Abstract
In recent years there has been a surge in methods to synthesize geometrically and chemically complex microparticles. Analogous to atoms, the concept of a “periodic table” of particles has emerged and continues to be expanded upon. Complementing the natural intellectual curiosity that drives the creation of increasingly intricate particles is the pull from applications that take advantage of such high-value materials. Complex particles are now being used in fields ranging from diagnostics and catalysis to self-assembly and rheology, where material composition and microstructure are closely linked with particle function. This is especially true of polymer hydrogels, which offer an attractive and broad class of base materials for synthesis. Lithography affords the ability to engineer particle properties a priori and leads to the production of homogenous ensembles of particles. This review summarizes recent advances in synthesizing hydrogel microparticles using lithographic processes and highlight a number of emerging applications. We discuss advantages and limitations of current strategies, and conclude with an outlook on future trends in the field.
Keywords: hydrogels, microgels, synthesis, lithography, microfluidics, assembly, delivery
1. Introduction
Cross-linked hydrophilic polymer gels, or “hydrogels”, have become an important class of materials for applications in nanotechnology, biotechnology, and medicine due to their unique material properties [1]. They can be prepared from a wide variety of natural and synthetic precursors ranging from commodity to designer chemicals, and as a result, can be readily commercialized [2]. Hydrogels are highly swelling in water and combine the ability to transport molecular and nano-scale species throughout the material while maintaining solid-like mechanical properties [3]. This feature has led to the widespread use of hydrogels as a scaffolding material in biomedical applications including drug delivery, tissue engineering, and wound healing [1,4–6]. Their microstructure and interfacial properties can also be rendered responsive to various stimuli through chemical and physical cues, as well as applied fields, resulting in “smart” materials which can respond to their local environment.
Hydrogels are typically prepared and processed as bulk materials such as monolithic structures or supported films. However, emerging applications require miniaturization and tailoring of the hydrogel architecture at increasingly small length scales for delivery and transport purposes in microscopic environments. This has spurred the development of various processes for the synthesis of colloidal and nanoparticle hydrogels, or “microgels”. The majority of these techniques are based on traditional batch polymerization methods such as dispersion and emulsion polymerization [7]. More recent techniques based on microfluidic methodologies provide a higher degree of control over the size distribution of the microgels [8]. In either case, droplets of a polymerizable phase are generated within an inert carrier phase and stabilized by a surfactant [9]. The droplets are subsequently polymerized, after which they must be transferred from the carrier fluid to an appropriate solvent depending on further processing or end-use application, eventually yielding a suspension of colloidal hydrogel particles.
Microgels serve as model “soft colloids”, as they are easily stabilized and their swelling and mechanical properties can be tuned using various physicochemical stimuli [7]. They have become an important class of materials for various aspects of fundamental colloid science, including colloidal interactions, phase behavior, and rheology [10–15]. Furthermore, microgels are being developed for a number of potential applications in nanomaterial synthesis [16], optics and photonics [17–19], and medicine [20]. Although utilized for a wide range of fundamental and applied studies, the traditional “bottom-up” microgel synthesis techniques mentioned above have generally been limited to the production of spherical or spheroidal particles with uniform microstructure and surface chemistry, as well as moderate polydispersity. As a result, these techniques are ill-suited for emerging applications that demand high degrees of both uniformity and complexity and that call for the use of chemically and morphologically anisotropic particle architectures [21].
These technological requirements have driven the development of advanced methodologies for the synthesis of “designer” colloidal hydrogels with a number of desired process and particle characteristics. The ideal process would be able to produce highly uniform particles with well-defined shape or chemical anisotropy, with primary particle sizes spanning the colloidal domain. Furthermore, it would be versatile enough to accommodate different hydrogel materials, including changes to the fundamental polymer chemistry, chemical functionalization, and encapsulation of functional materials. Finally, the ideal process would be scalable to quantities of commercial interest, while remaining cost-effective by efficiently consuming reagents.
Lithographic processing methods, in which hydrogel colloids are templated in a highly controlled and reproducible manner, have emerged as an attractive “top-down” alternative to traditional colloidal hydrogel synthesis techniques because they meet many of these requirements [22]. Existing capabilities in microfabrication methods such as imprint lithography, photolithography, and microfluidics have been combined to create designer colloids with tailored architectures and customizable internal microstructures that cannot be achieved with bottom-up processes. Lithographic synthesis has thereby significantly expanded the design space of colloidal hydrogels, enabling enhanced applications in self-assembled and stimuli-responsive materials, micromechanical systems, pharmaceuticals, and medical diagnostics. In this review, we highlight progress to date in the synthesis and application of complex hydrogel colloids by lithographic techniques. We begin by describing the materials and methods commonly used in lithography of colloidal hydrogels, noting advantages and limitations of current strategies. We then examine the most crucial design properties of colloidal hydrogels, and how these can be controlled using the various forms of lithography-based synthesis. Finally, we outline a number of applications enabled by complex structured hydrogel particles, and conclude with an outlook on future trends and opportunities.
2. Lithographic patterning of hydrogel colloids
2.1 Materials for cross-linked, functionalized, and composite hydrogels
In principle, lithographically-patterned hydrogel colloids can be prepared by any of the routes typically used to produce a cross-linked polymer network, including chemical cross-linking, physical association, and molecular self-assembly [9]. However, the most prevalent method of hydrogel formation in lithographic processing is through covalent cross-linking via polymerization reaction. Nearly all studies to date involve photoinitiated free radical polymerization due to controlled initiation and relatively fast propagation kinetics compared to other types of polymerization. The vast majority of these methods involve the processing of a hydrogel precursor which consists of, at minimum, a photoinitiator, a monomer or reactive polymer, and a cross-linking agent.
Hydrogel precursors used in lithographic processing are typically based on acrylic-functionalized species, including acrylates and methacrylates. The most commonly used precursors are based on poly(ethylene) glycols (PEGs) [23–26], as they are relatively inexpensive, available in a wide variety of molecular weights and derivative chemistries, biocompatible, and exhibit negligible cytotoxicity [27]. Other biocompatible polymers, such as polylactic acid (PLA) and polyglycolic acid (PGA), both of which exhibit tunable biodegradation [28], have also been utilized. These systems form a valuable complement of materials for use in a wide variety of applications in biotechnology and high-performance materials. Moreover, co-polymerization of multiple different hydrogel pre-cursors, either by the use of co-polymers in the precursor itself or by random co-polymerization during lithographic processing, provides an additional degree of flexibility in the synthesis of hydrogel colloids, and has been used to create particles with highly tunable equilibrium and dynamic properties, including swelling [29], biodegradation [28], and self-assembly [30].
For many applications, it is desirable to conjugate the hydrogel material with chemical functionality in order to carry out specific reactions within the hydrogel or at the particle surface after synthesis. In situ conjugation with acrylic-functional species can be used for covalent incorporation of functional species directly into the hydrogel matrix. For example, homogeneous or spatially-selective fluorescent labeling provides a particularly useful method for characterization of hydrogel colloids with complex internal structure and chemistry. Several methods have been developed to either covalently incorporate or otherwise encapsulate fluorescent species during lithographic processing, including molecular dyes such as rhodamine [31], fluorescein [32,33], and FITC-dextran [34], as well as fluorescent beads [28] and quantum dots [35].
Because lithographic production of hydrogel colloids typically occurs as a single-step synthesis, molecular and colloidal objects can be easily encapsulated into the resulting hydrogel particles, provided that these species are miscible and/or readily suspended in the hydrogel precursor. This can be accomplished by simply adding the material to be encapsulated into the precursor fluid, and provides a facile method to impart functionality and novel properties to colloidal hydrogels. For example, encapsulation of inorganic nanoparticles and colloids has previously allowed for the facile production of colloidal nanocomposites [36], while encapsulation of electrically or magnetically active species such as magnetic beads [37,38] has been used to create field-responsive colloids.
The encapsulation of biological and bioactive materials within colloidal hydrogels is of particular interest in biotechnology applications [1,9]. Lithography-based processing techniques provide significant advantages for this purpose, since the bioactive material only contacts the hydrogel precursor (and in some cases, the lithographic template). This is in stark contrast to traditional emulsion or microfluidic droplet-based methods for encapsulation in hydrogel colloids, where the process must be carefully designed in order to ensure that the bioactive material is compatible and non-interacting with process additives such as stabilizers, carrier fluids, and microfluidic materials [9]. Examples of bioactive materials which have been incorporated into colloidal hydrogels by lithographic processing include cells [5,27,39,40], enzymes [32], proteins [41,42], and small molecule therapeutics [34].
2.2 Lithographic synthesis processes for hydrogel colloids
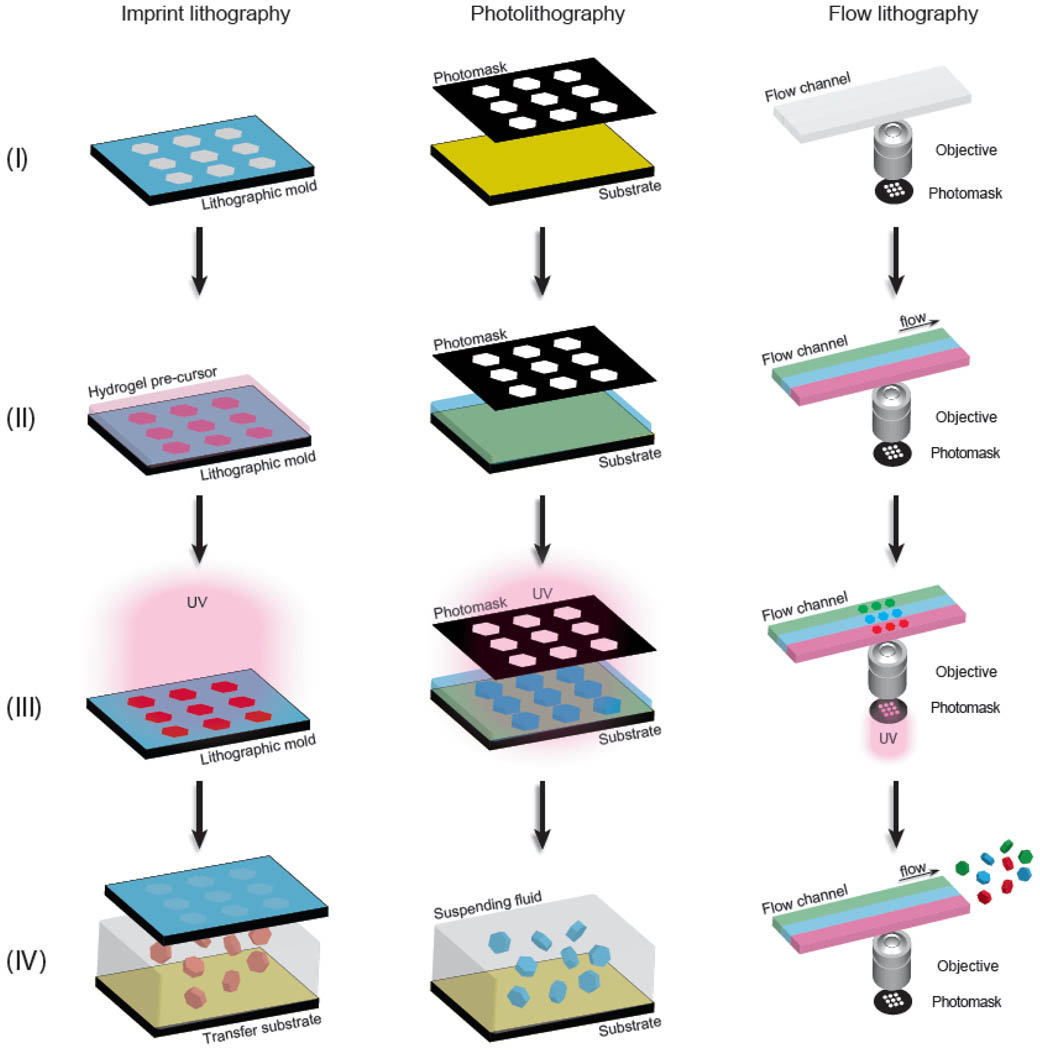
At its essence, lithographic synthesis of hydrogel colloids involves the transfer of a pre-designed template pattern, containing the various geometrical features of the particles to be synthesized, to the hydrogel precursor, followed by (or concomitant with) polymerization and/or cross-linking. The variety of emerging lithographic techniques for the synthesis of complex hydrogel colloids fall into three distinct categories: imprint lithography, photolithography, and flow lithography. Despite differences in format and processing details, these techniques generally share a common workflow methodology, which is illustrated in Figure 1.
Figure 1.
Schematic workflow diagram for lithographic synthesis of hydrogel colloids, illustrated here for imprint lithography (left), photolithography (center), and flow lithography (right). (I) The patterned template is prepared. (II) The fluid reservoir is filled with a hydrogel pre-cursor fluid(s). (III) Hydrogel colloids are synthesized by simultaneous pattern transfer and cross-linking reaction. (IV) Particles are recovered from the fluid reservoir.
Template design and production
First, the lithographic template is generated, which contains feature patterns that determine the size and shape of the particles to be synthesized (I). In imprint lithography, the lithographic template is a patterned mold, typically polymeric in nature, with negative features corresponding to the particles to be synthesized and which is prepared from a master template [43]. Imprint lithography of hydrogel colloids has developed as an extension of standard soft lithography techniques originally used for the production of patterned polymeric substrates such as microfluidic devices [44]. The first process to demonstrate imprint lithography of individual hydrogel colloids was the particle replication in non-wetting templates (PRINT) method developed by Desimone and co-workers [34]. Similar processes and variations have been presented in the literature [24,45]. In recent years, these processes have enjoyed significant enhancements in performance due to advances in microfabrication technology. For example, improved resists and write methods for master templates have enabled the generation of two- and three-dimensional patterns with feature sizes below 100 nm [46]. Furthermore, the ability to form template patterns from any free-standing object has led to the interesting ability to template various nanoscale objects and thus replicate them in the form of hydrogel colloids [47]. Unfortunately, the complexity of the hydrogel colloids generated by imprint lithography is constrained by practical limitations in the production of soft templates. Bending and buckling of lithographic template, for instance, limit the transfer of patterns with high-aspect ratios or internal features [48].
In photolithography and flow lithography, the template is light (or other radiation) incident on the material to be synthesized, which has been patterned through various optical elements. As a result, the minimum feature size for photolithography is limited by the wavelength of the light used in the diffraction-limiting case. Typically, the light source used is the same as that used to initiate photopolymerization, such that pattern transfer and hydrogel synthesis occur simultaneously. Common processes for photolithographic hydrogel synthesis use transparency masks to pattern the incident light. In most cases this approach limits the morphology of the structures formed to two-dimensional extruded shapes, where the thickness of the particle is determined by the height of the fluid that is exposed to the patterned light. However, more recent studies have demonstrated the ability to form nearly arbitrary three-dimensional hydrogel structures through photolithography, either through three-dimensional projection of patterned light with multi-photon [42,49], interference pattern [50], and holographic [51,52] sources. Alternatively, three-dimensional features can be achieved by pre-forming the hydrogel precursor into a three-dimensionally patterned or flexible mold [53,54], essentially combining the capabilities of photolithography with imprint lithography. Photolithographic techniques feature several attractive characteristics not offered by imprint lithography. For example, the design and preparation of light-patterning elements such as transparency masks are significantly less resource-intensive than microfabrication. In addition, recent variations have used digital micromirror devices (DMDs) in place of transparencies, resulting in the ability to dynamically change the template pattern during processing, as well as create highly complex and interconnected shapes [25,40,55] not achievable using imprint lithography.
Loading of hydrogel pre-cursor
Once the lithographic template is prepared, the hydrogel precursor is introduced into a reservoir that is aligned coincident with the lithographic template (II). For imprint lithography, the fluid reservoir is often the negative pattern features of the template itself, which are drop-filled either manually or automatically into the polymer mold [45]. Unfortunately, this approach often results in poor transfer of the template pattern to the hydrogel particles due to non-uniform filling. To prevent this, other methods use a fluid film that is cast onto a planar substrate [34]. The fluid film is then sandwiched between the planar substrate and soft template, the latter of which is filled by capillary forces [24,26]. A major advance introduced by the PRINT technique is the utilization of perfluoropolyether (PFPE) soft templates and substrates, which are highly non-wetting to both polar and non-polar organic solvents. This allows for the filling of multiple immiscible fluid layers within the template, thereby producing chemically anisotropic particles with simple shapes [26].
The simplest photolithographic methods involve pattern transfer into a stationary fluid film or reservoir [29,42,56] prepared in a manner similar to that used in imprint lithography. Recently, a number of so-called “flow lithography” techniques have been introduced, in which photolithography is performed in situ within a microfluidic environment [23]. Initial iterations of flow lithography involved continuous flow of the hydrogel precursor within a microfluidic channel, which was exposed at regular intervals to ultraviolet (UV) light patterned by a photomask to produce discrete particles [57]. Because of the interplay between polymerization and flow, this resulted in poor transfer of pattern features to the synthesized particles. This shortcoming led to the development of stop-flow lithography (SFL), in which the flow of precursor is stopped prior to patterned exposure, resulting in more precise pattern transfer for feature sizes approaching 1 µm [23,58].
Flow lithography involves the introduction of easily manipulated laminar microflows into photolithographic processing. As such, flow lithography techniques such as stop flow lithography (SFL) [23] and optofluidic maskless lithography [25] have led to a number of new motifs of hydrogel colloid synthesis previously unavailable using droplet-based microfluidic techniques [59]. For instance, the ability to create co-flowing layered streams allows for the facile production of hydrogels with multiple polymer chemistries on the same particle of arbitrary one-dimensional configuration [23]. Unlike imprint lithography, the different polymer chemistries need not be immiscible, providing for nearly arbitrary multi-functionality. Furthermore, if the co-flowing precursor streams are immiscible, the interfacial tension between the fluids can be used to induce three-dimensional curvature to the particle shape [60]. Initial studies were limited to co-flowing streams in the plane of the pattern transfer, lowering throughput and limiting chemical anisotropy to “striped” particles [31]. However, by stacking the different chemical layers vertically with respect to the incident irradiation, these limitations can be avoided, allowing for synthesis of particles with more complex designs of chemical “patchiness” [61]. As mentioned previously, the geometry of the microchannel in which the flow lithography is performed can also be adjusted in order to impart three-dimensional features to the hydrogel particles [40,53]. Combined with the ability to alternate flow of different precursor streams, this design strategy can be leveraged to impart highly sophisticated chemical patterns to a single hydrogel particle [53].
Polymerization and cross-linking
After aligning the fluid with the template features, the polymerization reaction is initiated (III), resulting in the formation of cross-linked particle structures within the fluid. In the common case of photopolymerization, this is done by exposure of the fluid to a light source for a pre-defined period of time dictated by the polymerization kinetics. This requires the fluid, fluid reservoir, and/or template to be transparent to the required wavelength(s) of radiation. Typically, the fidelity with which the patterned template features are transferred to the synthesized polymer structure depends critically on several factors relating to the polymerization kinetics and mass transport within the precursor fluid. In photolithography processes, the rate of diffusion of reacting species relative to the rates of propagation and termination defines the lower limit of the feature resolution [62]. Furthermore, the presence and transport of polymerization-quenching species such as oxygen will provide further limitations on the fidelity of pattern transfer, although such inhibition provides a particularly important role in flow lithography techniques, as it prevents adhesion of the particles to the microfluidic device walls [62].
Particle recovery
After the polymerization has completed (or is quenched), the particles are then recovered from the fluid reservoir (IV). This final step is often difficult in practice, as the newly synthesized particles can adhere to the template and/or fluid reservoir depending on the surface chemistry and wetting properties of the materials used, as well as the way in which the fluid is filled. In the case of imprint lithography, there is often a residual polymerized film which connects adjacent particles that must be eliminated before isolated particles can be recovered [24]. First, the particles and so-called “flash layer” are isolated from the patterned mold, typically by mechanical delamination. The flash layer is then eliminated by various physical or chemical means such as wet or dry etching [24]. In the case of the PRINT method and its variations, careful design of the mold chemistry results in a fluid seal which prevents the formation of a flash layer [34]. Even so, difficulties arise in removing newly formed particles from their mold features. More recent iterations of the PRINT method harvest particles from the mold by adhering a polymer that is wetting to the hydrogel particles but not to the PFPE mold, which is then used to mechanically delaminate the particles from the mold. Subsequent agitation of the adhesive allows for recovery of the particles [22]. In other cases, the mold material was cleverly chosen such that it can be dissolved or swollen in order to remove the synthesized hydrogel colloids [45].
Since photolithographic techniques do not require contact of the precursor fluid with the patterned photomask, particle recovery is much simpler than for imprint lithography. For stationary photolithography, the newly formed hydrogel particles can simply be washed from the film or fluid reservoir, provided that the precursor is non-wetting and the hydrogel does not adhere to the substrate. This can be ensured through proper choice of substrate chemistry [56]. In the case of flow lithography techniques, the presence of flow is advantageous in that the newly formed hydrogel particles can be freely advected away from the region of synthesis. Other specific features of conventional microfluidic fabrication and operation can also aid in particle recovery. As mentioned previously, in SFL, oxygen-induced inhibition of the polymerization reaction is heightened near the walls for oxygen-permeable materials such as poly(dimethylsiloxane) (PDMS) [62], thereby preventing newly formed hydrogel particles from adhering to the synthesis channel walls. In the case of hydrodynamic focusing lithography (HFL), the ability to co-flow non-polymerizable fluid layers between the particles and the channel walls enables the use of oxygen-impermeable microfluidic materials of construction such as glass [61]. For cases in which the microfluidic synthesis channel has three-dimensional features that are polymerized into or around, the particles can either be held in place in order to perform additional synthesis steps, or can be released upon application of a pressure drop capable of deforming the microchannel [53,63].
Polymeric materials synthesized by lithography-based processes typically require some form of purification after recovery in order to remove unreacted material or other impurities left over from the synthesis process. For hydrogel colloids, this is typically accomplished through a solvent exchange in which the particles receive several “washes” against a desired final suspending medium. Isolation of the particles during these wash steps can be aided by centrifugation, filtration, or magnetic separation [38]. However, since hydrogel materials are soft by nature, the conditions under which purification is performed must be carefully chosen to ensure that the hydrogel colloids are not adversely affected by extreme shear or compression. In the case of microfluidic-based flow lithography techniques, the particles can be transported and manipulated downstream under gentle laminar flow conditions, allowing for facile additional processing in situ after synthesis, such as purification [64], functionalization [65], or assembly [66].
Process throughput
The utility and eventual commercialization of particle synthesis technologies depend critically on process throughput and yield, as well as operating cost. Since traditional soft lithography techniques are now fairly routine, both imprint lithography and microfluidic-based flow lithography of hydrogel colloids present a minimal expense relative to material costs. However, because of limitations on contemporary microfabrication, both techniques have suffered from fairly low throughput. Imprint lithography suffers due to limitations on the size of molds that can be prepared from typical soft lithography methods. For example, the first demonstrations of the PRINT method reported throughputs on the order of several milligrams per hour of material [34]. Significant progress has been made recently in scaling up the process, which can now be performed via roll-to-roll processing with throughputs on the order of ten grams of material per hour [22]. In the case of photolithography, the primary limiting factor is the available field of irradiation. Since most photolithography-based processes to produce colloidal hydrogels are based on optical projection, throughput is limited to the field of view of the objective used [59]. In the case of SFL, this limits the throughput available by a single-objective, single-synthesis channel configuration to approximately one gram per hour, but the process can, in theory, be parallelized to multiple channels for multiple grams per hour.
3. Properties of colloidal hydrogels
3.1 Particle architecture: size, shape, and chemical anisotropy
As discussed previously, traditional microgel synthesis techniques typically produce uniform spherical or spheroidal colloids. In some cases, complicated multi-step synthetic pathways and processing methods can be used to create multi-functional or non-spherical colloids [66–68], but the types of structures that can be prepared are extremely limited. In contrast, lithographic processing techniques enable the creation of hydrogel colloids with complex size, shape, and chemical patterns simply by proper design of the lithographic template and synthesis conditions [59]. Moreover, due to current abilities in lithographic mask production and pattern transfer, the particles are highly monodisperse in both size and shape. For example, both the SFL and PRINT methods are capable of producing particles with coefficients of variation for particle features of less than 1% in most cases [31,69], compared to 10% or more for traditional droplet-based techniques [70].
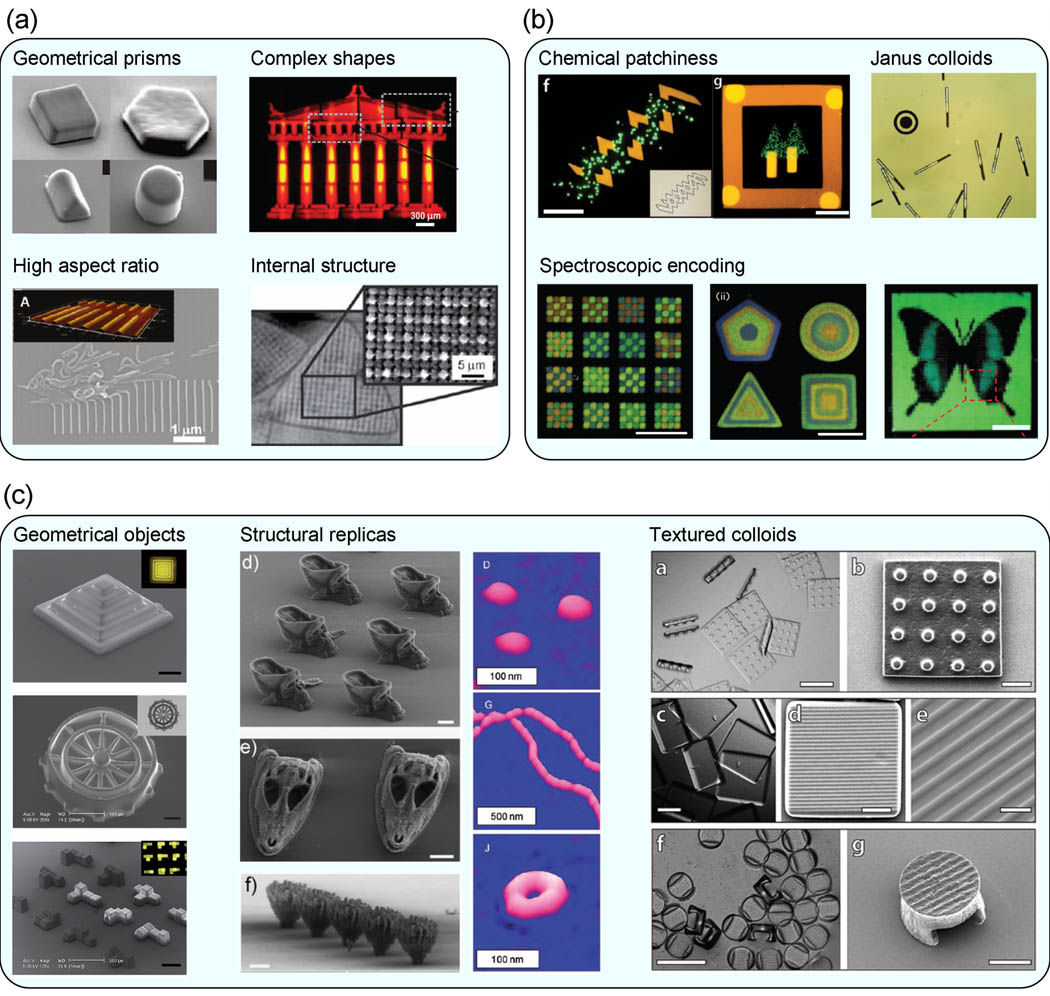
With this in mind, many reports of lithographic processing of hydrogel colloids involve the preparation of particles with novel shapes and morphologies, a small cross-section of which is illustrated in Figure 2. Because prototypical lithography techniques create extruded two-dimensional structures, a wide array of low-aspect ratio geometric solids have been prepared, including cubes and cuboids [5], discs [23], and other polygonal prisms [23,25]. Low-aspect ratio elongated objects are also fairly common, including cylinders [23], quasi-ellipsoids [57], and bars [31]. More complex quasi-two dimensional shapes include particles with various curved features [23,42]. High-aspect ratio objects, such as wire-like [71], thread-like [42,55], and other curvilinear structures [25,72], have also been synthesized, though control over their size and polydispersity is typically poor due to limitations in lithographic pattern transfer.
Figure 2.
Representative examples of complex hydrogel colloids prepared by lithographic synthesis. (a) Quasi-two dimensional particles such as geometrical prisms (reproduced with permission from [23]), complex shapes (reproduced with permission from [40]), high aspect ratio wormlike structures (reproduced with permission from [22], and internally structured particles (reproduced with permission from [50]). (b) Chemically anisotropic particles exhibiting chemical patchiness (reproduced with permission from [53]), amphiphilic Janus chemistry (reproduced with permission from [38]), and spectroscopic encoding (reproduced with permission from[35]). (c) Three-dimensional particles including geometrical objects (reproduced with permission from [54]), structural replicas (at left reproduced with permission from [55]), at right reproduced with permission from [47]), and textured colloids (reproduced with permission from [53]).
The development of advanced lithographic templating techniques has enabled the creation of complex three-dimensional particle morphologies. A number of studies have demonstrated the replication or miniaturization of structures from nature as hydrogel colloids [47,54,55]. Other studies have involved the generation of patterned three-dimensional motifs such as stepped [54,55] and helical [55,71] structures. These techniques also allow for the generation of particles with various surface textures, including ridges and pillars [53].
Lithographic processing also enables the production of colloidal hydrogels with uniform internal features, such as two-dimensional holes [31,36,45,47], three-dimensional channels [42,55], and various container-like structures [42,53,55]. The creation of hole structures with controlled size and shape along the width of a particle enables a simple yet versatile graphical encoding scheme with which individual particles can be identified [31,38,73–75]. The development of interference photolithography techniques, in which the templated light is further patterned using an interference mask, can be used to generate periodic voids within the interior of the hydrogel particle, whose density and configuration can be readily controlled by adjusting the periodic features of the interference mask [50].
As previously discussed, the unique aspects of lithographic synthesis as compared to traditional microgel synthesis methods enable the generation of particles with complex chemical anisotropy. The simplest motifs include amphiphilic “Janus” particles, in which patches of alternating hydrophilic and hydrophobic polymers are incorporated into the particle [26,30]. Other examples involve various chemical patterning schemes, such as localized fluorescent labeling of various particle features [26,31,53,71,76] as well as site-specific encapsulation of functional species [38,58]. Similar to hole features, chemical patterning has also been used to implement color-based barcoding of hydrogel colloids [76].
3.2 Particle microstructure: cross-linked networks and porosity
Hydrogels typically contain a relatively low volume fraction of polymer compared to other organic and inorganic colloids, and as such their microstructure is primarily governed by their porosity or pore structure. Because porosity controls a number of properties relevant to current applications of hydrogels, including mechanical properties [77], mass transport [78], swelling [1], and adsorption and reaction [58], it is a critical (if often overlooked) design variable for hydrogel colloids. Porosity is categorized based on the length scale of the pore structure that controls various properties of the material, including “micropores” (1–10 nm), “mesopores” (10–1000 nm), and “macropores” (1–100 µm).
The microporous structure of the hydrogel is governed by the configuration of the cross-linked polymer network, which is characterized by a correlation length for the distance between cross-links (i.e., the mesh size) [1]. For chemically cross-linked polymers, the mesh size can be controlled by tuning the composition of the hydrogel precursor. Specifically, the concentration and molecular weight of the monomer and/or cross-linking agent have been used to tune the mesh size of polymer hydrogels [29,58]. Although it is difficult (if not impossible) to directly observe the mesh size or microporous structure of the hydrogel, many indirect methods exist to estimate the mesh size of cross-linked polymers, including scattering [14], equilibrium swelling [1], and probe diffusivity measurements [58,75].
Microporosity is important to a number of properties relevant to the design of hydrogel colloids. First, because the cross-linked polymer network serves as a steric barrier to mass transport, microporosity can be used as a means to control the diffusion of molecular species into and out of the hydrogel interior. For example, adjustment of the mesh size of the polymer can result in size-selective exclusion of proteins and other unwanted contaminants in therapeutic and diagnostic applications [58]. Furthermore, because the viscoelasticity of the hydrogel can be fundamentally linked to the mesh size, control of the cross-linked microstructure of the polymer network at the microscale can be used to tune the flexibility. This, combined with control of particle shape, can be used to control the flow behavior of hydrogel colloids beyond what is typically possible with ordinary colloidal particles [74,79].
Mesoporous structure in hydrogels and other cross-linked polymers is accomplished by the addition of “porogens”, or pore-inducing non-polymerizable species. Common porogens include co-solvents which are miscible with the hydrogel precursor but are immiscible with the polymer. inducing phase separation during or immediately following the polymerization reaction. This results in the formation of pores of unpolymerized material, whose size and morphology depend on the type of porogen and the nature of the phase separation [80]. Unfortunately, pores induced by this method are often highly polydisperse and difficult to control. An alternative is the addition of inert pore-templating agents to the polymerizing fluid, which are encapsulated during polymerization and subsequently removed to yield pores of voids with similar size and shape of the templating agent. Examples of templating agents include colloidal particles [81], emulsions [82], and macromolecules [83]. A drawback of these templating agents is that they must be removed after particle synthesis, requiring additional purification, often by the addition of harsh buffers or co-solvents that may negatively affect material performance. As discussed above, interference photolithography allows for the synthesis of controlled, highly porous internal structure within hydrogel colloids without the use of templating agents or additives, and as such is an attractive method for controlling mesoporous structure. It should be noted, however, that the only study to use interference lithography for this purpose demonstrated a limited operating space in terms of pore morphology, density, and size, such that the potential of the technique in templating mesoporosity remains relatively unexplored.
Mesoporosity of hydrogels is important for a number of applications requiring high internal surface area for processes such as adsorption and reaction at the hydrogel-fluid interface. Mesoporous structure allows for control over the diffusion of colloidal-scale objects, such as nanoparticles and proteins, within the hydrogel interior. Bulk measurements of the internal surface area of colloidal particles is typically performed by techniques such as gas adsorption and porosimetry. However, these techniques require drying of the particles or immersion in nonaqueous solvents, and thus disturb the structure of the hydrogel, resulting in significant under-prediction of specific surface area due to de-swelling of the polymer network. Alternatively, mesopores can be directly observed by various microscopy methods, such as confocal microscopy [84], atomic force microscopy [85], and various cryogenic electron microscopy techniques [86].
Macroporous features on the micron-scale are easily produced through lithographic processing by imparting internal features into the lithographic template. As discussed previously, the induced macropores can either take the form of linear holes along one dimension of the particle, typical of projection photolithography, or as tortuous channels through the particle interior, which can be prepared by the multi-photon photolithography [55]. Because of their size, macropores provide significantly less internal surface area compared to mesopores. However, they do allow for significantly greater convection of fluid through the particle interior, which results in more efficient mass transfer from bulk solution to the particle interior.
3.3 Colloidal behavior and stability
Like most other colloids, the ability to render hydrogel colloids suspendable and colloidally stable is critical in processing, storage, and application. Small particles, typically below several microns in size, are easily suspended due to Brownian forces [34], whereas larger particles tend to sediment, which is problematic for bulk processing but is advantageous for their separation. Unlike most other colloids, however, the interior of the hydrogel is accessible to the solvent, which can either be advantageous or problematic depending on the solvent in which they are suspended. Typically, hydrogels are rendered colloidally stable by many of the same mechanisms as ordinary colloids. Steric stabilization can be imparted by the adsorption of non-ionic surfactants with oligomeric moieties such as ethoxylated alkanes. Electrostatic stabilization can be similarly imparted by adsorption of ionic surfactants, or through the incorporation of a polyelectrolyte within the hydrogel itself, such as poly(acrylic acid) [87].
Because of their highly porous cross-linked polymer microstructure, hydrogel colloids possess unique swelling characteristics that depend on the physicochemical conditions of the polymer in their suspending medium. Fundamentally, swelling of the colloid arises from a balance between the excluded volume due to polymer-solvent interactions and entropic elasticity of the cross-linked polymer chains [1]. Thus, changes in free volume of the polymer due to changes in solvent quality will give rise to changes in size of the hydrogel colloids. Swelling of hydrogel colloids is typically quantified by the swelling ratio, which is the ratio of the particle volume at the conditions of interest versus that in the unswollen or “relaxed” state (i.e., in a theta-solvent),. By assuming an appropriate model for the entropy of mixing of the polymer, the equilibrium swelling ratio can be used to calculate microstructural properties of the hydrogel network, such as the cross-link density and mesh size [1]. In the case of spherical particles, the swelling is typically isotropic (i.e., the particle size changes while retaining a roughly spherical shape). However, the ability to synthesize particles with shape and chemical anisotropy has enabled the design of colloidal hydrogels with novel swelling behaviors. For example, particles with alternating patches of non-ionic and polyelectrolyte polymers exhibit significant changes in shape triggered by changes in solution pH [53]. Gradients in particle thickness have been used to create bending and buckling instabilities, such as the bending of rod-like polyelectrolyte particles [42]. These abilities enable a whole new class of “shape-shifting” colloids whose shape and morphology can be finely tuned by small changes in physicochemical environment.
Since many of the applications of hydrogel colloids involve flows, either in suspension or in a microfluidic environment, their hydrodynamic and rheological properties are also of considerable importance. Hydrogel colloids exhibit significant deformability under relatively mild flows because of their relatively low modulus compared to other organic and inorganic materials [74]. This property, combined with the ability to produce anisotropic particles from lithographic techniques, has been utilized to generate hydrogel colloids whith biomimetic flow properties [79]. Furthermore, the ability to create elongated and high-aspect ratio particles allows for facile manipulation of particle orientation under flow, which is critical for certain applications [74]. The ability to create particles with highly uniform, complex shapes has also provided new insight into the role of particle shape in certain rheological phenomena, such as shear thickening [88].
4. Applications
4.1 Particle assembly
There is a growing interest in the fundamental science of anisotropic colloids as novel materials for directed and self-assembly [21]. However, most methods to produce colloids with shape anisotropy are limited to rods, ellipsoids, and spherical clusters [22]. Similarly, methods to produce chemical anisotropy are mostly limited to so-called “Janus” colloids, with two discrete chemical patches. These constraints reduce the diversity of structures that can be assembled. The many new types of complex particle architectures made available by lithographic processing thus offer significant advances in the area of colloidal assembly (Figure 3a).
Figure 3.
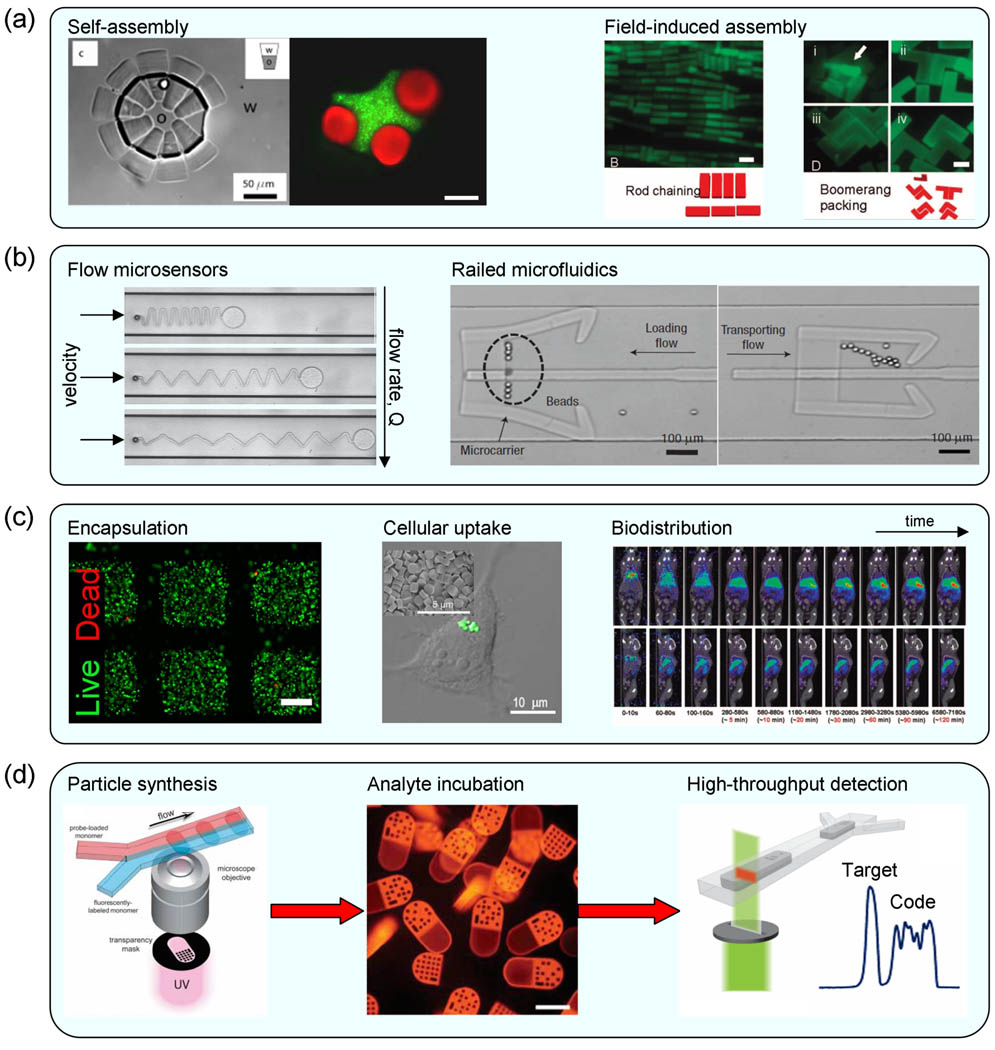
Applications of complex colloidal hydrogels from lithographic synthesis. (a) Examples of colloidal assembly. Left: amphiphilic self-assembly of colloidal “surfactants” and shape-specific assembly of lock-and-key colloids, reproduced with permission from [30] and [97], repsectively. Right: electric field-induced assembly of colloidal bars into mixtures of lateral and transverse chains (left) and colloidal “boomerangs” into densely packed structures, reproduced with permission from [94]. (b) Example of a micromechanical device, reproduced with permission from [72]. Left: operation of an in situ hydrogel flow sensor in a fluid microchannel. Right: Demonstration of a railed microfluidic transporter [40]. (c) Examples of encapsulation and delivery. Left: encapsulation and viability of NIH 3T3 fibroblasts in PEG-gelatin hydrogel cubes, reproduced with permission from [98]. Center: cellular uptake of hydrogel microparticles by HeLa cells, reproduced with permission from [99]. Right: Biodistribution imaging of the same particles in healthy mouse model after bolus tail injection, reproduced with permission from [100]. (d) Workflow diagram for high-throughput, multiplexed bioassays utilizing graphically-encoded hydrogel colloids, reproduced with permission from [31].
The most simple motif for colloidal self-assembly relies on the packing of colloidal particles, either in the bulk or at chemical interfaces. Khademhosseini and co-workers have utilized the packing of anisotropic PEG-based hydrogel colloids confined to interfaces to create densely packed hydrogel structures, which can be made permanent by subsequent chemical cross-linking. Geometric solids confined to an air-water interface were shown to self-assemble into tessellated colloidal crystals, whose growth rate can be controlled by adjusting aspects of the particle architecture such as thickness and cross-link density [89]. Conducting the assembly process from an evaporating film which has been deposited on shaped PDMS molds allows for bottom-up “masonry” of hollow three-dimensional objects, whose surface structure can be tuned by the shape of the hydrogel colloid sub-units [90]. Alternatively, confinement of box-shaped particles to evaporating emulsion drops yields self-assembled clusters with reproducible aggregation number and geometry based on the aspect ratio of the primary particles [91], similar to the method of Pine and co-workers of creating clusters of packed spheres [68]. Such structures are attractive for tissue engineering applications, as they enable tailoring and functionalization of the hydrogel environment at the cellular level.
Other methods of colloidal assembly involve the manipulation of amphiphilic interactions. Inspired by the molecular self-assembly of surfactants, Dendukuri et al. synthesized amphiphilic wedge-shaped “surfactant” hydrogels with differentially-shaped hydrophilic and hydrophobic blocks [30]. Adjusting the relative size and shapes of the two chemical blocks allowed for their self-assembly in suspension into a number of surfactant-mimetic structures (Figure 3a, left), including micelles, reverse micelles, and both oil-in-water and water-in-oil emulsions. The structure of the aggregates formed was accurately predicted using simple packing arguments typically used for surfactant micelles and emulsions. These results are particularly intriguing in the context of colloid-stabilized Pickering emulsions and may enable more rational design of colloids for the control of emulsion structure and stability [92].
An alternative motif for colloidal assembly is the use of electric and magnetic fields with field-conductive particles. The electric field-induced assembly of spheroidal particles was reviewed by Velev and co-workers [93]. For such particles, the range of self-assembled structures is limited to linear and branched chains as well as colloidal crystals. The synthesis of more complex-shaped particles for field-induced assembly allows for better control over the assembly process, and enables the generation of new types of structures (Figure 3a, right). For example, Herlihy et al. showed that dielectrophoretic assembly of bisymmetric-shaped colloids produced chains and ordered arrays with primary particles in multiple preferred orientations [94]. Furthermore, dielectrophoretic assembly of more complex “boomerang” shapes lead to highly-packed, less ordered structures due to the number of different packing configurations available to the primary particles. A number of studies have also involved the synthesis and assembly of magnetically-responsive hydrogel colloids with encapsulated magnetic beads. Hwang et al. demonstrated the magnetic field-induced assembly of dimpled spheroids and tablet-shaped particles into ordered chains [28]. More recently, the ability to template the encapsulation of the magnetic material in a localized and oriented manner has led to more robust control of the magnetic assembly process. For example, Nunes et al. synthesized beam-shaped particles where the magnetic material within each primary particle could be controllably patterned into stripes with orientation parallel or perpendicular to the beam axis [95]. This provided control of the magnetic field-induced assembly of the resulting hydrogel colloids into chains where the primary particle orientation is dictated by the orientation of the magnetic stripes.
4.2 Micromechanical systems
Lithographic processes provide the means to rapidly produce gel microstructures with high spatial resolution and reproducibility, an increasingly attractive basis for the development of enhanced integrated functional components in microfluidic devices. Beebe and coworkers have utilized photopatterning in microfluidic channels to create stimuli-responsive hydrogel structures capable of sensing and actuation [96]. The small length scale of the gels enables rapid swelling for effective flow control and sorting without the need for an external power source or complex assembly protocols. Using SFL, soft PEG-based microflow sensors with high chemical and mechanical robustness have been fabricated in situ within PDMS channels for the reversible measurement of flowrate over four orders of magnitude [72] (Figure 3b). Unlike conventional methods for the creation of micromechanical components that require complex sacrificial procedures and specific clean room equipment, this technique uses a simple, inexpensive prototyping strategy capable of producing soft, mobile structures with elastic moduli that are six orders of magnitude smaller than comparable components fabricated from silicon. Importantly, the performance of these soft sensors is relatively unaffected by changes in the chemical composition of the flow, making them a better option for analyzing biological fluids than sensors based on electrolysis and electrochemistry, which can be sensitive to chemical content. Stop-flow lithography has also been used to generate various complex morphologies of colloidal, glass, and silicon microcomponents by sintering and reducing particles produced from precursor mixtures containing silica microspheres, acrylamide monomer, DMSO, Darocur 1173 photoinitiator, and N,N-methylene bisacrylamide [36 2008].
The synthesis and reliable arrangement of large groups of three-dimensionally patterned hydrogel microparticles have been demonstrated with the “railed microfluidic” method of Kwon and coworkers [40]. PEG-based structures bearing raised fins are photopolymerized and then guided along rails built into PDMS microchannels to assemble intricate systems that can be subsequently fixed together in a second polymerization step. This in situ synthesis and assembly strategy provides a powerful new tool in the effort to fabricate complex functional components for micromechanical applications. In contrast to the probabilistic assembly of thermodynamically-driven methods, railed microfluidics offers a deterministic approach with near-zero assembly error and the ability to precisely construct a complex system with components synthesized from multiple precursor materials.
4.3 Targeted delivery and encapsulation of biologicals
The ability to customize and precisely tune the physical, chemical, and material properties of colloidal gels has led to applications in the delivery of therapeutics, biologicals, and imaging agents. In order to achieve the targeted delivery of a variety of entities with desired release characteristics, cargo-carrying particles must be highly configurable and offer independent control over a wide range of design parameters. The capacity for rationally engineering gel-based particles for cellular encapsulation and delivery has been extensively explored by the Desimone group (Figure 3c). Utilizing the PRINT method, the effects of particle morphology, chemical composition, porosity, flexibility, texture, and surface modifications were investigated in vivo for the delivery of diverse molecular cargos [33,69,100]. In contrast to the kinetic trapping employed with micellar and liposomal vectors, the direct incorporation of cargo molecules into the gel matrix provided a high degree of control over both load and release processes, and a variety of potential scaffold modifications offers a powerful toolbox for enhancing particle stability and improving targeting specificity. In cellular internalization studies, cylindrical 1 µm PRINT particles were readily taken up by several cell types, with no significant cytotoxicity observed and with an internalization rate controlled by the surface charge of the microgel [99].
Lithographic methods have been further used for the creation of cell-laden hydrogels for microscale tissue engineering applications. Hydrated gel matrices provide a natural three-dimensional environment in which to culture and proliferate cells with minimal risk of toxicity or eliciting immunological responses, and the modularity of colloidal particles offers exciting possibilities for the creation of complex meso- and macroscale biological milieus via directed self-assembly of micron-scale components. Stop-flow lithography has been utilized for the rapid microfluidic synthesis of large numbers of anisotropic PEG-based microgels containing mouse fibroblast cells [27]. By optimizing photoinitiator and cross-linker concentrations, the vast majority of encapsulated cells remained viable following particle synthesis, suggesting possible uses in multi-cell drug assays and immuno-isolation of cells for implantation. In other work, gelatin methacrylate (GelMA) microstructures with tunable mechanical and swelling properties have achieved homogeneous cell distribution with high long-term cell viabilities despite the use of photopolymerization [98]. It is important to note that cells encapsulated in standard UV crosslinkable PEG and hyaluronic (HA) gels typically cannot remodel their environments because of poor adherence to the surrounding scaffold. However, cells readily transported through, adhered to, and proliferated on both two- and three-dimensional GelMA microstructures, a result with powerful implications for the creation of complex biomimetic environments.
4.4 Molecular separations and multiplexed detection
The unique material properties of colloidal hydrogels have also been exploited in recent years for the capture and quantification of various molecular species. The ability to precisely define particle shapes and complex internal features via photolithography has enabled the creation of classes of encoded microgel particles that can serve as the basis for a suspension, or particle-based, array. In such a system particle morphology provides information on the identity of capture molecules immobilized within the gel matrix, allowing for the efficient multiplexed analysis of several target species in a single assay. This morphological encoding provides a significantly larger library for multiplexing than can be offered by solid-particle array systems that rely on optical encoding schemes constrained by spectral overlap. Moreover, the fabrication of these solid-particle systems requires multi-step, time-consuming protocols, whereas colloidal gels can be simultaneously synthesized, functionalized, and encoded in a single lithographic step [31].
In contrast to the solid-surface immobilization of capture molecules in conventional planar microarrays and polystyrene-based particle systems, the gel scaffold offers a three-dimensional, hydrated environment that more closely mimics solution-phase conditions and enables the loading of higher densities of capture species for more sensitive detection. PEG-based hydrogels are also known to be both non-fouling and biocompatible, features which are crucial for the specific detection of a variety of biological target molecules in complex samples. Importantly, gel immobilization has been shown to retain the biological activity of fragile entities such as cells, enzymes, and antibodies [27,39,75].
Shape-encoded hydrogel particles called MUFFINS (mesoscale unaddressed functionalized features indexed by shape) represent the first generation of colloidal microgel biosensors [29]. A single contact photolithography step was used to reproducibly synthesize batches of PEG-based colloids functionalized with either single stranded-DNA or cell-expressed antibody fragments for the capture of fluorescently-labeled complementary DNA sequence and hapten, respectively. Large quantities of individual particles could be self-assembled at an air-liquid interface via attractive lateral capillary forces to form random arrays for sensing applications.
Stop-flow lithography has been extensively employed for the creation of graphically-encoded PEG microparticles for the sensitive and specific detection of DNA, RNA, and proteins [31,58] [75,101] (Figure 3d). The versatile microfluidic synthesis method can produce >104 particles/hr using only minute amounts of reagents and offers the flexibility to incorporate multiple, spatially-segregated capture species on a single particle for “intrabead” multiplexing [31] [75,101]. An accompanying PDMS-based flow-through scanner is used to decode precisely-oriented processions of gel microparticles and quantify bound target in a high-throughput manner (25 particle/s) [75,101]. A recent 12-plex microRNA expression profiling study of four human cancer types using these gel microparticles achieved limits of detection more than two orders of magnitude better than those exhibited by solid-bead systems, and with shorter assay times than all other existing quantification platforms [101]. In addition to the magnetic barcoded microgels synthesized by Bong et al. for multiplexed detection [38], Kwon and coworkers have created color-encoded, magnetically-addressable gel microparticles that can be precisely handled and manipulated for efficient multiplexed DNA analysis [76].
4.5 Economic considerations
Structured and anisotropic hydrogel microparticles rely on more sophisticated fabrication schemes than their isotropic counterparts, and hence are intrinsically more expensive to produce. Given that the field is still in its inception, it is difficult to perform a complete economic analysis of the various synthesis approaches. However, it is worthwhile mentioning new small companies which are finding it economically worthwhile to add structure to gel particles. For example, Liquidia Technologies is using the PRINT technology to develop particle-based vaccines and therapeutics. Firefly Bioworks is using the Stop Flow Lithography technologies for multiplexed sensing applications. This is not surprising, since biosensing and pharmaceuticals are two areas where the added value of the end product compensates for the additional cost of processing.
5. Outlook
Lithographically patterned hydrogel microparticles are fascinating materials that hold great promise for both developing transformative technologies and enabling hitherto unimaginable fundamental studies. Several challenges and exciting opportunities exist within this field. First, modifying existing or developing new synthesis methods must take place in order to produce reasonable quantities of particles that can be harvested for use in applications. The PRINT technologies are a wonderful example of how scale-up of a new process can be achieved [22]. Synthesis processes have yet to take full advantage of inducing non-equilibrium structures via flows or fields (magnetic, electric, temperature) and capturing these states in microparticles. SFL generation of striped particles is a step in this direction [61], but this is just the beginning. Modeling (fluid dynamics, heat/mass transfer, reaction kinetics, statistical field theories) should play a more prominent role in the rational engineering and design of advanced synthesis processes. Chemists and material scientists will increasingly develop new precursor materials that are specifically designed for both lithography and downstream applications [102].
Beyond synthesis, advancement and development of new applications for structured microgel particles continues at a rapid pace. Biological applications are the most natural place where the added value of a structured gel particle can be fully leveraged, with possibilities in fields ranging from tissue engineering to drug delivery to sensing. The true advantage of a hydrogel scaffold will become increasingly evident as research transitions from model to real systems (e.g. moving from demonstrating sensing synthetic nucleic acids in buffer to sensing in messy samples like serum). Hydrogels are naturally soft materials and this property has generally been under-exploited in fundamental studies of lithographically patterned particles. In the future it will be interesting to explore “softness” further in terms of developing biomimetic particles (e.g. artificial cells [79]), shape changing colloids, or particles which evolve or move in time based on local chemical cues from each other or other entities (e.g. cells). Last, but not least, fundamental studies of passive and driven self-assembly of these materials will be increasingly explored as particles become available to a wider community.
Acknowledgements
We acknowledge support from the NSF grant DMR-1006147, the Novartis/MIT Center for Continuous Manufacturing, and grant R21EB008814 from the National Institute of Biomedical Imaging and Bioengineering, National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading*,**
* of special interest.
** of outstanding interest.
- 1.Peppas NA, Hilt JZ, Khademhosseini A, Langer R. Hydrogels in biology and medicine: From molecular principles to bionanotechnology. Advanced Materials. 2006;18(11):1345–1360. [Google Scholar]
- 2.Rosiak JM, Yoshii F. Hydrogels and their medical applications. Nuclear Instruments & Methods in Physics Research Section B-Beam Interactions with Materials and Atoms. 1999;151(1–4):56–64. [Google Scholar]
- 3.Peppas NA, Khare AR. Preparation, Structure and Diffusional Behavior of Hydrogels in Controlled-Release. Advanced Drug Delivery Reviews. 1993;11(1–2):1–35. [Google Scholar]
- 4.Langer R, Tirrell DA. Designing materials for biology and medicine. Nature. 2004;428(6982):487–492. doi: 10.1038/nature02388. [DOI] [PubMed] [Google Scholar]
- 5.Lee KY, Mooney DJ. Hydrogels for tissue engineering. Chemical Reviews. 2001;101(7):1869–1879. doi: 10.1021/cr000108x. [DOI] [PubMed] [Google Scholar]
- 6.Slaughter BV, Khurshid SS, Fisher OZ, Khademhosseini A, Peppas NA. Hydrogels in Regenerative Medicine. Advanced Materials. 2009;21(32–33):3307–3329. doi: 10.1002/adma.200802106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Saunders BR, Vincent B. Microgel particles as model colloids: theory, properties and applications. Advances in Colloid and Interface Science. 1999;80(1):1–25. This article gives a review of microgels as model colloidal particles, and includes discussions of their synthesis, microstructure, and colloidal stability. In particular, it gives a fairly complete list of synthetic polymers used for microgel synthesis.
- 8. Tumarkin E, Kumacheva E. Microfluidic generation of microgels from synthetic and natural polymers. Chemical Society Reviews. 2009;38(8):2161–2168. doi: 10.1039/b809915b. This paper gives an overview of various microfluidic-based techniques for the synthesis of isotropic, chemically anisotropic, and shape anisotropic microgel particles, primarily through droplet production methods.
- 9.Dubinsky S, Zhang H, Nie ZH, Gourevich I, Voicu D, Deetz M, Kumacheva E. Microfluidic synthesis of macroporous copolymer particles. Macromolecules. 2008;41(10):3555–3561. [Google Scholar]
- 10.Senff H, Richtering W. Influence of cross-link density on rheological properties of temperature-sensitive microgel suspensions. Colloid and Polymer Science. 2000;278(9):830–840. [Google Scholar]
- 11.Senff H, Richtering W. Temperature sensitive microgel suspensions: Colloidal phase behavior and rheology of soft spheres. Journal of Chemical Physics. 1999;111(4):1705–1711. [Google Scholar]
- 12.Wu JZ, Zhou B, Hu ZB. Phase behavior of thermally responsive microgel colloids. Physical Review Letters. 2003;90(4) doi: 10.1103/PhysRevLett.90.048304. [DOI] [PubMed] [Google Scholar]
- 13.Stieger M, Pedersen JS, Lindner P, Richtering W. Are thermoresponsive microgels model systems for concentrated colloidal suspensions? A rheology and small-angle neutron scattering study. Langmuir. 2004;20(17):7283–7292. doi: 10.1021/la049518x. [DOI] [PubMed] [Google Scholar]
- 14.Stieger M, Richtering W, Pedersen JS, Lindner P. Small-angle neutron scattering study of structural changes in temperature sensitive microgel colloids. Journal of Chemical Physics. 2004;120(13):6197–6206. doi: 10.1063/1.1665752. [DOI] [PubMed] [Google Scholar]
- 15.Cloitre M, Borrega R, Leibler L. Rheological aging and rejuvenation in microgel pastes. Physical Review Letters. 2000;85(22):4819–4822. doi: 10.1103/PhysRevLett.85.4819. [DOI] [PubMed] [Google Scholar]
- 16.Zhang JG, Xu SQ, Kumacheva E. Polymer microgels: Reactors for semiconductor, metal, and magnetic nanoparticles. Journal of the American Chemical Society. 2004;126(25):7908–7914. doi: 10.1021/ja031523k. [DOI] [PubMed] [Google Scholar]
- 17.Xu SQ, Zhang JG, Paquet C, Lin YK, Kumacheva E. From hybrid microgels to photonic crystals. Advanced Functional Materials. 2003;13(6):468–472. [Google Scholar]
- 18.Debord JD, Eustis S, Debord SB, Lofye MT, Lyon LA. Color-tunable colloidal crystals from soft hydrogel nanoparticles. Advanced Materials. 2002;14(9):658–662. [Google Scholar]
- 19.Serpe MJ, Kim J, Lyon LA. Colloidal hydrogel microlenses. Advanced Materials. 2004;16(2) 184-+ [Google Scholar]
- 20.Oh JK, Drumright R, Siegwart DJ, Matyjaszewski K. The development of microgels/nanogels for drug delivery applications. Progress in Polymer Science. 2008;33(4):448–477. [Google Scholar]
- 21. Glotzer SC, Solomon MJ. Anisotropy of building blocks and their assembly into complex structures. Nature Materials. 2007;6(8):557–562. doi: 10.1038/nmat1949. This work develops a conceptual framework for designing and describing different types of anisotropy that can be produced in colloidal particles, giving examples of syntheses which demonstrate the various ‘dimensions’ of anisotropy.
- 22. Merkel TJ, Herlihy KP, Nunes J, Orgel RM, Rolland JP, DeSimone JM. Scalable, Shape-Specific, Top-Down Fabrication Methods for the Synthesis of Engineered Colloidal Particles. Langmuir. 2010;26(16):13086–13096. doi: 10.1021/la903890h. This review gives an overview of various top-down methods for anisotropic particle fabrication, including hard templating, PRINT and other soft template methods, and microfluidics. It gives a particularly thorough comparison of the relative benefits and limitations of each of the techniques.
- 23. Dendukuri D, Gu SS, Pregibon DC, Hatton TA, Doyle PS. Stop-flow lithography in a microfluidic device. Lab on a Chip. 2007;7(7):818–828. doi: 10.1039/b703457a. This article gives the first presentation of Stop Flow Lithography, demonstrating its advantages in terms of particle anisotropy and feature resolution compared to other microfluidic techniques.
- 24.Glangchai LC, Caldorera-Moore M, Shi L, Roy K. Nanoimprint lithography based fabrication of shape-specific, enzymatically-triggered smart nanoparticles. Journal of Controlled Release. 2008;125(3):263–272. doi: 10.1016/j.jconrel.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 25. Chung SE, Park W, Park H, Yu K, Park N, Kwon S. Optofluidic maskless lithography system for real-time synthesis of photopolymerized microstructures in microfluidic channels. Applied Physics Letters. 2007;91(4) This paper gives the first demonstration of optofluidic maskless lithography, a variant of Stop Flow Lithography in which the photolithographic template can be dynamically varied to produce various particle shapes on demand.
- 26.Zhang H, Nunes JK, Gratton SEA, Herlihy KP, Pohlhaus PD, DeSimone JM. Fabrication of multiphasic and regio-specifically functionalized PRINT (R) particles of controlled size and shape. New Journal of Physics. 2009;11 [Google Scholar]
- 27.Panda P, Ali S, Lo E, Chung BG, Hatton TA, Khademhosseini A, Doyle PS. Stop-flow lithography to generate cell-laden microgel particles. Lab on a Chip. 2008;8(7):1056–1061. doi: 10.1039/b804234a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hwang DK, Oakey J, Toner M, Arthur JA, Anseth KS, Lee S, Zeiger A, Van Vliet KJ, Doyle PS. Stop-Flow Lithography for the Production of Shape-Evolving Degradable Microgel Particles. Journal of the American Chemical Society. 2009;131(12):4499–4504. doi: 10.1021/ja809256d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meiring JE, Lee S, Costner EA, Schmid MJ, Michaelson TB, Willson CG, Grayson SM. Pattern recognition of shape-encoded hydrogel biosensor arrays. Optical Engineering. 2009;48(3) [Google Scholar]
- 30. Dendukuri D, Hatton TA, Doyle PS. Synthesis and self-assembly of amphiphilic polymeric microparticles. Langmuir. 2007;23(8):4669–4674. doi: 10.1021/la062512i. This paper presents the amphiphilic self-assembly of chemical and shape anisotropic hydrogel microparticles prepared using lithographic processing, and demonstrates how the particle architecture can be varied to vary the structure of the resulting colloidal “micelles”.
- 31. Pregibon DC, Toner M, Doyle PS. Multifunctional encoded particles for high-throughput biomolecule analysis. Science. 2007;315(5817):1393–1396. doi: 10.1126/science.1134929. Using Stop Flow Lithography, this paper is the first to demonstrate the combination of graphical encoding and probe encapsulation on a single particle for molecular detection applications.
- 32.Lee W, Choi D, Kim JH, Koh WG. Suspension arrays of hydrogel microparticles prepared by photopatterning for multiplexed protein-based bioassays. Biomedical Microdevices. 2008;10(6):813–822. doi: 10.1007/s10544-008-9196-1. [DOI] [PubMed] [Google Scholar]
- 33.Gratton SEA, PohhauS PD, Lee J, Guo I, Cho MJ, DeSimone JM. Nanofabricated particles for engineered drug therapies: A preliminary Biodistribution study of PRINT (TM) nanoparticles. Journal of Controlled Release. 2007;121(1–2):10–18. doi: 10.1016/j.jconrel.2007.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rolland JP, Maynor BW, Euliss LE, Exner AE, Denison GM, DeSimone JM. Direct fabrication and harvesting of monodisperse, shape-specific nanobiomaterials. Journal of the American Chemical Society. 2005;127(28):10096–10100. doi: 10.1021/ja051977c. This paper gives the first presentation of the PRINT method, in which chemically homogeneous polymeric particles across a range of sizes and shapes are synthesized using non-wetting lithographic molds.
- 35. Kim H, Ge J, Kim J, Choi S, Lee H, Lee H, Park W, Yin Y, Kwon S. Structural colour printing using a magnetically tunable and lithographically fixable photonic crystal. Nature Photonics. 2009;3(9):534–540. This article presents the first synthesis of color-encoded hydrogel microparticles using optofluidic maskless lithography.
- 36.Shepherd RF, Panda P, Bao Z, Sandhage KH, Hatton TA, Lewis JA, Doyle PS. Stop-Flow Lithography of Colloidal, Glass, and Silicon Microcomponents. Advanced Materials. 2008;20(24) 4734-+ [Google Scholar]
- 37.Hwang DK, Dendukuri D, Doyle PS. Microfluidic-based synthesis of non-spherical magnetic hydrogel microparticles. Lab on a Chip. 2008;8(10):1640–1647. doi: 10.1039/b805176c. [DOI] [PubMed] [Google Scholar]
- 38.Bong KW, Chapin SC, Doyle PS. Magnetic Barcoded Hydrogel Microparticles for Multiplexed Detection. Langmuir. 2010;26(11):8008–8014. doi: 10.1021/la904903g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koh WG, Revzin A, Pishko MV. Poly(ethylene glycol) hydrogel microstructures encapsulating living cells. Langmuir. 2002;18(7):2459–2462. doi: 10.1021/la0115740. [DOI] [PubMed] [Google Scholar]
- 40.Chung SE, Park W, Shin S, Lee SA, Kwon S. Guided and fluidic self-assembly of microstructures using railed microfluidic channels. Nature Materials. 2008;7(7):581–587. doi: 10.1038/nmat2208. [DOI] [PubMed] [Google Scholar]
- 41.Kelly JY, DeSimone JM. Shape-specific, monodisperse nano-molding of protein particles. Journal of the American Chemical Society. 2008;130(16) doi: 10.1021/ja8014428. 5438-+ [DOI] [PubMed] [Google Scholar]
- 42.Kaehr B, Shear JB. Multiphoton fabrication of chemically responsive protein hydrogels for microactuation. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(26):8850–8854. doi: 10.1073/pnas.0709571105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gates BD, Xu QB, Stewart M, Ryan D, Willson CG, Whitesides GM. New approaches to nanofabrication: Molding, printing, and other techniques. Chemical Reviews. 2005;105(4):1171–1196. doi: 10.1021/cr030076o. [DOI] [PubMed] [Google Scholar]
- 44.McDonald JC, Duffy DC, Anderson JR, Chiu DT, Wu HK, Schueller OJA, Whitesides GM. Fabrication of microfluidic systems in poly(dimethylsiloxane) Electrophoresis. 2000;21(1):27–40. doi: 10.1002/(SICI)1522-2683(20000101)21:1<27::AID-ELPS27>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 45.Acharya G, Shin CS, McDermott M, Mishra H, Park H, Kwon IC, Park K. The hydrogel template method for fabrication of homogeneous nano/microparticles. Journal of Controlled Release. 2010;141(3):314–319. doi: 10.1016/j.jconrel.2009.09.032. [DOI] [PubMed] [Google Scholar]
- 46.Takei S, Ogawa T, Deschner R, Jen K, Nihira T, Hanabata M, Willson CG. Silicon-Containing Spin-on Underlayer Material for Step and Flash Nanoimprint Lithography. Japanese Journal of Applied Physics. 2010;49(7) [Google Scholar]
- 47.Maynor BW, Larue I, Hu Z, Rolland JP, Pandya A, Fu Q, Liu J, Spontak RJ, Sheiko SS, Samulski RJ, Samulski ET, DeSimone JM. Supramolecular nanomimetics: Replication of micelles, viruses, and other naturally occurring nanoscale objects. Small. 2007;3(5):845–849. doi: 10.1002/smll.200600507. [DOI] [PubMed] [Google Scholar]
- 48.Rogers JA, Paul KE, Whitesides GM. Quantifying distortions in soft lithography. Journal of Vacuum Science & Technology B. 1998;16(1):88–97. [Google Scholar]
- 49.Sun HB, Kawata S. Nmr - 3d Analysis - Photopolymerization. 2004;Vol. 170:169–273. [Google Scholar]
- 50.Jang JH, Dendukuri D, Hatton TA, Thomas EL, Doyle PS. A route to three-dimensional structures in a microfluidic device: Stop-flow interference lithography. Angewandte Chemie-International Edition. 2007;46(47):9027–9031. doi: 10.1002/anie.200703525. [DOI] [PubMed] [Google Scholar]
- 51.Lee SK, Park HS, Yi GR, Moon JH, Yang SM. Holographic Fabrication of Microstructures with Internal Nanopatterns Using Microprism Arrays. Angewandte Chemie-International Edition. 2009;48(38):7000–7005. doi: 10.1002/anie.200901166. [DOI] [PubMed] [Google Scholar]
- 52.Lee SK, Park H, Yi GR, Yang SM. Hierarchically Ordered Structures by Converging Holographic Lithography and Surfactant Templating. Chemistry of Materials. 2010;22(14):4117–4119. [Google Scholar]
- 53.Bong KW, Pregibon DC, Doyle PS. Lock release lithography for 3D and composite microparticles. Lab on a Chip. 2009;9(7):863–866. doi: 10.1039/b821930c. [DOI] [PubMed] [Google Scholar]
- 54. Lee SA, Chung SE, Park W, Lee SH, Kwon S. Three-dimensional fabrication of heterogeneous microstructures using soft membrane deformation and optofluidic maskless lithography. Lab on a Chip. 2009;9(12):1670–1675. doi: 10.1039/b819999j. This paper demonstrates the synthesis of 3D-patterned microgel particles by combining optofluidic maskless lithography with progressive pressure-induced membrane deflection in a microfluidic device.
- 55. Nielson R, Koehr B, Shear JB. Microreplication and Design of Biological Architectures Using Dynamic-Mask Multiphoton Lithography. Small. 2009;5(1):120–125. doi: 10.1002/smll.200801084. This paper demonstrates the use of holographic photolithography for the synthesis of highly complex 3D hydrogel structural replicas of naturally-occurring objects.
- 56.Oudshoorn MHM, Penterman R, Rissmann R, Bouwstra JA, Broer DJ, Hennink WE. Preparation and characterization of structured hydrogel microparticles based on cross-linked hyperbranched polyglycerol. Langmuir. 2007;23(23):11819–11825. doi: 10.1021/la701910d. [DOI] [PubMed] [Google Scholar]
- 57.Dendukuri D, Pregibon DC, Collins J, Hatton TA, Doyle PS. Continuous-flow lithography for high-throughput microparticle synthesis. Nature Materials. 2006;5(5):365–369. doi: 10.1038/nmat1617. [DOI] [PubMed] [Google Scholar]
- 58.Pregibon DC, Doyle PS. Optimization of Encoded Hydrogel Particles for Nucleic Acid Quantification. Analytical Chemistry. 2009;81(12):4873–4873. doi: 10.1021/ac9005292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Dendukuri D, Doyle PS. The Synthesis and Assembly of Polymeric Microparticles Using Microfluidics. Advanced Materials. 2009;21(41):4071–4086. This article gives a thorough review of microfluidic-based techniques for colloidal particle synthesis and assembly.
- 60.Panda P, Yuet KP, Hatton TA, Doyle PS. Tuning Curvature in Flow Lithography: A New Class of Concave/Convex Particles. Langmuir. 2009;25(10):5986–5992. doi: 10.1021/la8042445. [DOI] [PubMed] [Google Scholar]
- 61. Bong KW, Bong KT, Pregibon DC, Doyle PS. Hydrodynamic Focusing Lithography. Angewandte Chemie-International Edition. 2010;49(1):87–90. doi: 10.1002/anie.200905229. This paper presents the first synthesis of anisotropic particles with three-dimensional chemical patchiness using hydrodynamic focusing lithography, a variant of Stop Flow Lithography.
- 62.Dendukuri D, Panda P, Haghgooie R, Kim JM, Hatton TA, Doyle PS. Modeling of Oxygen-Inhibited Free Radical Photopolymerization in a PDMS Microfluidic Device. Macromolecules. 2008;41(22):8547–8556. [Google Scholar]
- 63.Panda P, Yuet KP, Dendukuri D, Hatton TA, Doyle PS. Temporal response of an initially deflected PDMS channel. New Journal of Physics. 2009;11 [Google Scholar]
- 64.Yamada M, Seki M. Hydrodynamic filtration for on-chip particle concentration and classification utilizing microfluidics. Lab on a Chip. 2005;5(11):1233–1239. doi: 10.1039/b509386d. [DOI] [PubMed] [Google Scholar]
- 65.Tornay RL, Braschler T, Demierre N, Steitz B, Finka A, Hofmann H, Hubbell JA, Renaud P. Dielectrophoresis-based particle exchanger for the manipulation and surface functionalization of particles. Lab on a Chip. 2008;8(2):267–273. doi: 10.1039/b713776a. [DOI] [PubMed] [Google Scholar]
- 66.Vanapalli SA, Iacovella CR, Sung KE, Mukhija D, Millunchick JM, Burns MA, Glotzer SC, Solomon MJ. Fluidic assembly and packing of microspheres in confined channels. Langmuir. 2008;24(7):3661–3670. doi: 10.1021/la703840w. [DOI] [PubMed] [Google Scholar]
- 67.Hong L, Cacciuto A, Luijten E, Granick S. Clusters of amphiphilic colloidal spheres. Langmuir. 2008;24(3):621–625. doi: 10.1021/la7030818. [DOI] [PubMed] [Google Scholar]
- 68.Manoharan VN, Elsesser MT, Pine DJ. Dense packing and symmetry in small clusters of microspheres. Science. 2003;301(5632):483–487. doi: 10.1126/science.1086189. [DOI] [PubMed] [Google Scholar]
- 69.Napier ME, Desimone JM. Nanoparticle drug delivery platform. Polymer Reviews. 2007;47(3):321–327. [Google Scholar]
- 70.Dubinsky S, Il Park J, Gourevich I, Chan C, Deetz M, Kumacheva E. Toward Controlling, the Surface Morphology of Macroporous Copolymer Particles. Macromolecules. 2009;42(6):1990–1994. [Google Scholar]
- 71.Seidlits SK, Schmidt CE, Shear JB. High-Resolution Patterning of Hydrogels in Three Dimensions using Direct-Write Photofabrication for Cell Guidance. Advanced Functional Materials. 2009;19(22):3543–3551. [Google Scholar]
- 72.Attia R, Pregibon DC, Doyle PS, Viovy JL, Bartolo D. Soft microflow sensors. Lab on a Chip. 2009;9(24):3604–3604. doi: 10.1039/b813860e. [DOI] [PubMed] [Google Scholar]
- 73.Meiring JE, Schmid MJ, Grayson SM, Rathsack BM, Johnson DM, Kirby R, Kannappan R, Manthiram K, Hsia B, Hogan ZL, Ellington AD, Pishko MV, Willson CG. Hydrogel biosensor array platform indexed by shape. Chemistry of Materials. 2004;16(26):5574–5580. [Google Scholar]
- 74.Chapin SC, Pregibon DC, Doyle PS. High-throughput flow alignment of barcoded hydrogel microparticles. Lab on a Chip. 2009;9(21):3100–3109. doi: 10.1039/b909959j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Appleyard DC, Chapin SC, Doyle PS. Multiplexed protein quantification with barcoded hydrogel microparticles. Analytical Chemistry. 2011 doi: 10.1021/ac1022343. In Press, DOI:10.1021/ac1022343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee H, Kim J, Kim H, Kim J, Kwon S. Colour-barcoded magnetic microparticles for multiplexed bioassays. Nature Materials. 2010;9:745–749. doi: 10.1038/nmat2815. [DOI] [PubMed] [Google Scholar]
- 77.Metters AT, Anseth KS, Bowman CN. Fundamental studies of a novel, biodegradable PEG-b-PLA hydrogel. Polymer. 2000;41(11):3993–4004. [Google Scholar]
- 78.Amsden B. Solute diffusion within hydrogels. Mechanisms and models. Macromolecules. 1998;31(23):8382–8395. [Google Scholar]
- 79.Haghgooie R, Toner M, Doyle PS. Squishy Non-Spherical Hydrogel Microparticles. Macromolecular Rapid Communications. 2010;31(2):128–134. doi: 10.1002/marc.200900302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dubinsky S, Petukhova A, Gourevich I, Kumacheva E. Hybrid porous material produced by polymerization-induced phase separation. Chemical Communications. 2010;46(15):2578–2580. doi: 10.1039/b924373a. [DOI] [PubMed] [Google Scholar]
- 81.Lee YJ, Braun PV. Tunable inverse opal hydrogel pH sensors. Advanced Materials. 2003;15(7–8):563–566. [Google Scholar]
- 82.Zhang HF, Cooper AI. Synthesis and applications of emulsion-templated porous materials. Soft Matter. 2005;1(2):107–113. doi: 10.1039/b502551f. [DOI] [PubMed] [Google Scholar]
- 83.Zhang H, Ju XJ, Xie R, Cheng CJ, Ren PW, Chu LY. A microfluidic approach to fabricate monodisperse hollow or porous poly(HEMA-MMA) microspheres using single emulsions as templates. Journal of Colloid and Interface Science. 2009;336(1):235–243. doi: 10.1016/j.jcis.2009.03.070. [DOI] [PubMed] [Google Scholar]
- 84.Fergg F, Keil FJ, Quader H. Investigations of the microscopic structure of poly(vinyl alcohol) hydrogels by confocal laser scanning microscopy. Colloid and Polymer Science. 2001;279(1):61–67. [Google Scholar]
- 85.Beines PW, Klosterkamp I, Menges B, Jonas U, Knoll W. Responsive thin hydrogel layers from photo-cross-linkable poly(N-isopropylacrylamide) terpolymers. Langmuir. 2007;23(4):2231–2238. doi: 10.1021/la063264t. [DOI] [PubMed] [Google Scholar]
- 86.Collier JH, Messersmith PB. Enzymatic modification of self-assembled peptide structures with tissue transglutaminase. Bioconjugate Chemistry. 2003;14(4):748–755. doi: 10.1021/bc034017t. [DOI] [PubMed] [Google Scholar]
- 87.Snowden MJ, Chowdhry BZ, Vincent B, Morris GE. Colloidal copolymer microgels of N-isopropylacrylamide and acrylic acid: pH, ionic strength and temperature effects. Journal of the Chemical Society-Faraday Transactions. 1996;92(24):5013–5016. [Google Scholar]
- 88.Brown E, Forman NA, Orellana CS, Zhang HJ, Maynor BW, Betts DE, DeSimone JM, Jaeger HM. Generality of shear thickening in dense suspensions. Nature Materials. 2010;9(3):220–224. doi: 10.1038/nmat2627. [DOI] [PubMed] [Google Scholar]
- 89.Zamanian B, Masaeli M, Nichol JW, Khabiry M, Hancock MJ, Bae H, Khademhosseini A. Interface-Directed Self-Assembly of Cell-Laden Microgels. Small. 2010;6(8):937–944. doi: 10.1002/smll.200902326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fernandez JG, Khademhosseini A. Micro-Masonry: Construction of 3D Structures by Microscale Self-Assembly. Advanced Materials. 2010;22(23):2538–2541. doi: 10.1002/adma.200903893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Du Y, Lo E, Vidula MK, Khabiry M, Khademhosseini A. Method of Bottom-Up Directed Assembly of Cell-Laden Microgels. Cellular and Molecular Bioengineering. 2008;1(2–3):157–162. doi: 10.1007/s12195-008-0020-z. This paper shows the interfacially-driven self-assembly of anisotropic microgel particles, and how the size and morphology of the resulting colloidal assemblies can be tuned through particle architecture.
- 92.Aveyard R, Binks BP, Clint JH. Emulsions stabilised solely by colloidal particles. Advances in Colloid and Interface Science. 2003;100:503–546. [Google Scholar]
- 93.Velev OD, Bhatt KH. On-chip micromanipulation and assembly of colloidal particles by electric fields. Soft Matter. 2006;2(9):738–750. doi: 10.1039/b605052b. [DOI] [PubMed] [Google Scholar]
- 94. Herlihy KP, Nunes J, DeSimone JM. Electrically driven alignment and crystallization of unique anisotropic polymer particles. Langmuir. 2008;24(16):8421–8426. doi: 10.1021/la801250g. This paper shows the field-driven assembly of complex shape anisotropic microgels prepared using the PRINT method, and the assembly of such particles leads to structures that cannot be formed using spheres or rods.
- 95.Nunes J, Herlihy KP, Mair L, Superfine R, DeSimone JM. Multifunctional Shape and Size Specific Magneto-Polymer Composite Particles. Nano Letters. 2010;10(4):1113–1119. doi: 10.1021/nl904152e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Beebe DJ, Moore JS, Bauer JM, Yu Q, Liu RH, Devadoss C, Jo BH. Functional hydrogel structures for autonomous flow control inside microfluidic channels. Nature. 2000;404(6778) doi: 10.1038/35007047. 588-+ [DOI] [PubMed] [Google Scholar]
- 97.Du YA, Lo E, Ali S, Khademhosseini A. Directed assembly of cell-laden microgels for fabrication of 3D tissue constructs. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(28):9522–9527. doi: 10.1073/pnas.0801866105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nichol JW, Koshy ST, Bae H, Hwang CM, Yamanlar S, Khademhosseini A. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials. 2010;31(21):5536–5544. doi: 10.1016/j.biomaterials.2010.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gratton SEA, Napier ME, Ropp PA, Tian SM, DeSimone JM. Microfabricated Particles for Engineered Drug Therapies: Elucidation into the Mechanisms of Cellular Internalization of PRINT Particles. Pharmaceutical Research. 2008;25(12):2845–2852. doi: 10.1007/s11095-008-9654-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gratton SEA, Williams SS, Napier ME, Pohlhaus PD, Zhou ZL, Wiles KB, Maynor BW, Shen C, Olafsen T, Samulski ET, Desimone JM. The Pursuit of a Scalable Nanofabrication Platform for Use in Material and Life Science Applications. Accounts of Chemical Research. 2008;41(12):1685–1695. doi: 10.1021/ar8000348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chapin SC, Appleyard DC, Pregibon DC, Doyle PS. Rapid microRNA profiling on encoded gel particles with a high-throughput microfluidic scanner. Angewandte Chemie-International Edition. doi: 10.1002/anie.201006523. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kloxin AM, Kasko AM, Salinas CN, Anseth KS. Photodegradable Hydrogels for Dynamic Tuning of Physical and Chemical Properties. Science. 2009;324(5923):59–63. doi: 10.1126/science.1169494. [DOI] [PMC free article] [PubMed] [Google Scholar]