Abstract
We have reacted [Pt(dien)Cl]Cl, [Pt(en)(D2O)2]2+, and [Pt(Me4en)(D2O)2]2+ [Me4en = N,N,N′,N′-tetramethylethylenediamine] with selenomethionine (SeMet). When [Pt(dien)Cl]Cl is reacted with SeMet, [Pt(dien)(SeMet-Se)]2+ is formed; two Se-CH3 resonances are observed due to the different chiralities at the Se atom upon platination. In a reaction of [Pt(dien)Cl]Cl with an equimolar mixture of SeMet and Met, the SeMet product forms more quickly though a slow equilibrium with approximately equal amounts of both products is reached. [Pt(Me4en)(D2O)2]2+ reacts with SeMet to form [Pt(Me4en)(SeMet-Se)(D2O)]2+ initially but forms [Pt(Me4en)(SeMet-Se,N)]+ ultimately. One stereoisomer of the chelate, assigned to the R chirality at the Se atom, dominates within the first few minutes of reaction. [Pt(en)(D2O)2]2+ forms a variety of products depending on reaction stoichiometry; when one equivalent or less of SeMet is added, the dominant product is [Pt(en)(SeMet-Se,N)]+. In the presence of excess SeMet, [Pt(en)(SeMet-Se)2]2+ is the dominant initially, but displacement of the en ligand occurs leading to [Pt(SeMet-Se,N)2] as the eventual product. Displacement of the en ligand from [Pt(en)(SeMet-Se,N)]+ does not occur. In reactions of K2PtCl4 with two equivalents of SeMet, [Pt(SeMet-Se,N)2] is formed, and three sets of resonances are observed due to different chiralities at the Se atoms. Only the cis geometric isomers are observed by 1H and 195Pt NMR spectroscopy.
Keywords: nuclear magnetic resonance, ligand binding, anticancer, platinum, selenium
1. Introduction
Complexes of the type cis-PtA2X2, where A2 represents two unidentate or one bidentate amine ligand and X2 represents two monodentate or one bidentate leaving group, have been widely studied in part due to the anticancer activity of cisplatin, cis-Pt(NH3)2Cl2. Cisplatin’s anticancer activity has been attributed to the formation of a 1,2-intrastrand crosslink between two adjacent guanine residues in DNA.[1] However, interaction with proteins may be important in transport of platinum complexes into the cell.[2–4] Furthermore, [Pt(dien)Cl]Cl has been shown to react faster with methionine analogs than with 5′-GMP.[5, 6] Thus, a better understanding of the interaction of platinum complexes with amino acids is necessary.
Platinum complexes have a high affinity for methionine, a standard amino acid found in proteins. Depending on factors such as pH, stoichiometry, and the bulk of the amine ligands on the platinum, a variety of products result from the reaction of methionine or N-acetylmethionine with cis-PtA2X2 complexes. When methionine is present in excess, complexes of the type cis-PtA2(Met-S)2 may be observed.[7] Chelates in which the sulfur atom and either a carboxyl oxygen or the amine nitrogen are coordinated to the platinum are also known.[7–9] The trans effect of the methionine sulfur atom can lead to displacement of the ammine ligands of cisplatin[10, 11] and can sometimes lead to displacement of a nitrogen atom from a chelated diamine such as ethylenediamine (en).[8, 12]
Selenomethionine (SeMet) is a nonstandard amino acid in which the sulfur atom of methionine is replaced by selenium. Unlike selenocysteine, which is intentionally inserted into proteins such as glutathione peroxidase, selenomethionine may be randomly inserted into proteins in place of methionine.[13] In addition to the presence of nonstandard selenium-containing amino acids, some organoselenium compounds have been in clinical trials as cancer chemopreventative drugs. Selenomethionine was found to have protective effects against cisplatin-induced toxicity in rats and mice.[14] Both 5-methylselenocysteine and selenomethionine greatly increased survival in nude mice that were administered toxic doses of cisplatin and oxaliplatin.[15] Thus, previous studies have focused on the molecular interaction of cisplatin and analogs with selenomethionine.[16, 17]
Selenium, like sulfur, has a significant trans effect that can lead to rapid displacement of an ammine ligand of cisplatin. Mass spectrometry studies of the reaction between cisplatin and selenomethionine found that even at 1:1 SeMet:Pt ratios, displacement of one amine ligand occurs to a significant amount within 10 minutes.[17] For both cisplatin and carboplatin, a significant amount of [Pt(SeMet-Se,N)2]+ was detected by mass spectrometry within 24 h,[16, 17] indicating that both ammine ligands had been displaced.
In this study, we have utilized platinum complexes that contain chelated diamine and triamine ligands. We wanted to use these platinum complexes to characterize SeMet complexes that would be less prone to amine ligand displacement than the cisplatin analogs used previously.[16, 17] We also wanted to determine whether Met or SeMet would react preferentially with platinum complexes.
2. Experimental
K2PtCl4, diethylenetriamine, Pt(en)Cl2, selenomethionine, methionine, and silver nitrate were used as received. [Pt(dien)Cl]Cl and Pt(Me4en)Cl2 were prepared by methods described previously.[9, 18]
Pt(en)(NO3)2 and Pt(Me4en)(NO3)2 were prepared by reacting two equivalents of AgNO3 with the appropriate platinum(II) dichloride compound in H2O and stirring in an amber vial overnight. The samples were filtered to remove AgCl precipitate and evaporated to dryness. When added to D2O, [Pt(en)(D2O)2]2+ and [Pt(Me4en)(D2O)2]2+ would be expected as the dominant species due to the very small association constant reported for NO3 with platinum complexes.[19]
1H and 195Pt NMR spectra were acquired on a JEOL Eclipse 500 MHz NMR instrument. The 1H NMR spectra were referenced to the residual HOD signal relative to TSP, adjusted for temperature. The 195Pt NMR spectra were referenced relative to K2PtCl6. All spectra were collected at room temperature unless otherwise noted.
A Hitachi LaChrom Elite HPLC system with a cation-exchange column was utilized. Buffer A was 20 mM sodium phosphate at pH 6, while buffer B was 20 mM sodium phosphate with 0.5 M NaCl added at pH 6. The gradient was as follows: t = 0, 100% A; t = 2 min, 100% A; t = 20 min, 50% A.
Molecular mechanics calculations were performed according to methods described previously using a modified AMBER force field.[9]
3. Results
3.1 Reaction of [Pt(dien)Cl]Cl with SeMet
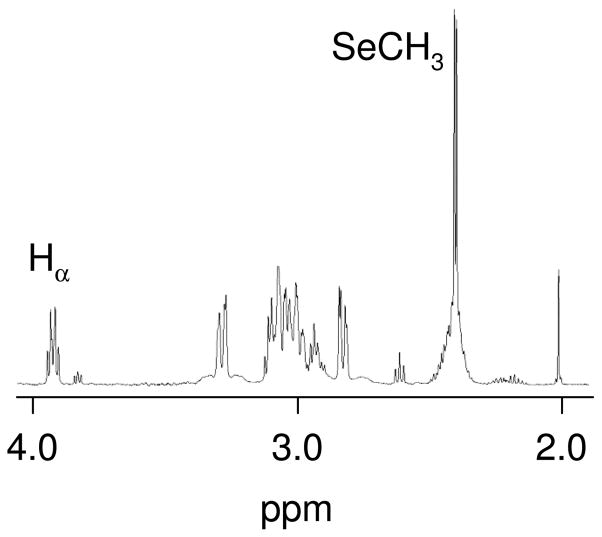
[Pt(dien)Cl]Cl and SeMet (10 mM each) were combined in D2O at pH 5. A 1H NMR spectrum acquired ~45 minutes later showed new resonances; two singlets at 2.41 and 2.42 ppm were observed; these singlets were ~0.4 ppm downfield of the singlet observed for unreacted SeMet (Figure 1). A downfield shift of similar magnitude has been previously observed for S-CH3 resonances of Met upon coordination of the Met to platinum via the sulfur atom,[9, 20, 21] and similar shifts were observed with previous complexes of platinum with selenomethionine.[16, 17] Thus, the singlets were assigned to the CH3 resonance of [Pt(dien)(SeMet-Se)]2+. A COSY spectrum was used to assign the additional product resonances (Table 1).
Figure 1.
Partial 1H NMR spectrum of the reaction of [Pt(dien)Cl]Cl with SeMet at pH ~4.5 at room temperature Initial concentrations of both are 10 mM.
Table 1.
1H and 195Pt NMR chemical shifts of SeMet complexes
| CH3 | Hα | Hβ | Hγ | 195Pt | |
|---|---|---|---|---|---|
| SeMet | 2.02 | 3.84 | 2.19, 2.24 | 2.63 | N/A |
| [Pt(dien)(SeMet-Se)]2+ | 2.44, 2.43 | 3.96 | 2.44 | 3.09, 2.98 | −3420 |
| [Pt(Me4en)(SeMet-Se,N)] R | 2.49 | 3.55 | 2.36, 2.79 | 2.91 | −3190 |
| [Pt(Me4en)(SeMet-Se,N)] S | 2.47 | 3.55 | 2.14, 2.87 | 3.17 | −3260 |
| [Pt(en)(SeMet-Se,N)]+ | 2.41, 2.43 | 3.48 | 2.32, 2.54 | 3.05, 2.93 | −3381, −3393 |
| [Pt(en)(SeMet-Se 2+)2] | 2.53, 2.54 | 3.86 | 2.25, 2.39 | 3.07, 3.18 | −3910 |
| cis-[Pt(SeMet-Se,N)2] RR | 2.55 | 3.73 | 2.36, 2.68 | 2.97, 3.04 | −3882 |
| cis-[Pt(SeMet-Se,N)2] RS | 2.50, 2.46 | 3.70, 3.76 | 2.28, 2.52a | a | −3837 |
| cis-[Pt(SeMet-Se,N)2] SS | 2.49 | 3.85 | 2.20, 2.44 | 3.09a | −3815 |
Incomplete assignment due to overlap with other signals
The presence of two CH3 resonances suggests that relatively slow interconversion of chirality about the Se atom is occurring. Only one CH3 resonance was observed previously for the analogous [Pt(dien)(Met-S)]2+ complex;[5] however, the presence of two sets of resonances has been observed previously for a number of platinum complexes with sulfur-coordinated methionine by 1H and/or 195Pt NMR methods [9, 22, 23] and multiple sets of resonances were observed in a [1H, 15N] HSQC NMR spectrum of a product formed between carboplatin and SeMet.[16] Support for the assignment of two interconverting chiralities came from variable temperature experiments that showed the signals broadening by 60 °C and beginning to coalesce by 80 °C. A single peak at −3420 ppm was observed in the 195Pt NMR spectrum at room temperature.
3.2 Reaction of [Pt(dien)Cl]Cl with SeMet and Met
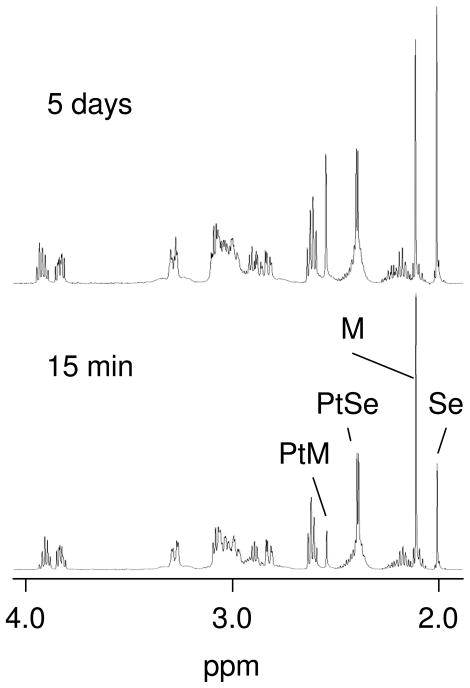
In order to determine the relative reactivities of SeMet and Met with a selected platinum complex, [Pt(dien)Cl]Cl was added to a mixture of SeMet and Met to give a solution that was 5 mM in all three components. During the first several hours of reaction, the 1H NMR spectrum showed signals corresponding to [Pt(dien)(SeMet-Se)]2+ and [Pt(dien)(Met-S)]2+; however, the signals of the former were notably larger than those of the latter (Figure 2). Moreover, the signals corresponding to unreacted Met were larger than those of unreacted SeMet. This result indicates that SeMet is the kinetically favored target of [Pt(dien)Cl]Cl. Previously, SeMet was reported to have a faster reaction rate than Met with carboplatin.[16]
Figure 2.
Partial 1H NMR spectra of the reaction of [Pt(dien)Cl] with a mixture of Met and SeMet at 15 min and 5 days at room temperature. Initial concentrations are 5 mM each. The methyl resonances of SeMet (Se), Met (M), [Pt(dien)(SeMet-Se)]2+ (PtSe), and [Pt(dien)(SeMet-Se)]2+ (PtM) are labeled.
After several days, the 1H NMR spectrum indicated that the amount of [Pt(dien)(Met-S)]2+ had increased (Figure 2). Furthermore, it was apparent that the amount of free SeMet had increased relative to the amount of [Pt(dien)(SeMet-Se)]2+. This result suggests that Met can slowly displace SeMet from the platinum complex.
In separate experiments, we added SeMet to [Pt(dien)(Met-S)]2+ and Met to [Pt(dien)(SeMet-Se)]2+; after several days, both 1H NMR spectra looked similar to the analogous spectrum in Figure 2. These results indicate that SeMet and Met can displace one another from platinum complexes and that thermodynamically both complexes are relatively similar in stability under these conditions.
3.3 Reaction of [Pt(Me4en)(D2O)2]2+ with SeMet
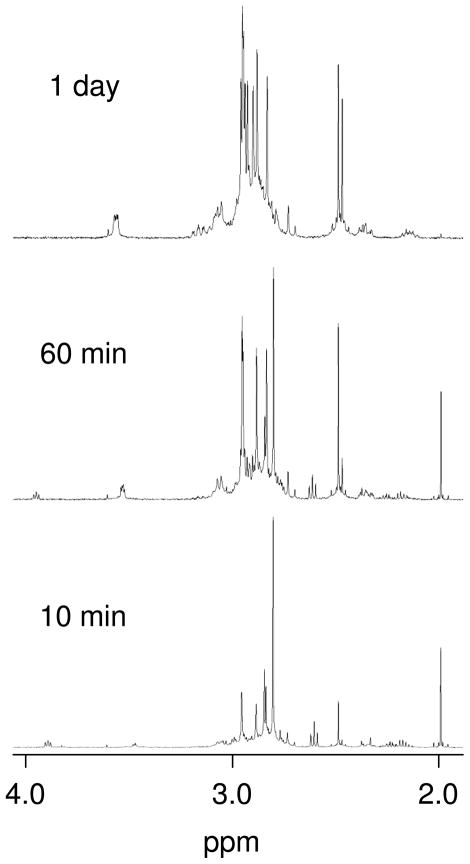
When [Pt(Me4en)(D2O)2]2+ was reacted with SeMet at pH ~4, new resonances were observed within ~10 minutes (Figure 3). Singlets in the ~2.3–2.4 ppm region and doublet of doublet signals around ~3.9–4.1 ppm appeared initially but had disappeared within 20 minutes, suggesting formation of [Pt(Me4en)(SeMet-Se)(D2O)]2+ complexes. A larger singlet was observed at 2.49 ppm within 10 minutes, and this resonance continued to increase over time. A small singlet was observed at 2.47 ppm within 10 minutes, and it slowly increased in intensity until it was approximately the same size as the 2.49 ppm resonance after 1 day. Two 195Pt NMR resonances were observed at −3190 and −3260 ppm after 1 day. The two sets of resonances that dominated after 1 day are assigned to a [Pt(Me4en)(SeMet-Se,N)]+ complex with different chiralities at the Se atom. Support for the presence of a Se,N chelate comes from the upfield shifts of the Hα signals (Table 1), which have been observed previously for S,N chelates.[9, 11, 24] A previous study found that [Pt(Me4en)(D2O)2]2+ reacted with Met to form [Pt(Me4en)(Met-S,N)]+, and two sets of resonances were likewise observed.[9]
Figure 3.
Partial 1H NMR spectra of the reaction of [Pt(Me4en)(D2O)2]2+ with SeMet at 10 min, 60 min, and 1 day at room temperature. Initial concentrations are 5 mM each.
We also mixed [Pt(Me4en)(D2O)2]2+ with an equimolar ratio of NaCl; this resulted in significant formation of [Pt(Me4en)(D2O)Cl]+ within ~30 minutes as evidenced by two large singlets at 2.84 and 2.85 ppm assigned to the two sets of methyl groups of the Me4en ligand in the non-C2-symmetrical compound. When SeMet was then added, we saw two resonances at 2.38 and 2.39 ppm that grew at approximately equal amounts. These resonances are assigned to the Se-CH3 groups of [Pt(Me4en)(SeMet-Se)Cl]+; after ~5 days, approximately one-third of the [Pt(Me4en)(SeMet-Se)Cl]+ had converted into [Pt(Me4en)(SeMet-Se,N)]+.
We have previously used molecular mechanics to study a number of platinum(II) diamine complexes with methionine.[9, 20] Since there are relatively few crystal structures of platinum(II) selenomethionine complexes, we instead examined models of energy-minimized [Pt(Me4en)(Met-S,N)]+ structures with both the R and the S stereochemistries at the sulfur atom. The energy-minimized structure with the R stereochemistry had a twist-boat conformation for the six-membered chelate ring that formed, as this conformation avoided steric clashes of the Se-CH3 group with the methyl groups of the Me4en ligand. However, the six-membered chelate ring had a chair conformation with the S chirality. The dihedral angles formed between the Hα and Hβ hydrogens were 151° and 33° in the R configuration and 176° and 66° in the S configuration. In examining the Hα coupling constants of the initial stereoisomer of [Pt(Me4en)(SeMet-Se,N)]+ that formed, they were found to be 3.0 and 5.5 Hz. The other stereoisomer had coupling constants of 2.6 and 8.9 Hz. The dihedral angles from the molecular mechanics structure in the R stereochemistry are more consistent with the coupling constants observed for the initially dominant stereoisomer, and thus we assign that stereoisomer to R.
3.4 Reaction of [Pt(en)(D2O)2]2+ with SeMet
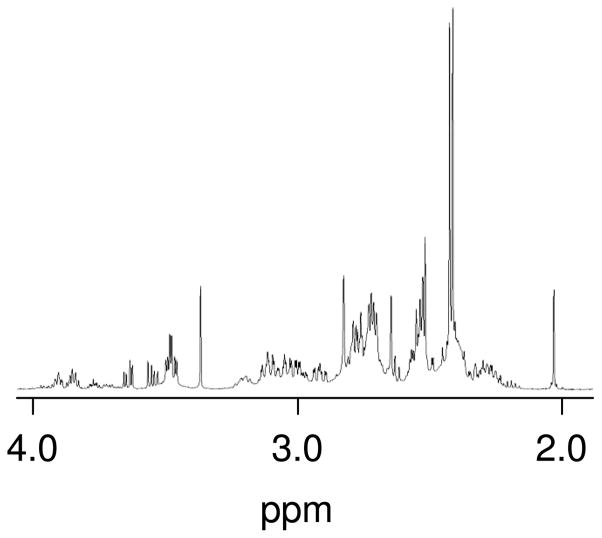
When [Pt(en)(D2O)2]2+ was reacted with SeMet, several new sets of resonances could be observed. The relative abundances of the signals depended heavily on the SeMet:Pt ratio. When low SeMet:Pt ratios (≤ were used, two sets of resonances were typically dominant with singlets observed at 2.41 and 2.43 ppm (Figure 4). An Hα resonance was observed at ~3.48 ppm; the upfield shift of this resonance suggests [Pt(en)(SeMet-Se,N)]+ is the dominant species,[9, 11] with differing chiralities at the Se atom accounting for the presence of two CH3 singlets.
Figure 4.
Partial 1H NMR spectrum of the reaction of [Pt(en)(D2O)2]2+ with SeMet at a ~1:1 Pt:Se ratio at room temperature. SeMet was added incrementally every 5 minutes for 5 additions, and the final concentrations are ~50 mM each. The spectrum was acquired ~2 h after initial addition of SeMet.
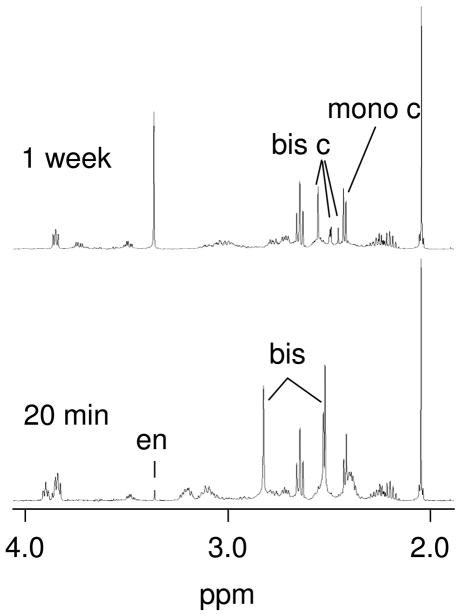
When SeMet was added in excess (2:1 or greater SeMet:Pt ratio), singlets at 2.53 and 2.54 ppm were observed within ~10 minutes; also, a singlet at ~2.83 ppm appeared concurrently (Figure 5). These resonances are assigned to [Pt(en)(SeMet-Se)2]2+. The singlet at 2.83 ppm is assigned to the en CH2 resonances; 1H-13C HMQC data showed the 13C NMR shift for this signal to be 47.8 ppm, and both 1H and 13C NMR data are consistent with en resonances in similar complexes.[7] The two singlets at 2.53 and 2.54 ppm are assigned to the CH3 resonances with chirality differences at the Se atom. Peaks due to [Pt(en)(SeMet-Se,N)]+ were also present though these signals are smaller.
Figure 5.
Partial 1H NMR spectra of the reaction of 10 mM [Pt(en)(D2O)2]2+ with excess (32 mM SeMet) at 20 min (bottom) and 1 week (top) at room temperature. Labeled peaks are from dissociated ethylendiamine (en), [Pt(en)(SeMet-Se)2]2+ (bis), [Pt(en)(SeMet-Se,N)]+ (mono c), and [Pt(SeMet-Se,N)2] (bis c).
As the reaction proceeded, the resonances corresponding to [Pt(en)(SeMet-Se)2]2+ decreased and new resonances appeared (Figure 5). A singlet at 3.38 ppm corresponds to uncoordinated ethylenediamine, indicating the en ligand has been displaced. Four new singlets in the 2.48–2.58 ppm range were observed and assigned to [Pt(SeMet-Se,N)2] species; two geometric isomers are possible, with three different diastereomers possible for each geometric isomer. Interestingly, even in the presence of excess SeMet, the signals due to [Pt(en)(SeMet-Se,N)]+ are still present. Similarly, when a sample that had been formed initially with a low Se:Pt ratio had excess SeMet added afterwards, we found that [Pt(en)(SeMet-Se,N)]+ signals that were present did not disappear even after 1 week. This result indicated that the formation of the [Pt(SeMet-Se,N)2] complex occurred via the [Pt(en)(SeMet-Se)2]2+ complex.
Support for the assignments of all of the complexes came from HPLC experiments using a cation exchange column. Samples containing [Pt(en)(SeMet-Se)2]2+ showed a peak with a retention time of 14.5 minutes, whereas samples containing [Pt(en)(SeMet-Se,N)]+ showed a peak with a retention time of 4.5 minutes. Samples of [Pt(SeMet-Se,N)2] showed no retention on the column and eluted in the void volume. Thus, the retention times correlate with the expected charges of the assigned species.
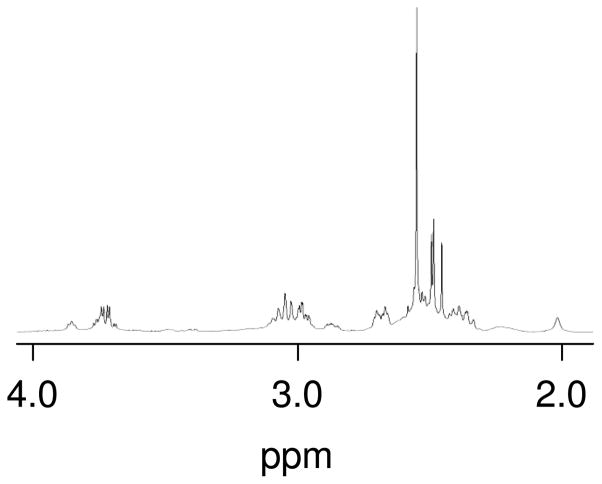
3.5 Reaction of K2PtCl4 with SeMet
In order to more fully characterize the [Pt(SeMet-Se,N)2] complexes, K2PtCl4 and SeMet were combined in a 1:2 molar ratio under conditions (heating to ~80 °C) similar to previous reactions between K2PtCl4 and Met that produced [Pt(Met-S,N)2].[23] Three new sets of resonances were observed (Figure 6). One set of resonances was dominant, with a large singlet at 2.58 ppm assigned to the S-CH3 resonance of the set. Resonances at 2.51 and 2.48 ppm were similar in size to one another, whereas a slightly larger resonance was observed at 2.52 ppm. The signals observed here are identical to those observed in the reactions of [Pt(en)(H2O)2]2+ with SeMet in which en displacement is observed, thus supporting their assignments to [Pt(SeMet-Se,N)2]. 195Pt NMR spectroscopy showed three major resonances in the range of −3800 to −3900 (Table 1).
Figure 6.
Partial 1H NMR spectrum of the reaction of 20 mM K2PtCl4 with 40 mM SeMet.
4 Discussion
A number of 195Pt NMR spectra of platinum(II) diamine complexes with methionine amino acids or methionine-containing peptides have been recorded; typically, a PtN2S2 environment gives shifts between −3600 and −3800 ppm whereas PtN3S environments give shifts of −3000 to −3300 ppm.[8, 9, 12] In the present study, we found that PtN2Se2 resonances were typically between −3800 and −4000 whereas PtN3Se resonances were approximately −3200 to −3400 ppm (Table 1), in each case ~200 ppm upfield of the range of analogous sulfur shifts. Other complexes containing PtN3Se coordination environments have been previously characterized and were found to have shifts in the same range as we observed.[25, 26]
Both SeMet[16, 17] and Met[24, 27, 28] have been shown previously to promote displacement of the ammine ligands from cisplatin and its analogs due to the trans effect from the selenium and sulfur ligation. The use of bidentate and tridentate amine ligands in the present study makes ligand displacement less prevalent. Nevertheless, we have found that an en ligand can be displaced from [Pt(en)(SeMet-Se)2]2+ as [Pt(SeMet-Se,N)2] complexes are formed. This conversion results in the displacement of one five- membered N,N chelate ring with two six-membered Se,N chelate rings. By comparison, [Pt(en)(N-AcMet-S)2] was found to be stable in D2O[7, 9] even though platinum complexes can coordinate to the amide nitrogen both in N-AcMet[7] and in peptides with internal methionine residues.[8] The amide nitrogen atom of N-AcMet is apparently less prone to displace the en ligand than the amine nitrogen atom of SeMet.
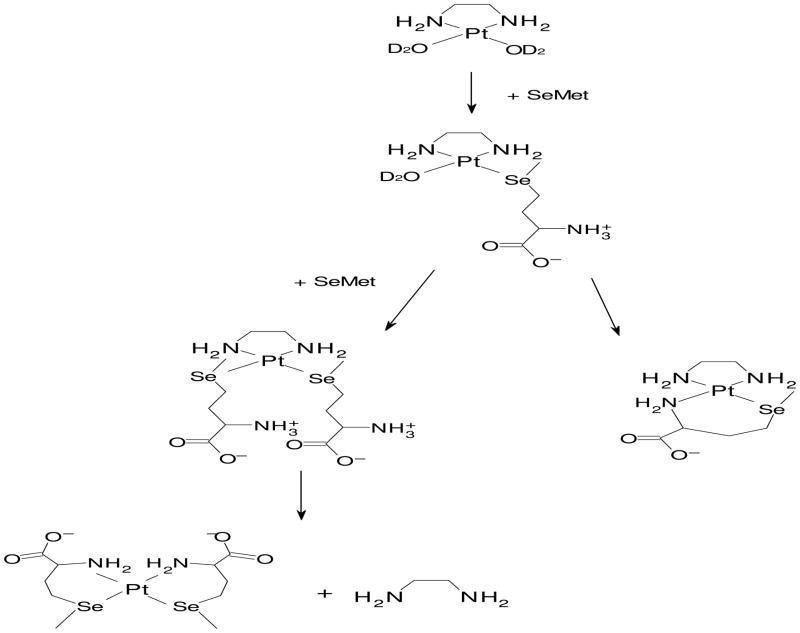
Reaction of SeMet with [Pt(en)(D2O)2]2+ can lead to multiple products (Figure 7). The initial product would be expected to be [Pt(en)(SeMet-Se)(D2O)]2+; however, this product was not directly observed. At high SeMet:Pt ratios, [Pt(en)(SeMet-Se)2]2+ is present initially; over time, this product disappears and signals from uncoordinated en and from [Pt(SeMet-Se,N)2] appear (Figure 5). At low SeMet:Pt ratios, [Pt(en)(SeMet-Se,N)]+ is dominant (Figure 4). When additional SeMet is present in a sample containing [Pt(en)(SeMet-Se,N)]+, little or no conversion of this product to [Pt(SeMet-Se,N)2] is observed. These results indicate that displacement of the en ligand occurs only when both coordination positions trans to the en N atoms are occupied by a selenium atom. In support of this observation, no displacement of the Me4en ligand was observed in the [Pt(Me4en)(SeMet-Se,N)]+ complexes. Previously, [Pt(en)(Met-S,N)]+ was found to be stable to substitution of the en ligand by 5′ough [Pt(NH3)2(Met-S,N)]+ led to displacement of the NH3 trans to the sulfur atom in the presence of 5′-GMP.[28]
Figure 7.
Proposed reaction scheme of [Pt(en)(D2O)2]2+ with SeMet.
Conversion of [Pt(en)(SeMet-Se)2]2+ to [Pt(SeMet-Se,N)2] presumably occurs through an intermediate in which one SeMet is chelated and the en ligand is coordinated in a monodentate fashion; formation of this intermediate would break a 5-membered N,N chelate ring but would form a 6-membered Se,N chelate ring. A monodentate en ligand was observed by 1H NMR spectroscopy previously in reactions with peptides that coordinated in a tridentate mode, though complete displacement of the en ligand was not observed in those complexes.[8, 12] We did not observe an intermediate with a monodentate en ligand in our complexes; thus, the complete displacement of the en ligand to form the second Se,N chelate in the present study may be occurring relatively rapidly.
The reaction of K2PtCl4 and SeMet could produce both cis- and trans-[Pt(SeMet-Se,N)2] complexes; furthermore, each geometric isomer could produce three diastereomers, with chiralities at the sulfur atoms of R,R; S,R; and S,S. Previously, the cis-[Pt(Met-S,N)2] geometric isomers were determined to be favored ~87:13 over the trans isomers at equilibrium,[23] and the latter had broader signals relative to the signals we observed in the SeMet complexes. Isomerization of the cis and trans forms of [Pt(Met-S,N)2] was very slow.[23] Furthermore, the resonances observed in the reaction of K2PtCl4 and SeMet are identical to those produced from the reaction of [Pt(en)(D2O)2]2+ with excess SeMet upon en displacement. Since the formation of [Pt(SeMet-Se,N)2] from [Pt(en)(SeMet-Se)2]2+ should produce the cis geometric isomers at least initially, we assign the [Pt(SeMet-Se,N)2] resonances observed to the three diasteromers with cis geometries. The 195Pt NMR spectra showed three major signals in the range of −3800 to −3900; [Pt(en)(SeMet-Se)2]2+, which has a cis-PtN2Se2 coordination environment, has a similar shift to those observed for [Pt(SeMet-Se,N)2] (Table 1). By comparison, the cis isomers of [Pt(Met-S,N)2] were ca. −3600 to −3700, whereas the trans isomers were significantly downfield of that at −3400 to −3500.[29]
The signals of identical size are assigned to the S, R isomer. The dominant isomer had a coupling constant of ~10 Hz between the Hα and one of the Hβ protons, which was true of the R stereochemistry in the analogous Met complexes.[23] Thus this isomer is assigned to the R,R stereochemistry and the remaining isomer to the S,S stereochemistry.
No signals corresponding to the trans geometric isomers of [Pt(SeMet-Se,N)2] were observed by 1H NMR spectroscopy; the 195Pt NMR spectrum showed three resonances between −3800 and −3900 ppm but no noticeable resonances ~200 ppm downfield as was observed for the analogous methionine complexes.[23] A preference for the cis geometric isomers of S-containing chelates has been noted previously and was rationalized by an increased π-accepting ability of the S atom relative to O.[30] The even greater preference for the cis isomer for SeMet complexes relative to Met complexes could be related to a stronger π-accepting ability of selenium compared to sulfur.
Previously, the reaction of carboplatin with SeMet was monitored at 310 K by both mass spectrometry and NMR spectroscopy.[16] The mass spectrometry data indicated that a [Pt(SeMet-Se,N)2]+ species was present in a significant amount in 3 h and dominant within 22 h. NMR spectroscopy showed resonances in the 2.55–2.65 ppm range that were significant at 5 h and 10 h. These resonances were not explicitly assigned to specific products, though the time of reaction would suggest [Pt(SeMet-Se,N)2] species as a possible assignment. We note that the pattern of these NMR resonances is very similar to those observed in our reaction of K2PtCl4 with SeMet, though with shifts slightly downfield of ours perhaps due to the temperature difference.
The reaction of [Pt(dien)Cl]Cl with SeMet produced two Se-CH3 resonances in the 1H NMR spectrum; however, the close spacing of the resonances and the broadening at higher temperatures suggest that the resonances are from a single complex with different chiralities at the Se atom. The presence of two diastereomers has been observed previously for complexes of platinum(II) with methionine[9, 22, 23] and selenomethionine.[16] Thus, the resonances were assigned to [Pt(dien)(SeMet-Se)]2+.
Competition experiments indicated that [Pt(dien)Cl]Cl reacts faster with SeMet than with Met (Figure 2). These results indicate that reaction with SeMet has a lower activation energy than reaction with Met, suggesting that the selenium atom has more favorable interactions in the transition state than the sulfur atom. This preference for SeMet is consistent with previous data[16] that indicated that carboplatin reacted with methionine slower than with selenomethionine.
Coordination of either sulfur or selenium to carboplatin led to displacement of the trans ammine ligand;[16] however, the tridentate dien ligand utilized in this study is inert to such substitution, and thus our competition studies can give insights about thermodynamic as well as kinetic preferences. When the reaction of [Pt(dien)Cl]Cl with SeMet and Met was monitored over several days, it was observed that approximately equal amounts of Met and SeMet products were produced. Moreover, when Met is added to a sample of [Pt(dien)(SeMet-Se)]2+ or SeMet is added to a sample of [Pt(dien)(Met-S)]2+, a similar distribution of Met and SeMet products is reached. Thus, the thermodynamic stabilities of the Met and SeMet products are apparently very similar, and a slow equilibrium is reached when both Met and SeMet are present.
Reaction of SeMet with [Pt(Me4en)(D2O)2]2+ led initially to two stereoisomers of [Pt(Me4en)(SeMet-Se)(D2O)]2+ and eventually to two stereoisomers of [Pt(Me4en)(SeMet-Se,N)]+. Formation of the chelates was expected, as analogous complexes have been previously found to form when Met was added to this platinum complex.[9] Interestingly, however, the selective formation of one stereoisomer of the SeMet chelates was observed initially. We assigned the initial product to the R stereochemistry at the Se atom based on an analysis of coupling constants and a molecular mechanics analysis of [Pt(Me4en)(Met-S,N)]+ chelates.
There are two possible explanations for the kinetic preference of one stereoisomer (assigned to R) in the chelate. One possibility is that formation of the R stereochemistry of the mono product is favored over S and that chelation occurs rapidly relative to isomerization of the mono products. Another possibility is that chelation occurs more readily for the R isomer than for the S isomer in SeMet. Signals from the [Pt(Me4en)(SeMet-Se)Cl]+ stereoisomers arise at approximately equal rates, and thus it is likely that the same would be true of [Pt(Me4en)(SeMet-Se)(D2O)]2+. Furthermore, isomerization occurs within the order of seconds at least for the mono product [Pt(dien)(SeMet-Se)]2+ according to variable temperature NMR experiments, suggesting that one stereoisomer would not build up in excess in the mono products. Thus, our experimental data is more consistent with the interpretation of faster chelation of the R isomer than the S isomer.
In summary, we have found that SeMet has several similarities but some notable differences compared with Met in terms of reactivity with platinum(II) complexes. Kinetically, SeMet is faster to react though both have similar thermodynamic stabilities; furthermore, SeMet and Met can displace one another from platinum(II) triamine complexes. In reactions with K2PtCl4, SeMet does not appear to form trans-[Pt(SeMet-Se,N)2] complexes whereas Met forms trans-[Pt(Met-S,N)2] complexes as minor species. The trans effect can lead to displacement of an ethylenediamine ligand in SeMet complexes when both remaining coordination positions have SeMet coordinated via selenium; the resulting substitution replaces one chelate ring with two chelate rings.
Acknowledgments
This research was supported by awards from Research Corporation (Cottrell College Science Award CC6464) and the NIH (1R15 GM074663-01). Stephanie Robey was supported as part of an award from the National Science Foundation (DBI-1004665).
Abbreviations
- SeMet
selenomethionine
- N-AcMet
N-acetylmethionine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wang D, Lippard SJ. Nat Rev Drug Discov. 2005;4:307–320. doi: 10.1038/nrd1691. [DOI] [PubMed] [Google Scholar]
- 2.Ishida S, Lee J, Thiele DJ, Herskowitz I. Proc Natl Acad Sci USA. 2002;99:14298–14302. doi: 10.1073/pnas.162491399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Song IS, Savaraj N, Siddik ZH, Liu P, Wei Y, Wu CJ, Kuo MT. Mol Cancer Ther. 2004;3:1543–1549. [PubMed] [Google Scholar]
- 4.Wu Z, Liu Q, Liang X, Yang X, Wang N, Wang X, Sun H, Lu Y, Guo Z. J Biol Inorg Chem. 2009;14:1313–1323. doi: 10.1007/s00775-009-0576-7. [DOI] [PubMed] [Google Scholar]
- 5.Barnham KJ, Djuran MI, Murdoch PD, Sadler PJ. J Chem Soc Chem Comm. 1994:721–722. [Google Scholar]
- 6.Djuran MI, Lempers ELM, Reedijk J. Inorg Chem. 1991;30:2648–2652. [Google Scholar]
- 7.Barnham KJ, Guo ZJ, Sadler PJ. J Chem Soc, Dalton Trans. 1996:2867–2876. [Google Scholar]
- 8.Fröhling CDW, Sheldrick WS. J Chem Soc, Dalton Trans. 1997:4411–4420. [Google Scholar]
- 9.Williams KM, Rowan C, Mitchell J. Inorg Chem. 2004;43:1190–1196. doi: 10.1021/ic035212m. [DOI] [PubMed] [Google Scholar]
- 10.Kasherman Y, Sturup S, Gibson D. J Biol Inorg Chem. 2009;14:387–399. doi: 10.1007/s00775-008-0456-6. [DOI] [PubMed] [Google Scholar]
- 11.Hahn M, Kleine M, Sheldrick WS. J Biol Inorg Chem. 2001;6:556–566. doi: 10.1007/s007750100232. [DOI] [PubMed] [Google Scholar]
- 12.Siebert AFM, Sheldrick WS. J Chem Soc, Dalton Trans. 1997:385–393. [Google Scholar]
- 13.Tinggi U. Envir Health Prev Med. 2008;13:102–108. doi: 10.1007/s12199-007-0019-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rao M, Rao MN. J Pharm Pharmacol. 1998;50:687–691. doi: 10.1111/j.2042-7158.1998.tb06906.x. [DOI] [PubMed] [Google Scholar]
- 15.Fakih M, Cao S, Durrani FA, Rustum YM. Clin Colorectal Cancer. 2005;5:132–135. doi: 10.3816/ccc.2005.n.026. [DOI] [PubMed] [Google Scholar]
- 16.Liu Q, Lin J, Jiang P, Zhang J, Zhu L, Guo Z. Eur J Inorg Chem. 2002;2002:2170–2178. [Google Scholar]
- 17.Liu Q, Zhang J, Ke X, Mei Y, Zhu L, Guo Z. J Chem Soc, Dalton Trans. 2001:911–916. [Google Scholar]
- 18.Annibale G, Brandolisio M, Pitteri B. Polyhedron. 1995;14:451–453. [Google Scholar]
- 19.Martin RB. In: Cisplatin: Chemistry and Biochemistry of a Leading Anticancer Drug. Lippert B, editor. Wiley-VCH; Weinheim: 1999. pp. 183–205. [Google Scholar]
- 20.Williams KM, Chapman DJ, Massey SR, Haare C. J Inorg Biochem. 2005;99:2119–2126. doi: 10.1016/j.jinorgbio.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 21.Hohage O, Manka S, Sheldrick WS. Inorg Chim Acta. 2009;362:953–966. [Google Scholar]
- 22.Gummin DD, Ratilla EMA, Kostić NM. Inorg Chem. 1986;25:2429–2433. [Google Scholar]
- 23.Murdoch PdS, Ranford JD, Sadler PJ, Berners-Price SJ. Inorg Chem. 1993;32:2249–2255. [Google Scholar]
- 24.Appleton TG, Connor JW, Hall JR. Inorg Chem. 1988;27:130–137. [Google Scholar]
- 25.Rothenburger C, Galanski M, Arion VB, Gorls H, Weigand W, Keppler BK. Eur J Inorg Chem. 2006;2006:3746–3752. [Google Scholar]
- 26.Beaty JA, Jones MM, Ma L. Chem Res Toxicol. 1992;5:647–653. doi: 10.1021/tx00029a009. [DOI] [PubMed] [Google Scholar]
- 27.Kasherman Y, Sturup S, Gibson D. J Biol Inorg Chem. 2009;14:387–399. doi: 10.1007/s00775-008-0456-6. [DOI] [PubMed] [Google Scholar]
- 28.Barnham KJ, Djuran MI, Murdoch PdS, Ranford JD, Sadler PJ. J Chem Soc, Dalton Trans. 1995;1995:3721–3726. [Google Scholar]
- 29.Norman RE, Ranford JD, Sadler PJ. Inorg Chem. 1992;31:877–888. [Google Scholar]
- 30.Kogan VA, Kharabaev NN, Osipov OA, Kochin SG. J Struct Chem. 1981;22:96–116. [Google Scholar]