Abstract
While many laboratory and field studies show that zooplankton are negatively affected when exposed to high intensities of ultraviolet radiation (UVR), most studies also indicate that zooplankton are well adapted to cope with large variations in their UVR exposure in the pelagic zone of lakes. The response mechanisms of zooplankton are diverse and efficient and may explain the success and richness of freshwater zooplankton in optically variable waters. While no single behavioural or physiological protection mechanism seems to be superior, and while several unexplained and contradictory patterns exist in zooplankton UVR ecology, recent increases in our understanding are consistent with UVR playing an important role for zooplankton. This review examines the variability in freshwater zooplankton responses to UVR, with a focus on crustacean zooplankton (Cladocera and Copepoda). We present an overview of UVR-induced damages, and the protection and recovery mechanisms freshwater zooplankton use when exposed to UVR. We review the current knowledge of UVR impact on freshwater zooplankton at species and community levels, and discuss briefly how global change over the last three decades has influenced the UVR milieu in lakes.
Keywords: UV radiation: zooplankton, lakes, damage, protection, recovery
Introduction
Solar ultraviolet radiation (UVR) has many deleterious effects on aquatic biota as a result of its highly energetic short wavelengths. UVR is the shortest wavelength band reaching the Earth’s surface. It is arbitrarily divided into two wavebands: wavelengths from 280 nm to 320 nm are referred as UV-B and those from 320 nm to 400 nm are referred to as UV-A. In most atmospheric situations, the UV-B range of the spectrum accounts for 0.1 %, UV-A 6 %, and the visible portion (400 nm to 700 nm) 50 % of the global radiation.
Zooplankton are present in the water column of most lakes throughout the world. In most lakes they occupy the intermediate trophic position in the food web, channelling resources from primary producers and heterotrophic microorganisms to invertebrate predators and fish. Apart from this very general classification, the role and life of zooplankton can be very different in diverse lakes. Zooplankton inhabit lakes with varying temperature, depth, colour, and composition of other species. These environmental characteristics contribute to defining the exposure and the protective response of zooplankton to UVR. A mid-latitude Daphnia in a 20 m deep brown water lake may face only negligible levels of UVR (Kirk, 1994), while another Daphnia, in a shallow clear water tundra pond, may be exposed to nearly continuous UVR for all of its life during polar summer (Rautio & Korhola, 2002a). The habitat also defines which UVR protection strategies are available. Accumulation of red carotenoids or synthesis of black melanin are efficient and safe protection mechanisms in lakes where visually hunting fish are absent, while vertical migration and other means of less visible protection are more likely in the presence of fish (Hansson, 2000; Hylander et al., 2009a; Williamson & Rose, 2009). Hence, organisms are adapted to their habitat and cope with UVR differently which results in great species-specific variability in UVR protection (Zagarese et al., 1997a; Tartarotti et al., 1999; Leech & Williamson, 2001; Hylander & Hansson, 2010). Recently, the adaptations and abilities of species to respond to UVR have been challenged with increases in UVR that are beyond the natural range in the animal’s habitat (ACIA, 2005). These increases have occurred mainly at high latitudes where the depletion of ozone in the stratosphere has caused greater intensities of UVR to reach the ground level.
Initially, the concern about UVR effects on aquatic ecosystems was directed particularly at Antarctic waters. The impact of the spring ozone hole on primary production and food chain processes in the marginal ice zone was predicted to range from negligible to catastrophic (Roberts, 1989). The first studies on the responses of marine and freshwater plankton to UVR dealt mainly with the survival of individual species and changes in photosynthesis (Siebeck, 1978; Lorenzen, 1979). These were soon followed by studies on different UVR protection strategies (e.g. Hebert & Emery, 1990; Sommaruga & Garcia-Pichel, 1999). During the last decade, the focus has shifted from individual species’ responses to ecosystem responses and changes in food webs and trophic cascades (Vinebrooke & Leavitt, 1998; Williamson et al., 1999; Wulff et al., 2000; Hylander & Hansson, 2010). Several studies have considered other global phenomena such as climate warming and acidification that can have a greater influence on the water column UVR regime (Schindler et al., 1996; Sommaruga et al., 1999; Williamson et al., 1999).
Research on solar ultraviolet radiation, and its ecological effects on zooplankton has expanded from few studies in the 1970s and earlier to several published papers per year in the first decade of the 21st century. We have chosen to include in this review papers that allow us to present a thorough overview of what is presently known about the responses of freshwater zooplankton to UV radiation and what is still unknown. Our aim is to give a holistic overview of the information published in research papers and reviews, which have either concentrated on a specific geographical region or on one or few aspects in UVR ecology, respectively. Most papers in this review relate to western alpine regions (reviewed by Zagarese et al., 2000; Sommaruga, 2001; Vinebrooke & Leavitt, 2005) and the Arctic (reviewed by Hessen, 2001; Perin & Lean, 2004) reflecting the geographical distribution of published UVR literature. Strict regulations on conduct of research and the lack of crustacean zooplankton in most lakes in the Antarctic (Rautio et al., 2008) reduce the UVR studies in this vast area that has been most influenced by ozone depletion and increases in UVR (but see Rocco et al., 2002). We address firstly the factors that define the geographical variability in the exposure of zooplankton to UVR, including the effects of climate change. We then review how to measure UVR-induced damage and what factors influence the damage. This is followed by the examination of types of protection and recovery mechanisms that are activated when freshwater zooplankton are exposed to UVR. Finally, we discuss UVR-induced changes at community level and comment on the state of understanding the ecology of UVR.
Geographical variability in freshwater zooplankton’s exposure to UVR
The exposure of zooplankton to UVR varies largely depending on the lake that they inhabit. Alpine lakes (e.g. Alps, Andes, Himalayas, Rockies), with the exception of those fed by turbid glacier streams, are among the most UVR-transparent aquatic ecosystems in the world, with diffuse attenuation coefficients (Kd) similar to those found in ice-covered Antarctic lakes (Morris et al., 1995; Sommaruga, 2001). At the other end of the range are UVR-protected humic-rich lakes on wetlands (Arts et al., 2000), while the rate of change in UVR attenuation is greatest in arctic lakes that are influenced by global change. The variability in lake optics is determined by lake location, catchment soil type, and the exposure of the lake to global change (changes in ozone layer, precipitation). The importance of these factors is briefly presented below, while for more extensive reading, reviews on factors affecting surface UVR and biological UVR exposure in aquatic ecosystems are recommended (e.g. Weatherhead et al., 2005; Vincent et al. 2007).
Zenith angles and altitude
There is a large latitudinal variation in ground level UVR irradiances (Fig. 1). Most of this variation is caused by seasonal variation in the solar zenith. Zenith angles are small in low-latitude regions leading to UV irradiances that are higher than those at higher latitudes. However, when daily integrated doses are compared, the length of polar summer days at high latitudes compensates for the effect of large solar zenith angles and the daily UVR doses approach those at the equator. UVR fluxes increase naturally with altitude, and have been measured to increase by 19 % per 1000 m in the Alps for the short wavelength UV-B radiation (Blumthaler et al., 1992). In a lake situated at 2000 m above sea level this can account for a substantial increase in the UVR exposure (Fig. 1). Small zenith angles and high elevation account for a great UVR exposure in mountain lakes at mid and low latitudes and zooplankton in these lakes are often observed to react strongly to UVR.
Fig. 1.
UVR in the water column is defined by lake location (latitude and altitude) and water column transparency (Kd = attenuation coefficient). The figure shows the surface and 1 m depth daily theoretical erythemal UV radiation dose (kJ m−2) for lakes in a) Great Plaines at 50 °N and 0 m a.s.l. with average lake KdUVB = 20 m−1 (30 lakes), b) Tyrolian Alps at 50 °N and 2000 m a.s.l. with average lake KdUVB = 0.56 m−1 (11 lakes), c) Northern Finland at 70 °N and 500 m a.s.l. with average lake KdUVB = 2 m−1 (12 lakes), and d) Ellesmere Island at 80 °N and 0 m a.s.l. with average lake KdUVB = 0.61 m−1 (3 lakes). Ground level daily dose data from ACIA (2005), lake Kd data from Laurion et al. (2000), Arts et al. (2000), and Rautio, unpublished. 1 m UV radiation has been calculated using the equation Ed(z) = Ed(0)e−Kdz (Kirk, 1994), and assuming a 19 % increase in UVR per 1000 m (Blumthaler et al., 1992).
Impact of the ozone layer and warmer climate
High latitude areas are most affected by ozone depletion and, therefore, experience the greatest increase in UVR, compared to the reference year 1979. The long-term trends in total column ozone obtained from satellite and ground-based data both indicate a latitudinal variation in the change of ozone concentration, with almost no change over the equator and substantial declines outside the 35 °S to 35 °N zone (ACIA, 2005). Although compounds such as chlorofluorocarbons and halons, which break down ozone and hence decrease the UVR absorption in the stratosphere, are mainly released from mid-latitudes, a combination of extreme cold and stratospheric circulation (the polar vortex) above the poles results in conditions that are favourable for ozone destruction (Anderson et al., 1991). In recent years, ozone loss rates in the Arctic region have reached values comparable to those recorded over the Antarctic although spring ozone levels over the Arctic often remain higher than those over the Antarctic (ACIA, 2005). Lower latitude zooplankton are not influenced by stratospheric ozone depletion in the same way as polar zooplankton, apart from some places in South America where evidence for Antarctic ozone ‘hole’ perturbation has been observed as far north as at 30 °S (Kirchhoff et al., 1996).
The increases in UVR irradiances occur primarily in spring, when ozone depletion reaches a maximum, and can result in spring UVR levels that are higher than those measured during the summer (ACIA, 2005). In the Scandinavian subarctic region, springtime irradiances of UV-B radiation reaching the surface of the Earth increased by 10–20 % between the late 1970s and 1995 (International Arctic Science Committee, 1995). Between 1979 and 2000, the trend in mean annual total column ozone over the Arctic was about −3 % per decade, while the trend in mean spring total column ozone was about −5 % per decade (ACIA, 2005). The observed changes in ozone depletion have not been symmetric around the North Pole. The greatest changes in ozone levels have been observed in the area between Siberia and Scandinavia where the climate is more continental, thus enhancing the formation of cold stratospheric clouds that promote ozone (O3) destruction.
Most polar lakes are still frozen at the time of elevated spring UVR irradiances and the zooplankton are therefore protected from solar damage. However, increases in CO2 also account for warmer temperatures and a shorter period of ice cover (Livingstone, 1997; Rouse et al., 1997). A simulation study by Elo et al. (1998) suggested that doubling of CO2 will lead to a 1–2 month earlier melting of the ice cover in Finnish lakes. Consequently, ice break-up in Finnish subarctic lakes would occur in May instead of late June, exposing the lake biota to the most intensive period of UVR. Historical observations of ice break-up times, including northern Lapland, and their comparison to present day measurements show that spring melting is occurring earlier (Magnuson et al., 2000). Alpine (Sommaruga-Wögrath et al., 1997) and Antarctic Peninsula regions (Quayle et al., 2002) are also being influenced by climate change-induced earlier melting of the ice, and subsequent increase in the underwater UVR exposure. Increased UV irradiance resulting from a combination of climatic warming and ozone depletion is likely to be lethal for zooplankton that are not able to adjust their protection against UVR.
Coloured dissolved organic matter (CDOM) and UV attenuation in water
The UVR regime in the water column differs from that reaching the ground. The penetration of UVR wavelengths into water is highly dependent on the colour of the water, which is defined by the amount of coloured dissolved organic matter (CDOM) in water and is estimated from the concentration of dissolved organic carbon (DOC). Typically, half of lake water DOC is composed of coloured compounds and DOC can therefore be used to predict UVR transparency, except in the lowest CDOM environments such as in some alpine lakes (Laurion et al., 1997; Sommaruga, 2001; Sommaruga & Augustin, 2006) where particles may also play a significant role in UVR attenuation, and in ‘white DOC’ lakes such as in Greenland where highly UVR-transparent lakes may have very high concentrations of DOC (> 100 mgC L−1) (Anderson & Stedmon, 2007). The clearest lakes (< 2 mgC L−1) are usually found above the tree line (Morris et al., 1995; Vincent & Pienitz, 1996; Rautio & Korhola, 2002a) with UV-B penetration to several metres depth (Schindler et al., 1996), while some humic lakes can have DOC concentration > 30 mgC L−1 and the UVR is absorbed within the first few centimetres (Kirk, 1994) (Fig. 1). The darkest lakes can be considered well-protected from UVR, however UV-irradiated DOC may promote the formation of reactive oxygen species (ROS) that in turn are harmful to toxic to organisms including zooplankton (Souza et al., 2007). Along with changes in DOC concentrations, there may also occur significant qualitative changes in DOM composition (McKnight et al., 2001), which is reflected in the optical properties of this material. CDOM above the tree line has a bigger component of autochthonous compounds which absorb short wavelength radiation less than allochthonous carbon (McKnight et al., 1994).
Many lakes, especially northern lakes that have had a stable low DOC concentration and hence have been exposed to UVR in the same manner for decades and even centuries, are now changing in their optical properties due to climate change-induced melting of permafrost soils and the subsequent release of DOC into the downstream receiving waters (Rosén et al., 2009). This process will release the biota from UVR stress. The magnitude of new DOC pulses to the lakes is dependent on temperature but also on precipitation which varies regionally. In some areas, e.g. in Svalbard (80 °N, 20 °E), there has been a significant rise in precipitation from the 1960s to the 1990s (Hanssen-Bauer & Forland, 1998), however, other regions have shown little change or decreases in precipitation (Vincent et al., 2001). With decreasing precipitation the runoff and hence the amount of UV-absorbing organic material into the Canadian water bodies has decreased, making the water clearer and consequently more exposed to UVR (Schindler et al., 1996).
The intensity of UVR in water is also related to anthropogenic acidification (Schindler et al., 1996; Williamson & Rose, 2009). Acidification increases transparency as a result of coagulation and removal of DOC by monomeric aluminium as well as by reduced productivity (Effler et al., 1985).
UVR impacts on zooplankton
UV radiation is likely to cause a variety of direct and indirect negative effects on zooplankton populations. The majority of studies concerning direct UV-induced damage on zooplankton have reported increased mortality rates (reviewed by Zagarese et al., 2003). In addition, there is a growing amount of literature on other negative UVR effects, including DNA damage (Malloy et al., 1997), reduced growth rates (de Lange et al., 1999), decreased fecundity (Williamson et al., 1994; Zellmer, 1996 & 1998; Huebner et al., 2006), increased UVR susceptibility in the presence of other environmental stressors (Hessen & Alstad Rukke, 2000; Cooke et al., 2006), and gut damage in zooplankton when fed with UVR pre-exposed algae (Zellmer et al., 2004). How and at what rate UVR influences zooplankton, however, varies greatly depending on the species in question, its physiological status, the environment it inhabits, and on its capability for UVR protection and recovery from damage.
Wavelength defines response
The negative effects of UVR on zooplankton, similar to all living organisms, are associated with the highly energetic short wavelengths. These UV wavelengths have low intensity at ground level but they can still cause the greatest biological damage due to the great energy content per photon (Frederik et al., 1989). The absorption maximum of DNA near 260 nm makes UV-B biologically the most dangerous waveband (Caldwell, 1979). However, the separation of solar radiation into UV-B and UV-A wavelengths is arbitrary, and full wavelength-dependent response curves need to be determined.
The effect or weight of a certain wavelength in solar damage can be quantified by biological weighting functions (BWF). BWFs are used to estimate the effects of different wavelengths on organisms by generating a function that provides a weighting coefficient for each individual wavelength (Neale & Kieber, 2000; Williamson et al., 2001a). Depending on the morphology of an organism, and on the presence or absence of photo-protective pigments, different BWFs may result (Tartarotti et al., 2000). Pigmented and non-pigmented clones of the same zooplankton species are different in their UVR susceptibility (Hessen, 1996) which affects the relationship between the UVR dose and, for example, mortality. Furthermore, zooplankton may not respond in a similar way to a UVR dose that is received in a single sunny day as to a dose that is received over a period of several cloudy days (law of reciprocity).
The existing zooplankton BWFs generally indicate the same result: the shortest wavelength UV-B radiation is in the order of a thousand times more damaging than is the longest wavelength UV-A (Kouwenberg et al., 1999; Tartarotti et al., 2000; Williamson et al., 2001a). Williamson et al. (2001a) showed that the most biologically damaging radiation for Daphnia pulicaria was in the 305 nm to 322 nm range. The contribution of UV-B to the mortality of D. pulicaria was 64 % while UV-A contributed 36 %. Other studies have also demonstrated that while UV-B causes the greatest damage, UV-A also induces mortality in zooplankton, including both copepods and cladocerans (Zellmer, 1998; Tartarotti et al., 2000; Rautio & Korhola, 2002a). The shape of the BWF curve of zooplankton closely resembles the action spectra for UV-induced erythema in human skin with the highest damage in the shortest UV-B wavelengths but some damage also occurring in the UV-A range (Tartarotti et al., 2000; Williamson et al., 2001a) (Fig. 2).
Fig. 2.

Biological weighting function for the mortality of the copepod Boeckella gracilipes (open circles) and action spectra for erythema in human skin (continuous line). Redrawn from Tartarotti et al. (2000).
Defining BWFs for zooplankton is laborious; hence, they have been used in only a small number of studies. Most studies have instead used a broad band (e.g. full sunlight, UV-B, UV-A) or single wavelength response of zooplankton to natural solar or artificial radiation sources. One of the first such studies is from Siebeck (1978) who demonstrated that Daphnia pulex, D. galeata and D. longispina survival increased when UVR was blocked out with a glass filter. More than 20 years later, Rautio & Korhola (2002a) performed a similar experiment with D. pulex, D. longispina and the copepod Eudiaptomus graciloides using four filters that block solar radiation differently. The three zooplankton species showed a similar ranking of response. Mortality of each species was highest in the full sunlight treatment and decreased with declining UV intensity, while not only the presence of UV-B but also UV-A resulted in high and rapid mortality. Similarly, Zagarese et al. (1994) showed how the intensity of UVR affects zooplankton survival. Significant in situ mortality of Daphnia was observed after two days of exposure at and above 73 % of surface radiation at 320 nm but not at 63 %.
Variability in damage
The vast number of studies conducted on zooplankton UVR tolerance clearly demonstrates that different species (Siebeck & Böhm, 1994; Hurtubise et al., 1998; Tartarotti et al., 1999; Leech & Williamson, 2000; Rautio & Korhola, 2002a), species from the same genus (Zagarese et al., 1997a; Rocco et al., 2002), or even the same species but during different seasons or from different lakes (Stutzman, 1999; Tartarotti et al., 1999), respond differently to UVR. This makes defining accurate UVR tolerance limits difficult if not impossible. Some general conclusions can, however, be drawn.
Most studies that have included age as a variable in UVR response have shown that adults tolerate UVR better than juvenile stages (Leech & Williamson, 2000; Vega & Pizarro, 2000; Ramos-Jiliberto et al., 2004; Huebner et al., 2006). One of the few documented exceptions from this pattern is the higher adult mortality in the rotifer Asplanchna girodi in comparison to its juveniles (Grad et al., 2003). Using four species of copepods (Diaptomus minutus, D. spatulocrenatus, Mesocyclops edax and Cyclops scutifer), Leech & Williamson (2000) showed that adults had up to 34 % higher LD50 UVR values than nauplii. Huebner et al. (2006) reported a similar age-dependent survival in Daphnia magna: after 12 h of UV-B exposure nearly 80 % of the oldest (4 days old) individuals were alive whereas less than 10 % of the 2 day olds survived and none of the one day olds did. Similarly, Ramos-Jiliberto et al. (2004) showed that Ceriodaphnia dubia less than 48 h of age were significantly more sensitive to UVR than adults of the same species. Most likely due to their greater sensitivity to UVR, photo-protective defences are also strongest among the young. Very high concentrations of UV-absorbing compounds (mycosporine-like amino acids, MAAs) have been measured in C. abyssorum tatricus populations from a UV-transparent high mountain lake, with eggs, nauplii, and young copepodid life stages having the highest contents (Tartarotti et al., 2001; Tartarotti & Sommaruga, 2006). Similar to MAAs, ontogenetic differences were found between nauplii and adult life stages of copepods, the former containing 3–10 times higher carotenoid concentrations (Hairston, 1978).
Previous exposure to UVR, i.e. experience of and acclimation to UVR, is an important factor determining the tolerance of zooplankton to UVR (Siebeck, 1978; Stutzman, 1999; Rautio & Korhola, 2002a; Zellmer et al., 2004). Daphnia pulex obtusa from a transparent mountain pond tolerated higher radiation doses than D. galeata and D. longispina which inhabit darker lowland lakes (Siebeck, 1978). In another study, D. pulex that originated from a clear pond were more UV-tolerant than D. longispina from a moderately humic and D. longispina from a very humic pond (Zellmer et al., 2004). These two studies compared different species of Daphnia which could explain the different tolerances to UVR. Other evidence, however, suggests that the same pattern also occurs within one species. Stutzman (1999) showed that populations of Diaptomus minutus routinely experiencing high levels of UVR in their natural environment were more tolerant to UVR than those that routinely experience low levels of UVR.
The study of Stutzman (1999) demonstrated that the UVR tolerance of the same population varies seasonally. Because of the high spatial and temporal variability within one species, only results from one lake and from one time can be compared when ranking species-specific UVR tolerances (as in Leech & Williamson, 2000). Studies that have made comparisons between species from different lakes are still valuable but rather than providing data for accurate ranking (as the species are most likely to have different previous UVR acclimation and protection strategies) they show the tremendous plasticity of zooplankton to cope with UVR under different environmental scenarios. Results from a 3-day in situ incubation in ultra-oligotrophic Lake Toncek, Argentina, showed that Boeckella gracilipes was highly vulnerable to UV-B and UV-A radiation (Zagarese et al., 1997b). In contrast, in another clear lake, Laguna Negra in the Chilean Andes, B. gracilipes was much more UV-tolerant and showed mortality only when exposed for 70 h to UVR (Tartarotti et al., 1999). Other species from this same genus (B. gibbosa, B. antique, B. poppei) have been reported to be highly UV-tolerant (Zagarese et al., 1997a; Rocco et al., 2002). The following conclusion made by Rocco et al. (2002) can be modified to include all zooplankton species: plasticity in UVR tolerance has probably been key to zooplankton success at colonising a great variety of UV environments, ranging from large oligotrophic lakes to ephemeral pools, and from sea level to high-elevation habitats.
Interactive effects of UVR and other environmental variables also modify the response of zooplankton to UVR. Dissolved organic carbon is the most studied variable in association with UVR and its influence is most often positive, arising from the efficient absorbance of UVR. Rautio & Korhola (2002a) showed that zooplankton survival increased > 50 % when DOC doubled from 5 mgC L−1 to 11 mgC L−1. Zagarese et al. (1994) reported a similar reduction in mortality for Daphnia populations, as did Cooke et al. (2006) for the fecundity of the copepod Leptodiaptomus ashlandi when DOC increased (Fig. 3). Food stimulation is another positive effect from the DOC-UV interaction. UVR breaks larger macromolecules into smaller units which are then more readily usable for microorganisms that metabolise DOC. They in turn can be an important nutritional source for zooplankton (Salonen & Hammer, 1986; de Lange et al., 2003). However, UV-irradiated DOC also promotes the formation of ROS that are harmful to zooplankton (Souza et al., 2007). Although no measurements exist for ROS in the zooplankton body, it is generally accepted that zooplankton are exposed to UV-induced ROS, formed either in surface waters of high-DOC lakes (Cooper et al., 1994) or inside the animals’ bodies by interaction between sensitive molecules and photons. Activation of photo-reactivation mechanisms, such as increased activity of antioxidant enzymes and the presence of carotenoid pigments, are considered as indices for the presence of ROS (Hessen, 1994; Borgeraas & Hessen, 2002a).
Fig. 3.
(a) Mean number of nauplii produced per female Leptodiaptomus ashlandi and (b) mean proportion of surviving females in the gravid state in +UVR and −UVR treatments with low levels of dissolved organic matter (DOM) (1.0 mg L−1, white bars) and when DOM was added (10.3 mg L−1, black bars). From Cooke et al., 2006 .
Other environmental variables that have been studied in relation to zooplankton UVR tolerance include temperature, oxygen, nutrients, water hardness, and pH (Borgeraas & Hessen, 2000; Hessen & Alstad Rukke, 2000; Williamson et al., 2001a; Cooke et al., 2006). It has been suggested that UVR is a more important stressor to zooplankton at colder temperatures than at warmer temperatures because enzymatic processes like UVR repair mechanisms (e.g. DNA repair) and detoxification of ROS are slower at colder temperatures (Hessen, 1996; Buma et al., 2001). Some experimental evidence supports this: for example, at colder temperatures, in comparison with warmer temperatures, copepods produced fewer nauplii in the presence of UVR than when UVR was excluded (Cooke et al., 2006). Other studies, however, have shown a different pattern. Leptodiaptomus ashlandi adult survival was higher in the presence than in the absence of UVR in the cold treatment (Persaud & Williamson, 2005), and survival tests of Borgeraas & Hessen (2000) at different temperatures showed that reduced temperatures significantly increased Daphnia magna survival in the presence of UVR. Interactions involving temperature are complex, influencing not only UVR repair mechanisms but also life span, generation time, metabolic activity and indirect influence from the food web. The acclimation of organisms to a certain temperature range is also likely to be a key factor that determines how zooplankton will react to the combination of different UV-temperature interactions. Daphnia longispina survival in the +UVR treatment was highest when exposing them to temperatures between 12 °C and 17 °C corresponding to the temperature in their natural habitat. At 6 °C as well as at 20 °C survival was significantly reduced with the highest mortality at 20 °C (Zellmer, personal communication).
Zooplankton UVR tolerance has been reported to be almost unaffected by ambient oxygen (Borgeraas & Hessen, 2000) and nutrient concentrations (Cooke et al., 2006) despite their potential role in ROS stress and in food availability through stimulation of the phytoplankton community. Water hardness, however, may be a major determinant for zooplankton UVR susceptibility among calcium (Ca) demanding zooplankton (Hessen & Alstad Rukke, 2000). Survival of Daphnia magna and D. tenebrosa under UVR exposure was strongly reduced at low Ca concentrations commonly found in soft-water localities in Canadian Shield lakes and major parts of Scandinavia (Hessen & Alstad Rukke, 2000). Anthropologic acidification may also have many adverse effects on zooplankton UVR tolerance. DOC declines with lake acidification and consequently increases UVR penetration (Schindler et al., 1996; Williamson et al., 1996).
UVR damage may also occur via trophic interactions. UVR has been reported to have negative effects on phytoplankton, some species of which provide a source of food to zooplankton, hence alterations in food quantity and quality may suppress zooplankton survival, growth and reproduction (Hessen et al., 1997). Some of the negative algal responses to UVR may, however, be beneficial for zooplankton. Leu et al. (2006) showed that in order to compensate for UVR stress, the chlorophyte Selenastrum capricornutum synthesised more polyunsaturated fatty acids (PUFAs) which are essential for zooplankton growth. The algae also increased phosphorus uptake which resulted in more favourable C:P and N:P ratios for zooplankton. Despite these positive UV-induced changes in nutritional quality of S. capricornutum, however, no significant effects on zooplankton growth or reproduction were detected (Leu et al., 2006). Other studies have reported negative effects on zooplankton that have been feeding on UV-exposed algae. A decline in growth rate and fecundity in Daphnia sp. feeding on UV-irradiated algae has been observed by Scott et al. (1999) and de Lange & van Reeuwijk (2003). Huebner et al. (2006) recently showed that this decline carries across two Daphnia generations. Both phytoplankton quality and quantity influence zooplankton UVR tolerance (Schönberger & Zellmer, 1994; Zellmer, 1996; Zellmer et al., 2004). Increasing food levels improved Daphnia survival rates and UV-B pretreatment of the algae enhanced the negative effects in Daphnia (Zellmer et al., 2004) (Fig. 4).
Fig. 4.
Daphnia pulex a) survival in different UV exposures when fed with and without UVR-pretreated algae, b) proportion of gut that appeared green in color (0 = no green, 1 = entire gut is green), and c) prevalence of damaged intestine (% of live animals). From Zellmer et al. (2004). Copyright regents of the University of Colorado.
Protection mechanisms
Much of the variability in zooplankton responses to UVR is due to the extent the species or the population is able to use protection strategies against UVR (see Hansson & Hylander, 2009a for a review). Zooplankton have several defence mechanisms against UV damage including avoidance, screening, quenching and repair.
Physically moving away from damaging fluxes of UVR by undergoing vertical migration (VM) or staying deep in the water column during the day is a very effective strategy to minimise UVR exposure. Vertical migration of zooplankton is commonly explained as a strategy to avoid visual predators like fish (Zaret & Suffern, 1976; Stich & Lampert, 1981; Ringelberg, 1991); however, several authors have proposed that VM is a natural response of zooplankton to UVR (e.g. Damkaer, 1982; Hessen, 1993; 1994). Evidence of the important role of UVR in zooplankton vertical distribution was found in the migratory behaviour of zooplankton in habitats devoid of vertebrate predators (Hessen, 1993; Williamson et al., 2001b; Rautio et al., 2003; Aguilera et al., 2006; Kessler et al., 2008). Both laboratory and field experiments demonstrated that downward migration is a common UVR avoidance strategy in cladocerans like Daphnia (Storz & Paul, 1998; Leech & Williamson, 2001; Rhode et al., 2001; Leech et al., 2005a, b; Fischer et al., 2006; Hansson et al., 2007; Hansson & Hylander, 2009b). Active UVR avoidance, for example, was experimentally induced in several Daphnia clones (Hessen, 1994). UV-B radiation, however, is unlikely to be responsible for the deep vertical distribution during the day, as the main daytime distribution of most zooplankton is close to or below the 10 % attenuation depth of UV-B radiation (Zagarese et al., 1997b). Nevertheless, in transparent subalpine and alpine lakes with few or no fish, UVR has been suggested to be the primary driver in explaining the vertical distribution of zooplankton (Kessler et al., 2008). Those findings and others support the transparency-gradient hypothesis, indicating that UVR is more important than fish predation in determining the vertical distribution of zooplankton in clear lakes (Leech et al., 2005a; Kessler et al., 2008). Several studies have assessed zooplankton response to simultaneous UVR and predator threats (Ringelberg, 1999; Hansson, 2000; Leech & Williamson, 2001; Rautio et al., 2003; Hansson, 2004; Hansson, et al., 2007; Hylander et al., 2009a). A conflicting selective pressure for zooplankton vertical distribution, for instance, was found when exposing Daphnia simultaneously to the invertebrate predator Chaoborus and UVR (Boeing et al., 2004). Although Chaoborus itself does not respond to UVR (detection and/or avoidance) (Persaud et al., 2003), it modified the depth distribution of Daphnia relative to UVR (Boeing et al., 2004). A recent experimental field study by Hansson & Hylander (2009b) confirmed that UV was the major force behind the depth distribution of the zooplankton population (Daphnia), while there was no statistically significant effect of fish or interactions between fish and UVR. These responses, however, incur a physiological cost (Stich & Lampert, 1984; Dawidowicz & Loose, 1992) or increase the risk of predatory losses (Stich & Lampert, 1981; Gliwicz, 1986). Zooplankton may use PAR (photosynthetically active radiation) as a proxy for UVR to initiate downward migration, or they may detect changes directly in the UV irradiance. Photoreceptors specific to UV-B have not been described until now, but the cladoceran Daphnia magna has a photoreceptor with peak sensitivity in the UV-A wavelength range (348 nm) (Smith & Macagno, 1990). Visual UVR photosensitivity of the compound eye of Daphnia was already suggested in the early studies of Koehler (1924) and Merker (1930) as cited in Smith & Macagno (1990). Copepods, however, have often been reported to be less responsive to UVR (Leech & Williamson, 2001; Hansson, 2004; Leech et al., 2005a, b; Fischer et al., 2006), although avoidance of surface waters with higher UVR irradiance has been observed (Alonso et al., 2004). Recent studies show that copepods invest mainly in photo-protective pigmentation and less in VM when exposed to UVR (Hansson et al., 2007; Hylander et al., 2009b). In shallow, highly transparent water bodies, UVR avoidance may not be feasible. Moreover, wind-induced turbulence can result in strong vertical mixing of the water column which is an additional factor that may lower the effectiveness of the avoidance strategy (Zagarese et al., 1998). Thus, organisms living in UV-exposed systems with a small depth refuge need other strategies to receive substantial protection against UVR.
One important UVR defence strategy in zooplankton is the synthesis or accumulation of photo-protective compounds (Fig. 5) acting either as sunscreens or as antioxidants. Among the coloured sunscreens known in zooplankton is melanin, a tan-brown to black cuticular pigment, which is typically found in high-latitude and high-altitude cladocerans such as in Daphnia and Scapholeberis (Hebert & Emery, 1990; Hessen & Sørensen, 1990; Hobaek & Wolf, 1991; Rautio & Korhola, 2002b; Hansson et al., 2007; Rautio et al., 2009). Only recently, melanin was detected in Arctic fairy shrimps (Artemiopsis stefanssoni and Branchinecta paludosa), although both species lacked the typically characteristic dark coloration (Rautio et al., 2009). In Daphnia pulex, for example, melanin concentrations were genetically determined within populations and correlated to UVR sensitivity; pigmented animals survived better than pale individuals (Hessen, 1996). Several studies showed that melanised Daphnia have higher UVR tolerance as compared to non-melanised relatives (Luecke & O’Brian, 1983; Hebert & Emery, 1990; Zellmer, 1995; Hessen, 1996; Hessen et al., 1999; Rhode et al., 2001) and that melanisation is reduced when the UVR threat is removed (Luecke & O’Brien, 1983; Hessen, 1996; Hansson et al., 2007; Rautio, 2007). The only deviation from these observations is the recent finding of Connelly et al. (2009) whose D. middendorfiana population remained pigmented when kept in the laboratory for six months under PAR only, and was not better protected from UVR than hyaline species. Pigmentation is assumed to be metabolically costly. Melanised Daphnia show delayed age at first reproduction and smaller clutch size, resulting in an overall lower growth rate of pigmented individuals compared to non-melanic conspecies (Weider, 1987; Hessen, 1996). Moreover, after each moult Daphnia has to synthesise new melanin since the pigment is shed with the carapace (Hebert & Emery, 1990). This probably explains the failure of melanised clones to compete with transparent ones in less clear waters lacking predators (Hebert & Emery, 1990; Hessen, 1996; Rautio & Korhola, 2002b). Strong seasonal patterns in melanin contents were found in Daphnia. The subarctic cladoceran D. umbra synthesised pigments only during the summer months, starting directly after ice out (Rautio & Korhala, 2002b) (Fig. 6). In summary, melanin seems to be synthesised to increase survival of aquatic organisms under harmful UVR despite being energetically costly and increasing vulnerability to visual predators (Hessen, 1996; Hansson, 2000).
Fig. 5.
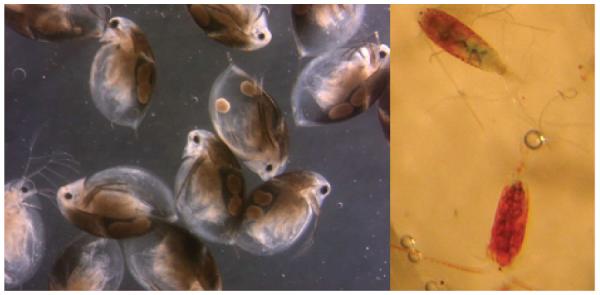
Pigmented zooplankton. Melanised Daphnia umbra (left) and Eudiaptomus graciloides with red carotenoids (right) from Kilpisjärvi, Finnish Lapland. Photographs Paavo Junttila and M.R.
Fig. 6.
Seasonal variation of a) melanin in subarctic Daphnia umbra and b) mycosporine-like amino acids (MAAs) in alpine Cyclops abyssorum. From Rautio & Korhola (2002b) and Tartarotti & Sommaruga (2006). Copyright (2002, 2006) by the American Society of Limnology, Inc.
A family of intracellular colourless UV-absorbing compounds, mycosporine-like amino acids (MAAs; absorption maxima between 309 nm and 360 nm), has been reported for several hundred species of marine organisms with their distribution ranging from the tropics to polar latitudes (see Karentz, 2001 and Shick & Dunlap, 2002 for reviews). Over the last years, these compounds have been described for several freshwater taxa including copepods, fairy shrimps, Chaoborus larvae, rotifers and ciliates (Sommaruga & Garcia-Pichel, 1999; Tartarotti et al., 2001; Gonçalves et al., 2002; Helbling et al., 2002; Rocco et al., 2002; Tartarotti et al., 2004; Moeller et al., 2005; Hansson et al., 2007; Persaud et al., 2007; Sonntag et al., 2007; Obertegger et al., 2008; Nagiller & Sommaruga, 2009; Rautio et al., 2009). Accumulation of MAAs does not seem to be a photo-protective strategy utilised by Daphnia or other cladocerans as they lack these compounds or show only trace amounts (Tartarotti et al., 2001; Gonçalves et al., 2002; Tartarotti et al., 2004; Hansson et al., 2007; Persaud et al., 2007). However, UV-absorbing compounds with properties similar to MAAs have been reported for Arctic Daphnia middendorffiana (Rautio et al., 2009). Variation in the qualitative and quantitative composition of MAAs in different copepod species has been found in lakes located across an altitude gradient (Central Alps, Austria) (Tartarotti et al., 2001). Apart from altitude, the variability in the MAA concentration of Cyclops populations was also strongly correlated with the lake transparency and the depth refuge. Although similar altitude and depth refuge relationships were observed in copepods from a set of Patagonian (Argentina) water bodies, lake transparency explained a lower percentage of the MAA variability compared with that found in Alpine lakes (Tartarotti et al., 2004). In a study by Persaud et al. (2007), copepods from higher UVR lakes located in Argentina, New Zealand and the USA tended to have higher MAA contents. Similar to melanin in cladocerans, MAA contents in copepods have been shown to vary seasonally in some lakes (Fig. 6). Concentrations of MAAs in C. abyssorum tatricus were on average three times higher in the summer compared to ice-cover periods (Tartarotti & Sommaruga, 2006). From these studies it is apparent that MAAs play an important role in UVR protection, and direct evidence of increased UVR tolerance was demonstrated for copepods (Leptodiaptomus minutus) rich in MAAs (Moeller et al., 2005). Since metazoans seem to lack the putative shikimate acid pathway needed to synthesise MAAs (Bentley, 1990), the UV-absorbing compounds found in zooplankton are thought to have a dietary origin. However, some recent evidence (García et al., 2008) shows that copepods are also able to accumulate these compounds even when maintained on a MAA-free diet. The source of MAAs in these copepods is likely to be the prokaryotic organisms that live on or in the animals (García et al., 2010). Laboratory experiments confirmed the dietary acquisition of MAAs by a freshwater zooplankter, L. minutus (Moeller et al., 2005). This experimental work is supported by a field survey showing that MAA concentrations in late copepodid to adult life stages of C. abyssorum tatricus were significantly correlated to those in phytoplankton when considering a time lag of 3–4 weeks between the synthesis and subsequent accumulation of these compounds (Tartarotti & Sommaruga, 2006). However, in laboratory conditions zooplankton MAA accumulation from food has been noted to occur at rates as fast as 3–4 days (Hylander & Jephson, 2010).
Another defence mechanism of many organisms against UVR is the possession of antioxidants like carotenoids, which quench photo-oxidative reactions, or enzymes such as superoxide dismutase that can counteract the oxidative nature of peroxides and other radicals. The strikingly red pigmentation of alpine and highly UV-exposed organisms has been known since the early work of Brehm (1938) and Gilchrist & Green (1962). Carotenoids (primarily free or esterified astaxanthin and canthaxanthin) have been found in several zooplankton taxa like copepods, cladocerans, fairy shrimps, and ciliates (Hairston, 1976; Byron, 1982; Ringelberg et al., 1984; Mostajir et al., 1998; Rautio et al., 2009), however with a greatly variable quantitative and qualitative composition. High carotenoid levels have been reported for copepod species from diverse alpine aquatic systems (Hairston, 1978; Hessen & Sørensen, 1990; Hessen, 1993; Tartarotti et al., 1999; Persaud et al., 2007; Sommaruga, 2010). Although to a much lesser extent, carotenoids are found in cladocerans, particularly in their ovaries and eggs (Siebeck et al., 1994). This discrepancy in carotenoid levels suggests that these compounds have different function in these taxa (Hessen & Sørensen, 1990; Siebeck et al., 1994). Since carotenoids can not be synthesised de novo by zooplankton, they are incorporated in the animals’ bodies by consumption of algal food (Andersson et al., 2003; Moeller et al., 2005). It has long been known that pigmented copepods are less UV-sensitive when exposed to UVR than unpigmented ones (Byron, 1982; Ringelberg et al., 1984). Furthermore, the content of carotenoids in these animals has been shown to increase with altitude and decrease with lake depth (Byron, 1982). All these findings support the physiological role of carotenoids as photoprotectants, although other functions were proposed such as metabolic benefit or the role as nutritional deposit (Ringelberg & Hallegraeff, 1976; Hairston, 1979; Byron, 1982). Further, observations of carotenoid-rich copepods under the ice in darkness also suggest that carotenoids have multiple roles in zooplankton (Hairston, 1976, 1981). In laboratory experiments, UVR did cause the copepod Leptodiaptomus minutus to accumulate carotenoids, but only if the diet lacked MAAs, which they otherwise accumulated instead of carotenoids (Moeller et al., 2005). Exposure to UVR, however, did not result in accumulation of carotenoids in cladocerans; their protective function seems to be restricted to the offspring (Hessen, 1994). High carotenoid levels do not necessarily imply high MAA contents as was shown in red pigmented calanoid copepods from North American lakes (Persaud et al., 2007). Comparable results have been observed in copepods from high altitude Himalayan lakes, concentrations of carotenoids were among the highest reported while content and diversity of MAAs were low (Sommaruga, 2010). Recent studies propose that MAAs and carotenoids are complementary photo-protective compounds in copepods, i.e. one is high when the other is low (Hylander et al., 2009b) (Fig. 7). Both cladocerans and copepods are able to increase or reduce pigmentation (i.e. melanin, carotenoids, and/or MAAs) in response to changed UVR and in response to predators (Hansson, 2000; Hansson, 2004; Tollrian & Heibl, 2004). Moreover, the size of the carotenoid reserves seems to be a plastic trait (van der Veen, 2005). A prominent seasonal pattern of higher carotenoid/low MAA content in spring, changing to low carotenoid/higher MAA content in summer, was found in calanoid copepods from relatively UV-transparent lakes (Moeller et al., 2005; Persaud et al., 2007). No clear seasonal pattern of photo-protective compounds (carotenoids and MAAs), however, was observed in the calanoid copepod Boeckella antiqua from a fishless shallow pond with low UV transparency (García et al., 2008). High contents of melanin, MAAs, and/or carotenoids probably provide zooplankton with substantial UV protection; however, there are other means that are useful for evading UV damage.
Fig. 7.
Concentrations (± 1 SD) of carotenoids (μg mg−1 dry wt) and MAAs (μg mg−1 dry wt) in subarctic, temperate, and dry-temperate copepods (n = 13, 12, and 12, respectively). From Hylander et al. (2009b). Copyright (2009) by the American Society of Limnology, Inc.
Only recently, scytonemin, an extracellular UV-screening pigment (in vivo absorption maximum at 370 nm) of cyanobacterial origin was detected in two crustacean species, Daphnia middendorffiana and the fairy shrimp Branchinecta paludosa, from Arctic (Canada, Alaska) freshwater bodies (Rautio et al., 2009). This yellow-brown pigment, which occurs in all sheathed cyanobacterial groups and increases the UVR tolerance of these species (Garcia-Pichel & Castenholz, 1991), has not been previously described in zooplankton. In the same study, four types of UVR protectants (carotenoids, melanins, scytonemin and MAAs) were studied simultaneously. Photoprotectants were detected in all Arctic crustacean species investigated and most populations had multiple pigments, suggesting a combination of defences in these organisms, including broadband screening of UVR and carotenoid quenching of ROS.
One important member of the cellular defence system against oxidative stress is the enzyme catalase (CAT) (Barata et al., 2005). This enzyme mainly assists in detoxifying hydrogen peroxide (H2O2) to oxygen and water. Glutathione transferase (GST) is assumed to work primarily at the intracellular level, neutralising peroxidised macromolecules and detoxifying breakdown products after lipid peroxidations (Hiratsuka et al., 1999), while the antioxidant enzyme superoxide-dismutase (SOD) is recognised to eliminate the superoxide radical. Antioxidant enzymes play a significant role in the UVR tolerance of microorganisms, plants, mammalian cells and skin tissues (Murali et al., 1988; Wang & Schellhorn, 1995; Bertling et al., 1996; Kerb et al., 1997). Relatively little is known about the protective function of these enzymes in pelagic freshwater organisms. In the cladoceran Daphnia magna, CAT activity was not affected by UVR, while GST activity was slightly influenced (Borgeraas & Hessen, 2000). Conversely, Vega & Pizarro (2000) found UV-B-increased CAT activity in another Daphnia species (D. longispina), suggesting an increased response to oxidative stress. Interestingly, no major differences in the concentrations of several enzymes (CAT, GST and SOD) were found between melanised and hyaline organisms in different species and populations of the cladoceran Daphnia (Borgeraas & Hessen, 2002b). Among the alpine populations of D. longispina examined, however, a significant positive relationship between absorbance (300 nm) of the pond water and CAT activity was found, which in turn could be related to ambient levels of photo-induced H2O2 production in these small water bodies (Borgeraas & Hessen, 2002b). A field study by Borgeraas & Hessen (2002a) showed a clear diel cycle in antioxidant enzyme activities in highly UV-exposed alpine Daphnia. In the hyaline morph, maxima in CAT and SOD concentrations were found at midday, while there was no elevated activity in the melanic morph at midday; on the contrary, it rather showed a midday minimum in GST contents. The higher enzyme activity in the hyaline Daphnia was explained by the need for photoprotection in these individuals compared to the more UV-protected pigmented animals (Borgeraas & Hessen, 2002a). A difference in antioxidant enzyme activity was found between copepods and cladocerans from Andean (Argentina) lakes (Souza et al., 2007). The CAT activity was significantly higher (4–5-fold) in the cladoceran Ceriodaphnia dubia than in the copepod Boeckella gracilipes, suggesting a differential importance of this mechanism probably associated with the absence of MAAs in cladocerans. The GST activity was similar in both crustacean groups; this enzyme detoxifies reactive species from different origins, this also including those not necessarily associated with UVR exposure. A comparison of enzyme activity in B. gracilipes populations from lakes of varying DOC levels showed variation in GST but not in CAT activity. The authors speculate that high GST activity was driven by both high (toxic-derived photoproducts) and low (increases in H2O2) DOC concentrations (Souza et al., 2007). Contrasting enzyme patterns were found in Arctic Daphnia along gradients of DOC and UVR exposure (Hessen et al., 2002). Activities of CAT were lower at low DOC, while the contrary was observed for GST. A negative relationship between CAT and solar radiation was found for the different DOC contents, indicating inhibition or inactivation of this enzyme by UVR, whereas there were no such effects in GST activities. Balseiro et al. (2008) found that low food quality (low P:C ratio) decreased the antioxidant response to UVR in Daphnia. The activities of GST and CAT were significantly affected by low food quality with strongly decreased enzyme activity in response to UVR for both enzymes.
Highly conserved polypeptides known as stress or heat shock proteins (Hsps) (see Sanders, 1993 and Feder & Hofmann, 1999 for reviews) may have physiological functions related to UVR protection. These proteins play an important role in maintaining protein homeostasis, and they act as molecular chaperones to stabilise and refold denatured proteins (Feder & Hofmann, 1999). Furthermore, the rapid up-regulation of heat shock proteins after a sublethal event can induce stress tolerance and protection against a subsequent stress. The most abundant and widely studied group of these chaperones is the 70 kDa heat shock protein family including constitutive (Hsc70) and stress-inducible forms (Hsp70). Induction of Hsps has been described in response to a variety of stresses including heat, hypoxia, toxins and UVR. While in mammalian cells the up-regulation of Hsps has been recognised as part of an adaptive cellular UV-protective mechanism (Trautinger et al., 1996), studies on the role of UVR in the synthesis of Hsps in aquatic organisms are limited. Induction of Hsps by UVR was found in UV-sensitive (but not in UV-tolerant) marine diatoms (Dohler et al., 1995), and in sea urchin embryos (Bonaventura et al., 2005, 2006), while responses in fish appear to be species-specific (Vehniäinen et al., 2003; Häkkinen et al., 2004). In the marine copepod Acartia tonsa, general protein synthesis was suppressed by high UVR intensities, while levels of constitutively expressed 70 kDa heat-shock proteins increased (Tartarotti & Torres, 2009). High incident solar radiation increased the levels of Hsp70 in adult females of the freshwater copepod Cyclops abyssorum tatricus (Tartarotti et al., unpublished data). Although this copepod species is very UV-resistant in terms of mortality (Tartarotti et al., 1999), at a sublethal molecular level female copepods seem to be more responsive than younger life stages. The rapid increase (within hours) in stress protein levels might be part of the suite of molecular and biochemical processes that can promote survival in highly UV-exposed aquatic organisms.
Recovery
When protective measures are not sufficient, zooplankton can repair UV-induced damage to some extent. Organisms generally have some kind of DNA repair system. In zooplankton there are primarily two repair processes: nucleotide excision repair (NER, dark repair) and photo-enzymatic repair (PER, light repair). Nucleotide excision repair is an energetically costly complex multi-protein, multi-step pathway, and is found in almost all taxa without being specific to UV-induced DNA damage (Mitchell & Karentz, 1993; Sancar, 1994a; Sinha & Häder, 2002), while PER uses the enzyme photolyase in the presence of longer wavelength UV-A and PAR (UV-A and PAR = photorepair radiation). This mechanism can reverse pyrimidine dimers (Sutherland, 1981; Mitchell & Karentz, 1993), and since it is a single-enzymatic process driven by photorepair radiation, it is less costly to the cell than is NER (MacFadyen et al., 2004). Although PER is specific to UV-induced DNA damage, it is not present in all taxa (Sancar, 1994b). The studies focusing on repair mechanisms in zooplankton show differences among taxa, species, and life stages (Siebeck & Böhm, 1991; Zagarese et al., 1997a; Grad et al., 2001, 2003; Gonçalves et al., 2002; Rocco et al., 2002; MacFadyen et al., 2004; Ramos-Jiliberto et al., 2004; Connelly et al., 2009). Survival of UV-stressed Daphnia increased in the presence of photo-repair radiation (Siebeck & Böhm, 1991; Grad et al., 2001; Williamson et al., 2001a, 2002; Huebner et al., 2006); this light-induced repair was assumed to rely on PER of DNA damage (Fig. 8). A study by MacFadyen et al. (2004) provided evidence of enzymatic photorepair in Daphnia at the molecular level. PER enhanced the repair of cytotoxic photoproducts (cyclobutane pyrimidine dimers, CPDs, and pyrimidine (6–4) pyrimidone photoproducts, (6–4)PDs), and was likely to be responsible for increases in Daphnia survival following UV-B exposure. Other zooplankters such as the rotifer Asplanchna girodi seem to utilise dark repair and have little to no PER (Sawada & Enesco, 1984; Grad et al., 2001). In juvenile A. girodi, however, evidence of photoreactivation has been found (Williamson et al., 2002; Grad et al., 2003). The importance of repair processes in copepods is not well understood. Zagarese et al. (1997a) found that photorepair accounted for the relatively high UV-B tolerance in red Boeckella gibbosa, while little evidence of PER was found in Boeckella gracilipes (Zagarese et al., 1997a; Tartarotti et al., 2000). Both the cyclopoid copepod Metacyclops mendocinus and the calanoid copepod Leptodiaptomus minutus showed high efficiency for photorepairing UV-B-induced DNA damage (Gonçalves et al., 2002; Williamson et al., 2002). PER and NER are temperature-dependent mechanisms (i.e. greater repair at higher temperatures), thus zooplankton such as Daphnia that rely heavily on DNA repair may be less able to survive high UVR intensities in low temperature environments (Williamson et al., 2002; MacFadyen et al., 2004). Surprisingly, when exposing four different Daphnia species to a single acute UV-B dose, higher survival and repair rates were found for the lower experimental temperature (10 °C compared to 20 °C), indicating that the enhanced rate of PER at the lower temperature contributed significantly to the recovery of these organisms (Connelly et al., 2009). The same authors confirm that photorepair is the primary mechanism to remove DNA lesions in Daphnia.
Fig. 8.
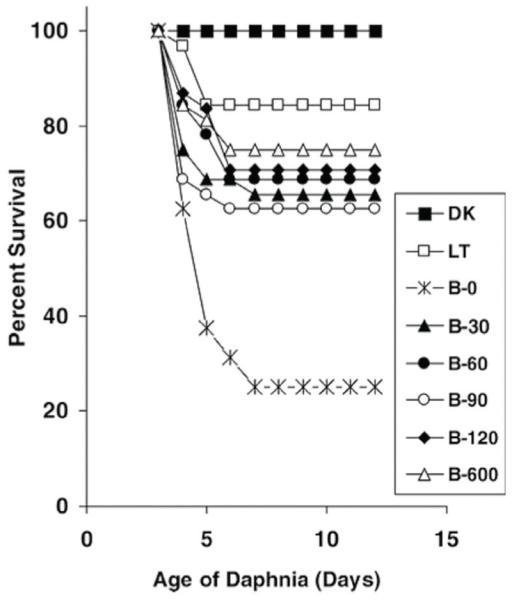
Mortality of Daphnia magna after a 6 h UV-B exposure followed by variable durations of photorepair radiation (UV-A and PAR). DK, LT: dark and light controls. B-0 B-600: UV-B exposure followed by 0–600 min of photorepair radiation. From Huebner et al. (2006).
Net community impact imposed by UV
At the species level, the net UV stress and damage imposed by UV is defined by the combination of photochemical degradation and the energetic costs of photoprotection (Vincent & Neale, 2000). The studies focusing on community responses show that with more interactions between species the responses to UVR become less clear (reviewed by Sommaruga, 2003).
De Lange et al. (1999) were not able to detect any changes in the abundance, species composition or biovolume of the phytoplankton or zooplankton communities, nor in the periphyton or macroinvertebrate community during an eight-week microcosm experiment. Cabrera et al. (1997) studied phytoplankton and zooplankton responses to UVR in a high-altitude Andean lake for a 30-day period in three different years. They showed different responses to different species ranging from positive, no effects, to negative effects. They concluded that although different species had different tolerances to UVR, the population fluctuations were most dependent on life cycles and the period of time the organisms were exposed to UVR. Vinebrooke & Leavitt (1999) studied littoral communities during a one-month period and showed that zoobenthos and zooplankton biomass were unaffected by UVR but species composition changed. The 4-month mesocosm study by Hylander & Hansson (2010) concluded that zooplankton communities were well buffered in response to UVR changes, due to efficient defence mechanisms, suggesting that future potential increases in UVR may have only a small impact on zooplankton population dynamics and community composition.
The above studies have extended the UV impact studies from single species to communities, and the study period to weeks and months from the more common day to week duration of an experiment. However, even a month scale is still a short period in terms of ecosystem dynamics, and precludes the detection of potential UV effects on the reproductive success of copepods, many of which require more than one year to complete a life cycle (Elgmork & Eie, 1989). Vinebrooke & Leavitt (2005) discuss how extrapolation of UVR results from small-scale laboratory and field experiments to larger spatial and temporal scales is a challenge. Damages in cell structures and avoidance response may take only hours to detect, but changes in zooplankton community composition and functioning may be seen only after months or years, while changes in optical properties of lakes may take decades.
There exist, however, some field data on zooplankton distribution in relation to UVR which indicate that zooplankton communities in lakes differ according to the presence of UVR in the water. A dataset that includes the above mentioned longer time-scales comes from Williamson et al. (2001b). They investigated changes in UVR attenuation and zooplankton community structure in a deglaciation chronosequence in Alaska. Terrestrial succession in the catchment area had resulted in increasing lake DOC content over time and decreases in UV attenuation depth. This attenuation depth (1 % of surface irradiance at 320 nm) was at 14 m in the youngest lake (10 years old), but only 0.6 m in the oldest (90 years old). Zooplankton community structure also changed across lakes of different ages. The pelagic cladocerans Ceriodaphnia quadrangula and Bosmina longirostris and the rotifer Asplanchna priodonta were absent from the clearest lakes, and it was shown that this was due to higher UVR in these lakes.
Further evidence of UVR effects on zooplankton community structure comes from a study of shallow ponds (< 0.5 m) in Finnish Lapland (Rautio & Korhola, 2002b) and from Patagonian lakes (Marinone et al., 2006). Daphnia in Finnish ponds were absent when DOC was < 5 mgC L−1, most likely because the ponds were too shallow to provide refuge from UVR. In Patagonia, zooplankton species richness and diversity decreased with high water column irradiance in lakes. Molot et al. (2004) also studied the distribution of zooplankton (Daphnia) in lakes in relation to UVR. They, however, showed that in > 250 Canadian lakes Daphnia distribution was not restricted by UV-B but their lake set did not include many very clear and shallow systems.
Most UVR studies to date have been done using a small number of species. Only about 50 crustacean zooplankton species have been studied for their UVR tolerance and 16 of these are from the genus Daphnia (data from papers referred in this review). More than half of all freshwater zooplankton UVR studies have been conducted with the genus Daphnia. Most studied species, including Daphnia, are also pelagic which skews the results. The vast number of littoral and benthic zooplankton, mostly belonging to Cladocera, in lakes with abundant littoral vegetation may experience a more sheltered and therefore more UV-protected environment than the pelagic zooplankton. Their need to respond to UVR is likely to be different and smaller than that of pelagic species. The few published results of the response of littoral communities to UVR are, however, contradictory. Vinebrooke & Leavitt (1999) showed that responses were taxon- and habitat-specific. UVR did not adversely affect motile taxa that could seek refuge in sediments but rotifers were significantly suppressed in UVR treatments. In another study (Cabrera et al., 1997), a common littoral cladoceran Chydorus sphaericus and the rotifer Lepadella were strongly inhibited by UV-B. However, in this study the zooplankton was kept in mesocosms near the surface and the species were not able to seek the shelter they would have in their natural environment.
Conclusions
Laboratory and short-term mesocosm experiments have convincingly shown that most species do better when UVR is excluded from the environment; however, the increased system complexity at the community level may compensate for some of the negative UVR effects observed at species level, or completely reverse species success. A UV-tolerant species can be excluded when the habitat becomes shielded from UVR if its superior competitor is released from UVR control (Jokiel, 1980). Furthermore, some longer-term mesocosm experiments and field observations have indicated no or very little zooplankton community response to UVR. However, some systems may be strongly controlled by UVR, showing that interactions at community level may vary greatly among systems. Vinebrooke & Leavitt (1999) have suggested that the importance of UVR for lake organisms is influenced by the system productivity and presence of other stressors. UVR effects may be more pronounced in extreme (i.e. alpine and polar) and anthropogenically-stressed (i.e. acidified) lakes in which abiotic regulation of communities is common. In more productive systems, increased biotic regulation of food-web structure may reduce the importance of direct UVR effects. Some evidence for this can be seen when studies in productive Dutch lakes (de Lange et al., 1999) were compared with studies in oligotrophic Andean lakes (Cabrera et al., 1997), with the latter community changing more in the UVR treatment.
The variability in zooplankton UVR responses may also result from the seasonally and ontogenetically changing inherent properties of different species, and create inconsistency among results. The physiological and developmental state of an organism and its prior history with UVR, as well as other environmental factors such as temperature, set limits or opportunities for the induction of different UV protections. For instance, although it is generally accepted that compounds such as MAAs and various pigments (e.g. melanin, carotenoids) have photo-protective roles, and should be synthesised or accumulated in zooplankton in the presence of UVR, it is also known that these compounds sometimes occur in the absence of UVR under the ice in winter (Hairston, 1979; Tartarotti & Sommaruga, 2006). Maybe they have been accumulated in advance to provide photoprotection at the time of ice break-up but it is likely that many mechanisms that have UV-protective effects are also induced for other ecological reasons. Carotenoids may have an important function in display and as they are bound to lipids they are likely to play a role in zooplankton energy metabolism, especially in low food winter conditions. Vertical migration is a well-known behavioural response induced by fish, perhaps more often than by UVR. Furthermore, many photo-protective strategies may also be complementary (Hylander et al., 2009b) and, hence, cannot be detected in all UVR situations. Lack of vertical migration in copepods in the absence of visually hunting predators may, for example, be explained by their high concentration of MAAs that allows an efficient enough shield from UVR for the copepods to stay in surface waters. Zooplankton may also use a broad band strategy to cope with UVR (Rautio et al., 2009). Species that accumulate multiple photo-protective pigments may have a lower concentration of a certain pigment for a given irradiance than its co-specimen that uses a single protective strategy. All above examples create inconsistency in UVR responses and without measuring multiple photo-protective strategies may underestimate the UVR impact on zooplankton.
Inconsistencies are also found when studying the effect of temperature on zooplankton UVR tolerance. Many papers have stated that cold lake water has an effect on the efficiency of species recovery from UVR damage because with lower temperatures enzymatic reactions should slow down, including the repair rate of photochemical damage. Some recent studies, however, have shown that in some systems enzyme activities are higher in the cold (Connelly et al., 2009) and species survival when exposed to UVR is also better in colder water (Borgeraas & Hessen, 2000). Some of these variations can be explained by scale differences. In some studies the temperature range tested has been 15 °C to 25 °C while in others it has been 5 °C to 15 °C. It is also very likely that the temperature in the species’ natural habitat has an influence. As the natural distribution of Daphnia middendorfiana is restricted to lakes with temperature < 15 °C (Patalas, 1990), it should not be a surprise that it tolerates UVR better in + 10 °C than in + 20 °C (Connelly et al., 2009).
To summarise, the relationship between UVR and the response of zooplankton is well documented for many damage (decreased survival and fecundity, reduced growth rates and offspring production) and protection (vertical migration, pigments, antioxidant enzymes) mechanisms, and there occurs a general consensus that UVR induces important behavioural or physiological responses in zooplankton. However, the accurate quantification of responses is more demanding given the range of variability in responses at species level, and especially the system complexity in natural communities which may either amplify or reduce direct UVR effects. The recent discoveries about the potential importance of heat shock proteins and scytonemin in zooplankton UVR protection, and the evidence that MAAs in zooplankton are not only accumulated from food but come also from associated bacteria, suggest that we do not yet know everything about the UV–zooplankton relationship. More studies are needed on both species and ecosystem levels to better understand the drivers that determine the multiple interactions in ecosystem responses to UVR, and to better predict how zooplankton respond to increases in UVR from climate change. Given the presence of UVR on the Earth since the beginning of life, and first in substantially higher intensities than presently, we predict that the diverse protection strategies observed in zooplankton may be enough to allow broad species distributions in future even in the clearest, most UV-exposed sites.
Acknowledgements
We are grateful to Colin Reynolds for inviting us to write this review and for all his help during the work. The ms. has also been improved by comments from R. Sommaruga. H.E. Zagarese and an anonymous reviewer are gratefully acknowledged. We thank all students and colleagues who have been involved with us in the UVR research in field and lab. M.R. especially thanks Dr Iris Zellmer for her enthusiasm in the field and the company in Kilpisjärvi biological station during the last 10 years. This research was supported by Finnish Academy of Science (grant 119205) to M.R. and Austrian Science Fund (FWF) grant T236-B17 to B.T.
Author Profile
Milla Rautio is an Academy Research Fellow in University of Jyväskylä, Finland, and has recently been awarded a Canada Research Chair in Boreal Aquatic Ecology at Université du Quebéc à Chicoutimi, Canada. Her main research interest is high-latitude freshwater ecology, especially the response of zooplankton to ultraviolet radiation, importance of non-phytoplankton food in aquatic food webs, and winter limnology.
Barbara Tartarotti is a postdoctoral research scientist in the Institute of Ecology at the University of Innsbruck, Austria, where she received her PhD in Limnology. Part of her PhD and postdoctoral work was done in South America (Argentina, Chile) and the USA. Her research interests include zooplankton ecology, ecological physiology of invertebrates, and effects of UV radiation and other stressors on aquatic organisms, with a special focus on high mountain lakes.
References
- ACIA . Arctic climate impact assessment. Cambridge University Press; Cambridge: 2005. p. 1042. [Google Scholar]
- Aguilera X, Crespo G, Declerck S, de Meester L. Diel vertical migration of zooplankton in tropical high mountain lakes (Andes, Bolivia) Polish Journal of Ecology. 2006;54:453–464. [Google Scholar]
- Alonso C, Rocco V, Barriga JP, Battini MA, Zagarese HE. Surface avoidance by freshwater zooplankton: field evidence on the role of ultraviolet radiation. Limnology and Oceanography. 2004;49:225–232. [Google Scholar]
- Anderson NJ, Stedmon CA. The effect of evapoconcentration on dissolved organic carbon concentration and quality in lakes of SW Greenland. Freshwater Biology. 2007;52:280–289. [Google Scholar]
- Anderson JG, Toohey DW, Brune WH. Free radicals within the Antarctic vortex: the role of CFCs in Antarctic ozone loss. Science. 1991;251:39–46. doi: 10.1126/science.251.4989.39. [DOI] [PubMed] [Google Scholar]
- Andersson M, van Nieuwerburgh L, Snoeijs P. Pigment transfer from phytoplankton to zooplankton with emphasis on astaxanthin production in the Baltic Sea food web. Marine Ecology Progress Series. 2003;254:213–224. [Google Scholar]
- Arts MT, Robarts RD, Kasai F, Waiser MJ, Tumber VP, Plante AJ, Rai H, de Lange HJ. The attenuation of ultraviolet radiation in high dissolved organic carbon waters of wetlands and lakes on the northern Great Plains. Limnology and Oceanography. 2000;45:292–299. [Google Scholar]
- Balseiro E, Souza MS, Modenutti B, Reissig M. Living in transparent lakes: low food P:C ratio decreases antioxidant response to ultraviolet radiation in Daphnia. Limnology and Oceanography. 2008;53:2383–2390. [Google Scholar]
- Barata C, Varo I, Navarro JC, Arun S, Porte C. Antioxidant enzyme activities and lipid peroxidation in the freshwater cladoceran Daphnia magna exposed to redox cycling compounds. Comparative Biochemistry and Physiology, Part C – Toxicology & Pharmacology. 2005;140:175–186. doi: 10.1016/j.cca.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Bentley R. The shikimate pathway: a metabolic tree with many branches. Critical Reviews in Biochemistry and Molecular Biology. 1990;25:307–384. doi: 10.3109/10409239009090615. [DOI] [PubMed] [Google Scholar]
- Bertling CJ, Lim F, Girotti AW. Role of hydrogen peroxide in the cytotoxic effects of UVA/B radiation on mammalian cells. Photochemistry and Photobiology. 1996;64:137–142. doi: 10.1111/j.1751-1097.1996.tb02433.x. [DOI] [PubMed] [Google Scholar]
- Blumthaler M, Ambach W, Rehwald W. Solar UV-A and UV-B radiation fluxes at two alpine stations at different altitudes. Theoretical and Applied Climatology. 1992;46:39–44. [Google Scholar]
- Boeing WJ, Leech DM, Williamson CE, Cooke S, Torres L. Damaging UV radiation and invertebrate predation: conflicting selective pressures for zooplankton vertical distribution in the water column of low DOC lakes. Oecologia. 2004;138:603–612. doi: 10.1007/s00442-003-1468-0. [DOI] [PubMed] [Google Scholar]
- Bonaventura R, Poma V, Costa C, Matranga V. UV-B radiation prevents skeleton growth and stimulates the expression of stress markers in sea urchin embryos. Biochemical and Biophysical Research Communications. 2005;328:150–157. doi: 10.1016/j.bbrc.2004.12.161. [DOI] [PubMed] [Google Scholar]
- Bonaventura R, Poma V, Russo R, Zito F, Matranga V. Effects of UV-B radiation on development and hsp70 expression in sea urchin cleavage embryos. Marine Biology. 2006;149:79–86. [Google Scholar]
- Borgeraas J, Hessen DO. UV-B induced mortality and antioxidant enzyme activities in Daphnia magna at different oxygen concentrations and temperatures. Journal of Plankton Research. 2000;22:1167–1183. [Google Scholar]
- Borgeraas J, Hessen DO. Diurnal patterns of antioxidant activity in alpine and arctic Daphnia under in situ UV-radiation. Archiv für Hydrobiologie. 2002a;156:83–95. [Google Scholar]
- Borgeraas J, Hessen DO. Variations of antioxidant enzymes in Daphnia species and populations as related to ambient UV exposure. Hydrobiologia. 2002b;477:15–30. [Google Scholar]
- Brehm V. Die Rotfärbung von Hochgebirgsorganismen. Biology Reviews. 1938;13:307–318. [Google Scholar]
- Buma AGJ, de Boer MK, Boelen P. Depth distributions of DNA damage in Antarctic marine phytoplankton and bacterioplankton exposed to summertime UV radiation. Journal of Phycology. 2001;37:200–208. [Google Scholar]
- Byron ER. The adaptive significance of calanoid copepod pigmentation – a comparative and experimental analysis. Ecology. 1982;63:1871–1886. [Google Scholar]
- Cabrera S, López M, Tartarotti B. Phytoplankton and zooplankton response to ultraviolet radiation in a high-altitude Andean lake: short- versus long-term effects. Journal of Plankton Research. 1997;19:1565–1582. [Google Scholar]
- Caldwell MM. Plant life and ultraviolet radiation: some perspective in the history of the Earth’s UV climate. BioScience. 1979;29:520–525. [Google Scholar]
- Connelly SJ, Moeller RE, Sanchez G, Mitchell DL. Temperature effects on survival and DNA repair in four freshwater cladoceran Daphnia species exposed to UV radiation. Photochemistry and Photobiology. 2009;85:144–152. doi: 10.1111/j.1751-1097.2008.00408.x. [DOI] [PubMed] [Google Scholar]
- Cooke SL, Williamson CE, Saros JE. How do temperature, dissolved organic matter and nutrients influence the response of Leptodiaptomus ashlandi to UV radiation in a subalpine lake? Freshwater Biology. 2006;51:1827–1837. [Google Scholar]
- Cooper WJ, Shao C, Lean DRS, Gordon AS, Scully FE., Jr. Factors affecting the distribution of H2O2 in surface waters. In: Baker LA, editor. Environmental Chemistry of Lakes and Reservoirs. American Chemical Society; Washington: 1994. pp. 391–422. [Google Scholar]
- Damkaer DM. Possible influences of solar UV radiation in the evolution of marine zooplankton. In: Calkins J, editor. The Role of Solar Ultraviolet Radiation in Marine Ecosystems. Plenum Press; New York: 1982. pp. 701–706. [Google Scholar]
- Dawidowicz P, Loose CJ. Metabolic costs during predator-induced diel vertical migration of Daphnia. Limnology and Oceanography. 1992;37:1589–1595. [Google Scholar]
- de Lange HJ, van Reeuwijk PL. Negative effects of UVB-irradiated phytoplankton on life history traits and fitness of Daphnia magna. Freshwater Biology. 2003;48:678–686. [Google Scholar]
- de Lange HJ, Verschoor AM, Gylstra R, Cuppen JGM, van Donk E. Effects of artificial ultraviolet-B radiation on experimental aquatic microcosms. Freshwater Biology. 1999;42:545–560. [Google Scholar]
- de Lange HJ, Morris DP, Williamsom CE. Solar ultraviolet photodegradation of DOC may stimulate freshwater food webs. Journal of Plankton Research. 2003;25:111–117. [Google Scholar]
- Dohler G, Hoffmann M, Stappel U. Pattern of proteins after heat-shock and UV-B radiation of some temperate marine diatoms and the Antarctic Odontella weissflogii. Botanica Acta. 1995;108:93–98. [Google Scholar]
- Effler SW, Schafran GC, Driscoll CT. Partitioning light attenuation in an acidic lake. Canadian Journal of Fisheries and Aquatic Sciences. 1985;42:1707–1711. [Google Scholar]
- Elgmork K, Eie JA. Two- and three-year life cycles in the planktonic copepod Cyclops scutifer in two high mountain lakes. Holoarctic Ecology. 1989;12:60–69. [Google Scholar]
- Elo A-R, Huttula T, Peltonen A, Virta J. The effects of climate change in the temperature conditions of lakes. Boreal Environment Research. 1998;2:137–150. [Google Scholar]
- Feder ME, Hofmann GE. Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annual Review of Physiology. 1999;61:243–282. doi: 10.1146/annurev.physiol.61.1.243. [DOI] [PubMed] [Google Scholar]
- Fischer JM, Nicolai JL, Williamson CE, Persaud AD, Lockwood RS. Effects of ultraviolet radiation on diel vertical migration of crustacean zooplankton: an in situ mesocosm experiment. Hydrobiologia. 2006;563:217–224. [Google Scholar]
- Frederik JE, Snell HE, Haywood EK. Solar ultraviolet radiation at the earth’s surface. Photochemistry and Photobiology. 1989;50:443–450. [Google Scholar]
- García PE, Pérez AP, Diéguez MC, Ferraro MA, Zagarese HE. Dual control of the levels of photoprotective compounds by ultraviolet radiation and temperature in the freshwater copepod Boeckella antiqua. Journal of Plankton Research. 2008;30:817–827. [Google Scholar]
- García PE, Diéguez MC, Ferraro MA, Zagarese HE, Pérez AP. Mycosporine-like amino acids in freshwater copepods: potential sources and some factors that affect their bioaccumulation. Photochemistry and Photobiology. 2010;86:353–359. doi: 10.1111/j.1751-1097.2009.00670.x. doi:10.1111/j.1751-1097.2009.00670.x. [DOI] [PubMed] [Google Scholar]
- Garcia-Pichel F, Castenholz RW. Characterization and biological implications of scytonemin, a cyanobacterial sheath pigment. Journal of Phycology. 1991;27:395–409. [Google Scholar]
- Gilchrist BM, Green J. Carotenoid pigments in Rotifera. Nature. 1962;195:905–907. [Google Scholar]
- Gliwicz ZM. Predation and the evolution of vertical migration in zooplankton. Nature. 1986;320:746–748. [Google Scholar]
- Gonçalves RJ, Villafañe VE, Helbling EW. Photorepair activity and protective compounds in two freshwater zooplankton species (Daphnia menucoensis and Metacyclops mendocinus) from Patagonia, Argentina. Photochemical & Photobiological Sciences. 2002;1:996–1000. doi: 10.1039/b208145h. [DOI] [PubMed] [Google Scholar]
- Grad G, Williamson CE, Karapelou DM. Zooplankton survival and reproduction responses to damaging UV radiation: a test of reciprocity and photoenzymatic repair. Limnology and Oceanography. 2001;46:584–591. [Google Scholar]
- Grad G, Burnett BJ, Williamson CE. UV damage and photoreactivation: timing and age are everything. Photochemistry and Photobiology. 2003;78:225–227. doi: 10.1562/0031-8655(2003)078<0225:udapta>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Hairston NG., Jr. Photoprotection by carotenoid pigments in the copepod Diaptomus nevadensis. Proceedings of the National Academy of Sciences of the United States of America. 1976;73:971–974. doi: 10.1073/pnas.73.3.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hairston NG., Jr. Carotenoid photoprotection in Diaptomus kenai. Verhandlungen der Internationale Vereingung für theoretische und angewandte Limnologie. 1978;20:2541–2545. [Google Scholar]
- Hairston NG., Jr. The adaptive significance of color polymorphism in two species of Diaptomus Copepoda. Limnology and Oceanography. 1979;24:15–37. [Google Scholar]
- Hairston NG., Jr. Williams WD, editor. The interaction of salinity, predators, light and copepod color. Hydrobiologia. 1981;81:151–158. Salt Lakes: proceedings of an International Symposium on Athalassic Salt Lakes. [Google Scholar]
- Häkkinen J, Vehniäninen E, Oikari A. High sensitivity of nothern pike larvae to UV-B but no UV-photoinduced toxicity of retene. Aquatic Toxicology. 2004;66:393–404. doi: 10.1016/j.aquatox.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Hanssen-Bauer I, Forland EJ. Long term trends in precipitation and temperature in the Norwegian Arctic: can they be explained by changes in atmospheric circulations patterns? Climate Research. 1998;10:143–153. [Google Scholar]
- Hansson L-A. Induced pigmentation in zooplankton: a trade-off between threats from predation and ultraviolet radiation. Proceedings of the Royal Society of London Series (B) 2000;267:2327–2331. doi: 10.1098/rspb.2000.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson L-A. Plasticity in pigmentation induced by conflicting threats from predation and UV radiation. Ecology. 2004;85:1005–1016. [Google Scholar]
- Hansson L-A, Hylander S. Effects of ultraviolet radiation on pigmentation, photoenzymatic repair, behaviour, and community ecology of zooplankton. Photochemical and Photobiological Sciences. 2009a;8:1266–1275. doi: 10.1039/b908825c. [DOI] [PubMed] [Google Scholar]
- Hansson L-A, Hylander S. Size-structured risk assessments govern Daphnia migration. Proceedings of the Royal Society (B) 2009b;276:331–336. doi: 10.1098/rspb.2008.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson L-A, Hylander S, Sommaruga R. Escape from UV threats in zooplankton: a cocktail of behavior and protective pigmentation. Ecology. 2007;88:1932–1939. doi: 10.1890/06-2038.1. [DOI] [PubMed] [Google Scholar]
- Hebert PDN, Emery CJ. The adaptative significance of cuticular pigmentation in Daphnia. Functional Ecology. 1990;4:703–710. [Google Scholar]
- Helbling EW, Zaratti F, Sala LO, Palenque ER, Menchi CF, Villafañe VE. Mycosporine-like amino acids protect the copepod Boeckella titicacae (Harding) against high levels of solar UVR. Journal of Plankton Research. 2002;24:225–234. [Google Scholar]
- Hessen DO. DNA-damage and pigmentation in alpine and arctic zooplankton as bioindicators of UV-radiation. Verhandlungen der Internationale Vereingung für theoretische und angewandte Limnologie. 1993;25:482–486. [Google Scholar]
- Hessen DO. Daphnia responses to UV-light. Archiv für Hydrobiologie. Beiheft Ergebnisse der Limnologie. 1994;43:185–195. [Google Scholar]
- Hessen DO. Competitive trade-off strategies in Arctic Daphnia linked to melanism and UV-B stress. Polar Biology. 1996;16:573–576. [Google Scholar]
- Hessen DO. UV-radiation and Arctic freshwater zooplankton. In: Hessen DO, editor. UV radiation and Arctic Ecosystems. Springer-Verlag; 2001. pp. 157–184. Ecological Series 153. [Google Scholar]
- Hessen DO, Sørensen K. Photoprotective pigmentation in alpine zooplankton populations. Aqua Fennica. 1990;20:165–170. [Google Scholar]
- Hessen DO, Alstad Rukke NEW. Increased UV-susceptibility in Daphnia at low calcium concentrations. Limnology and Oceanography. 2000;45:1834–1838. [Google Scholar]
- Hessen DO, de Lange HJ, van Donk E. UV-induced changes in phytoplankton cells and its effects on grazers. Freshwater Biology. 1997;38:513–524. [Google Scholar]
- Hessen DO, Borgeraas J, Kessler K, Refseth UH. UV-B susceptibility and photoprotection of Arctic Daphnia morphotypes. Polar Research. 1999;18:345–352. [Google Scholar]
- Hessen DO, Borgeraas J, Ørbæk JB. Responses in pigmentation and anti-oxidant expression in Arctic Daphnia along gradients of DOC and UV exposure. Journal of Plankton Research. 2002;24:1009–1018. [Google Scholar]
- Hiratsuka A, Saito H, Hirose K, Watabe T. Marked expression of glutathione S-transferase A4-4 detoxifying 4-hydroxy-2(E)-nonenal in the skin of rats irradiated by ultraviolet B-band light (UVB) Biochemical and Biophysical Research Communications. 1999;260:740–746. doi: 10.1006/bbrc.1999.0971. [DOI] [PubMed] [Google Scholar]
- Hobaeck A, Wolf HG. Ecological genetics of Norwegian Daphnia. II. Distribution of Daphnia longispina genotypes in relation to short-wave radiation and water colour. Hydrobiologia. 1991;225:229–243. [Google Scholar]
- Huebner JD, Young DLW, Loadman NL, Lentz VJ, Wiegand MD. Age-dependent survival, reproduction and photorepair activity in Daphnia magna (Straus, 1820) after exposure to artificial ultraviolet radiation. Photochemistry and Photobiology. 2006;82:1656–1661. doi: 10.1562/2006-05-03-RA-890. [DOI] [PubMed] [Google Scholar]
- Hurtubise RD, Havel JE, Little EE. The effects of ultraviolet-B radiation on freshwater invertebrates: experiments with a solar simulator. Limnology and Oceanography. 1998;43:1082–1088. [Google Scholar]
- Hylander S, Hansson L-A. Vertical migration mitigates UV effects on zooplankton community composition. Journal of Plankton Research. 2010 (in press). doi:10.1093/plankt/fbq037. [Google Scholar]
- Hylander S, Jephson T. UV protective compounds transferred from a marine dinoflagellate to its copepod predator. Journal of Experimental Marine Biology and Ecology. 2010 in press. [Google Scholar]
- Hylander S, Larsson N, Hansson L-A. Zooplankton vertical migration and plasticity of pigmentation arising from simultaneous UV and predation threats. Limnology and Oceanography. 2009a;54:483–491. [Google Scholar]
- Hylander S, Boing WJ, Granéli W, Karlsson J, Von Einem J, Gutseit K, Hansson L-A. Complementary UV protective compounds in zooplankton. Limnology and Oceanography. 2009b;54:1883–1893. [Google Scholar]
- International Arctic Science Committee . Effects of increased ultraviolet radiation in the Arctic. Norway: 1995. p. 56. IASC Report, n.2. [Google Scholar]
- Jokiel PL. Solar ultraviolet radiation and coral reef epifauna. Science. 1980;207:1069–1071. doi: 10.1126/science.207.4435.1069. [DOI] [PubMed] [Google Scholar]
- Karentz D. Chemical defenses of marine organisms against solar radiation exposure: UV-absorbing mycosporine-like amino acids and scytonemin. In: McClintock JB, Baker BJ, editors. Marine Chemical Ecology. CRC Press; 2001. pp. 481–519. [Google Scholar]
- Kerb R, Brockmöller J, Reum T, Roots I. Deficiency of glutathione S- transferases T1 and M1 as heritable factors of increased cutaneous UV sensitivity. Journal of Investigative Dermatology. 1997;108:229–232. doi: 10.1111/1523-1747.ep12335337. [DOI] [PubMed] [Google Scholar]
- Kessler K, Lockwood RS, Williamson CE. Vertical distribution of zooplankton in subalpine and alpine lakes: ultraviolet radiation, fish predation, and the transparency-gradient hypothesis. Limnology and Oceanography. 2008;53:2374–2382. [Google Scholar]
- Kirchhoff VWJH, Schuch NJ, Pinheiro DK, Harris JM. Evidence for an ozone hole perturbation at 30° south. Atmospheric Environment. 1996;30:1481–1488. [Google Scholar]
- Kirk JTO. Optics of UV-B radiation in natural waters. Ergebnisse der Limnologie. 1994;43:1–16. [Google Scholar]
- Koehler O. Über das Farbensehen von Daphnia magna Straus. Zeitschrift für vergleichende Physiologie. 1924;1:84–174. [Google Scholar]
- Kouwenberg JHM, Browman HI, Runge JA, Cullen JJ, Davis RF, St-Pierre J-F. Biological weighting of ultraviolet (280–400 nm) induced mortality in marine zooplankton and fish. II. Calanus finmarchicus (Copepods) eggs. Marine Biology. 1999;134:285–293. [Google Scholar]
- Laurion I, Vincent WF, Lean DRS. Underwater ultraviolet radiation: development of spectral models for northern high latitude lakes. Photochemistry and Photobiology. 1997;65:107–114. [Google Scholar]
- Laurion I, Ventura M, Catalan J, Psenner R, Sommaruga R. Attenuation of ultraviolet radiation in mountain lakes: factors controlling the among- and within-lake variability. Limnology and Oceanography. 2000;45:1274–1288. [Google Scholar]
- Leech DM, Williamson CE. Is tolerance to ultraviolet radiation in zooplankton related to body size, taxon, or lake transparency? Ecological Applications. 2000;10:1530–1540. [Google Scholar]
- Leech DM, Williamson CE. In situ exposure to ultraviolet radiation alters the depth distribution of Daphnia. Limnology and Oceanography. 2001;46:416–420. [Google Scholar]
- Leech DM, Moeller RE, Hargreaves BR. Effects of ultraviolet radiation on the seasonal vertical distribution of zooplankton: a database analysis. Archiv für Hydrobiologie. 2005a;162:445–464. [Google Scholar]
- Leech DM, Padeletti A, Williamson CE. Zooplankton behavioral responses to solar UV radiation vary within and among lakes. Journal of Plankton Research. 2005b;27:461–471. [Google Scholar]
- Leu E, Færøvig PJ, Hessen DO. UV effects on stoichiometry and PUFAs of Selenastrum capricornutum and their consequences for the grazer Daphnia magna. Freshwater Biology. 2006;51:2296–2308. [Google Scholar]
- Livingstone D. Break-up dates of alpine lakes as proxy data for local and regional mean surface air temperatures. Climatic Change. 1997;37:407–439. [Google Scholar]
- Lorenzen CJ. Ultraviolet radiation and phytoplankton photosynthesis. Limnology and Oceanography. 1979;24:1117–1120. [Google Scholar]
- Luecke C, O’Brian WJ. Photoprotective pigments in a pond morph of Daphnia middendorffianna. Arctic. 1983;36:365–368. [Google Scholar]
- MacFadyen EJ, Williamson CE, Grad G, Lowery M, Jeffrey WH, Mitchell DL. Molecular response to climate change: temperature dependence of UV-induced DNA damage and repair in the freshwater crustacean Daphnia pulicaria. Global Change Biology. 2004;10:408–416. [Google Scholar]
- Magnuson JJ, Robertson DM, Benson BJ, Wynne RH, Livingstone DM, Arai T, Assel RA, Barry RG, Card V, Kuusisto E, Granin NG, Prowse TD, Stewart KM, Vuglinski VS. Historical trends in lake and river ice cover in the Northern Hemisphere. Science. 2000;289:1743–1746. doi: 10.1126/science.289.5485.1743. [DOI] [PubMed] [Google Scholar]
- Malloy KD, Holman MA, Mitchell D, Detrich HW., III Solar UVB-induced DNA damage and photoenzymatic DNA repair in Antarctic zooplankton. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:1258–1263. doi: 10.1073/pnas.94.4.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinone MC, Menu Marque S, Añón Suárez D, Dieguex M, Perez P, De Los Rios P, Soto D, Zagarese HE. UVR radiation as a potential driving force for zooplankton community structure in Patagonian lakes. Photochemistry and Photobiology. 2006;82:962–971. doi: 10.1562/2005-09-09-RA-680. [DOI] [PubMed] [Google Scholar]
- McKnight DM, Andrews ED, Spaulding SA, Aiken GR. Aquatic fulvic acids in algal-rich antarctic ponds. Limnology and Oceanography. 1994;39:1972–1979. [Google Scholar]
- McKnight DM, Bover EW, Westerhoff PK, Doran PT, Kulbe T, Andersen DT. Spectrofluorometric characterization of dissolved organic matter for indication of precursor organic material and aromaticity. Limnology and Oceanography. 2001;46:38–48. [Google Scholar]
- Merker E. Sehen die Daphnien ultraviolettes Licht? Zoologische Jahrbücher. Abteilung für allgemeine Zoologie und Physiologie der Tiere. 1930;48:277–348. [Google Scholar]
- Mitchell DL, Karentz D. The induction and repair of DNA photodamage in the environment. In: Young AR, Björn LO, Moan J, Nultsch W, editors. Environmental UV photobiology. Plenum Press; 1993. pp. 345–371. [Google Scholar]
- Moeller RE, Shawna G, Williamson CE, Dee G, Sommaruga R. Dietary acquisition of photoprotective compounds (mycosporine-like amino acids, carotenoids) and acclimation to ultraviolet radiation in a freshwater copepod. Limnology and Oceanography. 2005;50:427–439. [Google Scholar]
- Molot LA, Keller W, Leavitt PR, Robarts RD, Waiser MJ, Arts MT, Clair TA, Pienitz R, Yan ND, McNicol DK, Prairie YT, Dillon PJ, Macrae M, Bello R, Nordin RN, Curtis PJ, Smol JP, Douglas MSV. Risk analysis of dissolved organic matter-mediated ultraviolet B exposure in Canadian inland waters. Canadian Journal of Fisheries and Aquatic Sciences. 2004;61:2511–2521. [Google Scholar]
- Morris DP, Zagarese H, Williamson CE, Balserio EG, Hargreaves B, Modenutti B, Moeller R, Queimalinos C. The attenuation of UV radiation in lakes and the role of dissolved organic carbon. Limnology and Oceanography. 1995;40:1381–1391. [Google Scholar]
- Mostajir B, Bustillos-Guzman J, Claustre H, Rassoulzadegan F. Pigment dynamics associated with the grazing of a ciliate and a flagellate feeding on a cyanobacterium. Oceanologica Acta. 1998;21:581–588. [Google Scholar]
- Murali NS, Teramura AH, Randall SK. Response differences between two soybean cultivars with contrasting UVB radiation sensitivities. Photochemistry and Photobiology. 1988;48:653–657. [Google Scholar]
- Nagiller K, Sommaruga R. Differential tolerance of UV radiation between Chaoborus species and role of photoprotective compounds. Journal of Plankton Research. 2009;31:503–513. [Google Scholar]
- Neale PJ, Kieber DJ. Assessing biological and chemical effects of UV in the marine environment: spectral weighting functions. In: Hester RE, Harrison RM, editors. Causes and Environmental Implications of Increased U.V.-B. Radiation. Royal Society of Chemistry; 2000. pp. 61–83. Issues in Environmental Science and Technology, 14. [Google Scholar]
- Obertegger U, Flaim G, Sommaruga R. Multifactorial nature of rotifer water layer preferences in an oligotrophic lake. Journal of Plankton Research. 2008;30:633–643. [Google Scholar]
- Patalas K. Diversity of the zooplankton communities in Canadian lakes as a function of climate. Verhandlungen der Internationalen Vereinigung für theoretische und angewandte Limnologie. 1990;24:360–368. [Google Scholar]
- Perin S, Lean DRS. The effects of ultraviolet-B radiation on freshwater ecosystems of the Arctic: influence from stratospheric ozone depletion and climate change. Environmental Review. 2004;12:1–70. [Google Scholar]
- Persaud AD, Williamson CE. Ultraviolet and temperature effects on planktonic rotifers and crustaceans in northern temperate lakes. Freshwater Biology. 2005;50:467–476. [Google Scholar]
- Persaud AD, Arts MT, Yan ND. Photoresponses of late instar Chaoborus punctipennis larvae to UVR. Aquatic Ecology. 2003;37:257–265. [Google Scholar]
- Persaud AD, Moeller RE, Williamson CE, Burns CW. Photoprotective compounds in weakly and strongly pigmented copepods and co-occurring cladocerans. Freshwater Biology. 2007;52:2121–2133. [Google Scholar]
- Quayle WC, Peck LS, Peat H, Ellis-Evans J-C, Harrigan PR. Extreme esponses to climate change in Antarctic lakes. Science. 2002;295:645. doi: 10.1126/science.1064074. [DOI] [PubMed] [Google Scholar]
- Ramos-Jiliberto R, Dauelsberg P, Zúñiga LR. Differential tolerance to ultraviolet-B light and photoenzymatic repair in cladocerans in a Chilean lake. Marine Freshwater Research. 2004;55:193–200. [Google Scholar]
- Rautio M. The use of Cladocera in paleolimnology. In: Elias SA, editor. Encyclopedia of Quaternary Sciences. Elsevier, B.V.; 2007. pp. 2031–2039. [Google Scholar]
- Rautio M, Korhola A. Effects of ultraviolet radiation and dissolved organic carbon on the survival of subarctic zooplankton. Polar Biology. 2002a;25:469–473. [Google Scholar]
- Rautio M, Korhola A. UV-induced pigmentation in subarctic Daphnia. Limnology and Oceanography. 2002b;47:295–299. [Google Scholar]
- Rautio M, Korhola A, Zellmer ID. Vertical distribution of Daphnia longispina in a shallow subarctic pond: does the interaction of ultraviolet radiation and Chaoborus predation explain the pattern? Polar Biology. 2003;26:659–665. [Google Scholar]
- Rautio M, Bayly I, Gibson J, Nyman M. Zooplankton and zoobenthos in high-latitude water bodies. In: Vincent WF, Laybourn-Parry J, editors. High Latitude Lake and River Ecosystems. Oxford University Press; 2008. pp. 231–247. [Google Scholar]
- Rautio M, Bonilla S, Vincent WF. UV photoprotectants in arctic zooplankton. Aquatic Sciences. 2009;7:93–105. [Google Scholar]
- Rhode SC, Pawlowski M, Tollrian R. The impact of ultraviolet radiation on the vertical distribution of zooplankton of the genus Daphnia. Nature. 2001;412:69–72. doi: 10.1038/35083567. [DOI] [PubMed] [Google Scholar]
- Ringelberg J. A mechanism of predator-mediated induction of diel vertical migration in Daphnia hyalina. Journal of Plankton Research. 1991;13:83–89. [Google Scholar]
- Ringelberg J. The photobehaviour of Daphnia spp. as a model to explain diel vertical migration in zooplankton. Biological Reviews of the Cambridge Philosophical Society. 1999;74:397–423. [Google Scholar]
- Ringelberg J, Hallegraeff CM. Evidence for a diurnal variation in the carotenoid content of Acanthodiaptomus denticornis (Crustacea, Copepoda) in Lac Pavin (Auvergne, France) Hydrobiologia. 1976;51:113–118. [Google Scholar]
- Ringelberg J, Keyser AL, Flik BJG. The mortality effect of ultraviolet radiation in a translucent and in a red morph of Acanthodiaptomus denticornis (Crustacea, Copepoda) and its possible ecological relevance. Hydrobiologia. 1984;112:217–222. [Google Scholar]
- Roberts L. Does the ozone hole threaten Antarctic life? Science. 1989;244:288–289. doi: 10.1126/science.244.4902.288. [DOI] [PubMed] [Google Scholar]
- Rocco VE, Oppezzo O, Pizarro R, Sommaruga R, Ferraro M, Zagarese HE. Ultraviolet damage and counteracting mechanisms in the freshwater copepod Boeckella poppei from the Antarctic Peninsula. Limnology and Oceanography. 2002;47:829–836. [Google Scholar]
- Rosén P, Cunningham L, Vonk J, Karlsson J. Effects of climate on organic carbon and the ratio of planktonic to benthic primary producers in a subarctic lake during the past 45 years. Limnology and Oceanography. 2009;54:1723–1732. [Google Scholar]
- Rouse WR, Douglas MSV, Hecky RE, Hershey AE, Kling GW, Lesack L, Marsh P, McDonald M, Nicholson BJ, Roulet NT, Smol JP. Effects of climate change on the freshwaters of arctic and subarctic North America. In: Cushing C, editor. Freshwater Ecosystems and Climate Change in North America. John Wiley and Sons; Chichester: 1997. pp. 55–84. [Google Scholar]
- Salonen K, Hammer T. On the importance of dissolved organic matter in the nutrition of zooplankton in some waters. Oecologia. 1986;68:246–253. doi: 10.1007/BF00384795. [DOI] [PubMed] [Google Scholar]
- Sancar A. Mechanisms of DNA excision repair. Science. 1994a;266:1954–1956. doi: 10.1126/science.7801120. [DOI] [PubMed] [Google Scholar]
- Sancar A. Structure and function of DNA photolyase. Biochemistry. 1994b;33:2–9. doi: 10.1021/bi00167a001. [DOI] [PubMed] [Google Scholar]
- Sanders BM. Stress proteins in aquatic organisms: an environmental perspective. Critical Reviews in Toxicology. 1993;23:49–75. doi: 10.3109/10408449309104074. [DOI] [PubMed] [Google Scholar]
- Sawada M, Enesco HE. Effects of UV radiation on the lifespan of the rotifer Asplanchna brightwelli. Experimental Gerontology. 1984;19:289–296. doi: 10.1016/0531-5565(84)90001-9. [DOI] [PubMed] [Google Scholar]
- Schindler DW, Curtis PJ, Parker BR, Stainton MP. Consequences of climate warming and lake acidification for UV-B penetration in North American boreal lakes. Nature. 1996;379:705–707. [Google Scholar]
- Schönberger M, Zellmer ID. The influence of food on population dynamics and UV-tolerance of Daphnia obtuse Kurz in a mountain pond. Verhandlungen der Internationalen Vereinigung für theoretische und angewandte Limnologie. 1994;25:2388–2391. [Google Scholar]
- Scott JD, Chalker-Scott L, Foreman AE, D’Angelo M. Daphnia pulex fed UVB-irradiated Chlamydomonas reihardtii show decreased survival and fecundity. Photochemistry and Photobiology. 1999;70:308–313. [Google Scholar]
- Shick JM, Dunlap WC. Mycosporine-like amino acids and related gadusols: Biosynthesis, accumulation, and UV-protective functions in aquatic organisms. Annual Review of Physiology. 2002;64:223–262. doi: 10.1146/annurev.physiol.64.081501.155802. [DOI] [PubMed] [Google Scholar]
- Siebeck O. Ultraviolet tolerance of planktonic crustaceans. Verhandlungen der Internationalen Vereinigung für theoretische und angewandte Limnologie. 1978;20:2469–2473. [Google Scholar]
- Siebeck O, Böhm U. UV-B effects on aquatic animals. Verhandlungen der Internationalen Vereinigung für theoretische und angewandte Limnologie. 1991;24:2773–2777. [Google Scholar]
- Siebeck O, Böhm U. Challenges for appraisal UV-B effects upon planktonic crustaceans under natural radiation conditions with a non-migrating (Daphnia pulex obtusa) and a migrating cladoceran (Daphnia galeata) Archiv für Hydrobiologie, Supplement Ergebnisse der Limnologie. 1994;43:197–206. [Google Scholar]
- Siebeck O, Vail TL, Williamson CE, Vetter R, Hassen D, Zagarese H, Little E, Balseiro E, Modenutti B, Seva J, Shumate A. Impact of UV-B radiation on zooplankton and fish in pelagic freshwater ecosystems. Ergebnisse der Limnologie. 1994;43:101–114. [Google Scholar]
- Sinha RP, Häder D-P. UV-induced DNA damage and repair: a review. Photochemical and Photobiological Sciences. 2002;1:225–236. doi: 10.1039/b201230h. [DOI] [PubMed] [Google Scholar]
- Smith KC, Macagno ER. UV photoreceptors in the compound eye of Daphnia magna (Crustacea, Branchiopoda). A fourth spectral class in single ommatidia. Journal of Comparative Physiology. 1990;166:597–606. doi: 10.1007/BF00240009. [DOI] [PubMed] [Google Scholar]
- Sommaruga R. The role of solar UV radiation in the ecology of alpine lakes. Journal of Photochemistry and Photobiology B: Biology. 2001;62:35–42. doi: 10.1016/s1011-1344(01)00154-3. [DOI] [PubMed] [Google Scholar]
- Sommaruga R. Ultraviolet radiation and its effects on species interactions. In: Helbling W, Zagarese H, editors. UV Effects in Aquatic Organisms and Ecosystems. European Society for Photobiology, Royal Society of Chemistry; London: 2003. pp. 485–508. Comprehensive Series in Photosciences. [Google Scholar]
- Sommaruga R. Preferential accumulation of carotenoids rather than of mycosporine-like amino acids in copepods from high altitude Himalayan lakes. Hydrobiologia. 2010 Special Issue Global Change Impacts on Mountain Lakes. (in press). doi:10.1007/s10750-010-0141-y. [Google Scholar]
- Sommaruga R, Augustin G. Seasonality in UV transparency of an alpine lake is associated to changes in phytoplankton biomass. Aquatic Sciences. 2006;68:129–141. [Google Scholar]
- Sommaruga R, Garcia-Pichel F. UV-absorbing mycosporine-like compounds in planktonic and benthic organisms from a high-mountain lake. Archiv für Hydrobiologie. 1999;144:255–269. [Google Scholar]
- Sommaruga R, Psenner R, Schafferer E, Koinig KA, Sommaruga-Wögrath S. Dissolved organic carbon concentration and phytoplankton biomass in high-mountain lakes of the Austrian Alps: potential effects of climatic warming on UV underwater attenuation. Arctic Antarctic and Alpine Research. 1999;31:247–254. [Google Scholar]
- Sommaruga-Wögrath S, Koinig KA, Schmidt R, Sommaruga R, Tessadri R, Psenner R. Temperature effects on the acidity of remote alpine lakes. Nature. 1997;387:64–67. [Google Scholar]
- Sonntag B, Summerer M, Sommaruga R. Sources of mycosporine-like amino acids in planktonic Chlorella-bearing ciliates (Ciliophora) Freshwater Biology. 2007;52:1476–1485. [Google Scholar]
- Souza MS, Modenutti BE, Balseiro EB. Antioxidant defenses in planktonic crustaceans exposed to different underwater light irradiances and Andean lakes. Water Air and Soil Pollution. 2007;183:49–57. [Google Scholar]
- Stich HB, Lampert W. Predator evasion as an explanation of diurnal migration by zooplankton. Nature. 1981;293:396–398. [Google Scholar]
- Stich HB, Lampert W. Growth and reproduction of migrating and non-migrating Daphnia species under simulated food and temperature conditions of diurnal vertical migration. Oecologia. 1984;61:192–196. doi: 10.1007/BF00396759. [DOI] [PubMed] [Google Scholar]
- Storz UC, Paul RJ. Phototaxis in water fleas (Daphnia magna) is differently influenced by visible and UV light. Journal of Comparative Physiology. 1998;183:709–717. [Google Scholar]
- Stutzman PL. A comparative study of ultraviolet radiation in different populations of Diaptomus minutes. Journal of Plankton Research. 1999;21:387–400. [Google Scholar]
- Sutherland BM. Photoreactivation. Bioscience. 1981;31:439–444. [Google Scholar]
- Tartarotti B, Sommaruga R. Seasonal and ontogenetic changes of mycosporine-like amino acids in planktonic organisms from an alpine lake. Limnology and Oceanography. 2006;51:1530–1541. doi: 10.4319/lo.2006.51.3.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartarotti B, Torres JJ. Sublethal stress: impact of solar UV radiation on protein synthesis in the copepod Acartia tonsa. Journal of Experimental Marine Biology and Ecology. 2009;375:106–113. doi: 10.1016/j.jembe.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartarotti B, Cabrera S, Psenner R, Sommaruga R. Survivorship of Cyclops abyssorum tatricus (Cyclopoida, Copepoda) and Boeckella gracilipes (Calanoida, Copepoda) under ambient levels of solar UVB radiation in two high-mountain lakes. Journal of Plankton Research. 1999;21:549–560. [Google Scholar]
- Tartarotti B, Cravero W, Zagarese HE. Biological weighting function for the mortality of Boeckella gracilipes (Copepods, Crustacea) derived from experiments with natural solar radiation. Photochemistry and Photobiology. 2000;73:314–319. doi: 10.1562/0031-8655(2000)072<0314:bwfftm>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Tartarotti B, Laurion I, Sommaruga R. Large variability in the concentration of mycosporine-like amino acids among zooplankton from lakes located across an altitude gradient. Limnology and Oceanography. 2001;46:1546–1552. [Google Scholar]
- Tartarotti B, Baffico G, Temporetti P, Zagarese HE. Mycosporine-like amino acids in planktonic organisms living under different UV exposure conditions in Patagonian lakes. Journal of Plankton Research. 2004;26:753–762. doi: 10.1093/plankt/fbh073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollrian R, Heibl C. Phenotypic plasticity in pigmentation in Daphnia induced by UV radiation and fish kairomones. Functional Ecology. 2004;18:497–502. [Google Scholar]
- Trautinger F, KindasMugge I, Knobler RM, Honigsmann H. Stress proteins in the cellular response to ultraviolet radiation. Journal of Photochemistry and Photobiology. 1996;35:141–148. doi: 10.1016/s1011-1344(96)07344-7. [DOI] [PubMed] [Google Scholar]
- van der Veen IT. Costly carotenoids: a trade-off between predation and infection risk? Journal of Evolutionary Biology. 2005;18:992–999. doi: 10.1111/j.1420-9101.2005.00903.x. [DOI] [PubMed] [Google Scholar]
- Vega MP, Pizarro R. Lethal effect induced by ultraviolet-B in a planktonic copepod: role of the post-irradiation time on mortality measurements. Journal of Freshwater Ecology. 2000;15:1–5. [Google Scholar]
- Vehniäinen E-R, Häkkinen J, Oikari A. Photoinduced lethal and sublethal toxicity of retene, a polycyclic aromatic hydrocarbon derived from resin acid, to coregonid larvae. Environmental Toxicology and Chemistry. 2003;22:2295–3000. doi: 10.1897/02-569. [DOI] [PubMed] [Google Scholar]
- Vincent WF, Neale PJ. Mechanisms of UV damage to aquatic organisms. In: Demers MS, Vernet M, editors. The Effects of UV Radiation in the Marine Environment. Cambridge University Press; Cambridge: 2000. pp. 149–176. [Google Scholar]
- Vincent WF, Pienitz R. Sensitivity of high latitude freshwater ecosystems to global change: temperature and solar ultraviolet radiation. Geoscience Canada. 1996;23:231–236. [Google Scholar]
- Vincent WF, Gibson JAE, Jeffries MO. Climate change, ice shelf collapse and habitat loss in the Canadian High Arctic. Polar Research. 2001;37:133–42. [Google Scholar]
- Vincent WF, Rautio M, Pienitz R. Climate control of underwater UV exposure in polar and alpine aquatic ecosystems. In: Orbaek JB, Kallenborn R, Tombre I, Hegseth E, Falk-Petersen A, Hoel AH, editors. Arctic Alpine Ecosystems and People in a Changing Environment. Springer; Berlin: 2007. pp. 227–249. [Google Scholar]
- Vinebrooke RD, Leavitt PR. Direct and interactive effects of allochthonous dissolved organic matter, inorganic nutrients, and ultraviolet radiation on an alpine littoral food web. Limnology and Oceanography. 1998;43:1065–1081. [Google Scholar]
- Vinebrooke RD, Leavitt PR. Differential responses of littoral communities to ultraviolet radiation in an alpine lake. Ecology. 1999;80:223–237. [Google Scholar]
- Vinebrooke RD, Leavitt PR. Mountain lakes as indicators of the cumulative impacts of ultraviolet radiation and other environmental stressors. In: Huber UM, Bugmann KM, Reasoner MA, editors. Global Change and Mountain Regions – A State of Knowledge Overview. Springer-Verlag; 2005. pp. 497–509. [Google Scholar]
- Wang P, Schellhorn HE. Induction of resistance to hydrogen peroxide and radiation in Deinococcus radiodurans. Canadian Journal of Microbiology. 1995;41:170–176. doi: 10.1139/m95-023. [DOI] [PubMed] [Google Scholar]
- Weatherhead B, Tanskanen A, Stevermer S, Andersen A, Arola A, Austin J, Bernhard G, Browman H, Fioletov V, Grewe V, Hermna J, Josefsson W, Kylling A, Kyrö E, Lindfors A, Shindell D, Taalas P, Tarasick D. ACIA 2005, Arctic Climate Impact Assessment. Cambridge University Press; 2005. Ozone and ultraviolet radiation; pp. 151–162. Chapter 5. [Google Scholar]
- Weider LJ. Life-history variation among low-arctic clones of obligately parthenogenetic Daphnia-pulex – a diploid-polyploid complex. Oecologia. 1987;73:251–256. doi: 10.1007/BF00377515. [DOI] [PubMed] [Google Scholar]
- Williamson CE, Rose KC. Ultraviolet insights: Attempting to resolve enigmatic patterns in pelagic freshwaters – the historical context and a view to the future. International Review of Hydrobiology. 2009;94:129–142. [Google Scholar]
- Williamson CE, Zagarese HE, Schulze PC, Hargreaves BR, Seva J. The impact of short-term exposure to UV-B readiation on zooplankton communities in north temperate lakes. Journal of Plankton Research. 1994;16:205–218. [Google Scholar]
- Williamson CE, Stemberger RS, Morris DP, Frost TM, Paulsen SG. Ultraviolet radiation in North American lakes: attenuation estimates from DOC measurements and implications for plankton communities. Limnology and Oceanography. 1996;41:1024–1034. [Google Scholar]
- Williamson CE, Hargreaves BR, Orr PS, Lovera PA. Does UV play a role in changes in predation and zooplankton community structure in acidified lakes? Limnology and Oceanography. 1999;44:774–783. [Google Scholar]
- Williamson CE, Neale PJ, Grad G, de Lange HJ, Hargreaves BR. Beneficial and detrimental effects of UV on aquatic organisms: implications of spectral variation. Ecological Applications. 2001a;11:1843–1857. [Google Scholar]
- Williamson CE, Olson OG, Lott SE, Walker ND, Engström DR, Hargreaves BR. Ultraviolet radiation and zooplankton community structure following deglaciation in Glacier Bay, Alaska. Ecology. 2001b;82:1748–1760. [Google Scholar]
- Williamson CE, Grad G, de Lange HJ, Gilroy S, Karapelou DM. Temperature-dependent ultraviolet responses in zooplankton: implications of climate change. Limnology and Oceanography. 2002;47:1844–1848. [Google Scholar]
- Wulff A, Wängberg S-Å, Sundbäck K, Nilsson C, Underwood GJC. Effects of UVB radiation on a marine microphytobenthic community growing on a sand-substratum under different nutrient conditions. Limnology and Oceanography. 2000;45:1144–1152. [Google Scholar]
- Zagarese HE, Williamson CE, Mislivets M, Orr P. The vulnerability of Daphnia to UV-B radiation in the northeastern United States. Archiv of Hydrobiologie Beiheft. 1994;43:207–216. [Google Scholar]
- Zagarese HE, Feldman M, Williamson CE. UV-B induced damage and photoreactivation in three species of Boeckella (Copepods, Calanoida) Journal of Plankton Research. 1997a;19:357–367. [Google Scholar]
- Zagarese HE, Williamson CE, Vail TL, Olsen O, Queimaliños C. Long-term exposure of Boeckella gibbosa (Copepoda, Calanoida) to in situ levels of solar UVB radiation. Freshwater Biology. 1997b;37:99–106. [Google Scholar]
- Zagarese HE, Tartarotti B, Cravero W, Gonzalez P. UV damage in shallow lakes: the implications of water mixing. Journal of Plankton Research. 1998;20:1423–1433. [Google Scholar]
- Zagarese HE, Diaz M, Pedrozo F, Ubeda C. Mountain lakes in northwestern Patagonia. Verhandlungen der Internationale Vereingung für theoretische und angewandte Limnologie. 2000;27:533–538. [Google Scholar]
- Zagarese HE, Tartarotti B, Añón Suárez A. The significance of ultraviolet radiation for aquatic organisms. In: Ambasht RS, Ambasht NK, editors. Modern Trends in Applied Aquatic Ecology. KluwerAcademic/Plenum Press; New York, USA: 2003. pp. 173–200. [Google Scholar]
- Zaret TM, Suffern JS. Vertical migration in zooplankton as a predator avoidance mechanism. Limnology and Oceanography. 1976;21:804–813. [Google Scholar]
- Zellmer ID. UV-B-tolerance of alpine and arctic Daphnia. Hydrobiologia. 1995;307:153–159. [Google Scholar]
- Zellmer ID. The impact on UV-B tolerance and recovery from UV-B damage in Daphnia pulex. Hydrobiologia. 1996;319:87–92. [Google Scholar]
- Zellmer ID. The effects of natural UVA and UVB on subarctic Daphnia pulicaria in its natural habitat. Hydrobiologia. 1998;379:55–62. [Google Scholar]
- Zellmer ID, Arts MT, Abele D, Humbeck K. Evidence of sublethal damage in Daphnia (Cladocera) during exposure to solar UV radiation in subarctic ponds. Arctic Antarctic and Alpine Research. 2004;36:370–377. [Google Scholar]







