Abstract
Study Objectives:
To examine the association between sleep-related factors and memory impairment.
Design:
Cross-sectional study
Setting:
Community-based study in Guangzhou, China.
Participants:
28,670 older Chinese (20,776 women and 7,894 men) aged 50 to 85 years.
Measurements and Results:
Demographic and socioeconomic data, sleep-related factors, and cognitive function were collected by face-to-face interview. Potential confounders, such as employment and occupational status, smoking, alcohol and tea use, physical activity, self-rated health, anthropometry, blood pressure, and fasting plasma glucose and lipids were measured. After adjusting for multiple potential confounders, an inverted U-shaped association between sleep duration and delayed word recall test (DWRT) score, a validated measure of memory impairment, was found, with 7 to 8 h of habitual sleep duration showing the highest score (P-values for trend from 3 to 7 h and from 7 to ≥ 10 h were all ≤ 0.001). Compared to sleep duration of 7 h, the adjusted odds ratio for memory impairment from the sleep duration of 3 to 4 or ≥ 10 h was 1.29 (95% confidence interval 1.07-1.56) and 1.52 (1.25-1.86), respectively. Subjects with daily napping, morning tiredness, or insomnia had significantly lower DWRT scores than those without (P ranged from < 0.001 to 0.01).
Conclusions:
Short or long sleep duration was an important sleep-related factor independently associated with memory impairment and may be a useful marker for increased risk of cognitive impairment in older people.
Citation:
Xu L; Jiang CQ; Lam TH; Liu B; Jin YL; Zhu T; Zhang WS; Cheng KK; Thomas GN. Short or long sleep duration is associated with memory impairment in older Chinese: the Guangzhou Biobank Cohort Study. SLEEP 2011;34(5):575-580.
Keywords: Sleep, sleep duration, napping, insomnia, memory impairment
INTRODUCTION
Several epidemiology studies have found an association between self-reported sleep duration with health-related conditions such as obesity, diabetes, hypertension, cardiovascular events or mortality.1–3 Both long (> 8 h) and short (< 6 h) habitual sleep durations are associated with unfavorable health outcomes.1,4,5
Associations derived from epidemiological studies between sleep duration and cognitive function showed inconsistent results.6–10 A recent study from Spain showed that long, but not short, sleep duration, was associated with decreased cognitive function.7 In the Health 2000 Survey, sleep duration was associated with decreased cognitive function in 5171 subjects aged ≥ 30 years.6 However, in a stratified analysis of a subgroup of 2201 subjects who did not report any illness and who also reported good, or relatively good subjective health showed that after adjusting for socioeconomic factors, the association between sleep duration and cognitive function was not observed. This suggested that self-reported sleep duration may not be causally associated with the objective decline of cognitive function. Moreover, some studies showed that sleep quality, but not longer or shorter sleep duration, was associated with cognitive performance in Western populations.8–10
To better understand the association of sleep-related factors, such as sleep duration, napping, morning tiredness or insomnia, and memory impairment, we analyzed the baseline data of the Guangzhou Biobank Cohort Study, which includes about 30,000 Chinese people aged ≥ 50 years.
METHODS
We used the baseline date of the Guangzhou Biobank Cohort Study (GBCS) recruited from Phases 1 to 3 in September 2003 to January 2008. Detailed information describing the sampling frame, selection strategy, and measurement of the GBCS has been reported elsewhere.11 In brief, subjects included in the GBCS were randomly selected from an unofficial organization that was affiliated with the local government: the “Guangzhou Health and Happiness Association for the Respectable Elders” (GHHARE), which is an association with membership open to older people aged 50 or above for a nominal monthly fee of 4 RMB (1 USD = 7 RMB). More than 95% of the selected people were eligible and gave consent to participate in the GBCS.11 This is a large social and welfare association with more than 100,000 older Guangzhou permanent residents. Subjects were ambulatory, capable of consenting, and not receiving treatment modalities of life-threatening diseases.
Subjects gave informed consent before the questionnaire-based face-to-face interview. Information describing demographic and socioeconomic status, lifestyle factors, occupational exposure, cognitive function, and disease history was collected by full-time trained nurses at the Guangzhou Number 12 Hospital. Sleep-related factors including habitual sleep duration per day, frequency of daytime napping and morning tiredness, and presence of insomnia were also assessed. Subjects were asked whether they had trouble falling asleep, waking up too early and not falling asleep again, or needed to take medicine (including herbal or sleeping pills) at least once a week to help sleep within the last month, and were classified as having insomnia if they reported having any of these sleep problems lasting ≥ 2 weeks. Health status was assessed by a subjective rating scale (very good, good, poor, or very poor). The reproducibility of the questionnaire was assessed in 200 subjects by re-interview after 1 month, and the result was very satisfactory.11 Fasting blood samples were drawn for measuring conventional risk factors of vascular disease, such as lipids, glucose, and inflammatory markers.
The modified Consortium to Establish a Registry for Alzheimer's Disease Delayed Word Recall Test (DWRT)12 was used to assess memory impairment in all subjects in Phase 1 to Phase 3 of GBCS. During the interview, 10 simple Chinese words (soy sauce, arm, letter, chairman, ticket, grass, corner, stone, book, and stick) were read out to the subjects one by one, pausing for one second between each. Subjects were asked to recall the words they heard immediately after the last word. This procedure was repeated 3 times, and then after 5 minutes, the subjects were asked to recall as many of the words as possible. Subjects who successfully recalled one word were given a score of 1, with a maximum score of 10, which was used as an outcome variable in this test. The detail of this test has been described elsewhere.13 Memory impairment was diagnosed according to the DWRT score (out of 10). Memory impairment was defined as DWRT score < 4, corresponding to 1 standard deviation (SD) below the mean (mean = 5.5 and SD = 1.8).
In Phase 3 of GBCS, we added the Mini-Mental State Examination (MMSE)14 to assess cognitive function. Thus, 9,237 subjects (6,829 women and 2,408 men) had the MMSE from September 2006 to January 2008. MMSE was scored from 0 to 30, with higher scores indicating better cognitive performance.
In the present analysis, 174 subjects with self-reported mental illnesses or neurological disease including depression, confused speech, schizophrenia, dementia, or Alzheimer disease (AD) were excluded. For self-reported sleep duration, 53 subjects with extremely short or long duration (< 3 h or > 15 h per day) were also excluded7 (sensitivity analysis by including them showed similar results). Thus, the final analysis included 28,670 subjects (20,776 women and 7,894 men) with all variables of interest. The study was approved by the Guangzhou Medical Ethics Committee of the Chinese Medical Association in Guangzhou, China.
Pearson χ2 test was used to compare categorical variables, and one-way analysis of variance (ANOVA) for continuous variables. Multivariable linear regression was used to study the association of sleep-related factors with DWRT score with adjusted mean and 95% confidence interval (CI) reported. Logistic regression was used to study the association of the sleep-related factors with memory impairment. Potential confounders (Table 1) were age, sex, employment status, occupation, education level, smoking and drinking status, physical activity, tea consumption, waist circumference, high-density lipoprotein- (HDL-) and low-density lipoprotein- (LDL-) cholesterol, triglycerides, systolic blood pressure (SBP), diastolic blood pressure (DBP), and fasting plasma glucose. Potential confounders were included in the multivariable models. Sensitivity analysis excluding subjects with poor self-rated health was also performed to overcome potential reverse causation. Data analysis was done using STATA/IC 10.1 (Stata Corp LP, College Station, TX, USA).
Table 1.
Demographic and clinical characteristics and sleep-related
| Memory impairment |
P | ||
|---|---|---|---|
| No (n = 24,966) | Yes (n = 3,704) | ||
| Age, years | 61.5 ± 7.0 | 65.3 ± 7.2 | < 0.001 |
| Sex, % men | 27.2 | 29.6 | 0.003 |
| Education, % primary or below | 39.1 | 65.9 | < 0.001 |
| Employment, % current | 4.5 | 2.3 | < 0.001 |
| Longest occupation, % manual | 47.0 | 62.2 | < 0.001 |
| Smoking, % current | 9.8 | 11.7 | < 0.001 |
| Drinking, % current | 31.4 | 22.1 | < 0.001 |
| Physical activity, % active | 51.7 | 47.8 | < 0.001 |
| Tea consumption, % regular | 40.5 | 40.8 | 0.73 |
| Poor self-rated health | 17.0 | 21.1 | < 0.001 |
| Waist circumference, cm | 78.7 ± 9.0 | 79.7 ± 9.2 | < 0.001 |
| High-density lipoprotein cholesterol, mmol/L | 1.66 ± 0.41 | 1.64 ± 0.40 | 0.007 |
| Low-density lipoprotein cholesterol, mmol/L | 3.27 ± 0.70 | 3.21 ± 0.73 | < 0.001 |
| Triglycerides, mmol/L | 1.68 ± 1.26 | 1.70 ± 1.30 | 0.33 |
| Systolic blood pressure, mm Hg | 130 ± 22 | 134 ± 23 | < 0.001 |
| Diastolic blood pressure, mm Hg | 73.6 ± 11.2 | 73.7 ± 11.4 | 0.48 |
| Fasting plasma glucose, mmol/L | 5.73 ± 1.63 | 5.89 ± 1.84 | < 0.001 |
| Sleep duration, hours per day | 6.94 ± 1.32 | 6.82 ± 1.48 | < 0.001 |
| Daytime napping, % daily | 39.0 | 42.1 | < 0.001 |
| Morning tiredness, % daily | 3.3 | 4.6 | < 0.001 |
| Insomnia, % yes | 17.0 | 17.9 | 0.21 |
Data were expressed as percentage (%) or mean ± standard deviation. P for one-way analysis of variance or chi-square test.
RESULTS
Table 1 shows that 3,704 (12.9%) subjects were identified as having memory impairment. Memory impairment was significantly associated with older age, male sex, lower education level, unemployment, manual occupation, smoking, physical inactivity, poor self-rated health, frequently napping and morning tiredness, higher levels of waist circumference, SBP, and fasting plasma glucose, and lower levels of LDL and HDL cholesterol and sleep duration.
Table 2 shows an inverted U-shaped association between sleep duration and DWRT score, with the peak at 7 to 8 h of habitual sleep, after adjusting for multiple potential confounders. Both shorter (3-4 and 5 h) and longer (9 and ≥ 10 h) duration showed lower DWRT scores compared to 7 h (both P-values for trend from 3-4 to 7 h and from 7 to ≥ 10 h ≤ 0.001). The adjusted mean difference (95% CI) for 3-4, 5, 9, and 10 h compared to 7 h was –0.17 (–0.30 to –0.06), –0.07 (–0.15 to –0.005), –0.11 (–0.20 to –0.008), and –0.32 (–0.44 to –0.20) words, respectively. If this risk estimate is correct, the decreased cognitive function in those with 3-4, 5 or 9, and 10 h sleep duration equals to a 4-, 2-, and 7-year age increment, respectively, relative to those with 7 h sleep duration.
Table 2.
Mean differences in the 10-word recall test score (95% confidence interval) by groups of sleep duration
| Sleep duration, hours per day |
P for linear trend (3 to 7 h) | P for linear trend (7 to ≥ 10 h) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 3–4 | 5 | 6 | 7 | 8 | 9 | ≥ 10 | |||
| No. of subjects | 1,074 | 2,768 | 7,016 | 8,598 | 6,734 | 1,546 | 934 | – | – |
| Crude model | −0.51 (−0.62 to −0.39)*** | −0.32 (−0.40 to −0.25)*** | −0.17 (−0.23 to −0.11)*** | Ref. | 0.02 (−0.04 to 0.08) | −0.15 (−0.25 to −0.05)** | −0.45 (−0.57 to −0.32)*** | < 0.001 | < 0.001 |
| Model 1 | −0.22 (−0.34 to −0.11)*** | −0.09 (−0.17 to −0.01)* | −0.03 (−0.09 to −0.02) | Ref. | 0.03 (−0.03 to 0.08) | −0.12 (−0.22 to −0.02)* | −0.34 (−0.46 to −0.22)*** | < 0.001 | < 0.001 |
| Model 2 | −0.17 (−0.30 to −0.06)** | −0.07 (−0.15 to −0.005) | −0.03 (−0.09 to 0.03) | Ref. | 0.03 (−0.03 to 0.09) | −0.11(−0.20 to −0.008)* | −0.32 (−0.44 to −0.20)*** | 0.001 | < 0.001 |
Model 1: adjusting for age and sex, employment, occupation, education, smoking, drinking, physical activity, tea consumption and self-rated health.
Model 2: adjusting for age, sex, employment, occupation, education, smoking, drinking, physical activity, tea consumption, self-rated health, waist circumference, HDL- and LDL-cholesterol, fasting plasma glucose, systolic blood pressure, daytime napping, feeling tired in the morning and insomnia.
P < 0.05,
P < 0.01,
P < 0.001.
Table 3 shows that increasing frequency of daytime napping was significantly associated with decreased DWRT score (P for trend = 0.005). Compared to those with never or < 1 day/week of daytime napping, subjects with 4-6 days/week or daily napping had significantly lower DWRT score (adjusted mean difference: –0.07 [–0.14 to –0.001] and –0.06 [–0.11 to –0.007], respectively).
Table 3.
Mean differences in the 10-word recall test score (95% confidence interval) by frequency of daytime napping
| Daytime napping |
P for trend | ||||
|---|---|---|---|---|---|
| Never to < 1 day/week | 1–3 days/week | 4–6 days/week | Daily | ||
| No. of subjects | 10,353 | 4,043 | 2,984 | 11,290 | – |
| Crude model | Ref. | 0.06 (−0.004 to 0.13) | −0.18 (−0.25 to −0.10)*** | −0.15 (−0.20 to −0.10)*** | < 0.001 |
| Model 1 | Ref. | 0.04 (−0.02 to 0.11) | −0.08 (−0.15 to −0.003)* | −0.07 (−0.12 to −0.02)** | 0.001 |
| Model 2 | Ref. | 0.04 (−0.03 to 0.10) | −0.07 (−0.14 to −0.001)* | −0.06 (−0.11 to −0.007)* | 0.005 |
Model 1: adjusting for age and sex, employment, occupation, education, smoking, drinking, physical activity, tea consumption and self-rated health
Model 2: adjusting for age, sex, employment, occupation, education, smoking, drinking, physical activity, tea consumption, self-rated health, waist circumference, HDL- and LDL-cholesterol, fasting plasma glucose, systolic blood pressure, sleeping duration, feeling tired in the morning and insomnia
P < 0.05,
P < 0.01,
P < 0.001.
Table 4 shows a significant dose-response relation between frequency of morning tiredness and DWRT score (P for trend < 0.001). Subjects who felt tired in the morning almost daily had a significantly lower DWRT score than those who never or seldom (< 1-2 days/month) felt so (adjusted mean difference: –0.23 [–0.34 to –0.12]). Moreover, subjects with insomnia had a lower DWRT score than those without insomnia (P = 0.01), independent of multiple potential confounders (Table 5).
Table 4.
Mean differences in the 10-word recall test score (95% confidence interval) by frequency of morning tiredness
| Frequency of morning tiredness |
P for trend | ||||
|---|---|---|---|---|---|
| Never to < 1–2days/month | 1–2 days/week | 3–4 days/week | Almost daily | ||
| No. of subjects | 25,411 | 1,644 | 615 | 1,000 | – |
| Crude model | Ref. | −0.05 (−0.14 to 0.04) | −0.13 (−0.27 to 0.02) | −0.36 (−0.47 to −0.24)*** | < 0.001 |
| Model 1 | Ref. | −0.07 (−0.16 to 0.02) | −0.15 (−0.29 to −0.005)* | −0.25 (−0.36 to −0.13)*** | < 0.001 |
| Model 2 | Ref. | −0.07 (−0.16 to 0.02) | −0.12 (−0.26 to 0.02) | −0.23 (−0.34 to −0.12)*** | < 0.001 |
Model 1: adjusting for age and sex, employment, occupation, education, smoking, drinking, physical activity, tea consumption and self-rated health
Model 2: adjusting for age, sex, employment, occupation, education, smoking, drinking, physical activity, tea consumption, self-rated health, waist circumference, HDL- and LDL-cholesterol, fasting plasma glucose, systolic blood pressure, sleeping duration, napping and insomnia.
P < 0.05,
P < 0.001.
Table 5.
Mean differences in the 10-word recall test score (95% confidence interval) by insomnia
| Insomnia |
P | ||
|---|---|---|---|
| No | Yes | ||
| No. of subjects | 23,884 | 4,779 | – |
| Crude model | Ref. | −0.06 (−0.12 to −0.01) | 0.03 |
| Model 1 | Ref. | −0.10 (−0.16 to −0.05) | < 0.001 |
| Model 2 | Ref. | −0.08 (−0.14 to −0.02) | 0.01 |
Model 1: adjusting for age and sex, employment, occupation, education, smoking, drinking, physical activity, tea consumption, and self-rated health
Model 2: adjusting for age, sex, employment, occupation, education, smoking, drinking, physical activity, tea consumption, self-rated health, waist circumference, HDL- and LDL-cholesterol, fasting plasma glucose, systolic blood pressure, sleeping duration, napping, and morning tiredness.
Table 6 shows a U-shaped relation between duration of sleep and risk for memory impairment, with the nadir at 7 to 8 h per day was found. Subjects with 3-4 or ≥ 10 hours of sleep duration had significantly increased risk of memory impairment (adjusted OR = 1.29 [1.07-1.56] and 1.52 [1.25-1.86], respectively). As expected, increased frequency of morning tiredness was also associated with increasing risk of memory impairment (P for trend = 0.003). No significant association between napping or insomnia and memory impairment was found.
Table 6.
Adjusted odds ratios (95% confidence interval) of memory impairment by usual sleep duration and frequency of daytime napping
| Memory impairment |
P | |||
|---|---|---|---|---|
| N (% cases) | Adjusted OR (95% CI) | |||
| Sleep duration, hours per day | ||||
| 3–4 | 1,074 (19.0) | 1.29 (1.07–1.56) | 0.007 | |
| 5 | 2,768 (15.8) | 1.12 (0.98–1.28) | 0.09 | |
| 6 | 7,016 (13.4) | 1.00 (0.90–1.11) | 0.96 | |
| 7 | 8,598 (11.7) | Ref. | ||
| 8 | 6.734 (11.1) | 0.94 (0.84–1.04) | 0.23 | |
| 9 | 1546 (13.3) | 1.09 (0.91–1.29) | 0.34 | |
| ≥ 10 | 934 (18.2) | 1.52 (1.25–1.86) | < 0.001 | |
| P for trend (3 to7 h) | 0.005 | |||
| P for trend (7 to ≥ 10 h) | 0.003 | |||
| Daytime napping | ||||
| Never to < 1 day/week | 10,331 (12.4) | Ref. | ||
| 1–3 days/week | 4,048 (11.2) | 0.95 (0.84–1.07) | 0.38 | |
| 4–6 days/week | 2,987 (13.8) | 1.06 (0.932–1.20) | 0.39 | |
| Daily | 11,304 (13.8) | 1.07 (0.98–1.16) | 0.15 | |
| P for trend | 0.09 | |||
| Tired in the morning | ||||
| Never to < 1–2days/month | 25,411 (12.7) | Ref. | ||
| 1–2 days/week | 1,644 (13.8) | 1.18 (1.01–1.39) | 0.04 | |
| 3–4 days/week | 615 (13.8) | 1.09 (0.84–1.41) | 0.51 | |
| Almost daily | 1,000 (16.9) | 1.27 (1.05–1.53) | 0.01 | |
| P for trend | 0.003 | |||
| Insomnia | ||||
| No | 23,747 (12.8) | Ref. | ||
| Yes | 4,916 (13.5) | 1.10 (1.00–1.22) | 0.06 | |
N (% cases): total number of subjects in each group (% with memory impairment). Adjusting for age, sex, employment, occupation, education, smoking, drinking, physical activity, tea consumption, self-rated health, waist circumference, HDL- and LDL-cholesterol, fasting plasma glucose and systolic blood pressure.
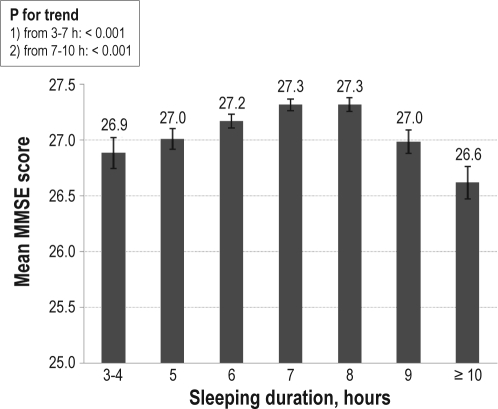
Sensitivity analysis showed that after excluding those with poor or very poor self-rated health, both short or long sleep duration, and morning tiredness were significantly associated with DWRT score. However, no association between napping or insomnia and DWRT was found (Table not shown). Consistent with those using DWRT score, an inverted U-shaped relationship between MMSE score and sleep duration was observed, with the peak at 7-8 h of sleep (Figure 1). Subjects who felt tired in the morning almost daily had significantly reduced MMSE score compared with those who never or seldom felt so (P for trend = 0.006), and no association between napping or insomnia and MMSE score was found (Table not shown).
Figure 1.
Adjusted mean MMSE test score (95% confidence interval) by groups of sleep duration (n = 9,237). Adjusting for age, sex, employment, occupation, education, smoking, drinking, physical activity, tea consumption, self-rated health, waist circumference, HDL- and LDL-cholesterol, fasting plasma glucose, systolic blood pressure, napping, morning tiredness, and insomnia factors by memory impairment.
DISCUSSION
To the best of our knowledge, this is the largest community-based study to date examining the association between sleep related factors and memory impairment in older Chinese. Memory impairment, as assessed by DWRT score, was significantly associated with duration of sleep, frequency of daytime napping, and morning tiredness and insomnia. An inverted U-shaped association between sleep duration and score of word delayed test was found, with the peak at 7 to 8 hours. Among the four selected sleep-related factors, short or long duration of sleep was the most important factor, significantly associated with reduction of memory impairment (as assessed by DWRT and MMSE) after controlling for multiple potential confounders.
Short or long sleep duration has been associated with cognitive impairment in earlier epidemiological or experimental studies.8,15–17 Two recent studies found that self-reported sleep duration was associated with cognitive function, as assessed objectively by verbal fluency, encoding, and retaining verbal material or MMSE.6,7 In Faubel's study on 3212 Spanish people aged > 60 years, the risk of cognitive impairment increased across sleep duration groups from 7 to ≥ 11 hours, while no association was found between short sleep duration and cognitive impairment.7 However, sleep factors have been suggested to account for only a small proportion of the variance in the objectively measured cognitive function, but for a larger proportion of the variation in subjectively measured cognitive function.6 A study of 88 healthy postmenopausal women showed that self-reported low sleep quality, but not objectively assessed sleep, was associated with decreased cognitive test performance and difficulty in concentrating.18 Different from the cross-sectional studies above, the Nurses' Health Study on 1,852 US women showed that neither long nor short sleep duration was associated with decline of cognitive function, as measured by category fluency, verbal memory, and global score after a 2-year follow-up. The results from existing prospective studies were inconsistent. The Maastricht Ageing Study followed up 838 middle-aged adults for 3 years and found that having any subjective sleep problem at baseline or follow-up was significantly associated with cognitive decline as assessed by MMSE. However, this association became statistically insignificant after further controlling for depression.19 In our study, after excluding those with self-reported depression, dementia, or Alzheimer disease, both short and long sleep duration showed independent and significant association with worse memory impairment.
Daytime sleepiness has been associated with cognitive impairment and dementia in earlier epidemiological studies.17,20 The Honolulu-Asia Aging Study on 2,346 elderly people found that daytime sleepiness, but not insomnia, was independently associated with cognitive decline or incidence of dementia after a 3-year follow-up.20 A study including 1,026 subjects aged 60 years or older in Paris found that daytime napping was an important risk factor for cognitive decline, and might result in longer total sleep duration.17 However, daytime napping was dichotomized into presence or absence in the two studies above,17,20 in which dose-response relation of daytime napping and cognitive performance could not be assessed. Regular napping is a common practice in all ages of Chinese compared to the north European populations.21,23 In the present study, we found that increasing frequency of daytime napping was associated with memory impairment, independent of total sleep duration and other potential confounders. A possible explanation for the predictive value of daytime napping is that other disorders, such as an organic or mental pathologic condition, or obstructive sleep apnea syndrome (OSAS), cause increasing frequency of daytime napping. OSAS has been shown to associate with cognitive impairment because breathing pause during sleep may cause repeated anoxia, leading to the cerebral white matter change and cognitive decline.24 Another possible explanation is that daytime napping has been associated with metabolic disorders, such as obesity or type 2 diabetes,25 which may increase the risk for cognitive impairment.26,27
Our study also found that morning tiredness and insomnia was also significantly associated with memory impairment with a linear dose-response pattern, and that those with daily morning tiredness had almost significantly increased risk for memory impairment compared with those who seldom felt tired. It is possible that those who had short sleep duration might also feel tired in the morning and have insomnia. However, after controlling for sleep duration, the associations between tiredness in the morning or insomnia and DWRT score remained unchanged. There is also evidence in different populations showing that insomnia was associated with cognitive impairment.28,29 Nevertheless, given the cross-sectional design of these studies and the lack of prospective studies, a causal relation between morning tiredness/insomnia and cognitive function cannot be established. It is possible that cognitive function may alter individuals' perception of fatigue or insomnia. The mechanism of how these factors influence cognitive impairment is not clear, and further studies are warranted.
The present study had several limitations. Firstly, given its cross-sectional design, the causal relationship between the sleep-related factors and cognitive impairment cannot be confirmed. Secondly, because information on disease history or sleep-related factors was collected by self-report, those with AD or dementia might not be able to accurately recall their disease status or sleep-related factors. Thus in future studies, objectively measured factors including physician diagnosed mental illnesses or neurological diseases and polysomnography to assess sleep duration would be needed. Another limitation is that we measured habitual sleep duration per day, and thus nocturnal sleep duration and daytime sleep duration could not be distinguished, although including daytime napping, morning tiredness, and insomnia might partly account for this limitation. Fourthly, our subjects were unlikely to be completely representative of the older adults in Guangzhou, and general population data were not available for checking of representativeness. Subjects were randomly selected from the GHHARE, and those with severe Alzheimer disease, dementia, or depression might not participate. Because the participants might have had better social and cognitive function, the “healthy volunteer bias” could not be ruled out, which could result in more conservative estimates of the strength of the association. Depression or depressive symptoms are often underreported and underdiagnosed in China,30 and some of the subjects included could have had undiagnosed depression. Further studies on more representative samples are warranted. Finally, residual confounders could partly explain the association of cognitive function with long/short sleep duration and insomnia. Depression or low socioeconomic position has been identified as important risk factors of long sleep duration.31 In the present study, we excluded the subjects with self-reported depression and other mental illnesses to minimize such confounding. However, because depression was assessed based on self-reported data, underreporting of depression could not be ruled out.
In conclusion, our results suggest that there are independent associations between sleep-related factors, such as sleep duration, daytime napping, morning tiredness and insomnia, and memory impairment. Of these four factors, short or long sleep duration showed a stronger association and may be a useful marker for increased risk of cognitive impairment. Further studies with prospective design and objective assessment of sleep-related factors in addition to self-reported data are warranted.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This work was supported by The University of Hong Kong Foundation for Educational Development and Research, Hong Kong; the Guangzhou Public Health Bureau and the Guangzhou Science and Technology Bureau, Guangzhou, China; and the University of Birmingham, UK.
The Guangzhou Biobank Cohort Study investigators include: the Guangzhou No. 12 Hospital: WS Zhang, M Cao, T Zhu, B Liu, CQ Jiang (Co-PI); The University of Hong Kong: CM Schooling, SM McGhee, GM Leung, TH Lam (Co-PI); The University of Birmingham: GN Thomas, P Adab, KK Cheng (Co-PI).
REFERENCES
- 1.Ikehara S, Iso H, Date C, et al. Association of sleep duration with mortality from cardiovascular disease and other causes for Japanese men and women: the JACC study. Sleep. 2009;32:295–301. doi: 10.1093/sleep/32.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vgontzas AN, Liao D, Pejovic S, Calhoun S, Karataraki M, Bixler EO. Insomnia with objective short sleep duration is associated with type 2 diabetes: A population-based study. Diabetes Care. 2009;32:1980–5. doi: 10.2337/dc09-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vgontzas AN, Liao D, Bixler EO, Chrousos GP, Vela-Bueno A. Insomnia with objective short sleep duration is associated with a high risk for hypertension. Sleep. 2009;32:491–7. doi: 10.1093/sleep/32.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grandner MA, Hale L, Moore M, Patel NP. Mortality associated with short sleep duration: The evidence, the possible mechanisms, and the future. Sleep Med Rev. 2010;14:191–203. doi: 10.1016/j.smrv.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gallicchio L, Kalesan B. Sleep duration and mortality: a systematic review and meta-analysis. J Sleep Res. 2009;18:148–58. doi: 10.1111/j.1365-2869.2008.00732.x. [DOI] [PubMed] [Google Scholar]
- 6.Kronholm E, Sallinen M, Suutama T, Sulkava R, Era P, Partonen T. Self-reported sleep duration and cognitive functioning in the general population. J Sleep Res. 2009;18:436–46. doi: 10.1111/j.1365-2869.2009.00765.x. [DOI] [PubMed] [Google Scholar]
- 7.Faubel R, Lopez-Garcia E, Guallar-Castillon P, Graciani A, Banegas JR, Rodriguez-Artalejo F. Usual sleep duration and cognitive function in older adults in Spain. J Sleep Res. 2009;18:427–35. doi: 10.1111/j.1365-2869.2009.00759.x. [DOI] [PubMed] [Google Scholar]
- 8.Tworoger SS, Lee S, Schernhammer ES, Grodstein F. The association of self-reported sleep duration, difficulty sleeping, and snoring with cognitive function in older women. Alzheimer Dis Assoc Disord. 2006;20:41–8. doi: 10.1097/01.wad.0000201850.52707.80. [DOI] [PubMed] [Google Scholar]
- 9.Nebes RD, Buysse DJ, Halligan EM, Houck PR, Monk TH. Self-reported sleep quality predicts poor cognitive performance in healthy older adults. J Gerontol B Psychol Sci Soc Sci. 2009;64:180–7. doi: 10.1093/geronb/gbn037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blackwell T, Yaffe K, Ancoli-Israel S, et al. Poor sleep is associated with impaired cognitive function in older women: the study of osteoporotic fractures. J Gerontol A Biol Sci Med Sci. 2006;61:405–10. doi: 10.1093/gerona/61.4.405. [DOI] [PubMed] [Google Scholar]
- 11.Jiang C, Thomas GN, Lam TH, et al. Cohort profile: The Guangzhou Biobank Cohort Study, a Guangzhou-Hong Kong-Birmingham collaboration. Int J Epidemiol. 2006;35:844–52. doi: 10.1093/ije/dyl131. [DOI] [PubMed] [Google Scholar]
- 12.Welsh KA, Butters N, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part V. A normative study of the neuropsychological battery. Neurology. 1994;44:609–14. doi: 10.1212/wnl.44.4.609. [DOI] [PubMed] [Google Scholar]
- 13.Heys M, Schooling CM, Jiang C, et al. Childhood growth and adulthood cognition in a rapidly developing population. Epidemiology. 2009;20:91–9. doi: 10.1097/ede.0b013e3181880396. [DOI] [PubMed] [Google Scholar]
- 14.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 15.Schmutte T, Harris S, Levin R, Zweig R, Katz M, Lipton R. The relation between cognitive functioning and self-reported sleep complaints in nondemented older adults: results from the Bronx aging study. Behav Sleep Med. 2007;5:39–56. doi: 10.1207/s15402010bsm0501_3. [DOI] [PubMed] [Google Scholar]
- 16.Pilcher JJ, Walters AS. How sleep deprivation affects psychological variables related to college students' cognitive performance. J Am Coll Health. 1997;46:121–6. doi: 10.1080/07448489709595597. [DOI] [PubMed] [Google Scholar]
- 17.Ohayon MM, Vecchierini MF. Daytime sleepiness and cognitive impairment in the elderly population. Arch Intern Med. 2002;162:201–8. doi: 10.1001/archinte.162.2.201. [DOI] [PubMed] [Google Scholar]
- 18.Kavikondala S, Jiang CQ, Zhang WS, et al. Intergenerational ‘mismatch’ and adiposity in a developing population: the Guangzhou biobank cohort study. Soc Sci Med. 2010;70:834–43. doi: 10.1016/j.socscimed.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 19.Jelicic M, Bosma H, Ponds RW, Van Boxtel MP, Houx PJ, Jolles J. Subjective sleep problems in later life as predictors of cognitive decline. Report from the Maastricht Ageing Study (MAAS). Int J Geriatr Psychiatry. 2002;17:73–7. doi: 10.1002/gps.529. [DOI] [PubMed] [Google Scholar]
- 20.Foley D, Monjan A, Masaki K, et al. Daytime sleepiness is associated with 3-year incident dementia and cognitive decline in older Japanese-American men. J Am Geriatr Soc. 2001;49:1628–32. doi: 10.1046/j.1532-5415.2001.t01-1-49271.x. [DOI] [PubMed] [Google Scholar]
- 21.Hays JC, Blazer DG, Foley DJ. Risk of napping: excessive daytime sleepiness and mortality in an older community population. J Am Geriatr Soc. 1996;44:693–8. doi: 10.1111/j.1532-5415.1996.tb01834.x. [DOI] [PubMed] [Google Scholar]
- 22.Chen W, Shen YD, Chen R, Ding GX. [Investigation on sleep status of college and high school students] Zhonghua Yu Fang Yi Xue Za Zhi. 2005;39:48–50. [PubMed] [Google Scholar]
- 23.Liu X, Liu L. Sleep habits and insomnia in a sample of elderly persons in China. Sleep. 2005;28:1579–87. [PubMed] [Google Scholar]
- 24.Naegele B, Thouvard V, Pepin JL, et al. Deficits of cognitive executive functions in patients with sleep apnea syndrome. Sleep. 1995;18:43–52. [PubMed] [Google Scholar]
- 25.Lam KB, Jiang CQ, Thomas GN, et al. Napping is associated with increased risk of type 2 diabetes: the Guangzhou Biobank Cohort Study. Sleep. 2010;33:402–7. doi: 10.1093/sleep/33.3.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Etgen T, Bickel H, Forstl H. Metabolic and endocrine factors in mild cognitive impairment. Ageing Res Rev. 2010;9:280–8. doi: 10.1016/j.arr.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 27.Yaffe K, Weston AL, Blackwell T, Krueger KA. The metabolic syndrome and development of cognitive impairment among older women. Arch Neurol. 2009;66:324–8. doi: 10.1001/archneurol.2008.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haimov I, Hanuka E, Horowitz Y. Chronic insomnia and cognitive functioning among older adults. Behav Sleep Med. 2008;6:32–54. doi: 10.1080/15402000701796080. [DOI] [PubMed] [Google Scholar]
- 29.Fulda S, Schulz H. Cognitive dysfunction in sleep disorders. Sleep Med Rev. 2001;5:423–45. doi: 10.1053/smrv.2001.0157. [DOI] [PubMed] [Google Scholar]
- 30.Lee S, Tsang A, Huang YQ, et al. The epidemiology of depression in metropolitan China. Psychol Med. 2009;39:735–47. doi: 10.1017/S0033291708004091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patel SR, Malhotra A, Gottlieb DJ, White DP, Hu FB. Correlates of long sleep duration. Sleep. 2006;29:881–9. doi: 10.1093/sleep/29.7.881. [DOI] [PMC free article] [PubMed] [Google Scholar]