Synopsis
It is now well established that post-learning sleep is beneficial for human memory performance. At the same time, it has long been known that learning experiences influence the content of subsequent sleep mentation (i.e., “dreaming”). Here, we review evidence that newly encoded memories are reactivated and consolidated in the sleeping brain, and that this process is directly reflected in the content of concomitant sleep mentation, providing a valuable window into the mnemonic functions of sleep.
Keywords: sleep, memory, dreaming, mentation, cognition, consolidation, default network, resting states
During all stages of sleep, the mind and brain are working to process new memories, consolidating them into long-term storage and integrating recently acquired information with past experience. In recent years, an accumulating body of research evidence has definitively demonstrated that post-learning sleep is beneficial for human memory performance across a variety of tasks, including verbal learning [1–4], procedural skill learning [2,5–7], emotional memory [8,9], and spatial navigation [10,11]. At the same time, memories of recent experience appear nightly in the content of our dreams, while animal research demonstrates that pre-sleep experience is literally “replayed” on a cellular level during post-learning sleep. Sleep-dependent memory consolidation has been extensively examined from a variety of behavioral and neuroscientific perspectives – yet studies examining dream experience as an indicator of mnemonic activity in the sleeping brain are conspicuously absent. Here, we review evidence that the use of subjective report as a method for probing the activities of the mind and brain is critical for a comprehensive approach to understanding memory consolidation. Indeed, recent work suggests that dream experiences recalled from sleep are a direct reflection of concomitant memory processes in the brain.
Memories in the Sleeping Brain
The Reactivation and Consolidation of Memory during Sleep
There is strong evidence that at least one function of sleep is to “consolidate” fragile new memory traces into more permanent forms of long-term storage, integrating key features of recent experience with existing remote and semantic memory networks. Behavioral studies in humans have clearly demonstrated that post-learning sleep is beneficial for human memory performance in a variety of learning domains. Until relatively recently, much of this work focused on simple procedural tasks, demonstrating that basic motor and perceptual skills were optimally developed across post-training periods that included sleep, relative to equivalent periods of wakefulness. Accumulating data now also strongly implicate sleep in the consolidation of various forms of complex declarative memory, similarly demonstrating that relative to wakefulness, sleep following learning leads to superior memory performance at later tests.
Models of the brain processes supporting these mnemonic benefits of sleep have drawn heavily from animal literature demonstrating a neural-level “reactivation” of recent experience during periods of post-training sleep and quiet rest. Initially focusing on the hippocampus, this literature has now demonstrated that across a wide network of brain systems, patterns of neural activity first seen when waking animals are exploring an environment are later reproduced when these animals sleep. This reactivation has most consistently been observed during periods of NREM just after learning, within brief hippocampal “sharp-wave ripple” burst events (SPW-Rs1 [12,13]) (Figure 1A). This “replay” of memory in sleep may be critical to long-term memory consolidation. In direct support of this hypothesis, a recent study has demonstrated that the extent of neural pattern reactivation after learning predicts subsequent gains in memory performance [14]. Human neurophysiological studies, meanwhile, have linked consolidation to sleep-specific electrophysiological and neurochemical events, and have employed functional imaging technologies to demonstrate a systems-level reactivation of brain regions active in encoding new memories (Figure 1B), roughly analogous to that which has been seen in rodents.
Figure 1.
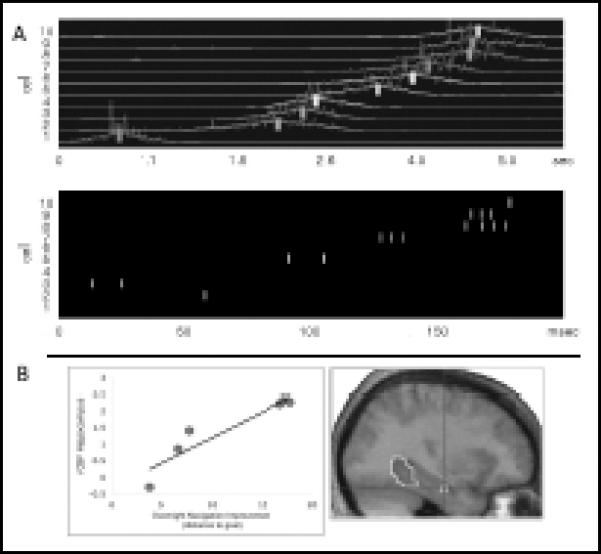
A. In animals, memory reactivation is seen as patterns of cell firings during waking exploration of an environment (Top) that are reiterated in subsequent NREM sleep (Bottom), albeit on a faster time scale. Vertical bars represent the time of peak firing for 10 individual cells demonstrating clear place fields in the training environment [79]. B. In humans, evidence of reactivation has been reported in imaging studies demonstrating that brain regions engaged during task encoding are again active during post-training sleep. In this example, “reactivation” of hippocampal activity during post-training sleep (Right) predicted overnight improvement in memory performance on a spatial learning task (Left). Adapted with permission from Lee AK & Wilson MA. Memory of Sequential Experience in the Hippocampus during Slow Wave Sleep. Neuron 2002; 1183–94 & Peigneux P, Laureys S, Fuchs S, et al. Are spatial memories strengthened in the human hippocampus during slow wave sleep? Neuron 2004; 535–45.
Classically, the consolidation of memory has been conceptualized as a process of “strengthening” an initially labile memory trace, such that the new memory is rendered increasingly resistant to interference across time. Much of the literature on sleep and memory has thus focused on simple quantitative measures of memory “strength” (e.g., the number of words recalled, or the number of motor sequences completed). Increasingly however, it is becoming clear that the role of sleep is much more complex than simply “strengthening” memories in their original forms. To the contrary, recent studies have demonstrated, that sleep is important for such complex processes as the integration of new information into established cortical memory networks [17,18], the extraction of meaning [16], and the development of insight [15]. Recent studies by Dumay & Gaskell [18] and Tamminen et al. [17] elegantly illustrate the first of these concepts. As newly learned words become integrated into neocortical lexical networks over time, competition with previously known words develops, and this competition can be measured as slowed reaction times to those well-known words that are similar to the newly acquired words. In a “lexical integration” paradigm, participants learn a set of pseudowords that are phonemically similar to well-known words (e.g. “cathedruke” is a pseudoword similar to the word “cathedral”) and response times to the new and old words measured. Fascinatingly, Dumay & Gaskell [18] have reported that competition between new and existing words emerges only after a period of sleep, thus suggesting that newly learned words are integrated into neocortical lexical networks by sleep-dependent processes. Using the same task, Tamminen et al. [17] found that the degree of lexical integration over a night of sleep is associated with sleep spindles2.
A recent study from our own laboratory suggests that sleep functions to transform memories such that the critical “gist” of an experience is retained, while specific details of the material are discarded. In the Deese-Roediger-McDermott (DRM) paradigm, participants learn several lists of semantically related words. At a delayed test, when participants are asked to recall these words, often they also report having seen “gist” words, which describe the general theme of the memorized word lists, but which were not themselves present in the list. Remarkably, sleep preferentially benefited (false) memory for these “gist” words, suggesting that one function of sleep-dependent memory processing is to extract meaningful generalities from large collections of related memories [16]. In summary, these and other recent studies suggest that sleep functions not simply to “strengthen” memories, but additionally to transform memory traces by integrating them into mnemonic networks and preferentially maintaining the general meaning or “gist” of the larger experience.
In parallel with this behavioral work, humans brain imaging studies have described a sleep-dependent reorganization of the network of brain structures supporting subsequent recall [19–22]. For example, for hippocampus-dependent declarative memories, retrieval-associated activity in the hippocampal complex decreases following sleep, while related activation in cortical structures, particularly medial prefrontal areas, increases [19,20]. Such evidence supports models of systems-level declarative memory consolidation that propose that memory retrieval, while initially dependent on the hippocampus for retrieval, becomes increasingly less reliant on the hippocampal system and more reliant on cortical structures over time [e.g. 23], and that this developing hippocampal independence may occur during sleep. Other fMRI studies have described an analogous sleep-dependent reorganization of emotional memory, such that medial prefrontal structures become more engaged at delayed retrieval when participants have been allowed to sleep immediately following encoding, in concert with increased retrieval-related functional connectivity between cortical and subcortical regions involved in emotional memory processing [21,22]. This type of functional memory reorganization, in which hippocampally dependent memories are gradually re-encoded into cortical networks that rely on strongly overlapping and related representations, could underlie the ability of sleep to facilitate both the integration of recent memory with past experience, and the abstraction of general concepts from specific stimulus material.
Linking Sleep-Dependent Memory Processing with Dream Experience
Given that fact that humans dream, the neurophysiological and fMRI evidence that memories are reactivated during sleep suggests that “replay” of experience in the sleeping brain could be related to the conscious experience of dreaming. Indeed, several key features of sleep-dependent memory reactivation and consolidation strongly parallel the form in which recently encoded memory appears in sleep mentation. These parallels will be introduced here and expanded on in later sections.
First, qualitatively different types of memory appear to be processed preferentially during different stages of human sleep. Hippocampus-dependent memory, for example, seems to benefit particularly from NREM sleep, perhaps especially slow wave sleep (SWS), while memory for emotional material may be preferentially enhanced by rapid eye movement (REM) sleep. Mirroring these proposed mnemonic functions of REM and NREM sleep, characteristics of dream experience vary similarly as a function of sleep stage. For example, subjective reports elicited from NREM sleep stages are more likely to contain episodic memory sources (a hippocampus-dependent form of memory) than are reports from REM [24], while dream experiences from REM are unique in the presence of particularly intense emotions [e.g., 25].
Secondly, a prominent feature of memory reactivation in rodent models is that, within NREM sleep, the strength of neural-level memory replay decays quickly across time [26,27]. Similarly, it seems that sleep mentation may be most strongly related to recent memories early in the sleep phase [24,28,29], with this association to recent experience decreasing over time.
Above, we also introduced the concept that sleep “transforms” human memories, rather than veridically “strengthening” them. Both rodent neural reactivation studies and data on human dreaming support this notion that recent experience is not faithfully replayed in videotape-like fashion during sleep. Neuronal firing sequences established during wake are re-expressed only intermittently during rodent NREM sleep, with relatively low fidelity and on a faster time scale than the original experience [30,31] Similarly, only intermittent fragments of recent episodic memories appear in sleep mentation, intermingled with remote and semantically related material [29,32].
Finally, there are several key features of the organization of the sleeping brain thought to play a critical role in supporting both offline memory processing and the formation of dream experience. As we fall asleep, the brain quickly undergoes dramatic changes in functional activation patterns, and in the composition of the neurochemical/neurohormone milieu driving the system. During all stages of sleep, regional cerebral blood flow to a network of frontal areas is dramatically decreased, relative to waking [33] while activity in a network of memory-related areas, including the hippocampal complex, medial prefrontal cortex, and anterior cingulate, remains relatively elevated, during both REM and NREM sleep stages. Meanwhile, as we enter early-night NREM-dominated sleep, levels of acetylcholine are dramatically reduced relative to waking, so that monoaminergic neurotransmitters dominate the system. These low levels of acetylcholine during NREM sleep have been hypothesized to facilitate the offline consolidation of hippocampus-dependent memory [34], during a time at which recent episodic memories are most likely to be appearing in concomitant dream experience [24,29]. Reduced levels of cortisol during early night, SWS-dominated sleep have also been hypothesized to both facilitate hippocampal-cortical interactions underlying memory consolidation [35,36], and to promote brain dynamics supporting NREM-type dream experience [36].
Recent papers in sleep and memory have liberally speculated on a possible connection between sleep-dependent memory processing on the one hand, and the imagery, thoughts, and feelings comprising dream experience on the other [e.g., 30,36,37,38–40]. Most recently, observations that the “replay” of memory in sleeping rodents occurs not only in the hippocampus, but in sensory cortices as well, have seemed to offer empirical evidence that “the expression of these reactivated memory traces in sensory cortex may directly relate to the perceptual imagery experienced during sleep and dream states” [30]. However, the presence of memory-related brain activity during sleep does not necessarily imply that this activity will be consciously experienced, and hence, does not imply a relationship to dream experiences recalled from this period of sleep. Until recently, very little empirical work has attempted to directly test the hypothesis that dream experience reflects the reactivation and consolidation of specific mnemonic content in the sleeping brain. But before describing these studies, we will consider the nature of incorporation of recent experiences into sleep mentation more generally, and describe how these observations can inform our approach to understanding offline memory consolidation processes.
The Incorporation of Recent Experience into Dreaming
It has long been known that recent memories constitute a significant component of sleep mentation. In 1900, Freud famously coined the term “day residue” to describe the presence of recent life experience in dream content, a phenomenon which he viewed as only secondary in relevance to the true “latent” meaning of a dream [41]. But as the notion that dreams harbor a “secret meaning” decipherable only by trained psychoanalysts has fallen into disfavor, it has become increasingly clear that the appearance of newly encoded information in our daydreams, mental imagery, thoughts, and dreams may be an observation of paramount importance to understanding the activities of mind and brain during both wake and sleep. Indeed, the form in which memories are incorporated into dream experience in many ways parallels what we know about memory consolidation during sleep, and may help us to understand the process of long-term memory ”consolidation”.
Effects of Pre-Sleep Experience on Sleep Mentation
In the 1960's and 70's, a considerable amount of research effort was devoted to understanding the relation of waking events to dream content by experimentally manipulating participants' presleep experience. Despite methodological weaknesses which plagued much of this literature, several useful conclusions can be drawn from this early work. Most notable is the extreme difficulty of manipulating dream content, even when highly emotional stimuli are introduced prior to sleep. Of the dozens of such studies performed during this time [e.g., 42,43–47], almost none demonstrated unambiguous, statistically significant effects of an experimentally introduced pre-sleep stimulus on subsequent dream content. Interestingly, the most consistently observed effect of waking experience on laboratory-collected sleep mentation has been a powerful influence of the laboratory setting itself. For example, in an analysis of 813 REM mentation reports collected across several studies, Dement et al. [48] report that 22% of reports unambiguously incorporated either isolated elements of the laboratory situation (i.e. the experimenter, electrodes, etc.) or more complete representations of the experimental setting (i.e. a combination of the previously-mentioned elements). In retrospect, given the salience of sleeping in a strange place and being awakened during the night to report dreams, it is hardly surprising that the laboratory environment would overshadow the effect of any particular film or activity introduced as part of an experimental protocol. In contrast, more recent data from our own laboratory, discussed below, demonstrate that experimental introduction of intensive and engaging learning experiences can dramatically influence the content of dreaming, at least early in the night.
Still, it is important to note that other, non-experimental approaches provide solid empirical evidence that the content of sleep mentation often references new memories and recent experiences. For example, in an analysis comparing 299 home-collected dream reports with possible memory sources from a diary of waking events, Fosse and colleagues demonstrated that fragments of recent experience are very often seen in dreams [32]. Although exact replication of any particular waking experience was rare, 51% of reports were judged to contain at least one dream element bearing strong similarity to a recent waking experience. Other research has similarly prompted participants to connect dream reports with likely memory sources from waking experience in the prior days and weeks [24,49–51], demonstrating that such memory sources are readily identified with high-confidence, and correspond with the ratings of blind judges [49]. We will later return to this notion that recent experience appears in sleep mentation in a fragmentary form, rather than as an exact video-tape-like “replay” of an event.
Other lines of work have explored the more general correspondence between sleep mentation and waking life. In collaboration with Robert Van de Castle, Calvin Hall pioneered the use of content analysis methods to quantitatively assess the content of large sets of mentation reports [52]. A response to the methodological pitfalls inherent to subjective interpretations of dream content employed by psychoanalysts, this meticulous classification system counts the occurrence of different types of characters, settings, objects, social interactions, activities, etc., explicitly described in the text of mentation reports. Using this system, Hall illustrated the transparent relationship between dream content and everyday life by creating surprisingly accurate profiles and histories of psychiatric patients based solely on blind content analysis of dream reports, and became amongst the first to champion the notion that quantitative methods could uncover straightforward and meaningful relationships between waking experience and dream content:
A large number of dreams reflect rather faithfully the daytime activities and preoccupations of the dreamer. Skiers dream of skiing, surfers dream of surfing, and mountain climbers dream of climbing mountains. Teachers dream of classroom situations, bankers dream of banking activities, and nurses dream about their patients [53].
More recent applications of Hall's system of content analysis have reported consistent, statistically significant differences in dream content of groups of individuals with divergent waking experience (i.e. between males and females, children and adults, blind and sighted individuals [for a review, see 54]). These investigations have contributed to our understanding of the dreaming process by reminding us that, while attempts to predict or control dream content have often failed, a broad correspondence between sleep mentation and waking experience is nonetheless transparently obvious.
Determinants of the Memory Sources of Dreaming
But what determines which memories will contribute to the content of dreaming on a particular night? Far from being a haphazard process, the incorporation of waking experience into sleep mentation appears to follow a set of predictable patterns, modulated by both sleep stage and temporal distance from a waking event. Contrary to the entrenched popular belief that “REM sleep = dreaming”, we dream during all stages of sleep [55–58]. Although reports of mental experiences from NREM sleep stages 2, 3 & 4 are often shorter and less emotional than REM reports, there is considerable overlap in the cognitive characteristics of reports from different stages of sleep [for example, see 59]. In fact, late-night NREM dreaming can be just as vivid, bizarre, and story-like as the typical dream from REM sleep [57,60]. Yet one consistent difference between dream reports from different stages of sleep appears to be the nature of participant-identified memory sources. Coinciding with the notion that NREM dreams tend to be more realistic and mundane than reports from REM sleep, mentation from NREM stages are characterized by a large proportion of episodic memory sources [24], derived from memories of specific autobiographical events which occurred at a particular place and time. Dream reports from REM sleep, on the other hand, incorporate more abstract and semantic memory sources, unrelated to specific life events. Thus, during NREM sleep a participant might report a dream concerning a friend she saw the previous afternoon, while dreams during later REM sleep would likely be more bizarre and not obviously connected to any specific pre-sleep experience.
The incorporation of recent experience into dream content also seems to follow an organized temporal pattern. Very recent events are more often incorporated into mentation occurring early in the sleep phase, with remote experiences appearing only later in the night. Though only a small handful of studies have examined time-of-night effects on incorporation in this manner, both the recency of subject-identified memory sources [61] and the similarity of sleep mentation reports to presleep thought [62] have been reported to correlate negatively with time since sleep onset. Meanwhile, work from our own laboratory using engaging video-game learning tasks has demonstrated that incidence of direct, unambiguous incorporation of task-related imagery at sleep onset declines linearly as a function of time since the initiation of sleep [28,29]. On a broader timescale, it appears that recent life events are most likely to appear in dream content, relative to more remote experiences from years past [63,64]. Meanwhile several studies also suggest that waking experiences tend to be incorporated into dream mentation either immediately following the experience, or else about a week later (the “dream lag” effect [49,65]).
In summary, these observations lead us to conclude that NREM early in the night is the state of sleep most likely to contain “replay” of recent memory. As described above, this notion is supported by the animal literature, in which neuronal-level memory reactivation has typically been observed during periods of NREM sleep immediately following learning, with the strength of this replay decaying rapidly across time. In contrast, only a single study has reported similar reactivation of memory during REM [39]. Our recent work examining the effects of learning tasks on sleep mentation have thus focused on periods of Stage 1 and 2 NREM sleep immediately following sleep onset.
The Effects of Intensive Learning Experiences on Sleep Onset Mentation
Studies in our own laboratory have examined NREM sleep mentation following intensive training on engaging, video-game-like tasks. Concentrating on periods of early-night NREM sleep, this research demonstrates that salient, interactive learning tasks can exert a dramatic influence on subsequent sleep mentation. In one such study, participants played the video game “Tetris” extensively several hours prior to sleep [28]. When mentation reports were then repeatedly elicited following short intervals of sleep, 64% of participants reported unambiguous game-related images in at least one sleep onset report. In a related investigation utilizing a downhill skiing arcade game [29], 30% of all post-training mentation reports directly incorporated the game (Figure 2). The frequency of direct incorporation in these sleep onset studies, dramatically higher than that observed in any previous overnight investigation, suggests that the first minutes of sleep provide ideal conditions for the cognitive-level reactivation of waking experience. Furthermore, it may be that interactive learning experiences are more likely to be reactivated as participants fall asleep than are passively viewed experimental stimuli.
Figure 2.
Following training on an engaging downhill skiing arcade game, 30% of 386 sleep onset mentation reports contained task-related imagery or thoughts [29]. Representation of the game primarily took the form of sensory imagery as opposed to thought, and most often bore a direct, unambiguous relationship to the game.
Interleaved Fragments of Experience
Despite the strong influence of waking experience on subsequent sleep mentation, dreams rarely consist of an exact “replay” of a life event. Instead, sleep mentation incorporates isolated elements of a waking episode, intermingled with fragments of other recent memories, as well as remote and semantic memory material, thus creating novel and sometimes bizarre scenarios which do not faithfully represent any particular waking event. For example, the following illustrates a sleep onset dream report which clearly incorporates fragments of a waking experience, but without replicating the original context in which these fragments were embedded (data from [32]):
Waking Experience: “When I left [work at] Starbucks, we had so many leftover pastries and muffins to throw away or take home. I couldn't decide which muffins to take and which to toss …”
Corresponding Sleep Mentation: “My dad and I leave to go shopping. We go from room to room, store to store. One of the stores is filled with muffins, muffins, muffins from floor to ceiling, all different kinds, I can't decide which one I want …”
In our own studies of sleep-onset and Stage 2 NREM reports after playing video games [28] [29], we have similarly observed that, rather than faithfully reiterating a learning task, mentation reports integrated elements of the learning experience into a narrative which included related material drawn from remote and semantic memory. For example, after training on a downhill skiing arcade game [29], one participant reported at sleep onset, “I was picturing stacking wood this time … I felt like I was doing it at… at a ski resort that I had been to before, like five years ago maybe.”. Similarly, following training on a virtual maze navigation task [66], a participant reported “I was thinking about the maze and kinda having people as check points, I guess, and then that led me to think about when I went on this trip a few years ago and we went to see these bat caves, and they're kind of like, maze-like”. Thus, when incorporated into a dream, the various components of a wake episode do not appear to remain bound together in the way that characterizes the “mental time travel” of episodic memory recall in waking life.
This observation that dreams do not veridically “replay” waking experience has led some to conclude that such mental activity is incompatible with consolidation of memory [67,68]. To the contrary, emerging evidence suggests that the fragmentary form in which waking experience appears in dreams, intermingled with other memory traces, reflects a critical feature of the memory consolidation process. As described above, consolidation appears to be considerably more complex than the simple “strengthening” of memories in their original forms, and indeed, the neuronal-level memory reactivation described in animals does not consist of a precise, veridical reiteration of waking experience. Instead, patterns of neural activity which statistically resemble (but are not identical to) those established in waking experience are played out on a speeded timescale. Furthermore, when animals are exposed to two successive spatial experiences, reactivation of both patterns appears to be instantiated simultaneously during subsequent NREM sleep [26]. Compatible with this observation is the proposal that sleep functions to transform memory traces in part by slowly “interleaving” neural representations of recent experience into existing remote and semantic cortical networks [38,69]. In their influential Psychological Review paper, for example, McCelland, McNaughton & O'Reilly speculate that optimal consolidation of new memories requires the alternating reactivation of these memories and related remote memories during different stages of sleep:
Once a memory is stored in the hippocampal system, it can be reactivated and then reinstated in the neocortex … [R]einstatement provides the opportunity for an incremental adjustment of neocortical connections, thereby allowing memories initially dependent on the hippocampal system to gradually become independent of it. We assume that reinstatement also occurs in off-line situations, including active rehearsal, reminiscence, and other inactive states including sleep … Possibly, events reactivated in the hippocampus during slow wave sleep prime related neocortical patterns, so that these in turn become available for activation during REM sleep. This could permit both new and old information to be played back in closely interleaved fashion. [69]
Following this line of reasoning, we propose that even within a single dream experience3, sleep mentation reflects the interleaved reactivation of memory fragments from different recent and remote sources, allowing newly acquired information to become increasingly connected with related memory traces across time. By initiating LTP-like plasticity in mnemonic networks, this simple process of simultaneously activating new and old memory traces during sleep could account for behavioral data indicating that sleep facilitates the integration of new information with existing semantic networks [17,18], as well the extraction of meaning [16], which may require a similar process of relating new information to existing knowledge. However, despite many hypotheses relating the conscious experience of dreaming to memory processing, this notion is yet to be subjected to systematic empirical investigation.
The Empirical Study of Spontaneous Subjective Experience
For the most part, cognitive neuroscience has abandoned the behaviorist notion that conscious, subjective experience is not a suitable object of empirical investigation. Research in the last two decades has moved beyond the philosophical question of mind-brain relationship and begun in earnest to study the neural correlates, for example, of motivation, emotion, attention, mental imagery, and episodic memory. Very often, mapping the brain basis of these subjective concepts relies on taking participants' verbal reports of experience at face value. Yet surprisingly, neuroscience has been slow to formalize the study of spontaneous subjective experience during “offline” states, when responses to sensory stimuli no longer drive the system. While research on the “default” mode of brain function has brought attention to the importance of spontaneous brain activity occurring during periods of rest and sleep [70,71], surprisingly, virtually none of this work has examined participants' own reports of what is going through their mind at rest. Data on spontaneous cognition during sleep has been even more lacking. Neurophysiological studies seeking to shed light on the neural basis of dreaming have often relied merely on describing the physiology of REM, or else have dichotomized conscious experience as either “present” or “absent”, without exploring the actual content of this mentation.
Above, we argued that the specific content of conscious experience during sleep (whether termed “sleep mentation”, “dreaming”, or “hypnagogic imagery”) is clearly relevant to understanding memory consolidation. Yet despite widespread theoretical agreement that sleep-dependent memory processing may relate to dream experience [30,36–40,72], attempts to empirically address this hypothesis have been conspicuously absent. Why is this the case? Firstly, for historical reasons, dream experience has typically been presumed to be difficult or impossible to quantify in a scientifically rigorous manner. Psychoanalytic approaches entrenched in the popular imagination have characterized dreaming as a mysterious, symbolic form of mental activity which, unlike waking cognition, cannot be measured, classified, and quantitatively analyzed in a meaningful way. Meanwhile, pseudoscientific approaches to dreaming popularized in the media have created a perception that, when occurring during sleep, cognition is not a legitimate area of scientific inquiry. Fortunately, this is not the case. There is little, if any, evidence that conscious experience during sleep is particularly more inaccessible, complex, or symbolic than waking thought. Furthermore, reliable approaches to quantifying dream content have been available for decades [e.g., 52,59], and methods of data collection are straightforward and completely compatible with standard designs in memory research. Although memory for sleep mentation is typically more fleeting than for waking experience, all self-report measures, whether for waking or sleep mentation, rely on taking post-hoc reports of unverifiable data at face value (“Did you see the stimulus?” “What strategy did you use to encode the material?” “To what degree have the following symptoms bothered you in the past month?”). Resurgent studies of such subjective concepts as emotion”, mood, “memory, and belief, demonstrate the progress which can be made when one takes a simple and straightforward approach to subjective experience, casting obfuscating philosophical concerns aside.
Critically, the content of spontaneous subjective experience can provide information inaccessible via any other means. First and foremost, the use of subjective report is an ideal method to determine whether a specific memory is indeed being “reactivated” in the sleeping brain. Particularly in human research, there is no known measure of brain activity (EEG, fMRI, PET, etc.) which can convincingly demonstrate the activation of a specific memory. For example, increased regional cerebral blood flow to learning-related brain regions suggests that the same memory systems engaged at learning are doing something during sleep, but does not demonstrate the “reactivation” of a particular memory. Conscious retrieval of a recent memory, in contrast, definitely demonstrates that the neural networks encoding that particular memory have been reactivated. Subjective reports allow us a detailed view of the form in which a memory is retrieved. For example, as described above, reports of sleep mentation reveal that recent memory fragments are reactivated in an interleaved fashion with past experience and semantic knowledge. Such observations illuminate how the brain “transforms” memories over time by integrating recently acquired information into existing knowledge structures. Finally, reports of conscious experience are unique in enabling us to explore which memories of everyday waking experience are spontaneously reactivated during offline states of quiet rest and sleep.
Reactivation of Memory in Dream Content and Sleep-Dependent Memory Consolidation
Although empirical investigation of these questions has been largely lacking, a handful of studies provide noteworthy evidence for a link between learning, sleep-dependent memory consolidation, and dream experience. That dreaming might function to process prior experience is a hypothesis predating the current resurgence of interest in sleep-dependent memory consolidation, and several studies from the 1960s onwards have addressed this question. Fiss et al. [73] investigated the morning recall of short stories encoded the night before, finding a correlation between story-related words in dream reports and memory for these stories the following morning. deKonink and colleagues [74,75] have also examined the correlation between sleep mentation and verbal learning, exploring dream content as a corollary of language learning in an academic setting. Amongst students enrolled in a French-immersion language class, those who exhibited superior acquisition of the new language across a 6-week course tended to incorporate French into dream content more often than students who were less successful in the class [74,75]. Hypothesizing that REM-sleep dreaming is important for “emotional adaptation” to stressful events, the work of Rosalind Cartwright has examined dream content as a predictor of psychological outcomes in women following divorce, finding that characteristics of spouse-related dreams predict remission from depression [76]. Other work also supports a role for sleep in emotional adaptation to negative experiences [77,78].
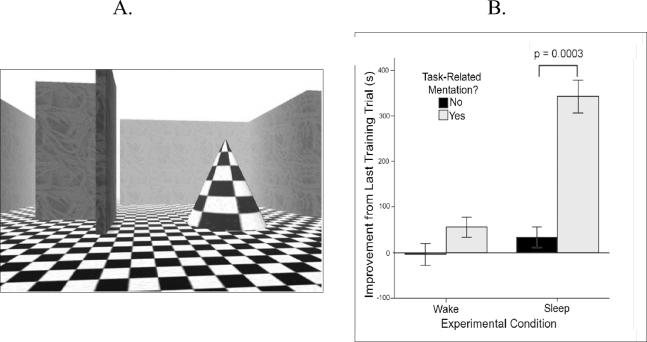
Recent work from our own laboratory has provided direct evidence that incorporation of a learning task into subsequent dream experience predicts enhanced sleep-dependent memory consolidation [66]. In this study, participants were trained on a 3D-style virtual maze task (Figure 3, Left) prior to a 1.5hr nap opportunity or else to an equivalent period of wake. During this period, all subjects were prompted three times to make open-ended verbal reports of “everything that was going through your mind”. We found that reports of task-related mentation was strongly associated with enhanced performance at subsequent retest (Figure 3, Right). In the Sleep group, participants who spontaneously referred to the maze task in their subjective reports improved ten-fold more at retest than Sleep participants who gave no task-related reports (p=.0003). In contrast, thinking of the maze while awake did not provide any performance benefit (condition × mentation interaction: p=.08). These findings demonstrate how reports of subjective experience can inform the study of memory consolidation, providing novel evidence that dream experience reflects the learning-induced reactivation of memory networks during sleep, and that such reactivation correlates with substantially enhanced memory performance.
Figure 3.
A,B. Dreaming of a spatial learning task is associated with enhanced navigation performance at delayed retest, while thinking of the task during wakefulness is unrelated to later performance [66]. Error bars represent ±S.E.M.
Conclusions
Recent advances in our understanding of long-term memory processing suggest that following learning, waking experience is reactivated in the sleeping brain, leading to a process of “consolidation” by which new, labile memory traces are reorganized into more permanent forms of long-term storage. Dream experiences recalled from sleep bear a transparent relationship to recently encoded information, and provide a useful window into consolidation-related activities of the sleeping brain. Indeed, recent work from our laboratory has established a direct relationship between the “replay” of recent experience in dream content, and enhanced memory performance in humans [66].
We have argued here that the study of spontaneous conscious experience has great potential for elucidating the mechanisms of offline memory processing, particularly by allowing an examination of precisely which memories from everyday experience are reactivated during offline states, and by providing detailed information on the activities of memory systems that is not available by any other means. It is our hope that future research will profitably focus on the quantification of subject experience during periods of quiet rest and sleep, relating neural and behavioral measures of memory consolidation to the particular form in which new information is incorporated into dreaming, interleaved with established remote and semantic memory networks.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Generated in the hippocampus during periods of immobility and sleep, SPW-Rs (sharp-wave ripples) are brief, high-amplitude population bursts associated with high-frequency “ripple” oscillations (>100Hz). SPW-R's are thought to support hippocampal-cortical communication involved in offline memory consolidation.
Sleep spindles are 12–15Hz rhythmic bursts in the cortex seen in the NREM sleep EEG.
If such a thing as a “single dream” can be said to exist. It is possible that mental experience is continuous throughout all sleep, only delimited by our ability to recall such experience upon awakening.
References
- 1.Ellenbogen JM, Hulbert JC, Stickgold R, et al. Interfering with theories of sleep and memory: sleep, declarative memory, and associative interference. Curr Biol. 2006;16:1290. doi: 10.1016/j.cub.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 2.Plihal W, Born J. Effects of early and late nocturnal sleep on declarative and procedural memory. Journal of Cognitive Neuroscience. 1997;9:534. doi: 10.1162/jocn.1997.9.4.534. [DOI] [PubMed] [Google Scholar]
- 3.Tucker MA, Hirota Y, Wamsley EJ, et al. A daytime nap containing solely non-REM sleep enhances declarative but not procedural memory. Neurobiol Learn Mem. 2006;86:241. doi: 10.1016/j.nlm.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 4.Schabus M, Gruber G, Parapatics S, et al. Sleep spindles and their significance for declarative memory consolidation. Sleep. 2004;27:1479. doi: 10.1093/sleep/27.7.1479. [DOI] [PubMed] [Google Scholar]
- 5.Stickgold R, Whidbee D, Schirmer B, et al. Visual discrimination task improvement: A multi-step process occurring during sleep. J Cogn Neurosci. 2000;12:246. doi: 10.1162/089892900562075. [DOI] [PubMed] [Google Scholar]
- 6.Walker MP, Brakefield T, Morgan A, et al. Practice with sleep makes perfect: sleep-dependent motor skill learning. Neuron. 2002;35:205. doi: 10.1016/s0896-6273(02)00746-8. [DOI] [PubMed] [Google Scholar]
- 7.Huber R, Ghilardi MF, Massimini M, et al. Local sleep and learning. Nature. 2004;430:78. doi: 10.1038/nature02663. [DOI] [PubMed] [Google Scholar]
- 8.Wagner U, Gais S, Born J. Emotional memory formation is enhanced across sleep intervals with high amounts of rapid eye movement sleep. Learn Mem. 2001;8:112. doi: 10.1101/lm.36801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nishida M, Pearsall J, Buckner RL, et al. REM sleep, prefrontal theta, and the consolidation of human emotional memory. Cerebral Cortex. 2009;19:1158. doi: 10.1093/cercor/bhn155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peigneux P, Laureys S, Fuchs S, et al. Are spatial memories strengthened in the human hippocampus during slow wave sleep? Neuron. 2004;44:535. doi: 10.1016/j.neuron.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 11.Wamsley EJ, Tucker MA, Payne JD, et al. A brief nap is beneficial for human route-learning: The role of navigation experience and EEG spectral power. Learning and Memory. 2010;17:332. doi: 10.1101/lm.1828310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buzsaki G. Memory consolidation during sleep: a neurophysiological perspective. J Sleep Res. 1998;7(Suppl 1):17. doi: 10.1046/j.1365-2869.7.s1.3.x. [DOI] [PubMed] [Google Scholar]
- 13.Buzsaki G. Hippocampal sharp waves: their origin and significance. Brain Res. 1986;398:242. doi: 10.1016/0006-8993(86)91483-6. [DOI] [PubMed] [Google Scholar]
- 14.Dupret D, O'Neill J, Pleydell-Bouverie B, et al. The reorganization and reactivation of hippocampal maps predict spatial memory performance. Nature Neuroscience. 13:995. doi: 10.1038/nn.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wagner U, Gais S, Haider H, et al. Sleep inspires insight. Nature. 2004;427:352. doi: 10.1038/nature02223. [DOI] [PubMed] [Google Scholar]
- 16.Payne JD, Schacter DL, Propper RE, et al. The role of sleep in false memory formation. Neurobiology of Learning and Memory. 2009;92:327. doi: 10.1016/j.nlm.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tamminen J, Payne JD, Stickgold R, et al. Sleep spindle activity is associated with the integration of new memories and existing knowledge. Journal of Neuroscience. doi: 10.1523/JNEUROSCI.3028-10.2010. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dumay N, Gaskell MG. Sleep-associated changes in the mental representation of spoken words. Psychological Science. 2007;18:35. doi: 10.1111/j.1467-9280.2007.01845.x. [DOI] [PubMed] [Google Scholar]
- 19.Takashima A, Nieuwenhuis IL, Jensen O, et al. Shift from hippocampal to neocortical centered retrieval network with consolidation. Journal of Neuroscience. 2009;29:10087. doi: 10.1523/JNEUROSCI.0799-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takashima A, Petersson KM, Rutters F, et al. Declarative memory consolidation in humans: a prospective functional magnetic resonance imaging study. Proc Natl Acad Sci U S A. 2006;103:756. doi: 10.1073/pnas.0507774103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Payne JD, Kensinger EA. Sleep Leads to Changes in the Emotional Memory Trace: Evidence from fMRI. Journal of Cognitive Neuroscience. doi: 10.1162/jocn.2010.21526. [DOI] [PubMed] [Google Scholar]
- 22.Sterpenich V, Albouy G, Darsaud A, et al. Sleep promotes the neural reorganization of remote emotional memory. Journal of Neuroscience. 2009;29:5143. doi: 10.1523/JNEUROSCI.0561-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frankland PW, Bontempi B. The organization of recent and remote memories. Nat Rev Neurosci. 2005;6:119. doi: 10.1038/nrn1607. [DOI] [PubMed] [Google Scholar]
- 24.Baylor GW, Cavallero C. Memory sources associated with REM and NREM dream reports throughout the night: a new look at the data. Sleep. 2001;24:165. [PubMed] [Google Scholar]
- 25.Smith MR, Antrobus JS, Gordon E, et al. Motivation and affect in REM sleep and the mentation reporting process. Conscious Cogn. 2004;13:501. doi: 10.1016/j.concog.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 26.Kudrimoti HS, Barnes CA, McNaughton BL. Reactivation of hippocampal cell assemblies: effects of behavioral state, experience, and EEG dynamics. J Neurosci. 1999;19:4090. doi: 10.1523/JNEUROSCI.19-10-04090.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilson MA, McNaughton BL. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265:676. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]
- 28.Stickgold R, Malia A, Maguire D, et al. Replaying the game: hypnagogic images in normals and amnesics. Science. 2000;290:350. doi: 10.1126/science.290.5490.350. [DOI] [PubMed] [Google Scholar]
- 29.Wamsley EJ, Perry K, Djonlagic I, et al. Cognitive replay of visuomotor learning at sleep onset: temporal dynamics and relationship to task performance. Sleep. 2010;33:59. doi: 10.1093/sleep/33.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ji D, Wilson MA. Coordinated memory replay in the visual cortex and hippocampus during sleep. Nat Neurosci. 2007;10:100. doi: 10.1038/nn1825. [DOI] [PubMed] [Google Scholar]
- 31.Nadasdy Z, Hirase H, Czurko A, et al. Replay and time compression of recurring spike sequences in the hippocampus. J Neurosci. 1999;19:9497. doi: 10.1523/JNEUROSCI.19-21-09497.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fosse MJ, Fosse R, Hobson JA, et al. Dreaming and episodic memory: a functional dissociation? J Cogn Neurosci. 2003;15:1. doi: 10.1162/089892903321107774. [DOI] [PubMed] [Google Scholar]
- 33.Braun AR, Balkin TJ, Wesenten NJ, et al. Regional cerebral blood flow throughout the sleep-wake cycle. An H2(15)O PET study. Brain. 1997;120:1173. doi: 10.1093/brain/120.7.1173. [DOI] [PubMed] [Google Scholar]
- 34.Hasselmo ME. Neuromodulation: acetylcholine and memory consolidation. Trends Cogn Sci. 1999;3:351. doi: 10.1016/s1364-6613(99)01365-0. [DOI] [PubMed] [Google Scholar]
- 35.Plihal W, Born J. Memory consolidation in human sleep depends on inhibition of glucocorticoid release. Neuroreport. 1999;10:2741. doi: 10.1097/00001756-199909090-00009. [DOI] [PubMed] [Google Scholar]
- 36.Payne JD, Nadel L. Sleep, dreams, and memory consolidation: the role of the stress hormone cortisol. Learn Mem. 2004;11:671. doi: 10.1101/lm.77104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cai DJ, Mednick SA, Harrison EM, et al. REM, not incubation, improves creativity by priming associative networks. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:10130. doi: 10.1073/pnas.0900271106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paller KA, Voss JL. Memory reactivation and consolidation during sleep. Learn Mem. 2004;11:664. doi: 10.1101/lm.75704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Louie K, Wilson MA. Temporally structured replay of awake hippocampal ensemble activity during rapid eye movement sleep. Neuron. 2001;29:145. doi: 10.1016/s0896-6273(01)00186-6. [DOI] [PubMed] [Google Scholar]
- 40.Walker MP, van der Helm E. Overnight therapy? The role of sleep in emotional brain processing. Psychological Bulletin. 2009;135:731. doi: 10.1037/a0016570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Freud S. The Interpretation of Dreams. :1900. [Google Scholar]
- 42.Cartwright R. The relation of daytime events to the dreams that follow. In: Hartmann E, editor. Sleep and Dreaming. Little, Brown and Company; Boston: 1970. p. 227. [PubMed] [Google Scholar]
- 43.Baekeland F. Laboratory studies of effects of presleep events on sleep and dreams. Int Psychiatry Clin. 1970;7:49. [PubMed] [Google Scholar]
- 44.Breger L, Hunter I, Lane RW. The effect of stress on dreams. Vol 7. International Universities Press; New York: 1971. [PubMed] [Google Scholar]
- 45.Foulkes D, Rechtschaffen A. Presleep Determinants of Dream Content: Effect of Two Films. Percept Mot Skills. 1964;19:983. doi: 10.2466/pms.1964.19.3.983. [DOI] [PubMed] [Google Scholar]
- 46.Goodenough DR, Witkin HA, Koulack D, et al. The effects of stress films on dream affect and on respiration and eye-movement activity during Rapid-Eye-Movement sleep. Psychophysiology. 1975;12:313. doi: 10.1111/j.1469-8986.1975.tb01298.x. [DOI] [PubMed] [Google Scholar]
- 47.Witkin HA, Lewis HB. The relation of experimentally induced presleep experiences to dreams. A report on method and preliminary findings. J Am Psychoanal Assoc. 1965;13:819. doi: 10.1177/000306516501300406. [DOI] [PubMed] [Google Scholar]
- 48.Dement WC, Kahn E, Roffwarg HP. The Influence of the Laboratory Situation on the Dreams of the Experimental Subject. J Nerv Ment Dis. 1965;140:119. doi: 10.1097/00005053-196502000-00002. [DOI] [PubMed] [Google Scholar]
- 49.Nielsen TA, Kuiken D, Alain G, et al. Immediate and delayed incorporations of events into dreams: further replication and implications for dream function. J Sleep Res. 2004;13:327. doi: 10.1111/j.1365-2869.2004.00421.x. [DOI] [PubMed] [Google Scholar]
- 50.Cavallero C. Dream sources, associative mechanisms, and temporal dimension. Sleep. 1987;10:78. doi: 10.1093/sleep/10.1.78. [DOI] [PubMed] [Google Scholar]
- 51.Cavallero C, Foulkes D, Hollifield M, et al. Memory sources of REM and NREM dreams. Sleep. 1990;13:449. [PubMed] [Google Scholar]
- 52.Hall C, Van de Castle R. The Content Analysis of Dreams. Appleton-Century-Crofts; New York: 1966. [Google Scholar]
- 53.Hall C, Nordby V. The Individual and His Dreams. New American Library; New York: 1972. [Google Scholar]
- 54.Domhoff GW. The Scientific Study of Dreams. American Psychological Association; Washington, D.C.: 2002. [Google Scholar]
- 55.Pivik T, Foulkes D. NREM mentation: relation to personality, orientation time, and time of night. J Consult Clin Psychol. 1968;32:144. doi: 10.1037/h0025489. [DOI] [PubMed] [Google Scholar]
- 56.Foulkes D. Nonrapid eye movement mentation. Exp Neurol. 1967;(Suppl 4):28. doi: 10.1016/0014-4886(67)90154-9. [DOI] [PubMed] [Google Scholar]
- 57.Wamsley EJ, Hirota Y, Tucker MA, et al. Circadian and ultradian influences on dreaming: A dual rhythm model. Brain Research Bulletin. 2007;71:347. doi: 10.1016/j.brainresbull.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 58.Cavallero C, Cicogna P, Natale V, et al. Slow wave sleep dreaming. Sleep. 1992;15:562. doi: 10.1093/sleep/15.6.562. [DOI] [PubMed] [Google Scholar]
- 59.Antrobus J. REM and NREM sleep reports: comparison of word frequencies by cognitive classes. Psychophysiology. 1983;20:562. doi: 10.1111/j.1469-8986.1983.tb03015.x. [DOI] [PubMed] [Google Scholar]
- 60.Antrobus J, Kondo T, Reinsel R, et al. Dreaming in the late morning: summation of REM and diurnal cortical activation. Conscious Cogn. 1995;4:275. doi: 10.1006/ccog.1995.1039. [DOI] [PubMed] [Google Scholar]
- 61.Verdone P. Temporal Reference of Manifest Dream Content. Percept Mot Skills. 1965;20(SUPPL):1253. doi: 10.2466/pms.1965.20.3c.1253. [DOI] [PubMed] [Google Scholar]
- 62.Baekeland F, Resch R, Katz D. Presleep mentation and dream reports. I. Cognitive style, contiguity to sleep, and time of night. Arch Gen Psychiatry. 1968;19:300. doi: 10.1001/archpsyc.1968.01740090044005. [DOI] [PubMed] [Google Scholar]
- 63.Natale V, Battaglia D. Temporal dating of autobiographical memories associated to REM and NREM dreams. Imagination, Cognition, and Personality. 1990–1991;10:279. [Google Scholar]
- 64.Grenier J, Cappeliez P, St-Onge M, et al. Temporal references in dreams and autobiographical memory. Mem Cognit. 2005;33:280. doi: 10.3758/bf03195317. [DOI] [PubMed] [Google Scholar]
- 65.Nielsen TA, Powell RA. The 'dream-lag' effect: a 6-day temporal delay in dream content incorporation. Psychiatr J Univ Ott. 1989;14:561. [PubMed] [Google Scholar]
- 66.Wamsley EJ, Tucker M, Payne JD, et al. Dreaming of a learning task is associated with enhanced sleep-dependent memory consolidation. Current Biology. 2010;20:850. doi: 10.1016/j.cub.2010.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Siegel JM. The REM sleep-memory consolidation hypothesis. Science. 2001;294:1058. doi: 10.1126/science.1063049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vertes RP. Memory consolidation in sleep; dream or reality. Neuron. 2004;44:135. doi: 10.1016/j.neuron.2004.08.034. [DOI] [PubMed] [Google Scholar]
- 69.McClelland JL, McNaughton BL, O'Reilly RC. Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychol Rev. 1995;102:419. doi: 10.1037/0033-295X.102.3.419. [DOI] [PubMed] [Google Scholar]
- 70.Spreng RN, Grady CL. Patterns of brain activity supporting autobiographical memory, prospection, and theory of mind, and their relationship to the default mode network. Journal of Cognitive Neuroscience. 22:1112. doi: 10.1162/jocn.2009.21282. [DOI] [PubMed] [Google Scholar]
- 71.Schacter DL, Addis DR, Buckner RL. Remembering the past to imagine the future: the prospective brain. Nature Reviews Neuroscience. 2007;8:657. doi: 10.1038/nrn2213. [DOI] [PubMed] [Google Scholar]
- 72.Winson J. The biology and function of rapid eye movement sleep. Current Opinion in Neurobiology. 1993;3:243. doi: 10.1016/0959-4388(93)90217-m. [DOI] [PubMed] [Google Scholar]
- 73.Fiss H, Kremer E, Lichtman J. The mnemonic function of dreaming. Sleep Research. 1977;6:122. [Google Scholar]
- 74.De Koninck J, Christ G, Hebert G, et al. Language learning efficiency, dreams and REM sleep. Psychiatr J Univ Ott. 1990;15:91. [PubMed] [Google Scholar]
- 75.DeKoninck J, Christ G, Rinfret N, et al. Dreams during language learning: when and how is the new language integrated. Psychiatr J Univ Ott. 1988;13:72. [PubMed] [Google Scholar]
- 76.Cartwright R, Agargun MY, Kirkby J, et al. Relation of dreams to waking concerns. Psychiatry Res. 2006;141:261. doi: 10.1016/j.psychres.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 77.Lara-Carrasco J, Nielsen TA, Solomonova E, et al. Overnight emotional adaptation to negative stimuli is altered by REM sleep deprivation and is correlated with intervening dream emotions. Journal of Sleep Research. 2009;18:178. doi: 10.1111/j.1365-2869.2008.00709.x. [DOI] [PubMed] [Google Scholar]
- 78.De Koninck JM, Koulack D. Dream content and adaptation to a stressful situation. J Abnorm Psychol. 1975;84:250. doi: 10.1037/h0076648. [DOI] [PubMed] [Google Scholar]
- 79.Lee AK, Wilson MA. Memory of sequential experience in the hippocampus during slow wave sleep. Neuron. 2002;36:1183. doi: 10.1016/s0896-6273(02)01096-6. [DOI] [PubMed] [Google Scholar]