Abstract
Observational studies have shown that inflammatory cells accumulate within the thrombus and surrounding vein wall during the natural history of venous thrombosis. More recent studies have begun to unravel the mechanisms that regulate this interaction and have confirmed that thrombosis and inflammation are intimately linked. This review outlines our current knowledge of the complex relationship between inflammatory cell activity and venous thrombosis and highlights new areas of research in this field. A better understanding of this relationship could lead to the development of novel therapeutic targets that inhibit thrombus formation or promote its resolution.
Keywords: venous thrombosis, inflammation, leukocytes, resolution
Introduction
Deep Vein Thrombosis (DVT) is a common condition affecting 1-2% of the population with an annual incidence of 1 in 5001. DVT can lead to death through pulmonary embolism (PE) and many patients subsequently suffer from venous reflux, which can lead to the post-thrombotic syndrome (PTS). This condition is characterised by pain, swelling and chronic leg ulceration. Around one quarter of patients develop PTS within 1 year of the episode of thrombosis2. DVT is therefore potentially fatal, can cause significant patient morbidity and has become an economic burden for health care services in the developed world.
Treatments for DVT such as anticoagulation prevent thrombus propagation and extension, but have little effect on existing thrombi, which resolve naturally through a process of organisation and vein recanalisation3. Rapid natural resolution is associated with less valvular damage, reduced venous hypertension, and fewer post thrombotic complications4-6. Treatments that remove thrombus rapidly such as thrombolysis and mechanical removal are, however, associated with increased morbidity, have significant haemorrhagic side effects and increase the risk of re-thrombosis7. An organised thrombus (usually those which have been present for more than 14 days), a history of stroke, or a recent operation, are contraindications to the use of thrombolytic therapy8. Alternative forms of treatment, which prevent thrombosis or accelerate natural thrombus resolution without haemorrhagic side effects, would therefore be attractive. It is likely that these will only be developed from a better understanding of the mechanisms that regulate thrombus formation and its resolution.
There is increasing evidence that inflammatory processes and DVT are intimately linked. This review outlines our current understanding of the complex relationship between inflammatory cell activity and venous thrombosis and highlights new areas of research in this field.
Venous thrombus formation
A triad of vessel wall injury, venous stasis and blood hypercoagulability have historically been considered to predispose to venous thrombosis9. Venous thrombi arise in both the vein valve pockets and dilated sinuses of the lower limbs, are fibrin and red cell rich, and form on the surface of the endothelium10-12. They have a laminar structure consisting of layers of platelets, leukocytes and fibrin (‘lines of Zahn’) that encompass the main erythrocyte mass. This is unlike the amorphous structure of a ‘blood clot’, which consists predominantly of erythrocytes within a fine fibrin mesh3.
Studies in the 1970s using radiolabeled leukocytes have shown uptake of white blood cells into venous thrombi13, while accumulation of polymorphonuclear neutrophils (PMNs) on the abluminal side of the endothelium following occlusion of canine veins, first led to the speculation that ‘white-cell’ induced damage to the endothelium may be a contributing factor to venous thrombosis in man14 (Fig 1i). Exposure of the collagen rich wall is said to lead to platelet aggregation and further leukocyte sequestration, which results in a nidus for thrombus propagation15, 16. C-reactive protein (CRP), an inflammatory marker, has been shown to increase in patients suffering with DVT17. Inflammation is therefore considered an important mechanism for venous thrombus formation.
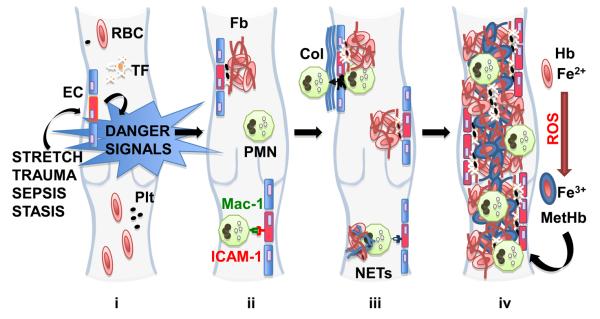
Fig 1. Inflammation in venous thrombogenesis.
(i) Activation of the endothelium (EC - red cell) generates intravascular danger signals, which guide leukocytes to areas of inflammation and induces tissue factor (TF) production. (ii) Upregulation of adhesion molecules on endothelium mediate PMN recruitment while tissue factor generates thrombin, activating platelet (Plt) deposition and converting fibrinogen to cross-linked fibrin (Fb) that entraps the main red blood cell (RBC) mass. (iii) PMN accumulation in the subendothelial layer and subsequent exposure of collagen (Col) causes platelet aggregation and further PMN sequestration establishing a nidus for thrombus formation (iii). PMN apoptosis in response to inflammatory stimuli releases neutrophil extracellular traps (NETs, blue strands) that provide a scaffold for further RBC capture (iii). Reactive oxygen species (ROS), released from the vessel wall and leukocytes, oxidise haemoglobin (Hb) to methaemoglobin (MetHb, blue RBC). As trapped RBCs lyse, Fe3+ contained in metHb is released and induces further RBC lysis. This leads to a positive feedback loop with increased areas of endothelial dysfunction resulting in thrombus propagation (iv).
Inflammation and the endothelium
The formation, propagation, and dissolution of venous thrombi represents a balance between coagulation and innate protective mechanisms, specifically the circulating inhibitors of coagulation (e.g. tissue factor pathway inhibitor, thrombomodulin, protein C, plasminogen activator inhibitors) and promoters of fibrinolysis (e.g. plasminogen activators)9.
Endothelial ‘microtears’ containing leukocytes have been demonstrated by electron microscopy in the deep veins following hip surgery in dogs18, leading to the suggestion that these may be the nidus for venous thrombus formation. Others have, however, found no major overt damage to the endothelium at sites of thrombosis in man10. Crushing the vein to cause endothelial damage does not lead to the formation of experimental venous thrombi19 and scanning electron microscopy reveals minimal endothelial damage immediately after thrombus formation in the rat20. Disturbance of the endothelium by mechanical (e.g. stretch or surgery) or chemical means (e.g. sepsis) can, however, cause activation of the endothelium resulting in increased expression of procoagulant proteins such as tissue factor (TF)21, cytokines and surface adhesion of molecules that promote leukocyte adhesion and initiate thrombosis22 (Fig 1i). Genetic knock-out of the adhesion molecules E- and P-selectin results in reduced thrombus size, and this is associated with altered leukocyte accumulation in the surrounding vein wall23. Neutralising P-selectin glycoprotein ligand-1 (PSGL-1) also reduces local inflammation and thrombus size, and could be a potential treatment for the prevention of DVT in the future24.
Circulating TF, in the form of microparticles (MPs) released by activated leukocytes, accumulate in areas of stasis such as the vein valve pockets21. Leukocyte MPs that express PSGL-1, bind to P-selectin both on platelets and activated endothelial cells25, 26, and this source of TF could sustain the production of thrombin on the forming thrombus, promoting its propagation27. The relative contribution of leukocyte MPs to venous thrombosis is, however, not clear, as adoptive transfer of bone marrow from mice expressing low levels of TF, into wild types does not inhibit thrombus formation28. It appears that the vessel wall is the most important source of TF that promotes thrombogenesis28, and therefore activation of the endothelium may be pivotal in the inflammatory processes that lead to thrombosis.
Polymorphonuclear neutrophils (PMNs)
PMNs are found in large numbers within the early venous thrombus20, 29. Recent studies on the mechanisms of PMN recruitment to sites of sterile inflammation have revealed that intravascular danger signals, including the activation of the Nlrp3 inflammasome, generation of a chemokine gradient and release of formyl-peptide signals, act as a guide to these sites30. PMN adhesion to endothelium is mediated by interactions between the integrin αMβ2 (Mac1) and its endothelial ligand intracellular adhesion molecule-1 (ICAM-1)30 (Fig 1ii). Whether a similar mechanism is involved in PMN accumulation during venous thrombosis, remains to be determined though it appears that the thrombogenic effects of antiphospholipid antibodies are mediated in part by ICAM-131.
Aside from the formation of a nidus for thrombus propagation, recent data have emerged which suggest that recruited PMNs may initiate thrombosis through the formation of neutrophil extracellular traps (NETs)32 (Fig 1iii). These extracellular DNA fibres, which comprise of histones and neutrophil antimicrobial proteins, form following a cell death programme in response to inflammatory stimuli (e.g. IL-8, reactive oxygen species) from cells in vitro33-35, and have been linked to small vessel vasculitis36 and pre-eclampsia37. DNA traps appear in the plasma and thrombus following induction of DVT in a baboon model and could provide a scaffold for thrombus formation, though their precise mechanism of action remains to be elucidated32.
The role of PMNs in the natural history of the venous thrombus is however complex. Selective antibody depletion of PMNs in a rat stasis model, led to larger venous thrombi suggesting that PMNs are not required for thrombus formation, but may be important in removal of forming thrombus38 (Fig 2). A similar finding was not, however, demonstrated in a murine model and CXCR2-dependent thrombus resolution appeared independent of the CXCR2 primary effector leukocyte, the PMN39. PMNs also show both pro and antifibrinolytic activity in an ex vivo thrombosis model40. These paradoxical results could, at least in part, be explained from functional heterogeneity in the neutrophil compartment. Circulating PMNs, express a variety of membrane receptors including CD11b, CD16, CXCR1, C5aR, FcγRII and TLR4. Differential expression of these receptors is known to confer functionally distinct roles for PMNs following LPS-induced inflammation in man; activated, differentiated PMNs mediate tissue damage41. Future studies into the role of the PMNs in venous thrombosis should consider the functional heterogeneity of this cell type. It is possible that given certain environmental cues, specific subsets of PMNs contribute to thrombus formation, while other distinct PMNs may be more important for its resolution.
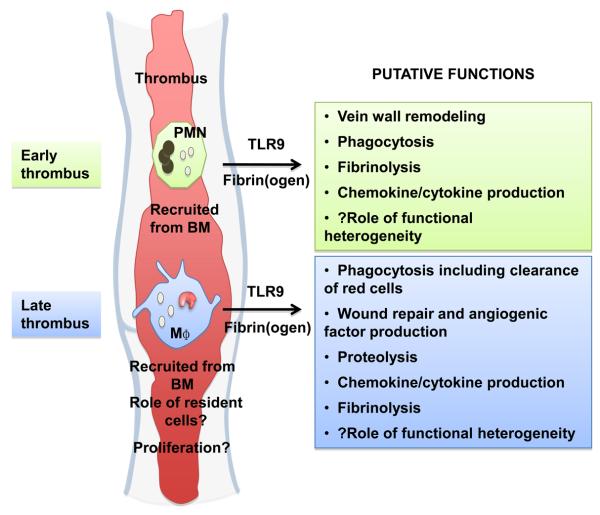
Fig 2. Putative functions of leukocytes in venous thrombus resolution.
Leukocytes accumulate in the venous thrombus during its resolution. Polymorphonuclear neutrophils (PMN) predominate in the early stages of resolution with mononuclear phagocytes (M?) predominating later. The origin of these cells appears to be from the bone marrow (BM), however the contribution of tissue resident macrophages or cells derived from the ‘splenic reservoir’, remains unknown. Leukocytes signal through a TLR9 mechanism and may be stimulated by fibrin(ogen) and its degradation products. They are speculated to have a number of functions important for thrombus resolution.
Red blood cells, inflammation and thrombosis
The contribution of red blood cells (RBCs) to venous thrombosis remains poorly understood, despite their abundance in the early venous thrombus and their contribution to platelet rich arterial thrombi42. The cytoplasm of RBCs is rich in iron, which when released into the circulation is highly inflammatory because of its oxidative effects on the endothelium. It has been hypothesised that reactive oxygen species, produced by leukocytes and vessel wall at the nidus of thrombosis, oxidises haemoglobin in RBCs that become trapped by cross-linked fibrin forming at this point. This results in the formation of methaemoglobin (metHb) containing Fe3+. The release of Fe3+ leads to a cascade of RBC lysis that results in further endothelial dysfunction43 and thrombus propagation (Fig 1iv). Natural antioxidants, such as hemeoxygenase-1 (HO-1)-derived carbon monoxide and superoxide dismutase, produced by cells within the vessel wall and thrombus, may limit these inflammatory effects. This putative mechanism by which RBCs may influence the development of venous thrombi is supported by the finding that propagation of venous thrombosis is enhanced in HO-1−/− mice44, while specific genetic variants (long GT-repeat alleles) in the HO-1 gene appear to confer increased risk of recurrent venous thromboembolism in man45.
The inflammatory nature of the early, oxidised, RBC-rich thrombus has been exploited in the development of imaging methods to identify acute venous thrombi in man46. MetHb exerts paramagnetic properties resulting from its five unpaired electrons, which shortens the nuclear magnetic resonance longitudinal relaxation time (T1) of the thrombus. It is therefore possible to accurately demonstrate contrast between young thrombi and surrounding tissues using MRI47. Advances in technology have allowed the rapid quantification of T1 relaxation time, suitable for a clinical setting48. When scanning thrombus in the same patient, T1 relaxation times are short in the young thrombus (800ms), but return to that of blood (1300ms) by six months, presumably because of the phagocytic action of cells that accumulate within the thrombus as it resolves and take up iron49. The in vivo characterisation of DVT in man, without the need for a contrast agent, could be used to predict clinical outcome following DVT and guide management. MRI identification of fresh thrombi, could help stratify patients into whom thrombolysis has the greatest potential. It could also act as a surrogate outcome measure when testing the efficacy of novel treatments in clinical trials. Validation of the relationship between T1 relaxation times and thrombus structure by comparing MR images with histology is, however, still required.
Venous thrombus resolution
Natural thrombus resolution is characterised by tissue organisational processes (including neovascularisation) reminiscent of those seen during wound healing3, 9. Organisation ultimately leads to varying degrees of thrombus resolution with consequent recanalisation of the vein lumen10, 11. As in wound healing, there is a temporal change in the leukocyte composition of the thrombus both in terms of cell types and their numbers during resolution20, 29 (Fig 2). The majority of the leukocytes in the early thrombus appear to be PMNs, which may have a role in vein wall remodeling50, while macrophages predominate in the later stages of resolution and are likely to be the most important effector cells of this process51-53. The chemotactic proteins interleukin-8 (IL-8) and Monocyte chemotactic protein-1 (MCP-1) that are produced within the thrombus, may be important stimuli for the recruitment of these cells54, 55. Our studies of thrombosis in mice with severe combined immunodeficiency (SCID) suggest that lymphocytes have no role in either thrombus formation or its resolution56.
Putative functions of inflammatory cells during thrombus resolution
The role of inflammation, and specifically leukocytes such as PMNs and the monocyte/macrophage, during thrombus resolution is not completely understood (Fig2). Studies in mice lacking the ets transcription factor Pu.1 suggest that inflammatory cell activity may not be a prerequisite for tissue repair57. Nevertheless, leukocytes comprise a significant proportion of the cells in the thrombus, and, interventions that lead to alterations in their accumulation lead to significant effects on subsequent thrombus resolution.
There is increased plasminogen activator content, both tissue-type (tPA) and urokinase-type (uPA) that co-localises with macrophages in thrombus formed in the rat58, 59. This has led to the speculation that fibrinolysis is important for thrombus resolution. Deletion of the tissue-type plasminogen activator gene (tPA−/−), however, has no effect on this process51. By contrast, deletion of the urokinase-type plasminogen activator gene (uPA−/−), prevents resolution and is associated with reduced macrophage numbers in the thrombus51. Adoptive transfer of wild-type bone marrow into uPA−/− mice rescues normal resolution51, 60-62, while upregulation of uPA in macrophages enhances this process56. Urokinase - urokinase receptor (uPAR) interaction is commonly thought to be a major regulatory mechanism for cell migration. Plasmin generation at the cell surface activates other proteases such as matrix metalloproteinases (MMPs, including MMP-2 and MMP-9) that degrade extracellular matrix, facilitating cell migration60-62. These data lead us to speculate that monocyte-associated urokinase-activity is important for venous thrombus resolution.
Neovascular channels appear around the thrombus wall junction and within the thrombus as resolution proceeds3, 10, 11. Histological studies in rodent models suggest that these channels are derived from the vein wall3, and they may also be derived from cells residing in the thrombus63. We have found that enhancing the levels of either VEGF alone, or simultaneously with a number of other angiogenic factors through upregulation of HIF1a within the thrombus, enhances its resolution64-67. These outcomes are linked to macrophage accumulation within the thrombus, which perhaps act as ‘cellular chaperones’ as recently described in the development of the vascular network68.
Leukocyte signaling during venous thrombus resolution
Fibrin(ogen) and its degradation products are present in abundance in the thrombus51, 69. These molecules are able to stimulate recruitment and activation of leukocytes, to produce cytokines (TNFα and IL1β) and chemokines (IL8 and MCP1) in inflammatory settings70, 71 and promote phagocytosis and cell migration in vitro72. Interaction of the fibrinogen γ chain residue 390-396 with Mac1 is thought to be an important pathway by which these molecules influence leukocyte activity72. Mononuclear cells may also interact with fibrinogen to produce chemokines through a TLR4 dependent mechanism73. Examination of thrombus formation and resolution in mice that carry mutations such as Fibγ 390-396A, may provide new insights into the role of fibrin(ogen) and its degradation products in the natural history of venous thrombi.
More recently, data have emerged that have provided new insights into the signaling mechanisms that regulate the functions of leukocytes during venous thrombus resolution. Deletion of toll-like receptor 9 gene (TLR9−/−) impairs resolution assessed at days 2 and 8 after induction, despite an increase in the numbers of both PMNs and Mac2+ macrophages in the thrombus74. These data suggest that TLR9 is important for leukocyte function during thrombus resolution. This effect was independent of MyD88 signalling (a major TLR signaling pathway), and was related to NOTCH ligand delta-like 4 pathways. TLR9−/− mice have reduced thrombus neovascularisation and decreased levels of the Th1 inflammatory cytokines IFNα, IL1α and IL2 in the vein wall, which appears to be important for venous thrombus resolution52. Further investigation is required to elucidate the role of TLR9 and MyD88 in later phases of thrombus resolution (beyond 8 days) and whether other TLRs are involved.
Mononuclear phagocytes
Macrophage accumulation within the thrombus is a hallmark of resolving thrombus in both man and experimental models3, 9, 49. Adoptive transfer experiments suggest that these cells are derived from the bone marrow (BM)75, however macrophages that are not of BM origin, may also have a role in thrombus resolution51. Macrophages that accumulate in the thrombus could be derived from cells that are resident within the vein wall, or may even arise from the ‘splenic reservoir’ that has recently been described76. The relative contribution of these sources for cells implicated in the resolution of venous thrombi remains unknown.
Although it is generally thought that the accumulation of macrophages is dependent on the recruitment of circulating monocytes, direct visualisation of these cells entering the thrombus has yet to be demonstrated. Renewal of certain resident mononuclear cells (microglia and Langerhans cells) in the steady state appears to be independent of BM77, 78. Adult LCs self renew in situ and proliferate during inflammation79. A demonstration that macrophages proliferate in the thrombus would change the current paradigm regarding the nature of their accumulation.
The function of mononuclear cells in the thrombus also remains to be fully elucidated. As they are phagocytic by definition, it seems reasonable to speculate that they contribute to the clearance of cells, nucleotides and matrix proteins within the thrombus. These cells may also promote fibrinolysis, are associated with angiogenesis and could regulate tissue remodeling80-82 - processes seemingly beneficial for thrombus resolution. To add further complexity, both monocytes and macrophages consist of heterogeneous populations of cells, which appear to have distinct functions83.
Circulating monocyte subsets can be distinguished on the basis of their expression of surface receptors84. Circulating ‘inflammatory’ monocytes, express Ly6C in the mouse, and are recruited into tissue where they undergo activation in a pathogen dependent response84, 85. In a model of spinal cord injury, recruitment of Ly6C+ monocytes appears important for tissue repair86 and these monocytes may also contribute to the fraction of myeloid derived suppressor cells (MDSCs) that promote tumour driven angiogenesis87. The contribution of Ly6C+ monocytes in the formation and resolution of venous thrombosis has yet to be established, but our previous studies suggest that recruitment of this subset may be important for thrombus resolution. Impaired resolution occurs in Ccr2−/− mice52, 53, and CCR2 is required for the exit of Ly6C+ monocytes from bone marrow88.
Ly6C− monocytes exhibit long range crawling over the endothelium of both arteries and veins89. It has been hypothesised that they are involved in the surveying of the vasculature and sensing of tissue damage such as dying or infected cells78, 90. In a model of myocardial infarction, Ly6C+ monocytes initially accumulate in the healing myocardium and may digest damaged tissue81. In a later reparative phase, Ly6C− monocytes predominate and are suggested to be involved in tissue repair by inducing myofibroblast accumulation, angiogenesis and collagen deposition81. The human equivalent of Ly6C− murine monocytes (CD14dim monocytes), have recently been implicated in the pathogenesis of autoimmune diseases such as lupus, and respond to viruses and nucleic acid-containing immune complexes via a pro-in?ammatory TLR7-TLR8-MyD88-MEK pathway90. Whether these patrolling cells have a role in venous thrombosis (initiation or resolution) remains to be determined, although we speculate that their patrolling function makes them an ideal candidate for the detection of endothelial dysfunction and possible initiation of coagulation.
When monocytes enter tissue they differentiate into macrophages91. Based on in vitro studies, these cells have been tentatively classified by some into two main phenotypes: those that promote inflammatory responses (M1 or classically activated – expressing inflammatory mediators such as TNFα and NOS2); and those that attenuate inflammatory responses (M2a-c or alternatively activated – expressing arginase, mannose receptor [MR] and the transcription factors - Fizz1 and Ym1/2). Others consider dividing macrophage populations based on their immunological or trophic roles in response to granulocyte/macrophage CSF (also known as CSF2) or macrophage CSF (also known as CSF1) respectively80. A rigid classification of macrophages probably represents the extremes of a continuous spectrum and may be too simplistic as these cells may exhibit characteristics of more than one phenotype81. M2-like macrophages have, however, been reported in the healing myocardium and injured skeletal muscle, where they are considered to be involved in tissue repair and wound resolution92, 93.
Clinical trials involving the therapeutic targeting of macrophages in other vascular diseases such as atherosclerosis has been largely unsuccessful94. This in part is because of a lack of understanding of their function95. Their roles in venous thrombosis require investigation, especially because different monocyte and macrophage phenotypes may have complimentary and contrasting functions83. This could be achieved through the use of functional reporter mice that express fluorescent proteins linked to cell specific genes96. We are currently developing these tools to examine cellular functions in thrombus resolution.
Inflammation is a central mechanism in both the genesis and resolution of venous thrombi. The temporal accumulation of leukocytes in the forming (PMNs) and resolving thrombus (macrophages) is part of a dynamic ‘intravascular wound healing process’ that results in either the early lysis of the thrombus, or its stabilisation and subsequent resolution. Enhancing our understanding of the cellular and molecular pathways that mediate sterile inflammation in the context of venous thrombosis, could lead to the development of novel therapeutic targets for: i) prevention of deep vein thrombosis in a manner that does not promote pathological bleeding; and ii) acceleration of natural thrombus resolution to reduce the incidence of post thrombotic complications. These may be achieved through developments in molecular and cellular imaging capable of delineating specific inflammatory processes that are currently on the horizon.
Acknowledgements
PS is funded by a Clinical Research Training Fellowship grant from the Welcome Trust. BM is funded through a Clinical Lectureship from the National Institute for Health Research. CEE is funded by a Studentship grant from the British Heart Foundation (BHF). AP is funded by a Clinical Training Fellowship grant from the BHF and OTAL is funded by an MRC Clinical Research Training Fellowship.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fowkes FJ, Price JF, Fowkes FG. Incidence of diagnosed deep vein thrombosis in the general population: Systematic review. Eur J Vasc Endovasc Surg. 2003;25:1–5. doi: 10.1053/ejvs.2002.1778. [DOI] [PubMed] [Google Scholar]
- 2.Prandoni P, Bernardi E, Marchiori A, Lensing AW, Prins MH, Villalta S, Bagatella P, Sartor D, Piccioli A, Simioni P, Pagnan A, Girolami A. The long term clinical course of acute deep vein thrombosis of the arm: Prospective cohort study. BMJ. 2004;329:484–485. doi: 10.1136/bmj.38167.684444.3A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Modarai B, Burnand KG, Humphries J, Waltham M, Smith A. The role of neovascularisation in the resolution of venous thrombus. Thromb Haemost. 2005;93:801–809. doi: 10.1160/TH04-09-0596. [DOI] [PubMed] [Google Scholar]
- 4.Meissner MH, Caps MT, Zierler BK, Polissar N, Bergelin RO, Manzo RA, Strandness DE., Jr Determinants of chronic venous disease after acute deep venous thrombosis. J Vasc Surg. 1998;28:826–833. doi: 10.1016/s0741-5214(98)70057-6. [DOI] [PubMed] [Google Scholar]
- 5.Meissner MH, Manzo RA, Bergelin RO, Markel A, Strandness DE., Jr Deep venous insufficiency: The relationship between lysis and subsequent reflux. J Vasc Surg. 1993;18:596–605. discussion 606-598. [PubMed] [Google Scholar]
- 6.Saarinen J, Kallio T, Lehto M, Hiltunen S, Sisto T. The occurrence of the post-thrombotic changes after an acute deep venous thrombosis. A prospective two-year follow-up study. J Cardiovasc Surg (Torino) 2000;41:441–446. [PubMed] [Google Scholar]
- 7.Patterson BO, Hinchliffe R, Loftus IM, Thompson MM, Holt PJ. Indications for catheter-directed thrombolysis in the management of acute proximal deep venous thrombosis. Arterioscler Thromb Vasc Biol. 2010;30:669–674. doi: 10.1161/ATVBAHA.109.200766. [DOI] [PubMed] [Google Scholar]
- 8.Hirsh J, Guyatt G, Albers GW, Harrington R, Schunemann HJ, American College of Chest P. Antithrombotic and thrombolytic therapy: American college of chest physicians evidence-based clinical practice guidelines (8th edition) Chest. 2008;133:110S–112S. doi: 10.1378/chest.08-0652. [DOI] [PubMed] [Google Scholar]
- 9.Wakefield TW, Myers DD, Henke PK. Mechanisms of venous thrombosis and resolution. Arterioscler Thromb Vasc Biol. 2008;28:387–391. doi: 10.1161/ATVBAHA.108.162289. [DOI] [PubMed] [Google Scholar]
- 10.Sevitt S. Organic canalisation and vascularisation of deep vein thrombi studied with dyed-micropaque injected at necropsy. J Pathol. 1970;100:Pi. [PubMed] [Google Scholar]
- 11.Sevitt S. The vascularisation of deep-vein thrombi and their fibrous residue: A post mortem angio-graphic study. J Pathol. 1973;111:1–11. doi: 10.1002/path.1711110102. [DOI] [PubMed] [Google Scholar]
- 12.Brooks EG, Trotman W, Wadsworth MP, Taatjes DJ, Evans MF, Ittleman FP, Callas PW, Esmon CT, Bovill EG. Valves of the deep venous system: An overlooked risk factor. Blood. 2009;114:1276–1279. doi: 10.1182/blood-2009-03-209981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Charkes ND, Dugan MA, Malmud LS, Stern H, Anderson H, Kozar J, 3rd, Maguire R. Letter: Labelled leucocytes in thrombi. Lancet. 1974;2:600. doi: 10.1016/s0140-6736(74)91930-8. [DOI] [PubMed] [Google Scholar]
- 14.Stewart GJ, Ritchie WG, Lynch PR. Venous endothelial damage produced by massive sticking and emigration of leukocytes. Am J Pathol. 1974;74:507–532. [PMC free article] [PubMed] [Google Scholar]
- 15.Wakefield TW, Strieter RM, Prince MR, Downing LJ, Greenfield LJ. Pathogenesis of venous thrombosis: A new insight. Cardiovasc Surg. 1997;5:6–15. doi: 10.1016/s0967-2109(96)00083-x. [DOI] [PubMed] [Google Scholar]
- 16.Schaub RG, Simmons CA, Koets MH, Romano PJ., 2nd Stewart GJ. Early events in the formation of a venous thrombus following local trauma and stasis. Lab Invest. 1984;51:218–224. [PubMed] [Google Scholar]
- 17.Bucek RA, Reiter M, Quehenberger P, Minar E. C-reactive protein in the diagnosis of deep vein thrombosis. Br J Haematol. 2002;119:385–389. doi: 10.1046/j.1365-2141.2002.03886.x. [DOI] [PubMed] [Google Scholar]
- 18.Stewart GJ, Alburger PD, Jr., Stone EA, Soszka TW. Total hip replacement induces injury to remote veins in a canine model. J Bone Joint Surg Am. 1983;65:97–102. [PubMed] [Google Scholar]
- 19.Thomas D. Venous thrombogenesis. Br Med Bull. 1994;50:803–812. doi: 10.1093/oxfordjournals.bmb.a072927. [DOI] [PubMed] [Google Scholar]
- 20.McGuinness CL, Humphries J, Waltham M, Burnand KG, Collins M, Smith A. Recruitment of labelled monocytes by experimental venous thrombi. Thromb Haemost. 2001;85:1018–1024. [PubMed] [Google Scholar]
- 21.Manly DA, Boles J, Mackman N. Role of tissue factor in venous thrombosis. Annu Rev Physiol. 2010 doi: 10.1146/annurev-physiol-042210-121137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stewart GJ. Neutrophils and deep venous thrombosis. Haemostasis. 1993;23(Suppl 1):127–140. doi: 10.1159/000216922. [DOI] [PubMed] [Google Scholar]
- 23.Sullivan VV, Hawley AE, Farris DM, Knipp BS, Varga AJ, Wrobleski SK, Thanapron P, Eagleton MJ, Myers DD, Fowlkes JB, Wakefield TW. Decrease in fibrin content of venous thrombi in selectin-deficient mice. J Surg Res. 2003;109:1–7. doi: 10.1016/s0022-4804(02)00041-0. [DOI] [PubMed] [Google Scholar]
- 24.Ramacciotti E, Myers DD, Jr., Wrobleski SK, Deatrick KB, Londy FJ, Rectenwald JE, Henke PK, Schaub RG, Wakefield TW. P-selectin/ psgl-1 inhibitors versus enoxaparin in the resolution of venous thrombosis: A meta-analysis. Thromb Res. 2010;125:e138–142. doi: 10.1016/j.thromres.2009.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Furie B, Furie BC. Mechanisms of thrombus formation. N Engl J Med. 2008;359:938–949. doi: 10.1056/NEJMra0801082. [DOI] [PubMed] [Google Scholar]
- 26.Falati S, Liu Q, Gross P, Merrill-Skoloff G, Chou J, Vandendries E, Celi A, Croce K, Furie BC, Furie B. Accumulation of tissue factor into developing thrombi in vivo is dependent upon microparticle p-selectin glycoprotein ligand 1 and platelet p-selectin. J Exp Med. 2003;197:1585–1598. doi: 10.1084/jem.20021868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giesen PL, Rauch U, Bohrmann B, Kling D, Roque M, Fallon JT, Badimon JJ, Himber J, Riederer MA, Nemerson Y. Blood-borne tissue factor: Another view of thrombosis. Proc Natl Acad Sci U S A. 1999;96:2311–2315. doi: 10.1073/pnas.96.5.2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Day SM, Reeve JL, Pedersen B, Farris DM, Myers DD, Im M, Wakefield TW, Mackman N, Fay WP. Macrovascular thrombosis is driven by tissue factor derived primarily from the blood vessel wall. Blood. 2005;105:192–198. doi: 10.1182/blood-2004-06-2225. [DOI] [PubMed] [Google Scholar]
- 29.Wakefield TW, Strieter RM, Wilke CA, Kadell AM, Wrobleski SK, Burdick MD, Schmidt R, Kunkel SL, Greenfield LJ. Venous thrombosis-associated inflammation and attenuation with neutralizing antibodies to cytokines and adhesion molecules. Arterioscler Thromb Vasc Biol. 1995;15:258–268. doi: 10.1161/01.atv.15.2.258. [DOI] [PubMed] [Google Scholar]
- 30.McDonald B, Pittman K, Menezes GB, Hirota SA, Slaba I, Waterhouse CC, Beck PL, Muruve DA, Kubes P. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science. 2010;330:362–366. doi: 10.1126/science.1195491. [DOI] [PubMed] [Google Scholar]
- 31.Pierangeli SS, Espinola RG, Liu X, Harris EN. Thrombogenic effects of antiphospholipid antibodies are mediated by intercellular cell adhesion molecule-1, vascular cell adhesion molecule-1, and p-selectin. Circ Res. 2001;88:245–250. doi: 10.1161/01.res.88.2.245. [DOI] [PubMed] [Google Scholar]
- 32.Fuchs TA, Brill A, Duerschmied D, Schatzberg D, Monestier M, Myers DD, Jr., Wrobleski SK, Wakefield TW, Hartwig JH, Wagner DD. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci U S A. 2010;107:15880–15885. doi: 10.1073/pnas.1005743107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.von Kockritz-Blickwede M, Goldmann O, Thulin P, Heinemann K, Norrby-Teglund A, Rohde M, Medina E. Phagocytosis-independent antimicrobial activity of mast cells by means of extracellular trap formation. Blood. 2008;111:3070–3080. doi: 10.1182/blood-2007-07-104018. [DOI] [PubMed] [Google Scholar]
- 34.Yousefi S, Gold JA, Andina N, Lee JJ, Kelly AM, Kozlowski E, Schmid I, Straumann A, Reichenbach J, Gleich GJ, Simon HU. Catapult-like release of mitochondrial DNA by eosinophils contributes to antibacterial defense. Nat Med. 2008;14:949–953. doi: 10.1038/nm.1855. [DOI] [PubMed] [Google Scholar]
- 35.Brinkmann V, Zychlinsky A. Beneficial suicide: Why neutrophils die to make nets. Nat Rev Microbiol. 2007;5:577–582. doi: 10.1038/nrmicro1710. [DOI] [PubMed] [Google Scholar]
- 36.Kessenbrock K, Krumbholz M, Schonermarck U, Back W, Gross WL, Werb Z, Grone HJ, Brinkmann V, Jenne DE. Netting neutrophils in autoimmune small-vessel vasculitis. Nat Med. 2009;15:623–625. doi: 10.1038/nm.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gupta AK, Hasler P, Holzgreve W, Gebhardt S, Hahn S. Induction of neutrophil extracellular DNA lattices by placental microparticles and il-8 and their presence in preeclampsia. Hum Immunol. 2005;66:1146–1154. doi: 10.1016/j.humimm.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 38.Varma MR, Varga AJ, Knipp BS, Sukheepod P, Upchurch GR, Kunkel SL, Wakefield TW, Henke PK. Neutropenia impairs venous thrombosis resolution in the rat. J Vasc Surg. 2003;38:1090–1098. doi: 10.1016/s0741-5214(03)00431-2. [DOI] [PubMed] [Google Scholar]
- 39.Henke PK, Varga A, De S, Deatrick CB, Eliason J, Arenberg DA, Sukheepod P, Thanaporn P, Kunkel SL, Upchurch GR, Jr., Wakefield TW. Deep vein thrombosis resolution is modulated by monocyte cxcr2-mediated activity in a mouse model. Arterioscler Thromb Vasc Biol. 2004;24:1130–1137. doi: 10.1161/01.ATV.0000129537.72553.73. [DOI] [PubMed] [Google Scholar]
- 40.Moir E, Robbie LA, Bennett B, Booth NA. Polymorphonuclear leucocytes have two opposing roles in fibrinolysis. Thromb Haemost. 2002;87:1006–1010. [PubMed] [Google Scholar]
- 41.Pillay J, Ramakers BP, Kamp VM, Loi AL, Lam SW, Hietbrink F, Leenen LP, Tool AT, Pickkers P, Koenderman L. Functional heterogeneity and differential priming of circulating neutrophils in human experimental endotoxemia. J Leukoc Biol. 2010;88:211–220. doi: 10.1189/jlb.1209793. [DOI] [PubMed] [Google Scholar]
- 42.Turitto VT, Weiss HJ. Red blood cells: Their dual role in thrombus formation. Science. 1980;207:541–543. doi: 10.1126/science.7352265. [DOI] [PubMed] [Google Scholar]
- 43.Woollard KJ, Sturgeon S, Chin-Dusting JP, Salem HH, Jackson SP. Erythrocyte hemolysis and hemoglobin oxidation promote ferric chloride-induced vascular injury. J Biol Chem. 2009;284:13110–13118. doi: 10.1074/jbc.M809095200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tracz MJ, Juncos JP, Grande JP, Croatt AJ, Ackerman AW, Katusic ZS, Nath KA. Induction of heme oxygenase-1 is a beneficial response in a murine model of venous thrombosis. Am J Pathol. 2008;173:1882–1890. doi: 10.2353/ajpath.2008.080556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mustafa S, Weltermann A, Fritsche R, Marsik C, Wagner O, Kyrle PA, Eichinger S. Genetic variation in heme oxygenase 1 (hmox1) and the risk of recurrent venous thromboembolism. J Vasc Surg. 2008;47:566–570. doi: 10.1016/j.jvs.2007.09.060. [DOI] [PubMed] [Google Scholar]
- 46.Orbell JH, Smith A, Burnand KG, Waltham M. Imaging of deep vein thrombosis. Br J Surg. 2008;95:137–146. doi: 10.1002/bjs.6077. [DOI] [PubMed] [Google Scholar]
- 47.Moody AR. Direct imaging of deep-vein thrombosis with magnetic resonance imaging. Lancet. 1997;350:1073. doi: 10.1016/s0140-6736(97)24041-9. [DOI] [PubMed] [Google Scholar]
- 48.Blume U, Orbell J, Waltham M, Smith A, Razavi R, Schaeffter T. 3d t(1)-mapping for the characterization of deep vein thrombosis. MAGMA. 2009;22:375–383. doi: 10.1007/s10334-009-0189-8. [DOI] [PubMed] [Google Scholar]
- 49.Nosaka M, Ishida Y, Kimura A, Kondo T. Time-dependent organic changes of intravenous thrombi in stasis-induced deep vein thrombosis model and its application to thrombus age determination. Forensic Sci Int. 2009;195:143–147. doi: 10.1016/j.forsciint.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 50.Henke PK, Varma MR, Deatrick KB, Dewyer NA, Lynch EM, Moore AJ, Dubay DA, Sukheepod P, Pearce CG, Upchurch GR, Jr., Kunkel SL, Franz MG, Wakefield TW. Neutrophils modulate post-thrombotic vein wall remodeling but not thrombus neovascularization. Thromb Haemost. 2006;95:272–281. doi: 10.1160/TH05-02-0099. [DOI] [PubMed] [Google Scholar]
- 51.Singh I, Burnand KG, Collins M, Luttun A, Collen D, Boelhouwer B, Smith A. Failure of thrombus to resolve in urokinase-type plasminogen activator gene-knockout mice: Rescue by normal bone marrow-derived cells. Circulation. 2003;107:869–875. doi: 10.1161/01.cir.0000050149.22928.39. [DOI] [PubMed] [Google Scholar]
- 52.Henke PK, Pearce CG, Moaveni DM, Moore AJ, Lynch EM, Longo C, Varma M, Dewyer NA, Deatrick KB, Upchurch GR, Jr., Wakefield TW, Hogaboam C, Kunkel SL. Targeted deletion of ccr2 impairs deep vein thombosis resolution in a mouse model. J Immunol. 2006;177:3388–3397. doi: 10.4049/jimmunol.177.5.3388. [DOI] [PubMed] [Google Scholar]
- 53.Ali T, Humphries J, Burnand K, Sawyer B, Bursill C, Channon K, Greaves D, Rollins B, Charo IF, Smith A. Monocyte recruitment in venous thrombus resolution. J Vasc Surg. 2006;43:601–608. doi: 10.1016/j.jvs.2005.10.073. [DOI] [PubMed] [Google Scholar]
- 54.Henke PK, Wakefield TW, Kadell AM, Linn MJ, Varma MR, Sarkar M, Hawley A, Fowlkes JB, Strieter RM. Interleukin-8 administration enhances venous thrombosis resolution in a rat model. J Surg Res. 2001;99:84–91. doi: 10.1006/jsre.2001.6122. [DOI] [PubMed] [Google Scholar]
- 55.Humphries J, McGuinness CL, Smith A, Waltham M, Poston R, Burnand KG. Monocyte chemotactic protein-1 (mcp-1) accelerates the organization and resolution of venous thrombi. J Vasc Surg. 1999;30:894–899. doi: 10.1016/s0741-5214(99)70014-5. [DOI] [PubMed] [Google Scholar]
- 56.Humphries J, Gossage JA, Modarai B, Burnand KG, Sisson TH, Murdoch C, Smith A. Monocyte urokinase-type plasminogen activator up-regulation reduces thrombus size in a model of venous thrombosis. J Vasc Surg. 2009;50:1127–1134. doi: 10.1016/j.jvs.2009.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Martin P, D'Souza D, Martin J, Grose R, Cooper L, Maki R, McKercher SR. Wound healing in the pu.1 null mouse--tissue repair is not dependent on inflammatory cells. Curr Biol. 2003;13:1122–1128. doi: 10.1016/s0960-9822(03)00396-8. [DOI] [PubMed] [Google Scholar]
- 58.Soo KS, Northeast AD, Happerfield LC, Burnand KG, Bobrow LG. Tissue plasminogen activator production by monocytes in venous thrombolysis. J Pathol. 1996;178:190–194. doi: 10.1002/(SICI)1096-9896(199602)178:2<190::AID-PATH454>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 59.Northeast AD, Soo KS, Bobrow LG, Gaffney PJ, Burnand KG. The tissue plasminogen activator and urokinase response in vivo during natural resolution of venous thrombus. J Vasc Surg. 1995;22:573–579. doi: 10.1016/s0741-5214(95)70041-2. [DOI] [PubMed] [Google Scholar]
- 60.Nosaka M, Ishida Y, Kimura A, Kondo T. Immunohistochemical detection of mmp-2 and mmp-9 in a stasis-induced deep vein thrombosis model and its application to thrombus age estimation. Int J Legal Med. 2010;124:439–444. doi: 10.1007/s00414-010-0484-y. [DOI] [PubMed] [Google Scholar]
- 61.Sood V, Luke CE, Deatrick KB, Baldwin J, Miller EM, Elfline M, Upchurch GR, Jr., Wakefield TW, Henke PK. Urokinase plasminogen activator independent early experimental thrombus resolution: Mmp2 as an alternative mechanism. Thromb Haemost. 2010;104:1174–1183. doi: 10.1160/TH10-03-0184. [DOI] [PubMed] [Google Scholar]
- 62.Deatrick KB, Elfline M, Baker N, Luke CE, Blackburn S, Stabler C, Wakefield TW, Henke PK. Postthrombotic vein wall remodeling: Preliminary observations. J Vasc Surg. 2010 doi: 10.1016/j.jvs.2010.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stirling GA, Tsapogas MJ, Girolami PL. Organization of thrombi. Br J Surg. 1966;53:232–235. doi: 10.1002/bjs.1800530318. [DOI] [PubMed] [Google Scholar]
- 64.Modarai B, Humphries J, Burnand KG, Gossage JA, Waltham M, Wadoodi A, Kanaganayagam GS, Afuwape A, Paleolog E, Smith A. Adenovirus-mediated vegf gene therapy enhances venous thrombus recanalization and resolution. Arterioscler Thromb Vasc Biol. 2008;28:1753–1759. doi: 10.1161/ATVBAHA.108.170571. [DOI] [PubMed] [Google Scholar]
- 65.Waltham M, Burnand K, Fenske C, Modarai B, Humphries J, Smith A. Vascular endothelial growth factor naked DNA gene transfer enhances thrombus recanalization and resolution. J Vasc Surg. 2005;42:1183–1189. doi: 10.1016/j.jvs.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 66.Waltham M, Burnand KG, Collins M, McGuinness CL, Singh I, Smith A. Vascular endothelial growth factor enhances venous thrombus recanalisation and organisation. Thromb Haemost. 2003;89:169–176. [PubMed] [Google Scholar]
- 67.Evans CE, Humphries J, Mattock K, Waltham M, Wadoodi A, Saha P, Modarai B, Maxwell PJ, Smith A. Hypoxia and upregulation of hypoxia-inducible factor 1{alpha} stimulate venous thrombus recanalization. Arterioscler Thromb Vasc Biol. 2010;30:2443–2451. doi: 10.1161/ATVBAHA.110.215038. [DOI] [PubMed] [Google Scholar]
- 68.Fantin A, Vieira JM, Gestri G, Denti L, Schwarz Q, Prykhozhij S, Peri F, Wilson SW, Ruhrberg C. Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of vegf-mediated endothelial tip cell induction. Blood. 2010;116:829–840. doi: 10.1182/blood-2009-12-257832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gossage JA, Humphries J, Modarai B, Burnand KG, Smith A. Adenoviral urokinase-type plasminogen activator (upa) gene transfer enhances venous thrombus resolution. J Vasc Surg. 2006;44:1085–1090. doi: 10.1016/j.jvs.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 70.Flick MJ, LaJeunesse CM, Talmage KE, Witte DP, Palumbo JS, Pinkerton MD, Thornton S, Degen JL. Fibrin(ogen) exacerbates inflammatory joint disease through a mechanism linked to the integrin alphambeta2 binding motif. J Clin Invest. 2007;117:3224–3235. doi: 10.1172/JCI30134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Szaba FM, Smiley ST. Roles for thrombin and fibrin(ogen) in cytokine/chemokine production and macrophage adhesion in vivo. Blood. 2002;99:1053–1059. doi: 10.1182/blood.v99.3.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Flick MJ, Du X, Degen JL. Fibrin(ogen)-alpha m beta 2 interactions regulate leukocyte function and innate immunity in vivo. Exp Biol Med (Maywood) 2004;229:1105–1110. doi: 10.1177/153537020422901104. [DOI] [PubMed] [Google Scholar]
- 73.Smiley ST, King JA, Hancock WW. Fibrinogen stimulates macrophage chemokine secretion through toll-like receptor 4. J Immunol. 2001;167:2887–2894. doi: 10.4049/jimmunol.167.5.2887. [DOI] [PubMed] [Google Scholar]
- 74.Henke PK, Mitsuya M, Luke CE, Elfline MA, Baldwin JF, Deatrick KB, Diaz JA, Sood V, Upchurch GR, Wakefield TW, Hogaboam C, Kunkel SL. Toll-like receptor 9 signaling is critical for early experimental deep vein thrombosis resolution. Arterioscler Thromb Vasc Biol. 2010 doi: 10.1161/ATVBAHA.110.216317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Modarai B, Burnand KG, Sawyer B, Smith A. Endothelial progenitor cells are recruited into resolving venous thrombi. Circulation. 2005;111:2645–2653. doi: 10.1161/CIRCULATIONAHA.104.492678. [DOI] [PubMed] [Google Scholar]
- 76.Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, Figueiredo JL, Kohler RH, Chudnovskiy A, Waterman P, Aikawa E, Mempel TR, Libby P, Weissleder R, Pittet MJ. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612–616. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chorro L, Geissmann F. Development and homeostasis of ‘resident’ myeloid cells: The case of the langerhans cell. Trends Immunol. 2010;31:438–445. doi: 10.1016/j.it.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 78.Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656–661. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chorro L, Sarde A, Li M, Woollard KJ, Chambon P, Malissen B, Kissenpfennig A, Barbaroux JB, Groves R, Geissmann F. Langerhans cell (lc) proliferation mediates neonatal development, homeostasis, and inflammation-associated expansion of the epidermal lc network. J Exp Med. 2009;206:3089–3100. doi: 10.1084/jem.20091586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pollard JW. Trophic macrophages in development and disease. Nat Rev Immunol. 2009;9:259–270. doi: 10.1038/nri2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Leor J, Rozen L, Zuloff-Shani A, Feinberg MS, Amsalem Y, Barbash IM, Kachel E, Holbova R, Mardor Y, Daniels D, Ocherashvilli A, Orenstein A, Danon D. Ex vivo activated human macrophages improve healing, remodeling, and function of the infarcted heart. Circulation. 2006;114:I94–100. doi: 10.1161/CIRCULATIONAHA.105.000331. [DOI] [PubMed] [Google Scholar]
- 83.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 84.Auffray C, Sieweke MH, Geissmann F. Blood monocytes: Development, heterogeneity, and relationship with dendritic cells. Annu Rev Immunol. 2009;27:669–692. doi: 10.1146/annurev.immunol.021908.132557. [DOI] [PubMed] [Google Scholar]
- 85.Serbina NV, Jia T, Hohl TM, Pamer EG. Monocyte-mediated defense against microbial pathogens. Annu Rev Immunol. 2008;26:421–452. doi: 10.1146/annurev.immunol.26.021607.090326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shechter R, London A, Varol C, Raposo C, Cusimano M, Yovel G, Rolls A, Mack M, Pluchino S, Martino G, Jung S, Schwartz M. Infiltrating blood-derived macrophages are vital cells playing an anti-inflammatory role in recovery from spinal cord injury in mice. PLoS Med. 2009;6:e1000113. doi: 10.1371/journal.pmed.1000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Murdoch C, Muthana M, Coffelt SB, Lewis CE. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer. 2008;8:618–631. doi: 10.1038/nrc2444. [DOI] [PubMed] [Google Scholar]
- 88.Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor ccr2. Nat Immunol. 2006;7:311–317. doi: 10.1038/ni1309. [DOI] [PubMed] [Google Scholar]
- 89.Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S, Sarnacki S, Cumano A, Lauvau G, Geissmann F. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007;317:666–670. doi: 10.1126/science.1142883. [DOI] [PubMed] [Google Scholar]
- 90.Cros J, Cagnard N, Woollard K, Patey N, Zhang SY, Senechal B, Puel A, Biswas SK, Moshous D, Picard C, Jais JP, D'Cruz D, Casanova JL, Trouillet C, Geissmann F. Human cd14dim monocytes patrol and sense nucleic acids and viruses via tlr7 and tlr8 receptors. Immunity. 2010;33:375–386. doi: 10.1016/j.immuni.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.van Furth R, Cohn ZA. The origin and kinetics of mononuclear phagocytes. J Exp Med. 1968;128:415–435. doi: 10.1084/jem.128.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, Libby P, Weissleder R, Pittet MJ. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007;204:3037–3047. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Arnold L, Henry A, Poron F, Baba-Amer Y, van Rooijen N, Plonquet A, Gherardi RK, Chazaud B. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med. 2007;204:1057–1069. doi: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Saha P, Modarai B, Humphries J, Mattock K, Waltham M, Burnand KG, Smith A. The monocyte/macrophage as a therapeutic target in atherosclerosis. Curr Opin Pharmacol. 2009;9:109–118. doi: 10.1016/j.coph.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 95.Woollard KJ, Geissmann F. Monocytes in atherosclerosis: Subsets and functions. Nat Rev Cardiol. 2010;7:77–86. doi: 10.1038/nrcardio.2009.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]