Abstract
Background
Antifibrinolytic therapy, such as the use of the serine protease inhibitor aprotinin, was a mainstay for hemostasis following cardiac surgery. However, aprotinin was empirically dosed, and while the pharmacological target was the inhibition of plasmin activity (PLact) this was never monitored, off-target effects occurred, and led to withdrawn from clinical use. The present study developed a validated fluorogenic-microdialysis method to continuously measure PLact and tested the hypothesis that standardized clinical empirical aprotinin dosing would impart differential and regional effects on PLact.
Methods/Results
Pigs (30 kg) were instrumented with microdialysis probes to continuously measure PLact in myocardial, kidney and skeletal muscle compartments (deltoid) and then randomized to High Dose aprotinin administration (2 mKIU load/ 0.5 mKIU/hr infusion; n=7), Low Dose aprotinin administration (1 mKIU load/ 0.250 mKIU/hr infusion; n=6). PLact was compared to time matched vehicle (n=4), and PLact was also measured in plasma by an in-vitro fluorogenic method. Aprotinin suppressed PLact in the myocardium and kidney at both High and Low Doses- indicative that both doses exceeded a minimal concentration necessary for PLact inhibition. However, differential effects of aprotinin on PLact were observed in the skeletal muscle indicative of different compartmentalization of aprotinin.
Conclusions
Using a large animal model and a continuous method to monitor regional plasmin activity, these unique results demonstrated that an empirical aprotinin dosing protocol causes maximal and rapid suppression in the myocardium and kidney and in turn would likely increase the probability of off-target effects and adverse events. Further, this proof of principle study demonstrated that continuous monitoring of determinants of fibrinolysis may provide a novel approach for managing fibrinolytic therapy.
Keywords: aprotinin, plasmin inhibition, fibrinolysis, plasma, interstitial, microdialysis probe
Introduction
Perioperative bleeding associated with cardiovascular surgery, particularly in the context of cardiopulmonary bypass (CPB) is a major cause for morbidity and mortality.1,2 Thus, strategies to reduce blood loss and transfusion requirements related to cardiovascular surgery, including the use of antifibrinolytics, are important therapeutic maneuvers.3–6 One prototypical antifibrinolytic which was commonly utilized for hemostatic control in the context of CPB was the serine protease inhibitor aprotinin. Clinical studies have reported potential adverse effects of aprotinin such as renal dysfunction; which ultimately resulted in the voluntary withdrawal of this antifibrinolytic. 1,2,6–11 However, other studies have failed to identify significant effects of aprotinin on renal function,12,13 whereas others have identified that these effects may be dose dependent.14 In general terms, causes of adverse drug effects include under/over dosing resulting in failure to achieve the desired target effects or inducing off-target effects respectively. While not directly measured in the clinical context, the putative target of aprotinin is plasmin, specifically plasmin activity (PLact).4,5 While the pharmacology has been studied previously,15 the basic regional and temporal effects of aprotinin on PLact remain unexplored. Direct and continuous measurement of PLact may provide mechanistic insight into how clinically utilized empiric aprotinin dosing regimens may have potentially contributed to off-targets effects and therefore associated adverse outcomes. Accordingly, the central hypothesis of this study was that temporal and regional PLact profiles following aprotinin administration are quantifiable and that empiric Hammersmith dosing regimens,14,16 cause temporal and regional heterogeneity in PLact. Consequently, the overall goal of this study was to quantify the effects of aprotinin on the regional and temporal PLact profiles in selected tissue compartments and plasma.
The homeostatic dynamics and kinetics of the coagulation and fibrinolytic pathways are uniquely modulated in a region specific manner.16–19 More specifically, regional and temporal heterogeneity in PLact has been shown to exist following the administration of commonly utilized antifibrinolytics.18,19 In order to explore the effects of aprotinin on the regional dynamics of PLact, a large animal model utilizing established microdialysis techniques was employed.18–20 Such microdialysis techniques, utilizing a fluorogenic substrate, allowed the detection of interstitial enzymatic activity, such as plasmin.18,19 Aprotinin has typically been employed using empirically based dosing regimens, of which the two most common are the full and half Hammersmith dosing schemes. Accordingly, the objectives of this study were two-fold. First, was to measure PLact in-vivo utilizing the half and full Hammersmith doses of aprotinin, in a clinically relevant porcine model. Second, was to determine whether and to what degree regional and temporal heterogeneity in PLact occurred with respect to these empirically based aprotinin regimens.
Methods
The present study was conducted in two stages. First, in-vitro validation studies were performed to develop a PLact measurement system using a plasmin specific fluorogenic substrate. This validated PLact measurement system was utilized to perform in-vivo PLact measurements, via microdialysis probes, within targeted regions. Then, aprotinin/vehicle was infused intravenously and PLact was continuously monitored within these regions. Finally, plasma aprotinin concentrations were measured.
Plasmin Substrate Validation
In-vitro validation studies were performed using a plasmin activatable fluorogenic substrate-based agent. In particular, this agent (VM3163, VisEn Medical, Inc., Bedford, MA) contained a validated fluorogenic peptide which, when cleaved by plasmin, yielded a fluorescent moiety with excitation/emission wavelengths of 670/700nm, respectively. Preliminary studies demonstrated the optimal concentration for VM3163 to be 5 μg/mL. This plasmin substrate was subjected to increasing concentrations of purified plasmin (3.1–31.3 μg/mL, Sigma-Aldrich, Cat # P1867) in diluted control porcine plasma and the fluorescence emissions, reflective of PLact, was measured. The fluorescence emissions values obtained demonstrated a directly proportional linear response to increasing concentrations of plasmin. A linear equation was matched to this data using regression analysis ( y{x}=48.43x, r2=0.9963, p=0.0019).
Therefore, these in-vitro studies established the optimal substrate concentration and demonstrated specificity of the substrate for plasmin. The development of this PLact measurement system was then translated to the in-vivo PLact studies described below.
Animal and Surgical Preparation
Yorkshire pigs (n=17, castrated male, 25–35 kg, Hambone Farms, Reevesville, SC) were instrumented to measure plasma and interstitial PLact. All animals were treated and cared for in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (National Institutes of Health, 1996). Approval of all animal care and use protocols was obtained from the Medical University of South Carolina Institutional Animal Care and Use Committee, AR# 2786.
After sedation with diazepam (100 mg po, Elkins-Sinn Inc (ESI), Cherry Hill, NJ), general inhalational anesthesia was induced using isoflurane (3%, Baxter Healthcare Corp., Deerfield, IL) mixed with oxygen and nitrous oxide (67:33%) and peripheral intravenous access obtained. A stable surgical plane of anesthesia was established and maintained throughout the protocol using sufentanil (2 μg/kg IV, ESI), etomidate (0.1 mg/kg IV, ESI), pancuronium (10mg IV bolus, 5 mg/hr IV infusion, Ben Venue Laboratories Inc, Bedford, OH), morphine sulfate(3 mg/kg/hr IV, ESI) and isoflurane (1%, Baxter Healthcare Corp.) Tracheal intubation was achieved via tracheostomy and mechanical ventilation established (Narkomed 2B, North American Drager, Telford, PA). Intravenous fluids (lactated Ringer's) were administered per established weight based protocols for maintenance fluids and estimated blood loss replacement. A single lumen catheter (8 Fr) was placed into the right external jugular vein for fluid and drug administration. An arterial line catheter (7 Fr) was placed into the right carotid artery to continuously monitor systemic blood pressures and obtain blood samples. Following a 60 minute stabilization and baseline period, each pig was randomly assigned to receive low dose aprotinin (1 million KIU IV over 30 minutes with a continuous infusion of 250,000 KIU/hour IV, Bayer Pharmaceuticals Corp.) or high dose aprotinin (2 million KIU IV over 30 minutes, with a continuous infusion of 500,000 KIU/hour IV, Bayer Pharmaceuticals Corp.) or vehicle (low dose = 200mL and high dose = 400mL over 30 minutes with a continuous infusion of 25mL/hour and 50mL/hour, normal saline respectively).
Microdialysis Techniques
Microdialysis probes (CMA/Microdialysis, North Chelmsford, MA) with a molecular weight cutoff of 20 kDa and an outer diameter of 0.5 mm were surgically placed interstitially in the anterior myocardium of the left ventricle, right lobe of the liver, lower pole of the right kidney and left deltoid muscle compartments. Placement of the microdialysis probes required a median sternotomy, a subxyphoid intra-abdominal incision, a subcostal flank incision and an anterior mid-shoulder incision with associated tissue dissections respectively.
The microdialysis probes were connected to precision infusion pumps and controller system (BASi, West Lafayette, IN). A flow rate of 5.0 μL/min was established and an iso-osmotic dialysis performed. Dialysate was infused for 30 minutes to allow for equilibration with each of the respective tissue compartments. The microdialysate infusion contained the validated fluorogenic agent VM3163 (5 μg/mL). Preliminary studies demonstrated this microdialysate concentration yielded a steady state fluorescence emission within 30 minutes of the initiation of dialysis, indicative of equilibration with the interstitial space of the target tissue. The fluorescence emission of the interstitial fluid collected from each of the microdialysis probes, which directly reflected PLact, was determined at steady-state baseline (time = 0), 30, 60, 90 and 120 minutes following the start of aprotinin/vehicle infusion using fluorescence measurement techniques previously described. In addition, plasma samples were collected at the specified time points and subjected to the same fluorogenic evaluation methods.
Plasma Aprotinin Concentration Estimations
Next, a series of in-vitro experiments were performed using a solution of referent normal porcine plasma which determined the aprotinin plasma concentration inhibition curve. This enzyme inhibition assay was used to determine aprotinin plasmin concentrations. Specifically, a plasmin specific fluorogenic substrate with the peptide sequence D-Ala-Leu-Lys-7-amido-4-methylcomarin (Sigma-Aldrich, Cat # A8171), at a fixed concentration of 3 μM, was mixed in a reaction buffer (MP Biomedicals, Solon OH, Cat # 2810305) containing a 1:10 dilution of porcine plasma and incubated at 37°C for 5 min in the presence of 6.7 μg/mL of plasmin (Sigma-Aldrich, Cat # P1867). The fluorescence emission of this reaction was measured utilizing a fluorogenic plate reader (Fluostar Galaxy, BMG Labtech Inc., NC) at excitation/emission wavelengths of 360/460 nm respectively. In addition, a set of standards were measured in which reference control porcine plasma was prepared in an identical manner as previously described in the presence and absence of increasing concentrations of aprotinin (0–266.4 KIU/mL, Bayer Pharmaceuticals Corp., West Haven, CT) to generate a standardized enzyme activity-inhibition curve. The fluorescence emission, reflective of PLact, decreased in response to increasing concentrations of aprotinin in a logarithmic concentration-dependent manner. A logarithmic equation was matched to this data using regression analysis (n=3, y(x) = 495.75 + 51.92*e−10.40*x + 90.61*e−0.55*x + 77.24*e−0.02*x, r2= 0.997, p<0.0001). The fluorescence emission was then measured on the 30 minute time point plasma samples for both high and low dose pigs as previously described. These fluorescence emissions values where used to extrapolate estimated aprotinin plasma concentration values utilizing the previously derived aprotinin plasma concentration inhibition curve.
Data Analysis
Comparisons for absolute change in PLact compared to baseline for all time points post infusion within each region were made using an analysis of variance (ANOVA) followed by pair-wise tests of individual time points means using PR-Comp. One-sample t-tests were performed on the computed absolute change in PLact compared to baseline for all time points within each region. All statistical procedures were performed using STATA statistical software (Intercooled STATA 8.0). Results are presented as mean±SEM with p values <0.05 considered to be statistically significant.
Results
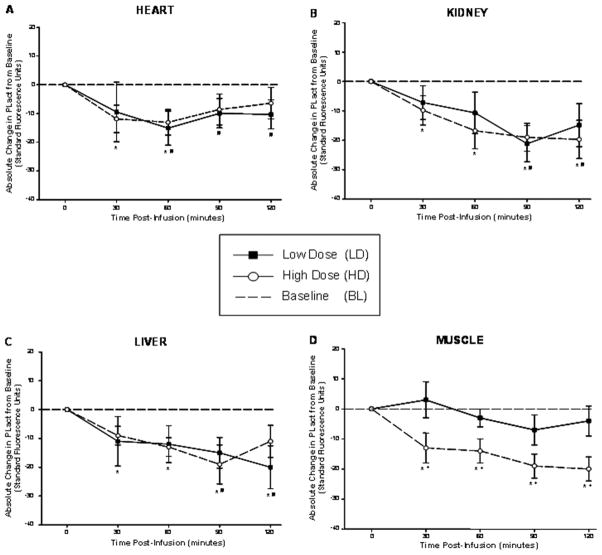
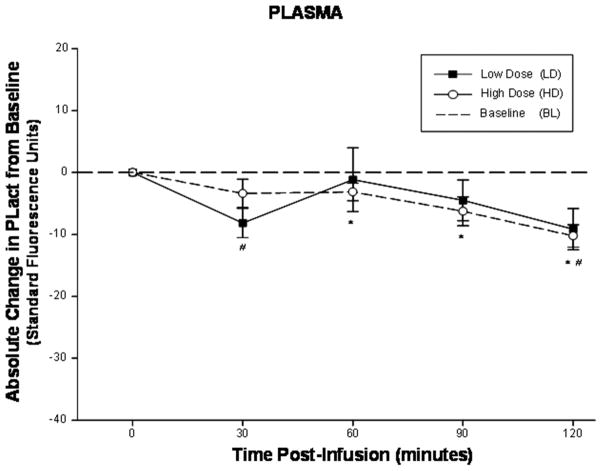
All animal preparations successfully completed the described protocols. The estimated aprotinin plasma concentrations at 30 minutes post aprotinin infusion for both low and high dose pigs were determined to be 2.6 KIU/mL (n=6, mean) and 16.5 KIU/mL (n=7, mean) respectively. The computed absolute changes in PLact from baseline, reflective of changes in PLact induced by the administration of aprotinin, were determined for each of the physiological compartments at the specified time points (Figure 1A–D). In all regions, with the exception of the shoulder, aprotinin induced reductions in PLact with respect to baseline values for all post infusion time points. More specifically, a nadir in PLact in the heart occurred at 60 minutes post infusion for both low and high dose groups which were significant with respect to baseline. In contrast, the kidney and liver demonstrated significant attenuations in PLact at 90 and 120 minutes post infusion for both the low and high dose groups. In addition, significant and progressive inhibition in PLact occurred in the shoulder compartment for all post infusion time points for the high dose regimen group. However, no significant suppression in PLact occurred in the shoulder region for the low dose group. When comparing induced changes in PLact within each region between low and high dose groups, only the shoulder compartment demonstrated significant differences which occurred for all time points. In comparison, plasma PLact initially declined at 30 minutes, followed by a relative transient rise and subsequent progressive decrease with significant inhibition in PLact at 120 minutes post infusion for both the low and high dose groups (Figure 2).
Figure 1.
(A) The computed absolute change in PLact in the heart compared to baseline, reflective of changes in PLact, following low/high dose aprotinin infusions demonstrates a nadir in PLact at 60 minutes post infusion for both low and high dose groups. (B) The computed absolute change in PLact in the kidney compared to baseline, reflective of changes in PLact, following low/high dose aprotinin infusions. Significant reductions in PLact occurred for all time points post infusion for the high dose group and at 90 and 120 minutes for the low dose group. (C) The computed absolute change in PLact in the liver compared to baseline, reflective of changes in PLact, following low/high dose aprotinin infusions. Significant decreases in PLact occurred for all time points post infusion for the high dose group and at 90 and 120 minutes for the low dose group. (D) The computed absolute change in PLact in the shoulder compared to baseline, reflective of changes in PLact, following low/high dose aprotinin infusions. Significant attenuation in PLact occurred for all time points post infusion for the high dose group only with significant differences in between high verses low dose responses for all time points (plotted values are mean±SEM, * p < 0.05 high dose verses baseline; + p < 0.05 high dose verses low dose).
Figure 2.
The computed absolute change in plasma PLact compared to baseline, reflective of changes in PLact, following low/high dose aprotinin infusions. Plasma PLact initially declined at 30 minutes, followed by a relative transient rise and subsequent progressive decrease with significant inhibition in PLact at 120 minutes post infusion for both the low and high dose groups (plotted values are mean±SEM, # p < 0.05 low dose verses baseline; * p < 0.05 high dose verses baseline).
Discussion
Perioperative bleeding is a significant cause for morbidity and mortality following major surgical procedures; particularly that of cardiovascular surgery.21–23 The serine protease inhibitor, aprotinin was a pharmacological mainstay in this clinical context by providing antifibrinolytic effects and therefore contributing to improved hemostasis. The dosing of aprotinin was most commonly performed using an empirical dosing formulation, termed the Hammersmith dosing protocol. While this dosing protocol uniformly demonstrated improved hemostasis and reduced blood product utilization in the context of cardiovascular surgery, retrospective and prospective studies have identified that this aprotinin dosing strategy may have been associated with adverse events such as renal dysfunction,1,2 and therefore this serine protease inhibitor was withdrawn for routine clinical use.24,25 One potential mechanism for the reported adverse events associated with aprotinin is that the dosing protocols were not developed through direct pharmacological targeting, and therefore may have resulted in off-target effects (inhibition of kallikrein, tissue factor, thrombin, protease-activated receptor-1, activated protein C and multiple cytokines).5,26–28 Specifically, the antifibrinolytic effects of aprotinin are likely due to the inhibition of plasmin activity (PLact), which in turn result in improved blood clot stabilization in the early postoperative period. On the other hand, higher concentrations of aprotinin can affect other enzyme systems and pathways, which in turn would alter biological processes independent of the effects on PLact. However, there have been no studies to date which have directly measured PLact on a continuous basis with the administration of aprotinin, and more importantly examined the regional and temporal changes in PLact induced by aprotinin. The present study addressed this issue through the use of a fluorogenic-microdialysis approach which allowed for continuous assessment of PLact in a large animal model. Using this methodology to continuously monitor PLact, in conjunction with the empirically based “high” and “low” Hammersmith aprotinin dosing regimens, the unique findings from this study were two fold. First, that interstitial and plasma PLact are differentially affected following aprotinin infusion in both a time and region dependent manner. For example, aprotinin induced temporally distinct changes in PLact within the heart, kidney, liver, muscle and the plasma compartments. Second, with the exception of the muscle compartment, PLact was significantly suppressed for both the high and low dose protocols suggesting that both regimens exceeded the minimal threshold necessary to adequately inhibit PLact. The temporal and regional heterogeneity in PLact induced by these empiric aprotinin dosing formulations and the lack of significant differences between the respective PLact profiles may be important considerations when managing fibrinolysis in the perioperative period. While future pre-clinical and clinical studies are necessary, this study provides the proof of concept that developing a definable pharmacologically based target for aprotinin dosing may be possible, which in turn would minimize off-target effects and optimally modulate fibrinolysis.
Antifibrinolytics Dosing Concerns
The dynamics and kinetics of the coagulation and fibrinolytic pathways are extensively localized and thus distinctively modulated in a region specific manner.16,18,19,30 For example, tissue plasminogen activator, the predominant pathway for the conversion of plasminogen into plasmin, is primarily produced by vascular endothelium as a result of tissue injury and subsequently rapidly metabolized in the liver.30 Thus, the coagulation/fibrinolytic balance is differentially influenced on a compartment specific basis. This intrinsic paradigm of regional modulation of fibrinolysis is a means by which hemostatic mechanisms are locally controlled to prevent adverse off-target effects such as remote thrombosis (venous, pulmonary artery, renal pelvic and artery, bladder and cerebral vascular). In the context of cardiovascular surgery, the causes of untoward effects related to antifibrinolytic therapy may be attributed to empirical based dosing regimens, regional pharmacodynamic heterogeneities, dynamic coagulation/fibrinolytic states, temporal alterations in organ function and patient specific genetic polymorphisms.6,7,18,19,26,27,30,31 In particular, these factors may accentuate the underlying mechanisms related to the potential adverse off-target effects of aprotinin, specifically renal dysfunction. As to whether and to what degree renal dysfunction associated with the use of aprotinin is a dose related phenomenon remains controversial.1,2,10–14 The proposed mechanisms by which aprotinin may induce renal dysfunction include alterations in prostaglandin and kinin pathways with subsequent alterations of intrarenal hemodynamics, the innate renal tubular processing of aprotinin and the compounding effects of co-administered pharmacological agents.10,11,32 The present study demonstrated that it is possible to directly measure in-vivo the regional and temporal effects of aprotinin on PLact in a continuous fashion and thus potentially provide a means by which to more carefully monitor the effects of antifibrinolytics.
Mechanisms of Action of Aprotinin and Potential Off-Target Pathways
Aprotinin is a nonspecific serine protease inhibitor which exerts its antifibrinolytic effects by directly binding to the active site of plasmin and forming reversible enzyme inhibitor complexes.5,15,26,27 In addition, aprotinin possesses several unique mechanisms of action which may also potentially play a beneficial role in the context of cardiovascular surgery.5,28 For example, aprotinin can reduce pro-inflammatory cytokines in the early post-cardiac surgery setting to a greater degree than the antifibrinolytic class of lysine analogues.5,28,33 Aprotinin has typically been employed using empirically based dosing regimens, of which the two most common are the full and half Hammersmith dosing schemes. Such empiric dosing regimens may be a significant contributing factor to the reported suspected adverse off-target effect of renal dysfunction associated with the use of aprotinin. Specifically, kallikrein is inhibited by low and high dose aprotinin regimens 5,34 which can cause alterations in intrarenal hemodynamics potentially leading to acute renal dysfunction. However, the present study did not simultaneously measure indices of renal function, which based upon the findings from the current study would be warranted in future investigations. In addition, kinins and the kallikrein system provide a critical renal protective role in the context of ischemia reperfusion injury commonly associated with cardiac surgery and cardiopulmonary bypass.35–37 The inhibition of the kinin and kallikrein pathways by aprotinin may suppress endogenous renal protective mechanisms and accentuate renal injury incurred during cardiac surgery, but this issue remains speculative and warrants further study.
Study Limitations and Conclusions
The primary potential limitations of the current study are two. First, the in-vivo investigations did not include the context of cardiopulmonary bypass or other heightened fibrinolytic states representative of typical clinical conditions under which aprotinin has been utilized. Second, the “low” and “high” aprotinin doses administered were based upon clinically established emperical dosing regimens, rather than using a weight based formulation. The primary objective of the current study was to quantify the regional and temporal effects of aprotinin on PLact in critical tissue compartments under de novo fibrinolytic conditions. Accordingly, this initial study was not performed in the context of cardiopulmonary bypass in order to limit the complexity of hemostatic interactions and pharmacokinetics which are significantly altered by cardiopulmonary bypass and requisite systemic heparinization. Studies by Iwata and colleagues demonstrated that distinct protective effects of aprotinin could be demonstrated in a piglet model of cardiopulmonary bypass.38 The extension of the current study findings provides a basis for the pursuit of similar PLact investigations involving a clinically relevant cardiopulmonary bypass model. This proof of concept pilot study was designed to determine if the predominant clinically relevant pharmacological effect of aprotinin, the inhibition of PLact relative to baseline, was quantifiable. A logical extension of the current study findings would be the pursuit of similar PLact investigations involving clinically relevant dose titrations that are designed to optimally inhibit PLact while minimizing off-target effects such as the suppression of the kinin and kallikrein pathways. These future dose-titration studies with simultaneous measurements of PLact would allow for identifying minimal threshold aprotinin dosing. Nevertheless, the present study provided a proof of concept approach for continuous and regional measurement of PLact, the fundamental pharmacological target for antifibrinolytic therapy, following aprotinin administration. The results of the present study provide a basis for future investigations whereby this continuous approach to measure interstitial PLact may provide a means to optimize and individualize antifibrinolytic therapy.
Acknowledgments
This study was supported in part by NIH grants HL059165, HL078650 and a Merit Award form the Veterans' Affairs Health Administration.
References
- 1.Fergusson DA, Hébert PC, Mazer CD, Fremes S, MacAdams C, Murkin JM, Teoh K, Duke PC, Arellano R, Blajchman MA, Bussiéres JS, Côté D, Karski J, Martineau R, Robblee JA, Rodger M, Wells G, Clinch J, Pretorius R. BART Investigators. A comparison of aprotinin and lysine analogues in high-risk cardiac surgery. N Engl J Med. 2008;29;358(22):2319–31. doi: 10.1056/NEJMoa0802395. [DOI] [PubMed] [Google Scholar]
- 2.Mangano DT, Tudor IC, Dietzel C. Multicenter Study of Perioperative Ischemia Research Group. Ischemia Research and Education Foundation. The risk associated with aprotinin in cardiac surgery. N Engl J Med. 2006;354(4):353–65. doi: 10.1056/NEJMoa051379. [DOI] [PubMed] [Google Scholar]
- 3.Mouton R, Finch D, Davies I, Binks A, Zacharowski K. Effect of aprotinin on renal dysfunction in patients undergoing on-pump and off-pump cardiac surgery: a retrospective observational study. Lancet. 2008;9;371(9611):475–82. doi: 10.1016/S0140-6736(08)60237-8. [DOI] [PubMed] [Google Scholar]
- 4.Davis R, Whittington R. Aprotinin. A review of its pharmacology and therapeutic efficacy in reducing blood loss associated with cardiac surgery. Drugs. 1995;49(6):954–83. doi: 10.2165/00003495-199549060-00008. [DOI] [PubMed] [Google Scholar]
- 5.McEvoy MD, Reeves ST, Reves JG, Spinale FG. Aprotinin in cardiac surgery: a review of conventional and novel mechanisms of action. Anesth Analg. 2007;105(4):949–62. doi: 10.1213/01.ane.0000281936.04102.9f. [DOI] [PubMed] [Google Scholar]
- 6.Beath SM, Nuttall GA, Fass DN, Oliver WC, Jr, Ereth MH, Oyen LJ. Plasma aprotinin concentrations during cardiac surgery: full- versus half-dose regimens. Anesth Analg. 2000;91(2):257–64. doi: 10.1097/00000539-200008000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Shaw A, Swaminathan M, Stafford-Smith M. Cardiac surgery-associated acute kidney injury: putting together the pieces of the puzzle. Nephron Physiol. 2008;109(4):p55–60. doi: 10.1159/000142937. [DOI] [PubMed] [Google Scholar]
- 8.Faulí A, Gomar C, Campistol JM, Alvarez L, Manig AM, Matute P. Kidney-specific proteins in patients receiving aprotinin at high- and low-dose regimens during coronary artery bypass graft with cardiopulmonary bypass. Eur J Anaesthesiol. 2005;22(9):666–71. doi: 10.1017/s0265021505001109. [DOI] [PubMed] [Google Scholar]
- 9.Schweizer A, Höhn L, Morel DR, Kalangos A, Licker M. Aprotinin does not impair renal haemodynamics and function after cardiac surgery. Br J Anaesth. 2000;84(1):16–22. doi: 10.1093/oxfordjournals.bja.a013374. [DOI] [PubMed] [Google Scholar]
- 10.Kincaid EH, Ashburn DA, Hoyle JR, Reichert MG, Hammon JW, Kon ND. Does the combination of aprotinin and angiotensin-converting enzyme inhibitor cause renal failure after cardiac surgery? Ann Thorac Surg. 2005;80(4):1388–93. doi: 10.1016/j.athoracsur.2005.03.136. discussion 1393. [DOI] [PubMed] [Google Scholar]
- 11.Seto S, Kher V, Scicli AG, Beierwaltes WH, Carretero OA. The effect of aprotinin (a serine protease inhibitor) on renal function and renin release. Hypertension. 1983;5(6):893–9. doi: 10.1161/01.hyp.5.6.893. [DOI] [PubMed] [Google Scholar]
- 12.Lindvall G, Sartipy U, Ivert T, van der Linden J. Aprotinin is not associated with postoperative renal impairment after primary coronary surgery. Ann Thorac Surg. 2008 Jul;86(1):13–9. doi: 10.1016/j.athoracsur.2008.03.033. [DOI] [PubMed] [Google Scholar]
- 13.Dietrich W, Spannagl M, Boehm J, Hauner K, Braun S, Schuster T, Busley R. Tranexamic acid and aprotinin in primary cardiac operations: an analysis of 220 cardiac surgical patients treated with tranexamic acid or aprotinin. Anesth Analg. 2008 Nov;107(5):1469–78. doi: 10.1213/ane.0b013e318182252b. [DOI] [PubMed] [Google Scholar]
- 14.Brown JR, Birkmeyer NJ, O'Connor GT. Meta-analysis comparing the effectiveness and adverse outcomes of antifibrinolytic agents in cardiac surgery. Circulation. 2007 Jun 5;115(22):2801–13. doi: 10.1161/CIRCULATIONAHA.106.671222. [DOI] [PubMed] [Google Scholar]
- 15.Kang HM, Kalnoski MH, Frederick M, Chandler WL. The kinetics of plasmin inhibition by aprotinin in vivo. Thromb Res. 2005;115(4):327–40. doi: 10.1016/j.thromres.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 16.McCarthy RJ, Tuman KJ, O'Connor C, Ivankovich AD. Aprotinin pretreatment diminishes postischemic myocardial contractile dysfunction in dogs. Anesth Analg. 1999;89(5):1096–100. [PubMed] [Google Scholar]
- 17.Mojcik CF, Levy JH. Aprotinin and the systemic inflammatory response after cardiopulmonary bypass. Ann Thorac Surg. 2001;71(2):745–54. doi: 10.1016/s0003-4975(00)02218-9. [DOI] [PubMed] [Google Scholar]
- 18.Reust DL, Reeves ST, Abernathy JH, 3rd, Dixon JA, Gaillard WF, Jr, Mukherjee R, Koval CN, Stroud RE, Spinale FG. Interstitial plasmin activity with epsilon aminocaproic acid: temporal and regional heterogeneity. Ann Thorac Surg. 2010;89(5):1538–45. doi: 10.1016/j.athoracsur.2010.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reust DL, Reeves ST, Abernathy JH, 3rd, Dixon JA, Gaillard WF, 2nd, Mukherjee R, Koval CN, Stroud RE, Spinale FG. Temporally and regionally disparate differences in plasmin activity by tranexamic acid. Anesth Analg. 2010;110(3):694–701. doi: 10.1213/ANE.0b013e3181c7eb27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deschamps AM, Zavadzkas J, Murphy RL, Koval CN, McLean JE, Jeffords L, Saunders SM, Sheats NJ, Stroud RE, Spinale FG. Interruption of endothelin signaling modifies membrane type 1 matrix metalloproteinase activity during ischemia and reperfusion. Am J Physiol Heart Circ Physiol. 2008;294(2):H875–83. doi: 10.1152/ajpheart.00918.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marietta M, Facchini L, Pedrazzi P, Busani S, Torelli G. Pathophysiology of bleeding in surgery. Transplant Proc. 2006 Apr;38(3):812–4. doi: 10.1016/j.transproceed.2006.01.047. [DOI] [PubMed] [Google Scholar]
- 22.Goodnough LT. Risks of blood transfusion. Anesthesiol Clin North America. 2005 Jun;23(2):241–52. doi: 10.1016/j.atc.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 23.Society of Thoracic Surgeons Blood Conservation Guideline Task Force; Society of Cardiovascular Anesthesiologists Special Task Force on Blood Transfusion. Ferraris VA, Ferraris SP, Saha SP, Hessel EA, 2nd, Haan CK, Royston BD, Bridges CR, Higgins RS, Despotis G, Brown JR, Spiess BD, Shore-Lesserson L, Stafford-Smith M, Mazer CD, Bennett-Guerrero E, Hill SE, Body S. Perioperative blood transfusion and blood conservation in cardiac surgery: the Society of Thoracic Surgeons and The Society of Cardiovascular Anesthesiologists clinical practice guideline. Ann Thorac Surg. 2007 May;83(5 Suppl):S27–86. doi: 10.1016/j.athoracsur.2007.02.099. [DOI] [PubMed] [Google Scholar]
- 24.Edmunds LH., Jr Managing fibrinolysis without aprotinin. Ann Thorac Surg. 2010;89:324–31. doi: 10.1016/j.athoracsur.2009.10.043. [DOI] [PubMed] [Google Scholar]
- 25.http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2007/ucm109021.htm.
- 26.Waxler B, Rabito SF. Aprotinin: a serine protease inhibitor with therapeutic actions: its interaction with ACE inhibitors. Curr Pharm Des. 2003;9(9):777–87. doi: 10.2174/1381612033455468. [DOI] [PubMed] [Google Scholar]
- 27.Taby O, Chabbat J, Steinbuch M. Inhibition of activated protein C by aprotinin and the use of the insolubilized inhibitor for its purification. Thromb Res. 1990 Jul 1;59(1):27–35. doi: 10.1016/0049-3848(90)90268-h. [DOI] [PubMed] [Google Scholar]
- 28.Greilich PE, Okada K, Latham P, Kumar RR, Jessen ME. Aprotinin but not epsilon-aminocaproic acid decreases interleukin-10 after cardiac surgery with extracorporeal circulation: randomized, double-blind, placebo-controlled study in patients receiving aprotinin and epsilon-aminocaproic acid. Circulation. 2001 Sep 18;104(12 Suppl 1):I265–9. doi: 10.1161/hc37t1.094781. [DOI] [PubMed] [Google Scholar]
- 29.Dubber AH, McNicol GP, Uttley D, Douglas AS. In vitro and in vivo studies with trasylol, an anticoagulant and a fibrinolytic inhibitor. Br J Haematol. 1968 Jan;14(1):31–49. doi: 10.1111/j.1365-2141.1968.tb01470.x. [DOI] [PubMed] [Google Scholar]
- 30.Hajjar Katherine A, Francis Charles W, Lichtman MA, Beutler E, Kipps TJ, Seligsohn U, Kaushansky K, Prchal JT. “Chapter 127. Fibrinolysis and Thrombolysis” (Chapter) Williams Hematology. :7e. http://www.accessmedicine.com/content.aspx?aID=2136025.
- 31.Duggan E, O'Dwyer MJ, Caraher E, Diviney D, McGovern E, Kelleher D, McManus R, Ryan T. Coagulopathy after cardiac surgery may be influenced by a functional plasminogen activator inhibitor polymorphism. Anesth Analg. 2007 Jun;104(6):1343–7. doi: 10.1213/01.ane.0000261267.28891.00. [DOI] [PubMed] [Google Scholar]
- 32.Kramer HJ, Moch T, von Sicherer L, Düsing R. Efects of aprotinin on renal function and urinary prostaglandin excretion in conscious rats after acute salt loading. Clin Sci (Lond) 1979 Jan;56(6):547–53. doi: 10.1042/cs0560547. [DOI] [PubMed] [Google Scholar]
- 33.Dorman BH, Stroud RE, Wyckoff MM, Zellner JL, Botta D, Leonardi AH, Ikonomidis JS, Spinale FG. Differential effects of epsilon-aminocaproic acid and aprotinin on matrix metalloproteinase release in patients following cardiopulmonary bypass. J Cardiovasc Pharmacol. 2008 Apr;51(4):418–423. doi: 10.1097/FJC.0b013e318168400a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bennett-Guerrero E, Sorohan JG, Howell ST, Ayuso L, Cardigan RA, Newman MF, Mackie IJ, Reves JG, Mythen MG. Maintenance of therapeutic plasma aprotinin levels during prolonged cardiopulmonary bypass using a large-dose regimen. Anesth Analg. 1996 Dec;83(6):1189–92. doi: 10.1097/00000539-199612000-00010. [DOI] [PubMed] [Google Scholar]
- 35.Bledsoe G, Shen B, Yao Y, Zhang JJ, Chao L, Chao J. Reversal of renal fibrosis, inflammation, and glomerular hypertrophy by kallikrein gene delivery. Hum Gene Ther. 2006 May;17(5):545–55. doi: 10.1089/hum.2006.17.545. [DOI] [PubMed] [Google Scholar]
- 36.Kakoki M, McGarrah RW, Kim HS, Smithies O. Bradykinin B1 and B2 receptors both have protective roles in renal ischemia/reperfusion injury. Proc Natl Acad Sci U S A. 2007 May 1;104(18):7576–81. doi: 10.1073/pnas.0701617104. Epub 2007 Apr 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wagener G, Lee HT. Aprotinin and urinary neutrophil gelatinase-associated lipocalin after cardiac surgery. Anesth Analg. 2008 May;106(5):1593. doi: 10.1213/ane.0b013e31816a31ad. [DOI] [PubMed] [Google Scholar]
- 38.Iwata Y, Okamura T, Ishibashi N, Zurakowski D, Lidov HG, Jonas RA. Optimal dose of aprotinin for neuroprotection and renal function in a piglet survival model. J Thorac Cardiovasc Surg. 2009 Jun;137(6):1521–9. doi: 10.1016/j.jtcvs.2008.06.049. discussion 1529. [DOI] [PMC free article] [PubMed] [Google Scholar]