SUMMARY
Septins are GTP-binding proteins that form ordered, rod-like multimeric complexes and polymerize into filaments, but how such supramolecular structure is related to septin function was unclear. In Saccharomyces cerevisiae, four septins form an apolar hetero-octamer (Cdc11–Cdc12–Cdc3–Cdc10–Cdc10–Cdc3–Cdc12–Cdc11) that associates end-to-end to form filaments. We show that septin filament assembly displays previously unanticipated plasticity. Cells lacking Cdc10 or Cdc11 are able to divide because the now-exposed subunits (Cdc3 or Cdc12, respectively) retain an ability to homodimerize via their so-called G interface, thereby allowing for filament assembly. In such cdc10Δ and cdc11Δ cells, the remaining septins, like wild-type complexes, localize to the cortex at the bud neck and compartmentalize non-septin factors, consistent with a diffusion barrier composed of continuous filaments in intimate contact with the plasma membrane. Conversely, Cdc10 or Cdc11 mutants that cannot self-associate, but “cap” Cdc3 or Cdc12, respectively, prevent filament formation, block cortical localization, and kill cells.
INTRODUCTION
Temperature-sensitive (ts) mutations in four genes (CDC3, CDC10, CDC11 and CDC12) identified by Hartwell (1971) cause the same terminal phenotype of failed cytokinesis, despite completion of nuclear division at the non-permissive temperature. At restrictive temperature, mutations in these four loci cause disappearance of a unique structure revealed by electron microscopy (EM)— a filamentous array subtending the plasma membrane (PM) at the bud neck (Byers & Goetsch, 1976a; ibid, 1976b). These four loci encode related proteins, dubbed septins, that localize to the bud neck (reviewed in McMurray & Thorner, 2008). Based on analysis of their dynamics, fluorescently-tagged septins in the “collar” at the bud neck are constrained with respect to mobility (Caviston, et al., 2003; Dobbelaere, et al., 2003) and relative orientation (Vrabioiu & Mitchison, 2006), consistent with highly organized structures. Native septins isolated from yeast (Frazier et al., 1998) and recombinant yeast septins purifed from bacteria (Versele et al., 2004; Farkasovsky et al., 2005) associate into complexes that can self-assemble into filaments in vitro, strongly suggesting that in the collar the four septins are co-assembled into filaments and required for cytokinesis.
Cytokinesis in S. cerevisiae involves coordinated contraction of an actomyosin ring and localized synthesis of a septum of cell wall material (Bi, 2001). These processes drive timely and orderly ingression of the PM and separation of the mother and daughter cells. Yeast cells still divide when either process is inoperative, but septin function is required for both, explaining the failure of cytokinesis in septin mutants (Bi, 2001). Specifically, septin localization at the incipient bud site is required for recruitment of actomyosin ring components (Bi, 2001; Lippincott & Li, 1998); but, once the contractile ring has assembled, septin structures are dispensable for ring stability and constriction (Dobbelaere & Barral, 2004). Similarly, enzymes involved in cell wall remodeling at the division site (e.g. Chs4/Skt5, an activator of chitin synthase III) are recruited in a septin-dependent manner early in the cell cycle, when septins are found as a diffuse ring before the prominent collar is established (DeMarini et al., 1997). Importantly, certain septin-associated factors remain co-localized with septins even in aberrant septin structures formed in response to experimental perturbations (Longtine et al., 1998; Roh et al., 2002). Thus, septin structures clearly serve as scaffolds to bind, and organize in space and time, machinery needed for cytokinesis.
To function, a scaffold requires temporal and/or spatial cues for its site-specific action and the capacity to recruit other factors. Septin arrival at the future bud site requires activation of the small GTPase Cdc42 and direct interaction with particular Cdc42 effector proteins (Iwase et al., 2006), as well as additional polarity factors. Moreover, in vitro, septins are able to interact with acidic phospholipids, consistent with direct PM contact in vivo, and PtdIns4,5P2, in particular, exerts rather profound effects on septin organization on model lipid monolayers (Bertin et al., 2010). For several proteins whose localization is septin-dependent (Gladfelter et al., 2001), direct binding to a septin (or bridged by another protein) has been convincingly demonstrated. For septins to fulfill a purely scaffold role, there is no strict requirement for filaments per se.
However, additional studies suggest a distinct, non-scaffold function for yeast septins. Septins at the bud neck restrict lateral diffusion of integral membrane proteins, thereby limiting the cortical distribution of factors that do not bind to the septins either directly or indirectly (Barral et al., 2000; Takizawa et al., 2000; Dobbelaere & Barral, 2004). A ring-like septin structure adjacent to the PM also is found in the midbody of dividing mammalian keratinocytes (Schmidt & Nichols, 2004), at the bases of dendritic spines (Tada et al., 2007; Xie et al., 2007), at the base of the primary cilium (Hu et al., 2010), and separating the head and tail in spermatozoa (Ihara et al., 2005; Kwitny et al., 2010). In each case, this structure is positioned to serve as a diffusion barrier that imposes compartmentation (Caudron & Barral, 2009). The simplest explanation for how septins establish a diffusion barrier is by forming continuous filaments tightly apposed to the PM. Such a “gasket” could prevent passage of integral membrane proteins by physically hindering their cytosolic domains, and/or by organizing PM lipids to impede lateral movement (Caudron & Barral, 2009). Thus, filament formation may be a critical, and perhaps conserved, feature of septin function.
Consistent with this concept, we ascertained that Cdc3, Cdc10, Cdc11 and Cdc12 stably co-assemble into linear rods with a defined composition (Cdc11–Cdc12–Cdc3–Cdc10–Cdc10–Cdc3–Cdc12–Cdc11) in the absence of any other yeast protein, and that these rods polymerize end-on-end in a highly cooperative fashion in low-salt solution to form long paired filaments (Bertin et al., 2008). However, viable yeast cells lacking Cdc10 or Cdc11 have been isolated in which neck structures reportedly were not detectable, from which it was concluded that organized filament arrays are not required for septin function (Frazier et al., 1998).
Our ultrastructural analysis of the supramolecular organization of the yeast septin hetero-octamer (Bertin et al., 2008), coupled with insights about the contacts involved in septin-septin interactions derived from the crystal structure of a mammalian hetero-hexameric septin complex (Sirajuddin et al., 2007; ibid, 2009), permitted design of experiments to test the relationship between filament assembly and septin function in budding yeast. We show here that septin complexes completely lacking certain subunits retain an ability to form filaments because loss of those subunits unmasks another subunit with an inherent capacity for self-association. In contrast, subunit alterations that abrogate the ability of complexes to form filaments prevent septin localization to the PM and cause cell death. Thus, filament-forming capacity, cortical septin localization, and cell survival are inextricably linked. Our findings demonstrate that, to execute at least one of its essential functions, the yeast septin complex must be able to polymerize into filaments.
RESULTS
Cdc10 and Cdc11 in Septin Filament Formation
In vitro polymerization of native or recombinant Cdc11–Cdc12–Cdc3–Cdc10–Cdc10–Cdc3–Cdc12–Cdc11 rods into filaments occurs at ≤150 mM salt (Farkasovsky et al., 2005; Frazier et al., 1998; Versele et al., 2004). Given their linear arrangement (Bertin et al., 2008), loss of any individual subunit should preclude filament assembly. Indeed, Cdc12–Cdc3–Cdc10–Cdc10–Cdc3–Cdc12 are stable, but did not form filaments in vitro under conditions in which wild-type (WT) complexes do (Bertin et al., 2008). In the absence of Cdc10, the remaining subunits produced Cdc11–Cdc12–Cdc3 trimers, but no detectable filaments at low salt (Bertin et al., 2008). Likewise, septin complexes isolated from cdc10Δ or cdc11Δ cells also failed to assemble into filaments in vitro (Frazier et al., 1998).
However, at sufficiently high protein concentration, recombinant Cdc10-less complexes self-associated, as detected by indirect immunofluorescence; but, in the EM, the structures formed were amorphous and disorganized, compared to WT filaments (Versele et al., 2004; Farkasovsky et al., 2005). Similar behavior was reported for Cdc11-less complexes (Farkasovsky et al., 2005). In our hands, at a protein concentration ≥150 μg-mL−1 and GTP (0.5 mM) present, Cdc11-less complexes associate into rudimentary filaments (Figure S1A). Under the same conditions, presence of a PtdIns4,5P2-containing lipid monolayer further enhances filament formation by Cdc11-less complexes and filamentous bundles by Cdc10-less complexes t(Bertin et al., 2010). The propensity of Cdc10-less and Cdc11-less complexes to display some degree of self-association in vitro prompted us to re-evaluate the conclusion that viability of cdc10Δ and cdc11Δ cells indicates that essential septin functions do not require filament assembly (Frazier et al., 1998).
Consequences of the Absence of Cdc10 or Cdc11
We removed the CDC10 or the CDC11 gene in the commonly-used strain BY4741 by direct deletion in a haploid (and by construction of a heterozygous diploid followed by sporulation to recover the null spores). Regardless of how a cdc10Δ mutation was introduced, haploid cells lacking Cdc10 exhibited severe defects in proliferation and morphology when initially cultivated at room temperature (~22°C; RT) on standard glucose (dextrose)-based rich medium (YPD) (Figure S2A). Upon serial repassaging, however, growth of cdc10Δ clones improved dramatically (Figure S2B and Supplemental Information), yet still displayed the ts behavior reported for other cdc10Δ derivatives (Versele et al., 2004). At 30° or below, these viable cdc10Δ haploids exhibited abnormal morphology (large cells with wide bud necks and often multiple buds) (Figure S2C); above 30°, they ceased growing and arrested as chains of unseparated cells.
In the course of this work, we found that loss of Cdc10 was tolerated better on galactose than on glucose medium (see Figure 2A and 3B). Consistent with some interplay between growth conditions, septin status, and transcription, others reported that a cdc10Δ mutation caused misexpression of certain genes in glucose-grown cells (Voronkova et al., 2006). Similarly, cdc11Δ derivatives of BY4741 were inviable on glucose medium at RT, as found in other strains (Versele et al., 2004), whereas on galactose medium, we were able to recover cdc11Δ derivatives (Figure S1B). Such viable cdc11Δ cells exhibited extremely slow growth and a highly elongated morphology, as described for viable cdc11Δ cells in an unrelated genetic background (Frazier et al., 1998). Propagation of cdc10Δ and cdc11Δ cells on galactose provided the opportunity to examine the contribution of the remaining septins to cell viability.
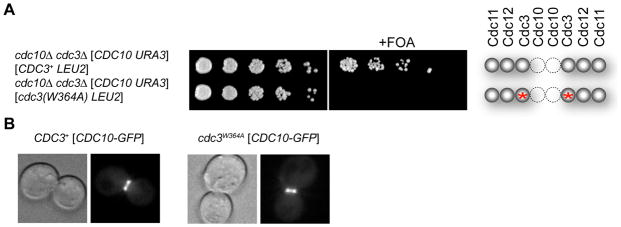
Figure 2. Survival of Cdc10-less Cells Requires Homodimerization of Cdc10 by Its G Interface.
(A) As in 1C, for cells of the indicated genotype; red asterisk, W364A mutation at G interface of Cdc3. (B) Morphology of representative cells of isogenic CDC3+ (left) or cdc3(W364A) strains (right) (derived from JTY5071) grown at 30°C, examined by differential interference contrast (DIC) microscopy (left panels) and co-expressing Cdc10-GFP (from plasmid pLA10), whose localization was determined by epifluorescence microscopy (right panels). See also Figure S2.
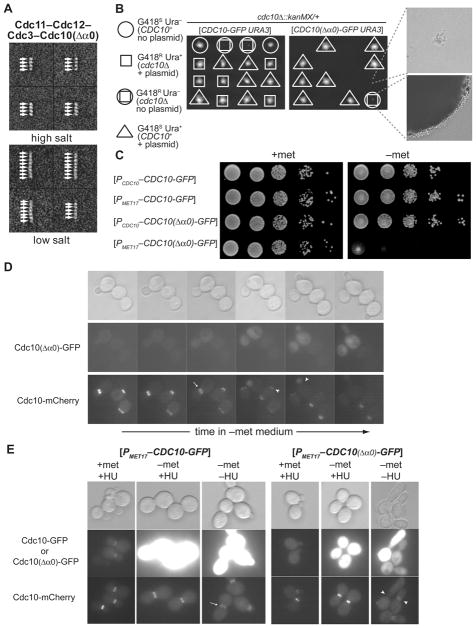
Figure 3. Blocking Filament Assembly is Lethal and Prevents Cortical Septin Localization.
(A) Representative class averages of negatively-stained particles of purified recombinant Cdc11–Cdc12–Cdc3–Cdc10(Δα0) complexes in 300 mM (top) and 60 mM (bottom) KCl buffer. (B) Growth of representative meiotic progeny of cdc10Δ::kanMX/CDC10+ cells (strain 4003482™) carrying either a URA3-marked plasmid expressing CDC10-GFP (left) or the same vector expressing cdc10(Δα0)-GFP (right) on YPD medium at 26° for 4 dy. Far right, images of representative clones of cdc10Δ spores either carrying (top) or lacking (bottom) the Cdc10(Δα0) plasmid. (C) Cultures of strain JTY3993 carrying URA3-marked plasmids expressing either CDC10-GFP or cdc10(Δα0)-GFP from either the CDC10 promoter (PCDC10) or the Met-repressible MET17 promoter (PMET17), as indicated, were grown in SCGlc-Ura and ten-fold serial dilutions were plated on the same medium either containing (top) or lacking (bottom) Met, and the plates were incubated at 26° C for 3 dy. (D) Strain JTY3993, which expresses CDC10-mCherry from the chomosomal CDC10 locus, and also carrying the URA3-marked PMET17-cdc10(Δα0)-GFP plasmid (pJT3456), was grown in SCGlc-Ura in the presence of Met, washed, spotted on an agarose pad containing the same medium lacking Met, incubated under a coverslip at RT, and examined periodically using DIC optics (top) and by epifluorescence microscopy using the appropriate filters to visualize GFP (middle) and mCherry (bottom). (E) JTY3993 cells carrying either the PMET17-CDC10-GFP plasmid (pJT2696) or the PMET17-cdc10(Δα0)-GFP plasmid (pJT3456) were grown in SCGlc-Ura in the presence of Met to mid-exponential phase, then exposed in the same medium to the ribonucleotide reductase (and DNA replication) inhibitor hydroxyurea (HU; 200 mM final concentration) and incubated for 2 h (left columns), then transferred to Met-free HU-containing medium and incubated for 7 h (center columns), and finally transferred to Met-free medium lacking HU for 1 h (right columns). Arrows, normal split rings; arrowheads, abnormal septin structures. See also Figure S3.
Cdc12 Homodimerization is Required for Survival of Cdc11-less Cells
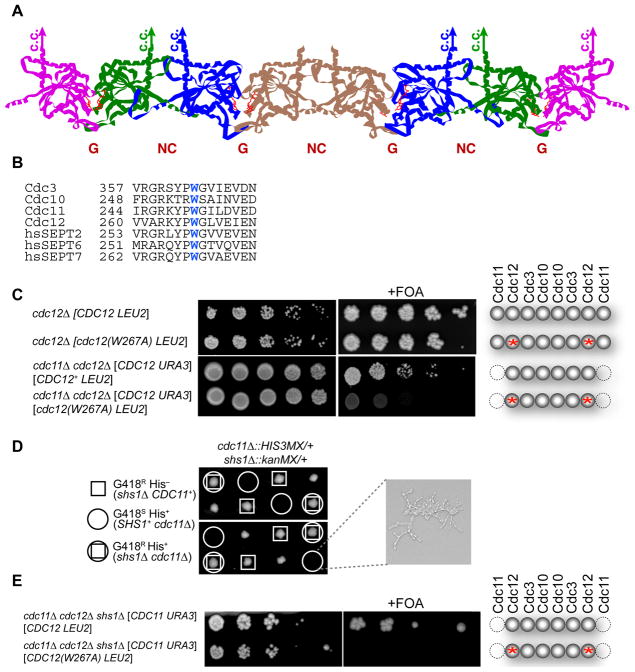
As deduced from the crystal structure of a human hetero-hexameric septin complex (SEPT7–SEPT6–SEPT2–SEPT2–SEPT6–SEPT7) (Sirajuddin et al., 2007), and confirmed for the yeast septin rod (Bertin et al., 2008), adjacent monomers in septin complexes interact by two alternative contact modes— a “G interface” (residues in and around the GTP-binding pockets) and an “NC interface” (residues in and around the N- and C-terminal segments of the GTP-binding domains) (Figure 1A). Cdc12, the penultimate subunit at each end of the yeast hetero-octamer, associates with the terminal Cdc11 subunit via a G interface. Formation of an NC interface between Cdc11 subunits on neighboring rods mediates filament formation (Bertin et al., 2008). Aside from the Cdc11-Cdc11 coupling that mediates rod-rod association, the only other homodimeric contact is the central Cdc10–Cdc10 doublet mediated by an NC interface. However, when expressed individually and purified from bacteria, each yeast septin exhibits a detectable degree of self-association (Versele et al., 2004). Likewise, human SEPT2 pairs with itself at the center of the SEPT7–SEPT6–SEPT2–SEPT2–SEPT6–SEPT7 heterohexamer via an NC interface, whereas when expressed alone, it can form homodimers via its G interface (Sirajuddin et al., 2007). Moreover, since the human heterohexamer polymerizes in vitro into filaments, yet is one subunit shorter at each end than the yeast hetero-octamer, a G interface must mediate SEPT7-SEPT7 interaction. We reasoned, therefore, that survival of cdc11Δ mutants might depend on homodimerization of the now-exposed Cdc12 subunit via its G interface, thereby permitting Cdc11-less complexes to polymerize into filaments.
Figure 1. Survival of Cdc11-less Cells Requires Homodimerization of Cdc12 by Its G Interface.
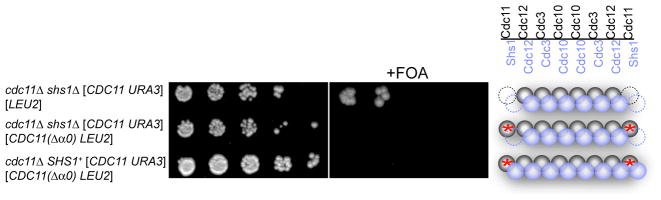
(A) Model of the subunit arrangement in the yeast septin hetero-octamer (McMurray & Thorner, 2008); Cdc3 (blue), Cdc10 (brown); Cdc11 (pink); Cdc12 (green). (B) Sequence conservation of the Trp contact residue in the G interface. (C) Cultures of the indicated genotypes were grown in YPGal, spotted in five-fold serial dilutions onto agar plates containing SCGal-Leu medium in the absence (left) or presence (right) of FOA to select against URA3-containing cells, and photographed after incubation at RT for 5–6 dy. Far right, schematic depiction of septin composition in cells that have lost the URA3 plasmid; red asterisk, W267A mutation at G interface of Cdc12. (D) Strains MJY201 and JTY3631 were crossed, generating (after loss of a URA3-marked plasmid) the indicated cdc11Δ0::HIS3MX/CDC11+ shs1Δ0::kanMX/SHS1+ diploid, which was subjected to sporulation and tetrad dissection. Shown are representative meiotic progeny of the indicated genotypes after incubation on YPD medium at 26° for 4 dy. Far right, representative image of the type of microcolony formed by the cdc11Δ SHS1+ spores, which all failed to form a viable clone. (E) As in (C), except that the cdc11Δ cells also lacked Shs1. See also Figure S1.
To test this idea, we mutated a conserved Trp in Cdc12 (W267) (Figure 1B). In SEPT2, the Trp at the corresponding position contributes significantly to the buried hydrophobic surface at the G interface and, when mutated to Ala, destroys the ability of SEPT2 to homodimerize via its G interface (Sirajuddin et al., 2007). The cdc12(W267A) mutation is tolerated in otherwise WT cells (Figure 1C, second row) because, as we demonstrated in vitro (Bertin et al., 2008), this substitution only slightly destabilizes the otherwise normal Cdc12–Cdc11 G interface. The presence of the corresponding conserved Trp in Cdc11 (W251) (Figure 1B) presumably makes an adequate contribution to the hydrophobic contact surface. Thus, as for SEPT2, the Cdc12(W267A) mutation should disrupt only the ability of Cdc12 to homodimerize via its G interface. Indeed, on galactose medium, unlike cdc11Δ mutants that express WT Cdc12, those expressing the cdc12(W267A) allele were inviable (Figure 1C, bottom row). The need for a Cdc12 G homodimer interface for survival of Cdc11-less cells is consistent with Cdc12–Cdc3–Cdc10–Cdc10–Cdc3–Cdc12 filament formation via Cdc12 self-association.
We previously proposed that septin Shs1 can replace Cdc11 in yeast hetero-octamers (Bertin et al., 2008), and have further evidence that such Shs1-tipped rods cannot polymerize end-to-end (data not shown). Also, others showed that SHS1 overexpression kills cdc11Δ cells, but not CDC11+ cells (Iwase et al., 2007). These observations suggest that, when no competing Cdc11 is present, Shs1 caps the Cdc12 subunit and prevents polymerization of Cdc12–Cdc3–Cdc10–Cdc10–Cdc3–Cdc12 complexes. If so, then a cdc11Δ shs1Δ double mutant should be viable. Indeed, we found that an shs1Δ mutation permitted the survival of cdc11Δ cells on glucose medium (Figure 1D). By contrast, cdc11Δ shs1Δ double mutants expressing Cdc12(W267A) as the sole source of Cdc12 are dead (Figure 1E), corroborating the conclusion that Cdc12-Cdc12 contact mediated via its G interface is required for viability of cdc11Δ shs1Δ cells.
Cdc3 Homodimerization is Required for Survival of Cdc10-less Cells
By similar reasoning, it seemed likely that cdc10Δ cells survive because homodimerization of the now-exposed Cdc3 subunits via their G interface assembles Cdc11–Cdc12–Cdc3–Cdc3–Cdc12–Cdc11 hexamers that maintain the capacity for filament formation via the normal Cdc11–Cdc11 interaction.
We tested this idea, first, by mutating the conserved Trp in Cdc3 (W364) (Figure 1B), which should cripple Cdc3 homodimer formation mediated by its G interface [but not the G interface in normal Cdc3-Cdc10 heterodimers]. Indeed, when the cdc3(W364A) allele was the sole source of Cdc3, cells lacking Cdc10 were dead under all conditions tested (Figure 2A). In contrast, when cdc3(W364A) was the source of Cdc3 in isogenic CDC10+ cells, they were viable at temperatures up to 30° and, in such cells, Cdc10-GFP was localized normally at the bud neck (Figure 2B).
Blocking Filament Assembly is Lethal and Prevents Cortical Septin Localization
Expression of Cdc10(Δ13–28), henceforth Cdc10(Δα0), a derivative of Cdc10 that has an intact G interface and is able to interact with Cdc3, but lacks a functional NC interface and is incapable of forming Cdc10 homodimers, should permit assembly of only Cdc11–Cdc12–Cdc3–Cdc10(Δα0) hetero-tetramers that are unable to polymerize into filaments. If filament formation is required for viability, then cells expressing Cdc10(Δα0) as the sole source of Cdc10 should be dead. To confirm that Cdc10(Δα0) has the expected properties, we prepared recombinant septin complexes containing Cdc3, Cdc11, Cdc12 and Cdc10(Δα0) and examined the resulting particles by EM. As expected, in high salt, only tetramers were observed (Figure 3A, top) and, in low salt, the largest particles were “inside-out” Cdc10(Δα0)–Cdc3–Cdc12–Cdc11–Cdc11–Cdc12–Cdc3–Cdc10(Δα0) complexes (Figure 3A, bottom), but never any filaments. Accordingly, upon meiosis and sporulation, a heterozygous cdc10Δ::kanMX/CDC10 diploid expressing Cdc10(Δα0) from a URA3-marked CEN plasmid (Figure 3B, right panel), yielded colony-forming G418R Ura− (cdc10Δ) spores, but no viable G418R Ura+ clone [cdc10Δ expressing Cdc10(Δα0)] (spores of this apparent genotype ceased growth after 3–4 divisions). By contrast, G418R Ura+ clones were readily recovered from the same diploid expressing WT CDC10+ from the same vector (Figure 3B, left panel). Lethality of Cdc10(Δα0) was due to lack of its NC interface, and not due to removal by the Δα0 mutation of basic residues thought to mediate interaction with PM phospholipids (Fig. S4A), because cells expressing a Cdc10 mutant in which all the basic residues are replaced by Ala were viable (Fig. S4B). By contrast, a Cdc10 Ile22Glu mutant, replacing a single non-polar residue predicted to make an important NC interface contact, did not support growth (Figure S4C; see also Supplemental Information). Thus, expressing Cdc10(Δα0) in the presence of Cdc3, Cdc11 and Cdc12 is lethal. Clearly, capping Cdc3 yields a decidedly worse phenotype than having no Cdc10 at all, consistent with preventing assembly of Cdc11–Cdc12–Cdc3–Cdc3–Cdc12–Cdc11 complexes and abrogating filament formation.
In further agreement with this view, high-level production of Cdc10(Δα0) from the MET17 promoter (Rönicke et al., 1997) was toxic to otherwise WT cells, whereas overproduction of normal Cdc10 had no effect (Figure 3C). Moreover, when septin filament assembly at the bud neck was visualized using chomosomally expressed Cdc10-mCherry, and production of MET17 promoter-driven Cdc10(Δα0)-GFP from a CEN plasmid was monitored in the same cells, there was an inverse correlation between the level of Cdc10(Δα0)-GFP present and the amount of Cdc10-mCherry incorporated at the bud neck (Figure 3D), as expected if Cdc10(Δα0) competes with normal Cdc10 for interaction with Cdc3 and thereby prevents septin filament assembly. High-level Cdc10(Δα0)-GFP also prevented each of the other septin subunits from localizing to the cortex (Figure S3), suggesting that septin complexes containing this mutant subunit were incapable of stable recruitment to the PM. Correspondingly, we found that Cdc10(Δα0) only had this effect if it was produced prior to the time when septin complexes normally assemble into filaments at the bud neck (Figure 3E). If cells were arrested, first, in S phase with hydroxyurea (HU), a cell cycle stage at which the filamentous septin collar has already formed (Versele & Thorner, 2004), subsequent high-level over-production of Cdc10(Δα0)-GFP did not displace Cdc10-mCherry from the bud neck, whereas the same level of Cdc10(Δα0)-GFP production in the absence of the HU block efficiently displaced Cdc10-mCherry from the neck and induced a highly elongated bud (Figure 3E), a hallmark of dysfunctional septin filaments (Shulewitz et al., 1999; Lew, 2003).
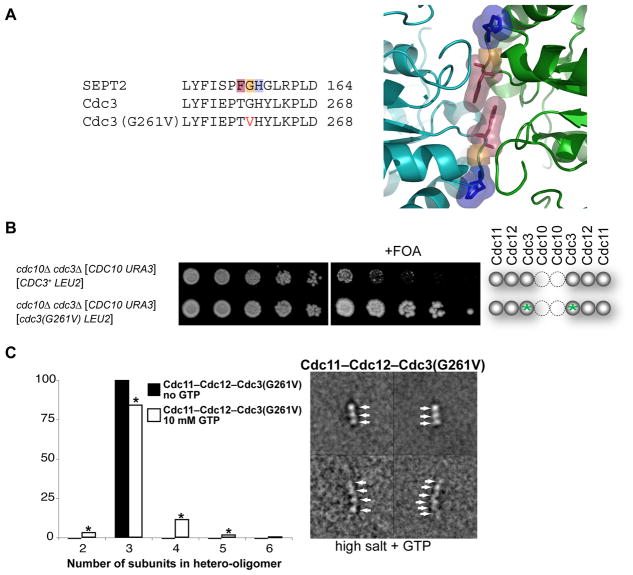
Stabilization of the Cdc3-Cdc3 G Interface Promotes Survival of Cdc10-less Cells
Although cells lacking Cdc10 survive, they exhibit wider-than-normal bud necks and are ts for growth. The abnormal morphology could arise from altered geometry imposed by filaments formed from Cdc11–Cdc12–Cdc3–Cdc3–Cdc12–Cdc11 hexamers and these structures may be unstable at higher temperatures because the non-canonical Cdc3-Cdc3 junction is non-optimal. To test the latter possibility, we selected at 37°C a spontaneous temperature-resistant variant of our cdc10Δ mutant, recovered the DNA, and sequenced the CDC3 locus from these cells. We found that codon 261 (Gly; GGA) in the CDC3 ORF was mutated to Val (GUA) (Figure 4A, left). Equivalently positioned side chains are located at the G interface between SEPT2 homodimers (Sirajuddin et al., 2007; ibid, 2009) (Figure 4A, right). We presume, therefore, that the Cdc3(G261V) mutation confers greater stability because, unlike Gly, Val contributes to the buried hydrophobic surface area at the G interface in the central homodimer of Cdc11–Cdc12–Cdc3–Cdc3–Cdc12–Cdc11 hexamers (and/or because this substitution stabilizes the conformation at the Cdc3 G interface most competent for self-association). Further genetic analysis showed that the cdc3(G261V) allele was necessary, but not sufficient, for the spontaneous temperature-resistant isolate of the cdc10Δ strain to grow at 37°C (Figure S2H), indicating that at least one other alteration was responsible for robust growth at the restrictive temperature. Nonetheless, our findings clearly indicated that G261V in Cdc3 is a major contributing factor because, even on glucose medium and at RT, cdc10Δ cells expressing Cdc3(G261V) displayed markedly better growth, relative to control cdc10Δ cells expressing normal Cdc3 (Figure 4B).
Figure 4. Mutation at the G Interface of Cdc3 Enhances Survival of Cdc10-less Cells.
(A) Sequence alignment (left) of human SEPT2 with WT Cdc3 and Cdc3(G261V). Highlighting (Phe, pink; Gly, orange; His, blue) refers to same residues shown (right) in the close-up of the G homodimer interface of GppNHp-bound mouse SEPT2 (PDB accession no. 3FTQ) (Sirajuddin et al., 2009). (B) As in 1C, except grown on SCGlc-Leu and incubated for 3 (left) or 5 dy (right); green asterisks, cdc3(G261V) mutation. (C) Purified recombinant Cdc11–Cdc12–Cdc3(G261V) complexes were examined by EM in 300 mM salt buffer in the absence (solid bars) and presence (open bars) of 10 mM GTP; frequencies of particle classes observed (representative examples on the right) plotted as histograms (left). All 824 particles (100%) in the absence of GTP were trimers; 3358 particles in the presence of GTP were 97 dimers (3%), 2831 trimers (84%), 376 tetramers (11%), 46 pentamers (1%), and 8 hexamers (0.2%). Asterisk, classes whose appearance depended on presence of GTP at a statistically significant level (P < 0.0001, according to a two-tailed Fisher’s exact test).
Furthermore, when expressed in and purified from bacterial cells, and examined in the EM in high-salt conditions (which obviate Cdc11-Cdc11 interaction) (Bertin et al., 2008), a detectable fraction of the recombinant septin complexes composed of Cdc3(G261V), Cdc11, and Cdc12 were larger than the trimers we exclusively observe when Cdc3 is WT [Bertin, et al. 2008]. When guanine nucleotide was present, the Cdc11–Cdc12–Cdc3(G261V) complex formed hexamers, albeit very rarely (Figure 4C), as expected for formation of a Cdc11–Cdc12–Cdc3(G261V)–Cdc3(G261V)–Cdc12–Cdc11 rod in which Cdc3 self-association is mediated by a G interface. As shown previously (Versele et al., 2004; Farkasovsky et al., 2005), such Cdc10-less complexes are able to assemble into bundled filaments under low-salt conditions.
Blocking Hetero-octamer Polymerization Causes Lethality
As another independent test of the concept that septin filament formation is required for yeast cell viability, we examined cells expressing Cdc11(Δα0), a mutant that lacks residues 2–12 of an α-helix critical for the NC interface that mediates Cdc11-Cdc11 interaction. Septin complexes containing Cdc11(Δα0) are stable hetero-octamers in vitro; but, these rods are incapable of end-to-end polymerization under low-salt conditions (Bertin et al., 2008). We reasoned that, in vivo, Cdc11(Δα0) should generate hetero-octamers capped with a subunit unable to mediate filament assembly. If filament formation is required for viability, this maneuver should kill the cells. Indeed, cells expressing the cdc11(Δα0) allele as the sole source of Cdc11 are inviable (Figure 5). Again, this lethality was not due to elimination of the basic residues within the Cdc11 α0 helix postulated to mediate interaction with acidic phospholipids, because a mutant allele of CDC11 in which these residues were replaced by Ala was not lethal (Figure S4D).
Figure 5. Blocking Hetero-octamer Polymerization is Lethal.
Cultures of the indicated genotype were grown in YPGal, spotted in five-fold serial dilutions onto agar plates containing SCGal-Leu medium in the absence (left) or presence (right) of FOA, and photographed after incubation at RT for 5–6 dy. Far right, schematic depiction of septin composition in cells that have lost the URA3 plasmid; red asterisk, Δα0 mutation at the NC interface of Cdc11; grey spheres, septin subunits of complexes where Cdc11 normally occupies the terminal position; blue spheres, septin subunits of complexes where Shs1 normally occupies the terminal position. See also Figure S4.
Consistent with the conclusion that rod-rod association is necessary for cell viability, high-level over-expression of either CDC11 or SHS1, but no other septin gene, is growth inhibitory and causes elongated buds (Iwase et al., 2007; Sopko et al., 2007). These effects are readily explained in light of our findings. For SHS1, displacement of the Cdc11 subunits from one or both the ends in hetero-octamers will abrogate filament formation. For CDC11, excess free Cdc11 will cap the ends of the rods by an NC interaction, blocking filament polymerization.
Localization and Diffusion Barrier Function are Maintained in Cdc10-less and Cdc11-less Cells
Our findings show that polymerization of septin hetero-octamers into filaments is required for viability and that mutants lacking Cdc10 and Cdc11 survive because the remaining septins can assemble into filaments via unconventional subunit-subunit interactions. Although the subunit arrangement in Cdc10-less or Cdc11-less septin complexes is aberrant due to lack of these components and, consequently, subunit order in the resulting filaments is abnormal, essential features of standard septin filaments must be preserved to a greater or lesser extent to account for the viability of cdc10Δ and cdc11Δ cells.
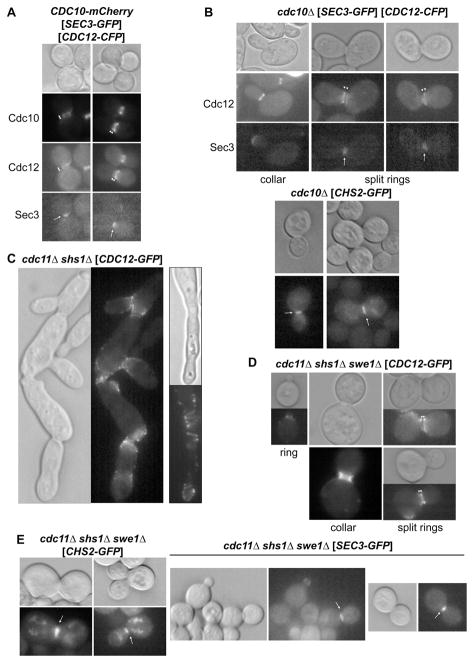
Indeed, as in CDC10+ cells (Figure 6A), septin complexes in Cdc10-less cells (marked with Cdc12-GFP) undergo the stereotypic pattern of cell cycle-dependent transitions (McMurray & Thorner, 2009): accumulation at the incipient bud site; assembly into an hourglass-shaped collar at the bud neck; and, formation of split rings during cytokinesis (Figure 6B). However, unlike WT split rings (in which septin deposition is equivalent on the mother and daughter sides of the bud neck), in the split rings in Cdc10-less cells, the distribution is biased toward the bud-side (Figure 6B). Importantly, at cytokinesis, and as in WT cells (Figure 6A), the septin structures at the bud neck in Cdc10-less cells trapped the exocytic vesicle targeting protein Sec3 (Figure 6B, top) and the chitin synthase Chs2 (Figure 6B, bottom), two factors required for cytokinesis and cell division that delineated the barrier function of the septins (Barral et al., 2000; Dobbelaere & Barral, 2004).
Figure 6. Septin Localization and Barrier Function in Cdc10-less and Cdc11-less Cells.
Cells of the genotype shown, expressing from either CEN plasmids (in brackets) or from the chomosomal locus (for CDC10-mCherry) the indicated fluorescently-tagged proteins, were grown to mid-exponential phase and examined by DIC (top or left) and by epifluorescence microscopy with appropriate filters to visualize either Cdc12-CFP, in presence and absence of Cdc10-mCherry, or Cdc12-GFP, and either Sec3-GFP or Chs2-GFP (bottom panels). Strains were JTY3992 (A), 4003482™ (B), FOA-resistant derivative of JTY4944 (C), and JTY5140 (D and E). Plasmids were pSEC3-GFP3-1, YCpH-CDC12-CFP, YCp-CHS2-iGFP, and pLP17 (CDC12-GFP). Arrowheads, split septin rings; Arrows, bud neck localization of non-septin proteins. See also Figure S5.
In contrast to the more normal morphology of cdc10Δ cells, Cdc11-less cells propagated on galactose medium were highly elongated and grew in chains with few obvious bud necks (Figure S5A), suggesting that most attempts at cytokinesis fail and that successful budding is infrequent, yet sufficient for survival of the clone. Consistent with this view, where occasional apparent buds were formed, Cdc12-GFP localized in collar-like structures at their necks (Figure S5A). At such locations, the septin structures in Cdc11-less cells compartmentalized Sec3-GFP (Figure S5B) and Chs2-GFP (Figure S5C). Given the pivotal role of Cdc11 in rod-rod interaction, it is not surprising that Cdc12–Cdc3–Cdc10–Cdc10–Cdc3–Cdc12 hexamers might assemble into filaments much less inefficiently than Cdc11–Cdc12–Cdc3–Cdc3–Cdc12–Cdc11 hexamers.
On the other hand, in cdc11Δ mutants, the Shs1 present could contribute to the behavior observed. Indeed, as evidenced by a near WT colony size at 26° on YPD (Figure 1D), cells lacking both Cdc11 and Shs1 grew more robustly, but still as chains of cells with grossly elongated morphologies (Figure 6C). Although Cdc12-GFP was localized predominantly at the constrictions corresponding to rudimentary bud necks in these cells, it was often organized into loose and rather elaborate spirals rather than tight collars or rings (Figure 6C). Thus, in the absence of Shs1, Cdc12–Cdc3–Cdc10–Cdc10–Cdc3–Cdc12 hexamers were capable of unrestrained, even luxuriant, filament formation.
Elongated bud morphology arises from persistent anisotropic bud growth during an extended G2 phase, and is characteristic of activation of a Swe1-mediated morphogenesis checkpoint that postpones mitosis if bud emergence is perturbed by defects in septin organization (Barral et al., 1999; Shulewitz et al., 1999; Lew, 2003). Indeed, deleting SWE1 markedly improved the morphology of cdc11Δ shs1Δ double mutants (Figure 6D), resulting in Cdc12-GFP-marked structures that were more compact and, although exhibiting certain nonuniformities (or “satellites”), were clearly arranged into single rings, collars, and split rings at appropriate stages of the cell cycle (Figure 6D). Also, in cdc11Δ shs1Δ swe1Δ cells, the septin structures at the bud neck compartmentalized Chs2-GFP (Figures 6E, left) and Sec3-GFP (Figure 6, right), and these locations corresponded to septin-containing collars in cells double-labeled with Cdc10-mCherry (Figure S5D). Thus, in Cdc11-less cells, as in Cdc10-less cells, the remaining septins generate structures that operate as diffusion barriers, compatible with a barricade formed by assembly of continuous septin filaments.
DISCUSSION
The filamentous collar at the bud neck of S. cerevisiae is the best understood septin-based structure in biology. Despite insights gained about its structure and function from genetic, physiological, biochemical and ultrastructural studies, it was unclear whether its filamentous nature per se is required to fulfill its biological roles, what structural consequences result from elimination or mutation of particular septins, and how defects in septin organization account for the observed physiological defects.
Given the defined linear order of subunits in the septin rod and that filaments assemble by end-to-end polymerization of rods (Bertin et al., 2008), the viability of Cdc10- or Cdc11-deficient cells seemed incompatible with a requirement for continuous filaments in essential septin functions (Frazier et al., 1998). However, our data show that, in both cdc10Δ and cdc11Δ mutants, the remaining subunits associate into hexameric rods that are able to assemble into filaments. In cells lacking Cdc11, the now exposed Cdc12 subunit at each end of a rod mediates end-to-end polymerization via its G interface. The reported ability of an analogous human SEPT7–SEPT6–SEPT2–SEPT2–SEPT6–SEPT7 hexamer to form filaments established an in vitro precedent for just such an interaction (Sirajuddin et al., 2007). At a G interface, a few residues from one septin make contact with the bound nucleotide in another (Sirajuddin, et al. 2007; ibid, 2009). The need for a septin subunit to maintain tight binding both to its own guanine nucleotide and to the nucleotide in the adjacent subunit presumably provides selective pressure to constrain the G interface against drift from its ancestral fold.
In cells lacking Cdc10, the “arms” of the standard hetero-octamer rejoin via an atypical Cdc3-Cdc3 interaction mediated by its G interface to form hexamers that polymerize via normal Cdc11-Cdc11 contact. The crucial role of Cdc3 self-association in sustaining the viability of cdc10Δ cells was most dramatically reinforced by the spontaneous mutation (G261V) in Cdc3 that clearly allowed cells lacking Cdc10 to proliferate more robustly. In vitro, the corresponding Cdc11–Cdc12–Cdc3(G261V)–Cdc3(G261V)–Cdc12–Cdc11 hexamers were more stable than those containing WT Cdc3. Thus, during evolution, the G interface of Cdc3 has partially lost its capacity for homodimerization, which was regained by imposing artificial selection in the lab. Interestingly, the filaments formed from Cdc11-less complexes clearly triggered the Swe1-dependent cell cycle checkpoint, whereas those formed by Cdc10-less complexes did not. Given that prominent filament spirals with “free” ends formed in Cdc11-less cells, it is possible that the morphogenesis checkpoint “senses” filament discontinuities, in analogy to the DNA breaks that trigger the DNA damage response.
Our findings implied that Cdc10-less and Cdc11-less cells survive because the septins In these cells still form continuous filaments. As an independent test of this idea, we designed mutants, namely Cdc10(Δα0) and Cdc11(Δα0), that fold and incorporate into complexes, but cannot form their NC interface, an interaction required for filament assembly. As anticipated, when expressed as the sole source of the corresponding septin, Cdc10(Δα0) and Cdc11(Δα0) were each lethal. The fact that a chain-terminating subunit compromises viability, whereas total absence of the same subunit does not, strongly validates the conclusion that formation of continuous filaments is essential for septin function.
Over-expression of Cdc10(Δα0) in WT cells blocked filament assembly, prevented cortical localization of all other septin subunits, and halted cell division. Coupling of filament assembly to stable membrane recruitment is not without precedent. We showed previously that over-expression of a Cdc3 or a Cdc12 mutant lacking its carboxy-terminal extension (CTE) prevented septin localization at the bud neck and stopped cell division (Versele et al., 2004). The CTEs of Cdc3 and Cdc12, while dispensable for hetero-octamer formation, are required, like the α0 helix in Cdc10, for filament assembly in solution (Bertin et al., 2010). Thus, conformational changes accompanying filament assembly may expose or properly orient residues in the subunits that mediate contact with the membrane, or filaments simply have greater membrane-binding avidity than individual rods with weaker membrane-binding propensity, or both. Indeed, at least in vitro, septin recruitment to a membrane mimic is tightly coupled to their capacity to polymerize into fllaments (Bertin, 2010). Thus, to execute their essential function in cell division, it is not sufficient for septin complexes merely to be present, but they must also be able to localize to the cortex, which requires their assembly into filaments.
The ability of Cdc10-less or Cdc11-less complexes to form filaments, albeit inefficiently, bespeaks unusual biological flexibility. Such plasticity in septin assembly in vivo is not idiosyncratic to S. cerevisiae (additional examples are discussed in Supplemental Information). Moreover, such organizational plasticity in the assembly of other oligomeric structures composed of multiple, structurally similar subunits is also not without precedent (an additional example is discussed in Supplemental Information).
In C. elegans, there are just two septins (UNC-59 and UNC-61), each essential (Finger et al., 2003), that assemble into UNC-59–UNC-61–UNC-61–UNC-59 hetero-tetramers, which can polymerize into filaments in vitro (John et al., 2007). In yeast lacking both Cdc10 and Cdc11, the remaining Cdc12–Cdc3–Cdc3–Cdc12 complex would be, in principle, identical in organization to worm UNC-59–UNC-61–UNC-61–UNC-59. However, we found that both cdc10Δ cdc11Δ double mutants and cdc10Δ cdc11Δ shs1Δ triple mutants were inviable on any medium tested (data not shown). Although recombinant Cdc3 and Cdc12 readily hetero-oligomerize, the resulting complexes do not form filaments in vitro (Versele et al., 2004; Farkasovsky et al., 2005). Thus, Inviability presumably reflects inability of Cdc3 and Cdc12 alone to form continuous filaments in vivo.
On the other hand, we also found that a cdc10Δ shs1Δ double mutant is inviable (data not shown). Based solely on filament formation, absence of Shs1 should not affect Cdc10-less cells, as long as all other septins are WT. However, a specialized role in recruiting certain non-septin factors directly involved in cytokinesis has been ascribed to Shs1, e.g., Myo1 (Iwase et al., 2007). Unlike loss of Shs1, absence of Cdc10 does not preclude localization of Myo1-GFP to the bud neck (Iwase et al., 2007; data not shown). If, however, Shs1 becomes essential for Myo1 neck localization when Cdc10 is absent, and Myo1 action is needed for such cells to divide, then inviability of cdc10Δ shs1Δ cells likely reflects crippling of cytokinesis rather than an effect on septin filament assembly per se. Indeed, unlike CDC10+ cells, a cdc10Δ mutant cannot grow when Myo1 is depleted (data not shown).
Does the biological function of septins generally require filament formation of the sort observed at the yeast bud neck? Based solely on fluorescence micrographs of other cell types, it is not possible to conclude that the septins present are actually organized into authentic filaments (despite frequent loose use of the term). In somes cases, the filamentous appearance seems attributable to association of septins with actin cables (Kinoshita et al., 2002) or microtubules (Vega and Hsu, 2003). In other cases, a scaffold function has been assigned to septin complexes in situations that do not demand filaments per se (Kremer et al., 2005; Spiliotis et al., 2005; Kremer et al., 2007). By comparison, and consistent with all of the data presented here, it is difficult to rationalize the appearance of the ring-shaped septin structures at the yeast bud neck and their role in compartmentalizing other cellular factors without envisioning that this organization and this function require the assembly of continuous closed filaments (Barral et al., 2000; Takizawa et al., 2000; Dobbelaere & Barral, 2004). Similarly, in the cytokinetic midbodies in cultured mammalian cells (Schmidt & Nichols, 2004), in the annulus separating the posterior and anterior tail segments of spermatozoa (Ihara et al., 2005), and in other situations where septin complexes appear to establish a barrier to diffusion (or co-localize with an apparent or demonstrated diffusion barrier), it seems highly likely that execution of this critical physiological function will require formation of ordered cortical septin filaments.
EXPERIMENTAL PROCEDURES
Strains and Plasmids
Yeast genetic techniques were according to Amberg et al. (2005). All yeast strains are BYB4741 derivatives (Table S1). Plasmids are described in Table S2. Sporulation medium was SPM (1% K+-acetate, 0.02% raffinose) containing nicotinamide (#N5535, Sigma). FOA medium was prepared according to Boeke et al. (1987). PCR of septin-encoding genes (followed, where necessary, by diagnostic cleavage with restriction enzymes) confirmed that FOA-resistant clones represented loss of the URA3-marked septin-expressing plasmid, and not acquisition of a ura3 mutation. For this analysis, genomic DNA was isolated from colonies of interest as described (McMurray & Gottschling, 2003) and appropriate primers were used to amplify the cognate septin ORFs. The cdc3(W364A) mutation introduces a PvuII site that CDC3+ lacks; hence, upon PvuII digestion, a WT CDC3 PCR product remains full-length, whereas that from cdc3(W364A) is cut. CDC12 contains a StyI site that the cdc12(W267A) mutation eliminates; thus, upon StyI digestion, the WT CDC12 PCR product is cut, whereas that from cdc12(W267A) remains full-length.
Microscopy
For imaging, cells were washed and resuspended in water or PBS, spotted on a freshly-made agarose (2%) pad on a microscope slide (or, occasionally, directly on the slide itself) in water, PBS or the appropriate SC-based medium, and placed under a coverslip, which was sealed around the edges with molten Aquaphor (Eucerin) to prevent drying when time-lapse was performed. Images were taken using a epifluorescence microscope (model BH-2, Olympus America) equipped with a 60X objective, with a TRITC filter (Chroma) to view mCherry, an eGFP filter (Chroma) to view GFP when no CFP-tagged protein was present or to view YFP-tagged proteins when CFP-tagged proteins were present, and a CFP filter (Chroma) to view CFP when GFP-tagged proteins were present, and captured digitally using a CCD camera (Optronics). Spore microcolonies were imaged directly on YPD dissection plates by standard light microcopy using a 20X objective. Images were cropped and adjusted for brightness/contrast using Photoshop (Adobe).
Preparation and EM Analysis of Recombinant Septin Complexes
Yeast septins were expressed in E. coli BL21(DE3), purified by sequential nickel affinity, size exclusion, and ion exchange chomatography and examined by EM as described in Bertin et al. (2008).
Supplementary Material
Acknowledgments
Supported by NIH K99 grant GM86603 (to M.A.M.), Jane Coffin Childs Postdoctoral Fellowship 61-1357 (to A.B.), an NSF Predoctoral Fellowship (to G.G.), the Agouron Foundation, DOE Office of Biological & Environmental Research, and Howard Hughes Medical Institute (to E.N.), and NIH R01 grant GM21841 (to J.T.). We thank Ho-leung Ng and Thomas C. Alber for their interest, advice and material assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amberg DC, Burke DJ, Strathern JN. Methods in Yeast Genetics: A Course Manual. 2005. Cold Spring Harbor Laboratory Press; 2005. [Google Scholar]
- Barral Y, Mermall V, Mooseker MS, Snyder M. Compartmentalization of the cell cortex by septins is required for maintenance of cell polarity in yeast. Mol Cell. 2000;5:841–851. doi: 10.1016/s1097-2765(00)80324-x. [DOI] [PubMed] [Google Scholar]
- Barral Y, Parra M, Bidlingmaier S, Snyder M. Nim1-related kinases coordinate cell cycle progression with the organization of the peripheral cytoskeleton in yeast. Genes Dev. 1999;13:176–187. doi: 10.1101/gad.13.2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertin A, McMurray MA, Grob P, Park SS, Garcia G, 3rd, Patanwala I, Ng HL, Alber T, Thorner J, Nogales E. Saccharomyces cerevisiae septins: supramolecular organization of heterooligomers and the mechanism of filament assembly. Proc Natl Acad Sci USA. 2008;105:8274–8279. doi: 10.1073/pnas.0803330105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertin A, McMurray MA, Thai L, Garcia G, 3rd, Votin V, Grob P, Allyn T, Thorner J, Nogales E. Phosphatidylinositol-4,5-bisphosphate promotes budding yeast septin filament assembly and organization. J Mol Biol. 2010;404:711–731. doi: 10.1016/j.jmb.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi E. Cytokinesis in budding yeast: the relationship between actomyosin ring function and septum formation. Cell Struct Funct. 2001;26:529–537. doi: 10.1247/csf.26.529. [DOI] [PubMed] [Google Scholar]
- Boeke JD, Trueheart J, Natsoulis G, Fink GR. 5-Fluoroorotic acid as a selective agent in yeast molecular genetics. Methods Enzymol. 1987;154:164–175. doi: 10.1016/0076-6879(87)54076-9. [DOI] [PubMed] [Google Scholar]
- Byers B, Goetsch L. Highly ordered ring of membrane-associated filaments in budding yeast. J Cell Biol. 1976a;69:717–721. doi: 10.1083/jcb.69.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers B, Goetsch L. Loss of filamentous ring in cytokinesis-defective mutants of budding yeast. J Cell Biol. 1976b;70:A35–A35. [Google Scholar]
- Caudron F, Barral Y. Septins and the lateral compartmentalization of eukaryotic membranes. Dev Cell. 2009;16:493–506. doi: 10.1016/j.devcel.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Caviston JP, Longtine M, Pringle JR, Bi E. The role of Cdc42p GTPase-activating proteins in assembly of the septin ring in yeast. Mol Biol Cell. 2003;14:4051–4066. doi: 10.1091/mbc.E03-04-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMarini DJ, Adams AE, Fares H, De Virgilio C, Valle G, Chuang JS, Pringle JR. A septin-based hierarchy of proteins required for localized deposition of chitin in the Saccharomyces cerevisiae cell wall. J Cell Biol. 1997;139:75–93. doi: 10.1083/jcb.139.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbelaere J, Barral Y. Spatial coordination of cytokinetic events by compartmentalization of the cell cortex. Science. 2004;305:393–396. doi: 10.1126/science.1099892. [DOI] [PubMed] [Google Scholar]
- Dobbelaere J, Gentry MS, Hallberg RL, Barral Y. Phosphorylation-dependent regulation of septin dynamics during the cell cycle. Dev Cell. 2003;4:345–357. doi: 10.1016/s1534-5807(03)00061-3. [DOI] [PubMed] [Google Scholar]
- Farkasovsky M, Herter P, Voss B, Wittinghofer A. Nucleotide binding and filament assembly of recombinant yeast septin complexes. Biol Chem. 2005;386:643–656. doi: 10.1515/BC.2005.075. [DOI] [PubMed] [Google Scholar]
- Finger FP, Kopish KR, White JG. A role for septins in cellular and axonal migration in C. elegans. Dev Biol. 2003;261:220–234. doi: 10.1016/s0012-1606(03)00296-3. [DOI] [PubMed] [Google Scholar]
- Frazier JA, Wong ML, Longtine MS, Pringle JR, Mann M, Mitchison TJ, Field C. Polymerization of purified yeast septins: evidence that organized filament arrays may not be required for septin function. J Cell Biol. 1998;143:737–749. doi: 10.1083/jcb.143.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladfelter AS, Pringle JR, Lew DJ. The septin cortex at the yeast mother-bud neck. Curr Opin Microbiol. 2001;4:681–689. doi: 10.1016/s1369-5274(01)00269-7. [DOI] [PubMed] [Google Scholar]
- Hartwell LH. Genetic control of the cell division cycle in yeast. IV. Genes controlling bud emergence and cytokinesis. Exp Cell Res. 1971;69:265–276. doi: 10.1016/0014-4827(71)90223-0. [DOI] [PubMed] [Google Scholar]
- Hu Q, Milenkovic L, Jin H, Scott MP, Nachury MV, Spiliotis ET, Nelson WJ. A septin diffusion barrier at the base of the primary cilium maintains ciliary membrane protein distribution. Science. 2010;329:436–439. doi: 10.1126/science.1191054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihara M, Kinoshita A, Yamada S, Tanaka H, Tanigaki A, Kitano A, Goto M, Okubo K, Nishiyama H, Ogawa O, et al. Cortical organization by the septin cytoskeleton is essential for structural and mechanical integrity of mammalian spermatozoa. Dev Cell. 2005;8:343–352. doi: 10.1016/j.devcel.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Iwase M, Luo JY, Bi E, Toh-e A. Shs1 plays separable roles in septin organization and cytokinesis in Saccharomyces cerevisiae. Genetics. 2007;177:215–229. doi: 10.1534/genetics.107.073007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwase M, Luo JY, Nagaraj S, Longtine M, Kim HB, Haarer BK, Caruso C, Tong ZT, Pringle JR, Bi E. Role of a Cdc42p effector pathway in recruitment of the yeast septins to the presumptive bud site. Mol Biol Cell. 2006;17:1110–1125. doi: 10.1091/mbc.E05-08-0793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John CM, Hite RK, Weirich CS, Fitzgerald DJ, Jawhari H, Faty M, Schlapfer D, Kroschewski R, Winkler FK, Walz T, et al. The Caenorhabditis elegans septin complex is nonpolar. EMBO J. 2007;26:3296–3307. doi: 10.1038/sj.emboj.7601775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita M, Field CM, Coughlin ML, Straight AF, Mitchison TJ. Self- and actin-templated assembly of mammalian septins. Dev Cell. 2002;3:791–802. doi: 10.1016/s1534-5807(02)00366-0. [DOI] [PubMed] [Google Scholar]
- Kremer BE, Adang LA, Macara IG. Septins regulate actin organization and cell-cycle arrest though nuclear accumulation of NCK mediated by SOCS7. Cell. 2007;130:837–850. doi: 10.1016/j.cell.2007.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer BE, Haystead T, Macara IG. Mammalian septins regulate microtubule stability though interaction with the microtubule-binding protein MAPP-1. Mol Biol Cell. 2005;16:4648–4659. doi: 10.1091/mbc.E05-03-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwitny S, Klaus AV, Hunnicutt GR. The annulus of the mouse sperm tail is required to establish a membrane diffusion barrier that is engaged during the late steps of spermiogenesis. Biol Reprod. 2010;82:669–678. doi: 10.1095/biolreprod.109.079566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew DJ. The morphogenesis checkpoint: how yeast cells watch their figures. Curr Opin Cell Biol. 2003;15:648–653. doi: 10.1016/j.ceb.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Lippincott J, Li R. Dual function of Cyk2, a cdc15/PSTPIP family protein, in regulating actomyosin ring dynamics and septin distribution. J Cell Biol. 1998;143:1947–1960. doi: 10.1083/jcb.143.7.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longtine MS, Fares H, Pringle JR. Role of the yeast Gin4p protein kinase in septin assembly and the relationship between septin assembly and septin function. J Cell Biol. 1998;43:719–736. doi: 10.1083/jcb.143.3.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurray MA, Gottschling DE. An age-induced switch to a hyper-recombinational state. Science. 2003;301:1908–1911. doi: 10.1126/science.1087706. [DOI] [PubMed] [Google Scholar]
- McMurray MA, Thorner J. Biochemical properties and supramolecular architecture of septin hetero-oligomers and septin filaments. In: Hall PA, Russell SEG, Pringle JR, editors. The Septins. Chicester, West Sussex, UK: John Wiley & Sons, Ltd; 2008. pp. 49–100. [Google Scholar]
- McMurray MA, Thorner J. Reuse, replace, recycle: specificity in subunit inheritance and assembly of higher-order septin structures during mitotic and meiotic division in budding yeast. Cell Cycle. 2009;8:195–203. doi: 10.4161/cc.8.2.7381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh DH, Bowers B, Schmidt M, Cabib E. The septation apparatus, an autonomous system in budding yeast. Mol Biol Cell. 2002;13:2747–2759. doi: 10.1091/mbc.E02-03-0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rönicke V, Graulich W, Mumberg D, Müller R, Funk M. Use of conditional promoters for expression of heterologous proteins in Saccharomyces cerevisiae. Methods Enzymol. 1997;283:313–322. doi: 10.1016/s0076-6879(97)83025-x. [DOI] [PubMed] [Google Scholar]
- Schmidt K, Nichols BJ. A barrier to lateral diffusion in the cleavage furrow of dividing mammalian cells. Curr Biol. 2004;14:1002–1006. doi: 10.1016/j.cub.2004.05.044. [DOI] [PubMed] [Google Scholar]
- Shulewitz MJ, Inouye CJ, Thorner J. Hsl7 localizes to a septin ring and serves as an adapter in a regulatory pathway that relieves tyrosine phosphorylation of Cdc28 protein kinase in Saccharomyces cerevisiae. Mol Cell Biol. 1999;19:7123–7137. doi: 10.1128/mcb.19.10.7123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirajuddin M, Farkasovsky M, Hauer F, Kuhlmann D, Macara IG, Weyand M, Stark H, Wittinghofer A. Structural insight into filament formation by mammalian septins. Nature. 2007;449:311–315. doi: 10.1038/nature06052. [DOI] [PubMed] [Google Scholar]
- Sirajuddin M, Farkasovsky M, Zent E, Wittinghofer A. GTP-induced conformational changes in septins and implications for function. Proc Natl Acad Sci USA. 2009;106:16592–16597. doi: 10.1073/pnas.0902858106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sopko R, Huang D, Smith JC, Figeys D, Andrews BJ. Activation of the Cdc42p GTPase by cyclin-dependent protein kinases in budding yeast. EMBO J. 2007;26:4487–4500. doi: 10.1038/sj.emboj.7601847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiliotis ET, Kinoshita M, Nelson WJ. A mitotic septin scaffold required for mammalian chomosome congression and segregation. Science. 2005;307:1781–1785. doi: 10.1126/science.1106823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada T, Simonetta A, Batterton M, Kinoshita M, Edbauer D, Sheng M. Role of septin cytoskeleton in spine morphogenesis and dendrite development in neurons. Curr Biol. 2007;17:1752–1758. doi: 10.1016/j.cub.2007.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takizawa PA, DeRisi JL, Wilhelm JE, Vale RD. Plasma membrane compartmentalization in yeast by messenger RNA transport and a septin diffusion barrier. Science. 2000;290:341–344. doi: 10.1126/science.290.5490.341. [DOI] [PubMed] [Google Scholar]
- Vega IE, Hsu SC. The septin protein Nedd5 associates with both the exocyst complex and microtubules and disruption of its GTPase activity promotes aberrant neurite sprouting in PC12 cells. Neuroreport. 2003;14:31–37. doi: 10.1097/00001756-200301200-00006. [DOI] [PubMed] [Google Scholar]
- Versele M, Gullbrand B, Shulewitz MJ, Cid VJ, Bahmanyar S, Chen RE, Barth P, Alber T, Thorner J. Protein-protein interactions governing septin heteropentamer assembly and septin filament organization in Saccharomyces cerevisiae. Mol Biol Cell. 2004;15:4568–4583. doi: 10.1091/mbc.E04-04-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versele M, Thorner J. Septin collar formation in budding yeast requires GTP binding and direct phosphorylation by the PAK, Cla4. J Cell Biol. 2004;164:701–715. doi: 10.1083/jcb.200312070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voronkova V, Kacherovsky N, Tachibana C, Yu D, Young ET. Snf1-dependent and Snf1-independent pathways of constitutive ADH2 expression in Saccharomyces cerevisiae. Genetics. 2006;172:2123–2138. doi: 10.1534/genetics.105.048231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrabioiu AM, Mitchison TJ. Structural insights into yeast septin organization from polarized fluorescence microscopy. Nature. 2006;443:466–469. doi: 10.1038/nature05109. [DOI] [PubMed] [Google Scholar]
- Xie Y, Vessey JP, Konecna A, Dahm R, Macchi P, Kiebler MA. The GTP-binding protein Septin 7 is critical for dendrite branching and dendritic-spine morphology. Curr Biol. 2007;17:1746–1751. doi: 10.1016/j.cub.2007.08.042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.