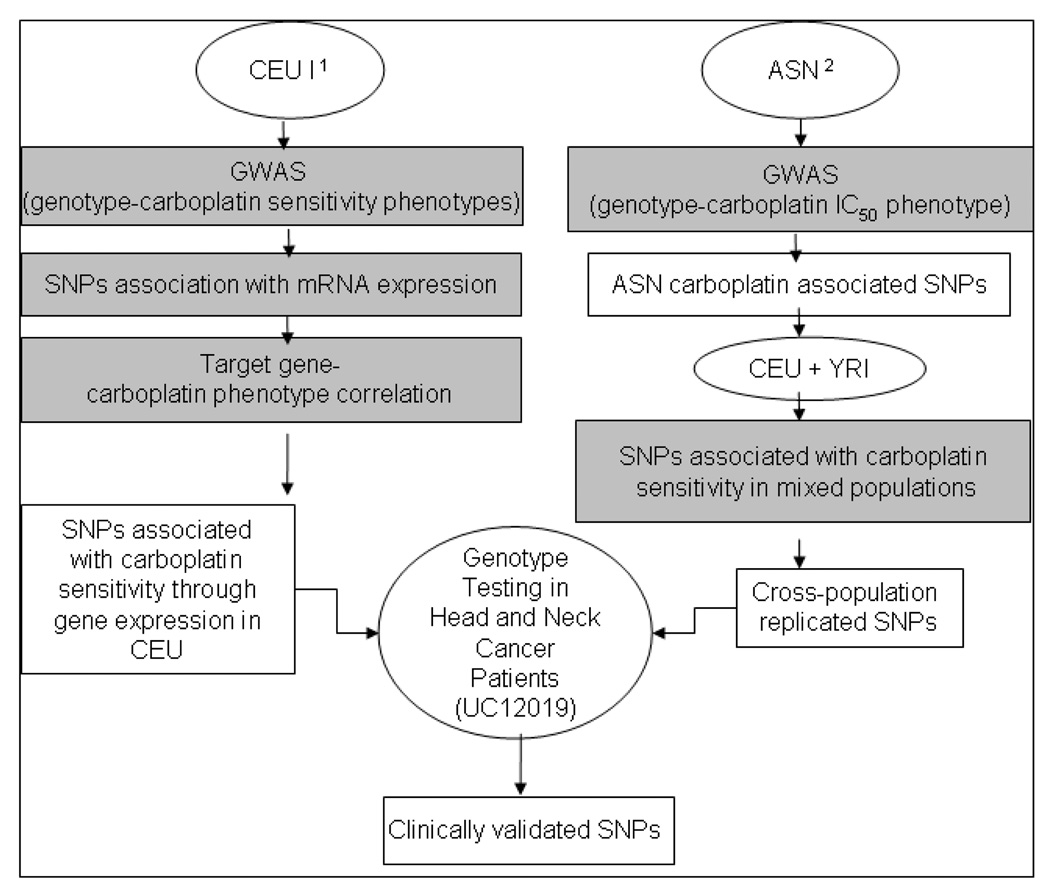
Figure 1. Flow chart of SNP discovery and validation methods used.
Pathway 1: The Triangle approach was used as described previously 13, which resulted in a list of SNPs associated with carboplatin sensitivity (p≤10−4) and also with baseline gene expression in these cell lines (p<3.8×10−6) and the gene expression of the “target gene” correlated back to carboplatin sensitivity (p<0.05). Pathway 2: Genome-wide association was performed on the “platinum sensitivity-enriched” ASN population (p<10−4) and the top ASN SNPs were replicated in a multi-ethnic population consisting of CEU and YRI samples (p<0.05) as described previously 15. SNPs from Pathways 1 and 2 were then combined and interrogated against clinically relevant phenotypes from a head and neck cancer phase II clinical trial involving carboplatin induction therapy. International HapMap lymphoblastoid cell lines (LCLs): CEU/CEPH are LCLs from Utah residents with northern and western European ancestry; YRI are LCLs from Yoruba people of Ibadan, Nigeria; ASN are LCLs from Asian individuals (Chinese in Beijing and Japanese in Tokyo). SNP = single nucleotide polymorphism.
Given the usually diverse clinical populations seen in the United States, in addition to identifying carboplatin sensitivity genetic predictors in samples derived from individuals with European ancestry (CEU), we expanded our SNP list to include SNPs identified through a cross-population approach in LCLs 15. This approach utilized a “platinum sensitivity-enriched” population (HapMap ASN) in an initial discovery setting. SNPs significantly associated with carboplatin sensitivity in these ASN samples were then independently validated in LCL samples derived from CEU and YRI populations.
Clinical Validation Testing
To test the clinical utility of SNPs identified through the cell-based LCL models, we evaluated clinical data generated from a phase II head and neck cancer (HNC) trial (University of Chicago Study IRB#12019 [UC12019]) 11. The trial received Institutional Review Board approval at The University of Chicago and was carried out according to the principles of the Declaration of Helsinki including informed consent. This study conforms to the relevant ethical guidelines for human and animal research. HNC patients enrolled in this trial had undergone treatment with carboplatin-containing regimens. Specifically, carboplatin and paclitaxel were used for induction therapy, followed by concomitant chemoradiotherapy with gefitinib, hydroxyurea and 5-fluorouracil (5-FU). The study required enrolled patients to have normal organ and marrow function at baseline, and a Karnovsky performance status ≥60%. Peripheral blood samples were obtained from study participants for DNA extraction. In addition, detailed baseline patient information and post-treatment response/toxicity data were available for all study participants. For the current exploratory pharmacogenomic analysis, we chose to evaluate four clinical endpoints: overall response post-carboplatin treatment, absolute count decreases in hemoglobin (Hgb), in white blood cells (WBC) and in platelets post-carboplatin-containing therapy.
SNP Genotyping in Patient Samples
Germline DNA was extracted from patients’ blood samples using the QIAamp Maxi Prep kit (Qiagen, Valencia, CA). The Sequenom MassARRAY iPLEX platform system was used for genotyping with 5–10 ng DNA used for each reaction. The iPLEX platform is a PCR-SBE (single base extension) genotyping assay that utilizes mass spectrometry to detect extended products. SNPs were processed using a multiplexed assay that utilized Sequenom’s Assay Design 3.1 program. The multiplexed PCR and iPLEX SBE reactions were arranged in a 384-well plate for all available patient samples and 30 HapMap controls, followed by the iPLEX Gold reaction protocol. PCR products were cleaned using an shrimp alkaline phosphatase reaction, followed by extension reactions in which 2 µl of iPLEX extend cocktail containing extend primer mix, iPLEX enzyme, termination mix and buffer was added to each multiplex PCR products. The extension primer mix for the moderate and high multiplexed reactions (2× 34-plex and 30-plex) was divided into 8/10/14µM based on the low/medium/high mass primers and for the low multiplexed reaction (20-plex) was divided into 7/14µM. The iPLEX reaction was performed using two-step 200 short cycles program in GeneAmp PCR System 9700 (Applied Biosystems, Carlsbad, CA). The SBE products were dispensed into a 384-sample SpectroCHIP and the CHIP was run on a MassARRAY Typer Workstation at The University of Chicago Genotyping Core Facility. Using matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry, the mass of the extended primer was determined for each SNP. The genotyping data was analyzed by Sequenom TYPER 4.0 software.
Statistical Analysis
Genotype-phenotype associations were performed using linear regression analysis. An additive genetic model was assumed, i.e. genotype was coded as the number of minor alleles for each SNP (0, 1, or 2). We used overall response after induction as the primary endpoint. In addition, decreases in Hgb, WBC and platelets post-carboplatin-containing therapy (calculated by subtracting post-induction complete blood counts [CBC] from baseline CBC) were also evaluated. All toxicity variables were log transformed in order to achieve approximate normality. We performed the analysis using the outcome as ordinal (proportional odds logistic regression, POLR) and as quantitative (simple linear regression). The results did not differ in any substantive way and we proceeded to treat the outcome as a quantitative variable to avoid convergence issues with the POLR that arose when the minor allele frequency was low. Given the relevance of carboplatin exposure on clinical response (Figure 2), we included carboplatin exposure as a covariate in the analysis. Adjusting for other covariates such as gender and race yielded similar results. False discovery rate (FDR) <0.05 was considered to be significant 16. All computations were performed using the R statistical software 17. FDR was computed using the qvalue package 18.
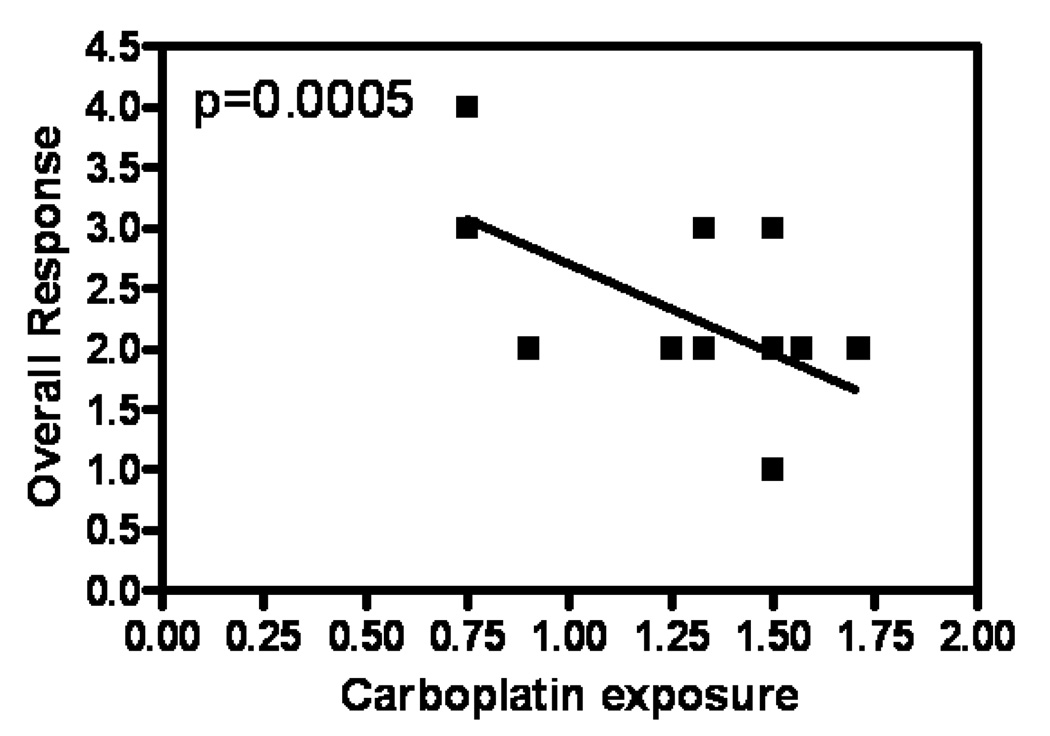
Figure 2. Delivered carboplatin dose correlates strongly with tumor response in head and neck cancer patients.
Linear regression analysis was performed between carboplatin exposure and response to drug in 52 head and neck cancer patients from the UC 12019 trial. The y-axis depicts overall response post-carboplatin treatment categorized into 4 groups; each was given a nominal value: 1 stands for complete response, 2 for partial response, 3 for stable disease and 4 for progressive disease. The x-axis, carboplatin exposure, was calculated by multiplying the desired carboplatin dose by the number of cycles of platinum given, divided by the time over which the drug was given. The negative correlation between carboplatin exposure and the four overall response categories suggests that the higher the carboplatin exposure, the better the response.
Results
Identifying Significant SNPs Associated with Carboplatin Sensitivity in Cell-based Models
Five cellular carboplatin sensitivity phenotypes (% survival after 10, 20, 40, 80 µM carboplatin treatment, and IC50) has been reported previously 19. These phenotypes were log-transformed and GWAS was performed between these phenotypes and more than 2 million CEU SNPs, independently. From the >2 million SNPs tested, we identified 997 as associated with carboplatin sensitivity for at least one individual phenotype (p≤ 0.0001). The number of SNPs associated with each phenotype is listed in Table 1. Using our previously-described SCAN database 14, we then identified which SNPs might be acting through gene expression, as a means to prioritize potentially functional SNPs (p<3.7×10−6) 20. Then, as a final filtering step, we interrogated whether the identified genes (associated with the remaining SNPs) were themselves correlated with carboplatin sensitivity phenotypes in the cell lines (p<0.05. Table 1). Via this cell-based triangular model, we identified a total of 62 SNPs that were associated with at least one carboplatin sensitivity phenotype through gene expression in the HapMap CEU samples. We performed linkage disequilibrium (LD) pruning on these 62 SNPs and tag SNPs were selected if a set of SNPs were in complete LD (defined as r2=1 in the HapMap CEU population).
Table 1.
Number of significant associations for each concentration of carboplatin.
| % survival phenotypes | |||||
|---|---|---|---|---|---|
| Approach | 10 µM | 20 µM | 40 µM | 80 µM | IC50 (µM) |
|
SNPs associated with phenotype (P≤0.0001) |
215 SNPs | 217 SNPs | 305 SNPs | 390 SNPs | 342 SNPs |
|
SNPs associated with gene expression (Pc<0.05) |
8 SNPs (16 genes) |
9 SNPs (14 genes) |
40 SNPs (44 genes) |
40 SNPs (40 genes) |
31 SNPs (29 genes) |
|
Gene expression correlated with phenotype (P<0.05) |
8 genes (6 SNPs) |
8 genes (8 SNPs) |
36 genes (33 SNPs) |
28 genes (30 SNPs) |
26 genes (31 SNPs) |
Pc = adjusted P value using Bonferroni correction.
After combining these with an additional 10 SNPs identified previously through a cross-population method (described fully in reference 15), we took forward 51 total SNPs to be genotyped in patients.
Clinical Replication Testing in HNC Patients
A total of 70 patients enrolled in the UC12019 trial, but only 60 had DNA available for analysis. The patient characteristics of those included in the analysis are shown in Table 2. Overall response post-carboplatin treatment data were available on 52 patients; decreases in Hgb, WBC and platelets were known for 59 patients.
Table 2.
Head and Neck Cancer Trial UC12019 – Patient Characteristics.
| Patients with Available DNA | n=60 | |
|---|---|---|
| Key Inclusion Requirements | Stage III or IV locally advanced disease | |
| Induction Therapy |
|
|
| Concomitant Chemoradiotherapy |
|
|
| Number of Patients (%) | ||
| Gender | Male | 42 (70.0) |
| Female | 18 (30.0) | |
| Race | Caucasian | 49 (81.7) |
| African-American | 10 (16.7) | |
| Asian | 1 (1.6) | |
| Age | Median (Range) | 55 yrs (30–77 yrs) |
| Metastatic Disease | 0 (0) | |
| Histologic Tumor Differentiation | Well | 4 (6.7) |
| Moderately well | 21 (35) | |
| Poorly | 22 (36.7) | |
| Undifferentiated | 4 (6.6) | |
| Unknown | 9 (15) | |
| PS | 0 | 43 (71.7) |
| 1 | 15 (25) | |
| 2 | 2 (3.3) | |
| Hematologic Toxicity ≥ grade 3* | Hgb | 6 (10) |
| WBC | 10 (16.7) | |
| ANC | 17 (28.3) | |
| Platelets | 3 (5) | |
RT, radiotherapy; PS, ECOG performance status; Hgb, hemoglobin; WBC, white blood cells; ANC, absolute neutrophil count;
according to CTC version 2 criteria.
Of the 51 SNPs selected for genotyping in patients, 45 were successfully genotyped using the iPLEX method. We found 3, 1, 4, and 8 (raw p<0.10) associations between genotype and each of the four clinical phenotypes (overall response, decreases in Hgb, WBC and platelets), respectively, after adjusting for carboplatin exposure (Table 3). Most notably, SNPs rs6870861 (p=0.004) and rs2551038 (p=0.005) were significantly associated (both FDR <0.05) with overall response (Table 3). It is likely that these two SNPs represent the same genomic signal since they are in high LD in CEU and ASN individuals (r2=0.89 in HNC patients). Based on HapMap data, rs2551038 is not polymorphic in Africans (YRI) while rs6870861 has a minor allele frequency (MAF) of 0.8% in these individuals. The MAFs of these 2 SNPs are similar at ~12% and ~21% in CEU and ASN, respectively.
Table 3.
Nominal associations between genotype and four carboplatin-related phenotypes: overall response, decreases in Hgb, WBC, and platelets (those associations satisfying raw p<0.10).
| SNP | P value | FDR | |
|---|---|---|---|
| Overall response | rs6870861 | 0.0040 | 0.046 |
| rs2551038 | 0.0049 | 0.0462 | |
| rs1426897 | 0.074 | 0.35 | |
| Decreases in Hgb | rs10798738 | 0.0511 | 0.179 |
| Decreases in WBC | rs7197684 | 0.040 | 0.627 |
| rs11994137 | 0.048 | 0.627 | |
| rs16882778 | 0.053 | 0.627 | |
| rs7134205* | 0.069 | 0.627 | |
| Decreases in platelets | rs7134205* | 0.0080 | 0.359 |
| rs11120986 | 0.024 | 0.415 | |
| rs11590447 | 0.037 | 0.415 | |
| rs115987056 | 0.0458 | 0.415 | |
| rs11120989 | 0.0511 | 0.415 | |
| rs6691275 | 0.0554 | 0.415 | |
| rs2333223 | 0.0780 | 0.480 | |
| rs1649942 | 0.089 | 0.480 |
The association analysis was performed with carboplatin dosage in the induction therapy as a covariate. Bold data are those with FDR<0.05.
Hgb, hemoglobin; WBC, white blood cells count; FDR, false discovery rate.
represents a SNP associated with both changes in WBC and platelets.
Regarding the carboplatin-related hematologic toxicities, while several SNPs were nominally associated with each clinical variable, none achieved statistical significance (FDR <0.05) after correction for multiple testing (Table 3). However, one SNP, rs7134205, was observed to be nominally associated with both decreases in WBC (p = 0.069) and platelets (p=0.008).
Discussion
Evaluating 45 novel SNPs derived from a cell-based genome-wide model, we found two significant SNPs as predictive genetic biomarkers of carboplatin-related outcomes in HNC patients treated with induction chemotherapy. The related SNPs rs6870861 and rs2551038 were notably associated with overall response to carboplatin-based therapy, showing, for the first time, that our cell-line derived, germline susceptibility SNPs not only can predict cell-based cytotoxicity-related phenotypes but also may represent tumor susceptibility biomarkers. Whether these genotype-outcome relationships are true for other settings in which cancer patients receive carboplatin is not known, but these findings appear to at least have potential utility for informing the use of carboplatin as a treatment in HNC.
These SNPs of interest, novel for their clinical association with carboplatin-mediated overall response in HNC, have some intriguing potential roles. While rs6870861 is not located within or near a known gene, rs2551038 is located in an intron of the gene HINT1 (histidine triad nucleotide binding protein 1, located on chromosome 5). HINT1 has interestingly been previously implicated as a tumor suppressor and an apoptosis mediator 21, 22 and variants within this gene have been associated with conditions as divergent as schizophrenia and nicotine dependence 23, 24. To our knowledge, this is the first time that a variant within HINT1 has been associated with platinum chemotherapy response. It also remains possible that the functional role of rs2551038 is actually exerted through regulation of a (perhaps distant) target gene. Indeed, in CEU HapMap cell lines, rs2551038 is a master regulator SNP 20associated with the baseline expression (p≤0.0001) of 20 different genes, adding credence to its possible importance. Two of the associated target genes are particularly striking. The first is SLC22A5 (also known as OCTN2), a member of the solute carrier 22 (organic cation/carnitine transporter) family. The SLC22A family has been under intensive investigation pertaining to platinum uptake and clearance in the kidney and gene family members have been shown to alter the pharmacokinetics of substrate drugs 25–27. The second target gene, SLCO4C1, is a kidney-specific organic anion transporter 28, and given that platinum compounds are primarily renally eliminated, one could hypothesize an obvious mechanism as to how variants affecting the expression of these genes could regulate platinum blood levels and thereby potentially influence anti-tumor platinum effects. These exciting findings therefore deserve further attention as a possible pharmacogenetic explanation of inter-individual differences in platinum response and susceptibility.
One additional SNP (rs7134205) was nominally associated with two different carboplatin-related hematologic toxicity phenotypes (WBC and platelet decreases). Of note, this SNP was previously reported by our lab in association with carboplatin susceptibility in LCLs from individuals representing multiple ethnicities 15, and importantly, this SNP was highly associated with the baseline expression (at least in Asian individuals’ LCLs) of HIST1H3A, a gene in the histone family which has logical mechanistic and demonstrated relevance for regulating platinum-adduct formation on DNA 29, 30. The potential association of this SNP with hematologic changes in HNC patients receiving carboplatin-based chemotherapy further increases its interest as a potentially important pharmacogenomic determinant.
It has long been recognized that increased drug dose often leads to increased treatment efficacy in the therapeutic range. This was supported by our clinical data that higher carboplatin exposure results in better response. Since the actual carboplatin exposure in the trial was determined after considering dose reduction due to toxicity, this suggests that identifying patients who will experience toxicity and require dose reduction a priori would be beneficial in the current clinical setting. Furthermore, treatment dosage has been shown to confound interpretation of other pharmacogenetic findings 31. Our study therefore used carboplatin exposure as a covariate, and the genetic variants we identified as associated with response should be interpreted as markers that affect treatment efficacy when an equivalent dose is given.
None of the typically-studied platinum candidate genes (ERCC1, ERCC2, XRCC1, ABCC2, ABCG2, MPO and GSTP) 32 were related to the validated SNPs in our study. This would further underscore the idea that a combined approach (genome-wide plus candidate gene methods) may likely ultimately yield the most comprehensive carboplatin pharmacogenomic evaluation in clinical trials. To date, there have been relatively few reports on predictive polymorphisms of platinum-based chemotherapy in HNC patients, and our study therefore provides a potentially useful companion to one study which previously examined cisplatin-specific SNPs in HNC patients 33.
Our study has several recognized limitations. The number of patients comprising the clinical study was admittedly relatively small, and beyond the limitation that this imposes from a statistical power standpoint, it would be clinically desirable and beneficial to further validate our finding in a much larger population of carboplatin-treated HNC patients to verify its predictive importance. Additionally, as previously discussed, although we attempted to isolate the pharmacogenomic associations of the replicated SNPs as specific to carboplatin, we cannot completely rule-out the possibility that the validated SNPs represent more general predictive markers of multi-drug or multimodality therapy susceptibility. However, because all of the tested SNPs were derived from carboplatin-specific methods using the pre-clinical cell-based model, and because we know that our genome-wide chemotherapy susceptibility findings are highly drug specific, this significantly increases our confidence that the clinically replicable findings are indeed related to a carboplatin-specific effect.
In summary, this study represents the first important translational validation of our genome-wide cell-based pharmacogenomic discovery method in a clinical setting. The findings deserve further replication and verification in a larger population of carboplatin-treated HNC patients but should be considered as a possible tool for incorporation in the decision-making process surrounding chemotherapy choices for HNC patients. The significantly-associated SNPs and other carboplatin susceptibility SNPs of interest also deserve testing in other clinical settings of patients with other cancers who are similarly receiving treatment with this widely-used anti-cancer drug.
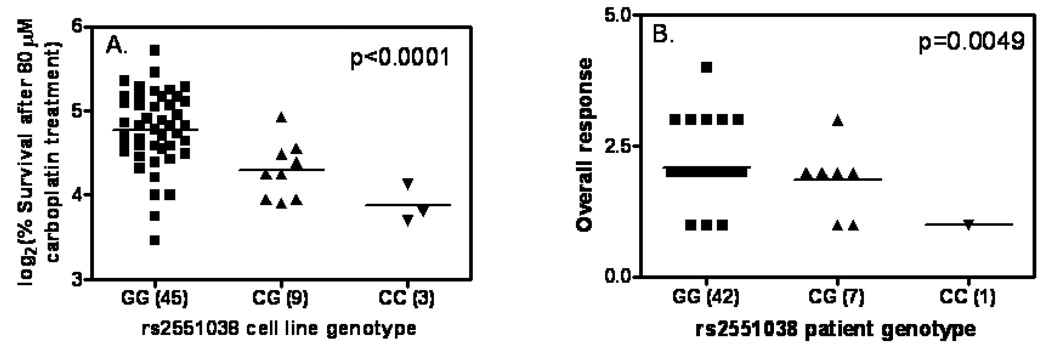
Figure 3. rs2551038 genotype is significantly associated with carboplatin sensitivity in vitro and in vivo.
A) Genotype and carboplatin sensitivity (depicted by % cellular survival after 80 µM carboplatin treatment) association in HapMap CEU samples. B) genotype and carboplatin sensitivity (depicted by tumor response post-carboplatin-containing induction therapy) association in UC12019 head and neck cancer patients. Overall response in 3B was categorized into 4 groups and each was given a nominal value: 1 stands for complete response, 2 for partial response, 3 for stable disease and 4 for progressive disease.
Acknowledgments
We thank the patients for consenting to the use of their clinical samples to perform the pharmacogenomic testing described herein. The clinical trial discussed in this study is registered with clinicaltrials.gov (study identifier NCT01172470).
This study is supported by NIH/NIGMS Pharmacogenomics of Anticancer Agents grant U01GM61393, the University of Chicago Breast Cancer SPORE grant P50 CA125183 and by NCI to the University of Chicago Comprehensive Cancer Center 01CA63187.
Abbreviations
- CEU
Centre d'Etude du Polymorphisme Humain (CEPH) people from Utah, USA
- YRI
Yoruba people from Ibadan, Nigeria
- LCLs
lymphoblastoid cell lines
- HNC
head and neck caner
- SNP
single nucleotide polymorphism
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no potential conflicts of interest to declare. The authors did not use editorial support for preparation of the manuscript.
References
- 1.Huang RS, Duan S, Bleibel WK, et al. A genome-wide approach to identify genetic variants that contribute to etoposide-induced cytotoxicity. PNAS. 2007;104:9758–9763. doi: 10.1073/pnas.0703736104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shukla S, Dolan M. Use of CEPH and non-CEPH lymphoblast cell lines in pharmacogenetic studies. Pharmacogenomics. 2005;6:303–310. doi: 10.1517/14622416.6.3.303. [DOI] [PubMed] [Google Scholar]
- 3.International HapMap Consortium. A haplotype map of the human genome. Nature. 2005;437:1299–1320. doi: 10.1038/nature04226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daly A. Genome-wide association studies in pharmacogenomics. Nat Rev Genet. 2010;11:241–246. doi: 10.1038/nrg2751. [DOI] [PubMed] [Google Scholar]
- 5.Marur S, Forastiere A. Challenges of integrating chemotherapy and targeted therapy with radiation in locally advanced head and neck squamous cell cancer. Curr Opin Oncol. 2010;22:206–211. doi: 10.1097/CCO.0b013e328338475c. [DOI] [PubMed] [Google Scholar]
- 6.Rosenthal D, Blanco A. Head and neck squamous cell carcinoma: optimizing the theraputic index. Expert Rev Anticancer Ther. 2005;5:501–514. doi: 10.1586/14737140.5.3.501. [DOI] [PubMed] [Google Scholar]
- 7.Choe K, Salama J, Stenson K, et al. Adjuvant chemotherapy prior to postoperative concurrent chemoradiotherapy for locoregionally advanced head and neck caner. Raidother Oncol. 2010 doi: 10.1016/j.radonc.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 8.Kies M, Holsinger F, Lee J, et al. Induction chemotherapy and cetuximab for locally advanced squamous cell carcinoma of the head and neck: results from a phase II prospective trial. Journal of Clinical Oncology. 2010;28:8–14. doi: 10.1200/JCO.2009.23.0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan A, Sanghera P, Choo B, et al. Hypofractionated Accelerated Raidotherapy with Concurrent Carboplatin for Locally Advanced Squamous Cell Carcinoma of the Head and Neck. Clin Oncol. 2010 doi: 10.1016/j.clon.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 10.Barkati M, Fortin B, Soulières D, et al. Concurrant chemoradiation with carboplatin-5-fluorouracil versus cisplatin in locallly advanced oropharyngeal cancers: is more always better? Int J Radiat Oncol Biol Phys. 2010;76:410–416. doi: 10.1016/j.ijrobp.2009.02.034. [DOI] [PubMed] [Google Scholar]
- 11.Cohen E, Haraf D, Kunnavakkam R, et al. Epidermal growth factor receptor inhibitor gefitinib added to chemoradiotherapy in locally advanced head and neck cancer. Journal of Clinical Oncology. 2010;28:3336–3343. doi: 10.1200/JCO.2009.27.0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang RS, Duan S, Kistner EO, Christine HM, Dolan ME. Genetic Variants Associated with Carboplatin-induced Cytotoxicity in Cell Lines derived from Africans Molecular Cancer Therapeutics. 2008;7:3038–3046. doi: 10.1158/1535-7163.MCT-08-0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang RS, Duan S, Shukla SJ, et al. Identification of Genetic Variants Contributing to Cisplatin-Induced Cytotoxicity using a Genome-wide Approach. Am J Hum Genet. 2007;81:427–437. doi: 10.1086/519850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gamazon ER, Zhang W, Konkashbaev A, et al. SCAN: SNP and copy number annotation. Bioinformatics. 2010;26:259–262. doi: 10.1093/bioinformatics/btp644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Donnell PH, Gamazon E, Zhang W, et al. Population differences in platinum toxicity as a means to identify novel genetic susceptibility variants. Pharmacogenet Genomics. 2010;20:327–337. doi: 10.1097/FPC.0b013e3283396c4e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Storey J, Tibshirani R. Statistical significance for genomewide studies. Proc Natl Acad Sci USA. 2003;100:9440–9445. doi: 10.1073/pnas.1530509100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dabney A, Storey JD, Warnes GR. qvalue: Q-value estimation for false discovery rate control. R package version 1.20.0. 2009 [Google Scholar]
- 18.Storey JD, Tibshirani R. Statistical significance for genome-wide studies. Proc Natl Acad Sci USA. 2003;100:9440–9445. doi: 10.1073/pnas.1530509100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shukla SJ, Duan S, Wu X, Badner JA, Kasza K, Dolan ME. Whole-genome approach implicates CD44 in cellular resistance to carboplatin. Hum Genomics. 2009;3:128–142. doi: 10.1186/1479-7364-3-2-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gamazon E, Huang RS, Cox NJ, Dolan ME. Chemotherapeutic Drug Susceptibility Associated SNPs are Enriched in Expression Quantitative Trait Loci. Proc Natl Acad Sci USA. 2010;107:9287–9292. doi: 10.1073/pnas.1001827107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y, Li H, Wu H, et al. Silencing of Hint1, a novel tumor suppressor gene, by promoter hypermethylation in hepatocellular carcinoma. Cancer Lett. 2009;275:277–284. doi: 10.1016/j.canlet.2008.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weiske J, Huber O. The histidine triad protein Hint1 triggers apoptosis independent of its enzymatic activity. Journal of Biological Chemistry. 2006;281:27356–27366. doi: 10.1074/jbc.M513452200. [DOI] [PubMed] [Google Scholar]
- 23.Jackson K, Chen Q, Chen J, Affen S, Kendler K, Chen X. Association of the histadine-triad nucleotide-binding protein-1 (HINT1) gene variants with nicotine dependence. Pharmacogenomics. 2010 doi: 10.1038/tpj.2010.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Q, Wang X, O'Neill F, Walsh D, Kendler K, Chen X. Is the histadine triad nucleotide-binding protein 1 (HINT1) gene a candidate for schizophrenia. Schizophr Res. 2008;106:200–207. doi: 10.1016/j.schres.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saito H. Pathophysical regulation of renal SLC22A organic ion transporters in acute kidney injury: pharmacological and toxicological implications. Pharmacol Ther. 2010;125:79–91. doi: 10.1016/j.pharmthera.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 26.Lancaster C, Hu C, Franke R, et al. Cisplatin-induced downregulation of OCTN2 affects carnitine wasting. Clin Cancer Res. 2010;16:4789–4799. doi: 10.1158/1078-0432.CCR-10-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Franke R, Kosloske A, Lancaster C, et al. Influence of Oct1/Oct2-deficiency on cisplatin-induced changes in urinary N-acetyl-beta-D-glucosaminidase. Clin Cancer Res. 2010;16:4198–4206. doi: 10.1158/1078-0432.CCR-10-0949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Toyohara T, Suzuki T, Morimoto R, et al. SLCO4C1 transporter eliminates uremic toxins and attenuates hypertension and renal inflammation. J Am Soc Nephrol. 2009;20:2546–2555. doi: 10.1681/ASN.2009070696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Galea A, Murray V. The interaction of cisplatin and analogues with DNA in reconstituted chromatin. Biochim Biophys Acta. 2002;1579:142–152. doi: 10.1016/s0167-4781(02)00535-3. [DOI] [PubMed] [Google Scholar]
- 30.Wu B, Dröge P, Davey C. Site selectivity of platinum anticancer therapeutics. Nat Chem Biol. 2008;4:110–112. doi: 10.1038/nchembio.2007.58. [DOI] [PubMed] [Google Scholar]
- 31.Hoskins J, Goldberg R, Qu P, Ibrahim J, McLeod H. UGT1A1*28 genotype and irinotecan-induced neutropenia: dose matters. J Natl Inst. 2007;99:1290–1295. doi: 10.1093/jnci/djm115. [DOI] [PubMed] [Google Scholar]
- 32.Marsh S, Paul J, King C, Gifford G, McLeod H, Brown R. Pharmacogenetic assessment of toxicity and outcome after platinum plus taxane chemotherapy in ovarian cancer: the Scottish Randomised Trial in Ovarian Cancer. J Clin Oncol. 2007;25:4528–4535. doi: 10.1200/JCO.2006.10.4752. [DOI] [PubMed] [Google Scholar]
- 33.Quintela-Fandino M, López J, Hitt R, et al. Breast cancer-specific mRNA transcripts presence in peripheral blood after adjuvant chemotherapy predicts poor survival among high-risk breast cancer patients treated with high-dose chemotherapy with peripheral blood stem cell support. Journal of Clinical Oncology. 2006;24:3611–3618. doi: 10.1200/JCO.2005.04.0576. [DOI] [PubMed] [Google Scholar]