Abstract
The design and synthesis of coordinative supramolecular polygons with open binding sites is described. Coordination-driven self-assembly of 2,6-bis(pyridin-4-ylethynyl)pyridine with 60° and 120° organoplatinum acceptors results in quantitative formation of a supramolecular rhomboid and hexagon, respectively, both bearing open pyridyl binding sites. The structures were determined by multinuclear (31P and 1H) NMR spectroscopy and electrospray ionization (ESI) mass spectrometry, along with a computational study.
Keywords: Coordination-Driven Self-Assembly, Open Binding Sites, Supramolecular Polygons
Coordination-driven self-assembly, the supramolecular architecture methodology relying on metal-ligand bonding interactions, has proven powerful for accessing well-defined coordinative supramolecules over the past two decades.1 The rational design of rigid molecular building blocks via coordination-driven self-assembly allows for the formation of discrete supramolecules of variable topologies, as demonstrated by the myriad of metallo-supramolecular grids, polygons, cages, and polyhedra.1a-l In general, supramolecules obtained by coordinative self-assembly are fully coordinated, wherein all coordinative binding sites of the building blocks are fully in use upon coordination.1 Such structures benefit from facile design principles and the potential for isomeric byproducts is minimized. Conversely, reports of coordinative supramolecules containing open binding sites are rare.2
Coordinative supramolecules with open binding sites are of interest due to the potential characteristics afforded by open binding sites. Recently, metal-organic frameworks (MOFs) bearing open binding sites have attracted considerable attention in the chemistry and material science literature.3 These sites enable post-synthetic modification and impart catalytic behavior and gas absorption properties to the MOFs possessing them. A similar effect was observed in a study of coordinative polymers bearing unsaturated metal centers, which could be used as catalysts by exploiting the properties of their open metal coordination sites.4 For finite coordinative supramolecules, Hupp and coworkers have prepared and studied the catalytic behavior of unsaturated metalloporphyrins.2a-c Recently, we presented a coordinative supramolecular rectangle capable of sensing transition metal ions, the key feature being open phenanthroline binding sites.2d
The major issue limiting the development of coordinative supramolecules with open binding sites is the synthetic difficulty resulting from open binding sites interrupting the assembly of the structural backbone, resulting in undesirable isomeric supramolecules as well as oligomers. Thus, in the reported examples,2 the binding moieties used in the self-assembly of the structural backbones are different from the open binding sites, to prevent side product formation. However, this method limits the diversity of potential structures and minimizes the number of usable ligands. In this work, we present the facile synthesis of supramolecular polygons bearing open binding sites, wherein the open substituent is the same species used for self-assembly of the backbone.
2,6-bis(pyridin-4-ylethynyl)pyridine 1 was prepared by the Sonogashira coupling reaction of 2,6-diacetylenepyridine5 and 4-bromopyridine (see Supporting Information). Ligand 1 contains two types of pyridyl moieties: terminal pyridine and central pyridine; the terminal pyridines are designed to direct self-assembly of the structural backbone and the central pyridine acts as the open binding site. According to the directional bonding model, as shown in Scheme 1, donor 1 can undergo [2 + 2] and [3 + 3] self-assemblies with 60° and 120° organoplatinum acceptors 26 and 3,7 to form a rhomboid8 and a hexagon9 4 and 5, respectively. The self-assembly of 1 with 2 or 3 was carried out by mixing the donor and acceptor in a 1:1 ratio in aqueous acetone solution (v/v 1:1). After heating at 75 °C for 5 h, the completed self-assembly was isolated by ion exchange with KPF6. The product was characterized by 31P and 1H multinuclear NMR spectroscopy and electrospray ionization mass spectrometry (ESI-MS), the results of which indicate that rhomboid 4 and hexagon 5, bearing open pyridyl cores, were formed quantitatively in the mixtures. No isomeric byproducts resulting from the coordination of the central pyridine of ligand 1 to Pt was observed in either case.
Scheme 1.
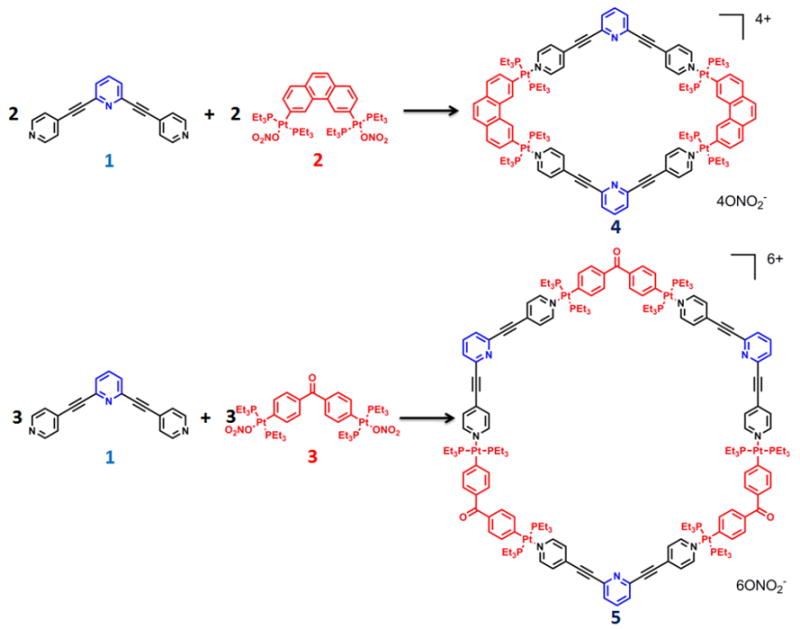
Representation of the self-assembly of supramolecular rhomboid 4 and hexagon 5 bearing open pyridyl binding sites.
As shown in Figure 1, the 31P{1H} NMR spectra of the reaction mixtures show only one singlet signal at 14.3 ppm for 4 and 13.9 ppm for 5 with contaminant 195Pt satellites, indicating that one highly symmetrical assembly was formed in each mixture. Likewise, in the 1H NMR spectra (Figure 2b and Figure S2 in Supporting Information), signals attributed to the terminal pyridyl protons of 1 can be found at 8.98 ppm (HPy-α) and 7.93 ppm (HPy-β) for rhomboid 4 and at 8.90 ppm (HPy-α) and 7.88 ppm (HPy-β) for hexagon 5. A comparison with the spectrum of 1 (Figure 2a) indicates that these signals are downfield shifted (Δδ = 0.2–0.3 ppm) upon coordination to the Pt metal centers. In contrast, the central pyridyl protons exhibit only minors shift (Δδ = 0.01 ppm for HPy-γ-Core; Δδ = 0.05 ppm for HPy-β-Core). These results are consistent with the terminal pyridines of 1 being involved in coordination-driven self-assembly, resulting in the formation of one discrete supramolecular polygon bearing open pyridyl cores.
Figure 1.

31P{1H} NMR spectra (121.4 MHz) of the coordinative supramolecular rhomboid (a) and hexagon (b) in acetone-d6/D2O = 1:1.
Figure 2.

1H NMR spectra (300 MHz) of free ligand 1 in acetone-d6 (a) and the coordinative supramolecular rhomboid 4 in acetone-d6/D2O = 1:1(b).
ESI mass spectral analysis further supports the formation of rhomboid 4 and hexagon 5. As shown in Figure 3 and Figure S3 in the Supporting Information, the ESI mass peaks corresponding to the consecutive loss of nitrate anions from rhomboid 4: m/z = 1382.3 [M - 2NO3-]2+ and m/z = 900.5 [M - 3NO3-]3+ are observed, as are those corresponding to the hexagon 5 at m/z = 1023.8 [M - 4NO3-]4+ and m/z = 806.7 [M - 5NO3-]5+. All of these signals are isotopically resolved and agree well with their theoretical distribution.
Figure 3.

Calculated (top, blue) and experimental (bottom, red) ESI-MS spectra of rhomboid 4 in acetone-d6/D2O (v/v 1:1).
During the self-assemblies described above, the thermodynamic stabilities of the supramolecular species play a significant role for controlling the coordination interactions, wherein only the terminal pyridines of ligand 1 are used for coordination. Supramolecular polygons 4 and 5 are the result of coordination between only the terminal pyridines and the organoplatinum acceptors. Using both terminal and central pyridines, the series of isomers 6–14 could be formed, as shown in Figure 4a. A computational study was carried out to estimate the energy difference between supramolecular polygons 4 and 5 and these isomers 6–14.10 All the structures were built within the input mode of the program Maestro v9.51.09 and subjected to a 1.0 ns molecular dynamics simulation (MMFF force field, gas phase, 300 K) to equilibrate the structures. The output of each simulation was then minimized to full convergence (see Supporting Information). The MMFF computational results are given in Figure 4b and show that in each case, the relative energies of the isomers 6–14 are significantly greater than those of rhomboid 4 and hexagon 5: E6-8 – E4 = 29–84 kcal/mol; E9-14 – E5 = 5–77 kcal/mol. The energy differences may be attributed to the improper directionality and steric hindrance of the central pyridine as compared to the terminal ones. Despite the relatively simple level of theory afforded by MMFF molecular force calculations, on account of the size of 4–14, these computational results strongly support the structures of 4 and 5 over their isomers 6–14, and therefore, only the terminal pyridines of ligand 1 are coordinated under equilibrium.
Figure 4.

(a) Graphical representation of supramolecular polygons 4 and 5 along with their isomers 6–14, and (b) graph of the energy differences between 4 and 5 and their isomers 6–14 obtained from molecular modeling. (Energies are expressed in kcal/mol. The lowest energy supramolecules 4 and 5 are taken as 0.0 kcal/mol, for each series of isomers.)
In conclusion, the facile synthesis of supramolecular polygons with open binding sites has been achieved by coordination-driven self-assembly of pyridyl ligand 1 and organoplatinum acceptors 2 and 3. Formation of these coordinative structures is well supported by the experimental evidence from NMR and ESI-MS, and explained by a computational study predicting the thermodynamic preference. By taking advantage of the open binding site embedded within the self-assembly, a variety of properties may be realized, ranging from post-modification to applications such as guest encapsulation and catalysis.
Supplementary Material
Acknowledgments
P.J.S. thanks the NIH (GM-057052) for financial support.
Footnotes
Dedicated to Professor Harry H. Wasserman on the occasion of his 90th birthday
Experimental details of the synthesis and characterization of ligand 1 and supramolecular hexagon 5. Computational details for modeling supramolecular polygon 4 and 5 as well as their isomers 6–14.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.(a) Leininger S, Olenyuk B, Stang P. J Chem Rev. 2000;100:853. doi: 10.1021/cr9601324. [DOI] [PubMed] [Google Scholar]; (b) Seidel SR, Stang P. J Acc Chem Res. 2002;35:972. doi: 10.1021/ar010142d. [DOI] [PubMed] [Google Scholar]; (c) Holliday BJ, Mirkin CA. Angew Chem, Int Ed. 2001;40:2022. [PubMed] [Google Scholar]; (d) Fujita M, Umemoto K, Yoshizawa M, Fujita N, Kusukawa T, Biradha K. Chem Commun. 2001:509. [Google Scholar]; (e) Fiedler D, Leung DH, Bergman RG, Raymond KN. Acc Chem Res. 2005;38:351. doi: 10.1021/ar040152p. [DOI] [PubMed] [Google Scholar]; (f) Fujita M, Tominaga M, Hori A, Therrien B. Acc Chem Res. 2005;38:369. doi: 10.1021/ar040153h. [DOI] [PubMed] [Google Scholar]; (g) Ruben M, Rojo J, Romero-Salguero FJ, Uppadine LH, Lehn J-M. Angew Chem, Int Ed. 2004;43:3644. doi: 10.1002/anie.200300636. [DOI] [PubMed] [Google Scholar]; (h) Severin K. Chem Commun. 2006:3859. doi: 10.1039/b606632c. [DOI] [PubMed] [Google Scholar]; (i) Nitschke JR. Acc Chem Res. 2007;40:103. doi: 10.1021/ar068185n. [DOI] [PubMed] [Google Scholar]; (j) Oliveri CG, Ulmann PA, Wiester MJ, Mirkin CA. Acc Chem Res. 2008;41:1618. doi: 10.1021/ar800025w. [DOI] [PMC free article] [PubMed] [Google Scholar]; (k) Ward MD. Chem Commun. 2009;30:4487. doi: 10.1039/b906726b. [DOI] [PubMed] [Google Scholar]; (l) Jin P, Dalgarno SJ, Atwood JL. Coord Chem Rev. 2010;254:1760. [Google Scholar]; (m) Pluth MD, Bergman RG, Raymond KN. Acc Chem Res. 2009;42:1650. doi: 10.1021/ar900118t. [DOI] [PubMed] [Google Scholar]; (n) Yoshizawa M, Klosterman JK, Fujita M. Angew Chem, Int Ed. 2009;48:3418. doi: 10.1002/anie.200805340. [DOI] [PubMed] [Google Scholar]; (o) Klosterman JK, Yamauchi Y, Fujita M. Chem Soc Rev. 2009;38:1714. doi: 10.1039/b901261n. [DOI] [PubMed] [Google Scholar]; (p) Northrop BH, Yang H-B, Stang PJ. Chem Commun. 2008;5896 doi: 10.1039/b811712h. [DOI] [PMC free article] [PubMed] [Google Scholar]; (q) Norhtrop BH, Zheng Y-R, Chi K-W, Stang PJ. Acc Chem Res. 2009;42:1554. doi: 10.1021/ar900077c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.(a) Slone RV, Hupp JT. Inorg Chem. 1997;36:5422. [Google Scholar]; (b) Merlau ML, Del Pilar Mejia M, Nguyen ST, Hupp JT. Angew Chem, Int Ed. 2001;40:4239. doi: 10.1002/1521-3773(20011119)40:22<4239::AID-ANIE4239>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]; (c) Lee SJ, Cho S-H, Mulfort KL, Tiede DM, Hupp JT, Nguyen ST. J Am Chem Soc. 2008;130:16828. doi: 10.1021/ja804014y. [DOI] [PubMed] [Google Scholar]; (d) Resendiz MJE, Noveron JC, Disteldorf H, Fischer S, Stang PJ. Org Lett. 2004;6:651. doi: 10.1021/ol035587b. [DOI] [PubMed] [Google Scholar]
- 3.(a) Lee JY, Farha OK, Roberts J, Scheidt KA, Nguyen ST, Hupp JT. Chem Soc Rev. 2009;38:1450. doi: 10.1039/b807080f. [DOI] [PubMed] [Google Scholar]; (b) Shultz AM, Farha OK, Hupp JT, Nguyen ST. J Am Chem Soc. 2009;131:4204. doi: 10.1021/ja900203f. [DOI] [PubMed] [Google Scholar]; (c) Horike S, Dinca M, Tamaki K, Long JR. J Am Chem Soc. 2008;130:5354. doi: 10.1021/ja800669j. [DOI] [PubMed] [Google Scholar]; (d) Dinca M, Long JR. Angew Chem, Int Ed. 2008;47:6766. doi: 10.1002/anie.200801163. [DOI] [PubMed] [Google Scholar]; (e) Bloch ED, Britt D, Lee C, Doonan CJ, Uribe-Romo FJ, Furukawa H, Long JR, Yaghi OM. J Am Chem Soc ASAP. doi: 10.1021/ja106935d. [DOI] [PubMed] [Google Scholar]; (f) Tanabe KK, Cohen SM. Angew Chem, Int Ed. 2009;48:7424. doi: 10.1002/anie.200903433. [DOI] [PubMed] [Google Scholar]; (g) Wang Z, Cohen SM. Chem Soc Rev. 2009;38:1315. doi: 10.1039/b802258p. [DOI] [PubMed] [Google Scholar]; (h) Wu H, Zhou W, Yildirim T. J Am Chem Soc. 2009;131:4995. doi: 10.1021/ja900258t. [DOI] [PubMed] [Google Scholar]; (i) Hwang YK, Hong D-Y, Chang J-S, Jhung SH, Seo Y-K, Kim J, Vimont A, Daturi M, Serre C, Ferey G. Angew Chem, Int Ed. 2008;47:4144. doi: 10.1002/anie.200705998. [DOI] [PubMed] [Google Scholar]
- 4.(a) Zhang J-P, Horike S, Kitagawa S. Angew Chem, Int Ed. 2007;46:889. doi: 10.1002/anie.200603270. [DOI] [PubMed] [Google Scholar]; (b) Cho S-H, Gadzikwa T, Afshari M, Nguyen ST, Hupp JT. Eur J Inorg Chem. 2007;31:4863. [Google Scholar]; (c) Zhang J-P, Kitagawa S. J Am Chem Soc. 2008;130:907. doi: 10.1021/ja075408b. [DOI] [PubMed] [Google Scholar]; (d) Yu L, Wang Z, Wu J, Tu S, Ding K. Angew Chem, Int Ed. 2010;49:3627. doi: 10.1002/anie.200906405. [DOI] [PubMed] [Google Scholar]
- 5.(a) Goto H, Heemstra JM, Hill DJ, Moore JS. Org Lett. 2004;6:889. doi: 10.1021/ol036376+. [DOI] [PubMed] [Google Scholar]; (b) Brunet E, Juanes O, Jimenez L, Rodriguez-Ubis JC. Tetrahedron Lett. 2009;38:5361. [Google Scholar]; (c) Munuera L, O’Reilly RK. Dalton Trans. 2010;2:388. doi: 10.1039/b912319a. [DOI] [PubMed] [Google Scholar]
- 6.Kryschenko YK, Seidel SR, Arif AM, Stang PJ. J Am Chem Soc. 2003;125:5193. doi: 10.1021/ja030018k. [DOI] [PubMed] [Google Scholar]
- 7.Yang H-B, Das N, Huang F, Hawkridge AM, Diaz DD, Arif AM, Finn MG, Muddiman DC, Stang PJ. J Org Chem. 2006;71:6644. doi: 10.1021/jo0608117. [DOI] [PubMed] [Google Scholar]
- 8.(a) Yang H-B, Hawkridge AM, Huang SD, Das N, Bunge SD, Muddiman DC, Stang PJ. J Am Chem Soc. 2007;129:2120. doi: 10.1021/ja066804h. [DOI] [PubMed] [Google Scholar]; (b) Zhao L, Ghosh K, Zheng Y-R, Stang PJ. J Org Chem. 2009;74:8516. doi: 10.1021/jo9019607. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Zheng Y-R, Yang H-B, Ghosh K, Zhao L, Stang PJ. Chem Eur–J. 2009;15:7203. doi: 10.1002/chem.200900230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang H-B, Das N, Huang F, Hawkridge AM, Muddiman DC, Stang PJ. J Am Chem Soc. 2006;128:10014. doi: 10.1021/ja063377z. [DOI] [PubMed] [Google Scholar]
- 10.(a) Zhao L, Northrop BH, Zheng Y-R, Yang H-B, Lee HJ, Lee YM, Park JY, Chi K-W, Stang PJ. J Org Chem. 2008;73:6580. doi: 10.1021/jo800957r. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Zheng Y-R, Northrop BH, Yang H-B, Zhao L, Stang PJ. J Org Chem. 2009;74:3554. doi: 10.1021/jo9002932. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Northrop BH, Chercka D, Stang PJ. Tetrahedron. 2008;64:11495. doi: 10.1016/j.tet.2008.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


