Abstract
Study Objectives:
To evaluate the effect of increasing the intensity and/or duration of exposure on light-induced changes in the timing of the circadian clock of humans.
Design:
Multifactorial randomized controlled trial, between and within subject design
Setting:
General Clinical Research Center (GCRC) of an academic medical center
Participants:
56 healthy young subjects (20-40 years of age)
Interventions:
Research subjects were admitted for 2 independent stays of 4 nights/3 days for treatment with bright or dim-light (randomized order) at a time known to induce phase delays in circadian timing. The intensity and duration of the bright light were determined by random assignment to one of 9 treatment conditions (duration of 1, 2, or 3 hours at 2000, 4000, or 8000 lux).
Measurements and Results:
Treatment-induced changes in the dim light melatonin onset (DLMO) and dim light melatonin offset (DLMOff) were measured from blood samples collected every 20-30 min throughout baseline and post-treatment nights. Comparison by multi-factor analysis of variance (ANOVA) of light-induced changes in the time of the circadian melatonin rhythm for the 9 conditions revealed that changing the duration of the light exposure from 1 to 3 h increased the magnitude of light-induced delays. In contrast, increasing from moderate (2,000 lux) to high (8,000 lux) intensity light did not alter the magnitude of phase delays of the circadian melatonin rhythm.
Conclusions:
Results from the present study suggest that for phototherapy of circadian rhythm sleep disorders in humans, a longer period of moderate intensity light may be more effective than a shorter exposure period of high intensity light.
Citation:
Dewan K; Benloucif S; Reid K; Wolfe LF; Zee PC. Light-induced changes of the circadian clock of humans: increasing duration is more effective than increasing light intensity. SLEEP 2011;34(5):593-599.
Keywords: Circadian rhythm sleep disorder (CRSD), phototherapy, phase shift, phase delay
INTRODUCTION
Circadian rhythm sleep disorders (CRSDs), which include shift work disorder (SWD), jet lag disorder (JLD), advanced sleep phase disorder (ASPD), delayed sleep phase disorder (DSPD), irregular sleep-wake rhythm (ISWR), and the non–24-hour sleep-wake syndrome (non-entrained type) or free-running disorder (FRD), involve a persistent or recurrent pattern of sleep disturbance due primarily to alterations in the circadian timekeeping system or a misalignment between endogenous circadian rhythms and exogenous factors that affect the timing or duration of sleep.1 Thus, either exogenous or endogenous factors, or often both, can contribute to the misalignment between timing of internal circadian rhythms and the desired or required time for sleep. Treatments for CRSDs focus on improving sleep and alertness when the internal and external schedules are misaligned (e.g., hypnotics, stimulants, and alerting agents), or aligning the internal circadian phase with the external social/work schedule (e.g., light and melatonin).
It is well established that the solar light-dark cycle is the primary environmental time cue for synchronizing the circadian system of most living organisms to the 24-h day. Previous studies have demonstrated robust melatonin suppression as well as phase shifting effects on the human circadian system with bright light exposure.2 In addition to being the primary synchronizing signal for the circadian pacemaker, light also has a direct alerting effect in humans.3 These findings have given rise to the use of timed light exposure as a treatment of CRSDs by both alerting (e.g., SWD) and changing the timing of the internal circadian clock when administered at the appropriate circadian phase.1
The appropriate timing of light exposure is determined by phase response curves (PRCs) to light, which are similar in both nocturnal and diurnal animals such as humans.4–6 PRCs have shown that exposure to light in the late afternoon through the first part of the night induces a delay in the time of the internal circadian pacemaker relative to external clock time, while light in the morning advances circadian rhythms to an earlier clock time. These changes in the internal circadian phase (phase shifts) can be measured by the physiological rhythms that the circadian pacemaker in the suprachiasmatic nucleus regulates, most commonly using the core body temperature rhythm (CBT) and the timing of nocturnal melatonin synthesis.7 In humans, the transition point on the PRC to light (i.e., when the response changes from delays to advances) occurs near the CBT minimum (CBTmin), which generally occurs around 05:00 in young, healthy adults.
Although the optimum timing of light exposure to induce phase advances or phase delays is well established, and some laboratory-based investigations of light intensity and duration have been performed, little is known about the intensity or duration of exposure needed for treatment of CRSDs in clinical practice. Recent practice parameters from the American Academy of Sleep Medicine (AASM) concluded that morning light exposure is indicated in the treatment of DSPD, but that optimal timing, duration, and dosing of morning light therapy for DSPD remain to be determined.1 The Practice Parameters provided a Guideline recommendation for timed light exposure in the work environment to decrease sleepiness and improve alertness during night shift work for SWD, although in the studies reviewed, light intensities ranged from 2,350 to 12,000 lux, and the duration ranged from 20 minutes during breaks to at least 50% of the shift. Timed light exposure for other CRSDs received only a weak recommendation (Option) due to limited evidence for efficacy. The equivocal results may be due to a lack of information on the intensities and durations of light exposure needed. In addition, due to limitations of artificial light, high intensity exposure (> 4,000 lux) usually requires remaining in close proximity of a specialized device for the entire exposure period.8,9 Thus, timed light exposure is inconvenient, and as with any lifestyle change, compliance may be a significant problem. Therefore, determining the most effective combination of intensity and duration of light exposure in humans may lead to improvements in the treatment of CRSDs. In the present study, we evaluated the effect of different intensities and durations of a single exposure to light on phase delays of the circadian clock in humans.
METHODS
Healthy young subjects, between 20 and 40 years of age, were recruited by advertisements and flyers posted at local universities. Volunteers were excluded from participation if any of the following were identified during an initial phone screening or the subsequent interview, including medical history and screening questionnaires: (1) cognitive, psychiatric, or other neurological disorders; (2) alcohol or substance abuse; (3) unstable or serious medical illness; (4) current use of psychoactive medications including antidepressants, anxiolytics, neuroleptics, anticonvulsants, hypnotics, or stimulant medications; (5) primary sleep disorder; (6) habitual sleep onset earlier than 21:00 or later than 24:00; (6) shift work; (7) daily caffeine intake > 200 mg; (8) use of tobacco; (9) travel across > 2 time zones within 90 days of the study; (10) pregnancy or the desire to become pregnant during the study period. This research study was approved by the institutional review board, and the subjects provided informed consent and were compensated for their participation. Sixty-two subjects completed both the bright light and control studies. Six subjects were excluded from the final analysis because of artifacts in the data that prevented the calculation of phase shifts. Thus, results from 56 subjects are presented here. The study population was 59.6% female. The average age of the participants was 29 ± 5.6 years.
The experimental protocol included two 4-night/3-day admissions in the General Clinical Research Center (GCRC). Bright light was administered on one of the 4-night/3-day admissions, while the other admission controlled for the experimental conditions. The 2 stays were spaced ≥ 3 weeks apart, and the order of the conditions was determined by a computer-generated random sequence. Seven of the subjects participated in a third stay as part of another study examining the effect of a calcium channel antagonist on light-induced phase shifts (not reported here). For 3 weeks prior to each admission, subjects were required to maintain a regular sleep/wake schedule (± 30 min of their habitual bedtime) and record their sleep and wake times with a sleep diary. An activity monitor (Actiwatch, Mini-mitter Inc, Bend, OR) was worn on the non-dominant wrist during the week prior to admission to verify compliance with the study protocol. The sleep and wake times over the pre-admission week were averaged from the actigraphic recordings with Actiware-Sleep software (Mini-Mitter, Inc.).
During each stay, light levels, activity, posture, and food intake were controlled to minimize masking of the circadian measures.10–12 Isocaloric snacks (150-250 kcal, depending on normal food intake, with 50% carbohydrate, 20% protein, and 30% fat) were provided every 2 h in place of regular meals. Caffeine intake during the stay was prohibited. Light levels were maintained at approximately 10 lux (equivalent to a small bedside lamp) during waking hours, with an 8-h enforced rest period in dark at their habitual bedtime. Throughout the 16-h wake period, the subjects remained seated or semi-reclined in bed, except for voiding and a daily shower. Subjects were allowed to engage in a variety of activities that allowed them to remain quietly awake in the dim light, including reading, watching TV or videos, talking on the telephone, or using a laptop computer.
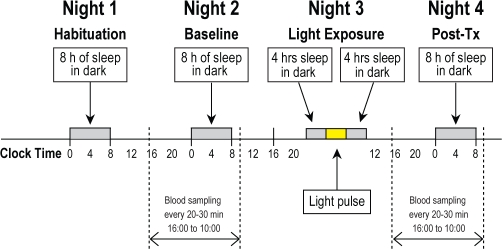
Each 4-night/3-day stay consisted of a habituation night, a 24-h baseline phase assessment, a treatment night, and a 24-h post-treatment phase assessment (Figure 1). Core body temperature was recorded with the use of a flexible rectal thermistor, which was connected to a lightweight data recording unit (Minilogger, Mini-Mitter Inc., Bend, OR). At 14:00 on the day of the baseline phase assessment, an intravenous catheter was inserted into the forearm vein. Blood collection began 2 h later (16:00) and continued throughout the night at intervals of 20 to 30 min (until 10:00 the following morning) to monitor both the rising and declining phases of the melatonin profile.7 During periods of wake, blood samples were taken via a stopcock attached directly to the intravenous catheter. During sleep periods, the blood samples were taken through tubing that extended into an adjacent room. The intravenous line was kept patent with a slow drip of heparinized saline (750 IU heparin in 9.0 g NaCl/L). The blood samples were centrifuged, and the plasma frozen at –80°C for subsequent measurement of melatonin. CBTmin on the second (baseline) night was calculated using both the Cleveland regression procedure and simple Cosine analysis from a 24-h recording period, with software provided by C. Eastman13,14
Figure 1.
Schematic diagram of the 4 night/3 day experimental protocol. Each admission consisted of (Night 1) a habituation night, (Night 2) baseline assessment of circadian temperature and melatonin rhythms, (Night 3) exposure to bright light (1, 2, or 3 hours at 2,000, 4,000, or 8,000 lux) or control (awake in dim light for the same period of time) with a midpoint of 3 h before the core body temperature minimum (CBTmin) measured on the baseline night, and (Night 4) post-exposure measurements of the timing of the nocturnl circadian melatonin rhythm.
On the third (treatment) night, the sleep period began earlier than normal, then subjects were awoken in the middle of the night for bright light exposure, or they remained awake for the same period of time under dim light conditions (10 lux). The bright light was provided by commercially available fluorescent light-boxes (Sunbox, Co. Gaithersburg, MD) at an intensity of 2000, 4000, or 8000 lux as measured by a photographic light meter at the level of the subject's eyes. The intervention consisted of 1, 2, or 3 hours of bright or dim light, with an additional 15 min at the beginning and end of the exposure period to ramp up from dark to bright light and then gradually decrease back down to complete dark. Subjects were assigned to the 9 different light conditions (3 intensities and 3 durations) by computer-generated block randomization. Initially the experimental protocol focused on 4,000 lux at 1, 2, or 3 hours; this was subsequently expanded to complete the 9 treatment conditions.
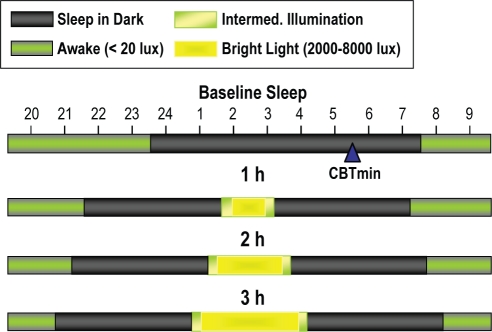
The light exposure period was centered 3 h before each subject's baseline CBTmin (Figure 2); a time known to induce phase delays. After awakening the subjects, staff elevated the head of the bed so that the subjects were semi-recumbent. The light-boxes (2 light-boxes for 2,000 or 4,000 lux, 4 light-boxes for 8,000 lux) were located adjacent to the side of the bed. One pair of lights was placed adjacent to the bed at the level of the elbow to knee, angled towards the subject (about 2 feet from the eyes). The second pair of lights, if needed, were angled towards the subject and placed adjacent to the bed at the level of the knee to foot at a distance of about 3 feet from the subject's eyes. The lights were increased over a period of 15 min with a 3-way switch until 2 or 3 (depending on the intensity) of the 3 fluorescent light bulbs in each light-box were illuminated. Adjustments were made in the proximity of the lights to the subject so that the light reached the target intensity as measured by a photographic light meter placed at the subject's forehead. A movie of the subject's choosing was shown on a video monitor at the foot of the bed to facilitate a stable direction of gaze during light exposure. Subjects were allowed to sleep for two 4-h periods before and after the light treatment. The timing of the sleep and wake periods were the same for each subject on their bright and dim light GCRC admissions (based on the first admission).
Figure 2.
Timing of light exposure, sleep and wake when the midpoint of light exposure (1, 2, or 3 h) was targeted to be 3 h before the core body temperature minimum (CBTmin). The CBTmin is at 05:30 for this example. Fifteen minutes of intermediate illumination and 4 h of sleep were scheduled before and after the bright light exposure.
Treatment-induced changes in the timing of the melatonin profile were assessed from blood samples collected on the fourth (post-treatment) night from 16:00 until 10:00 the following morning. Melatonin levels per milliliter of plasma were measured with a double-antibody radioimmunoassay using commercially available reagents (Stockgrand, Guilford, Surrey, UK).11 The lower limit of sensitivity of the assay was 2.5 pg/mL. The intra-assay coefficient of variation averaged 17.5% for values < 10 pg/mL, 8.6% in the range of 10 to 30 pg/mL, and 5.2% for values > 30 pg/mL. The inter-assay coefficient of variation averaged 20% for values < 10 pg/mL and 13.5% for values ≥ 10 pg/mL. All samples from the same subject were measured in the same assay.
Data Analysis
The time that melatonin levels rose to 50% of maximum (DLMO 50%) and the time that melatonin levels fell to 50% (DLMOff 50%) on the declining phase were selected as the phase markers for this analysis.15 Briefly, melatonin levels (in pg/mL) were converted to a percentage of the maximum level of melatonin obtained during the night (average of the 3 highest values). The data were then smoothed with the Lowess (Cleveland) curve-fitting procedure and interpolated at 1-min intervals (Graphpad Software). DLMO 50% and DLMOff 50% were recorded for each night of blood sampling.
Phase shifts were measured by the difference in the time of the phase markers for the baseline and post-treatment nights. The phase shift on the control stay (the same sleep/wake periods in dim light) was then subtracted from the phase shift on the light exposure stay to determine the effect of light alone on the magnitude of phase shifts. Aside from one subject who participated in 2 different conditions, each subject participated in only one of the 9 bright light conditions (2000, 4000, or 8000 lux of 1, 2, or 3 hours) along with a dim-light control stay. The magnitude of the phase changes due to light were compared for the 9 groups using 2-way ANOVA (intensity of the light and duration of exposure as the 2 factors) (Statistica). Comparisons beyond the main effects of intensity and duration were limited by the small number of subjects in some conditions and variability in the magnitude of phase shifts in response to light.
RESULTS
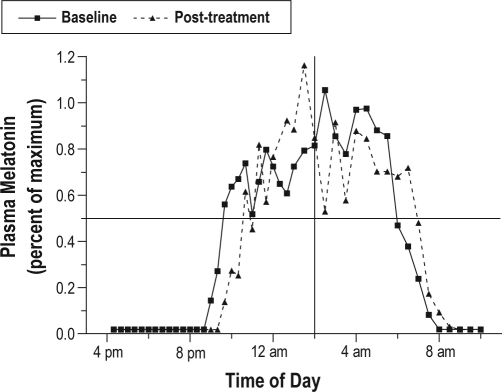
Under the control experimental conditions, the mean delay drift for all subjects (n = 56) was 11 min (0.19 ± 0.8 h) for DLMO 50% and 19 min (0.31 ± 0.85 h) for DLMOff 50%. The addition of bright light (1 to 3 h during 1 night) increased the magnitude of the phase delay of the melatonin onset by 49 min (0.82 ± 1.35 h) and the magnitude of the phase delay of the melatonin offset by 34 min (0.57 ± 1.07 h) over the dim light conditions. An example of a subject's baseline and post light-treatment melatonin profiles is shown in Figure 3.
Figure 3.
Baseline and post-treatment melatonin profiles from one subject showing a light-induced phase delay of the circadian melatonin rhythm. Blood samples were collected for 18 h on the night before and the night following light exposure (2000 lux light for 1 h). Note the rapid rise in melatonin levels between 21:00-23:00 (9:00-11:00 pm) and the rapid decline in melatonin between 06:00-08:00 (6:00-8:00 am). These periods of abrupt change, e.g., the time that melatonin levels rose to 50% of maximum (DLMO 50%), and the time when melatonin levels declined (DLMOff 50%), were used to mark the phase of the melatonin rhythm. Comparison of the time of these phase markers on the baseline and post-treatment nights showed a 101-min delay of the melatonin onset and a 59-min delay of the melatonin offset. The change during the subject's control GCRC stay (40 min and 20 min, respectively) was then subtracted to determine the phase delay attributable to the light (61 min for the DLMO and 39 min for the DLMOff). The midpoint of the melatonin rhythm on the baseline night is at the intersection of the horizontal and vertical lines.
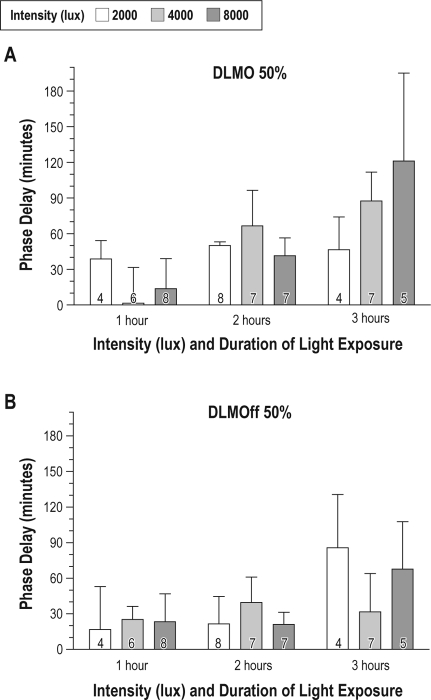
Figure 4A illustrates the magnitude of light-induced phase delays of DLMO 50% for the 9 treatment conditions. Two-way ANOVA revealed that increasing the duration of the light exposure from 1 to 3 h increased the magnitude of phase delays of DLMO 50% (F2,48 = 4.37, P = 0.01), but increasing the intensity of the light exposure did not (F2,48 = 0.88, P = 0.42). There was no interaction between the 2 factors (intensity and duration) in the overall analysis. As shown in Table 1, one hour of bright light exposure (minus the delay under control conditions) resulted in a mean phase delay of 10 min, 2 h of light exposure resulted in a phase delay of 53 min, while 3 h of light during the night delayed the melatonin onset by about 1.5 h (averaged across different intensities). There was a trend toward larger phase delays of the DLMOff 50% with 3 h of bright light (Figure 4)B, but the overall 2-way ANOVA did not show statistical significance for either intensity (F2,48 = 0.08, P = 0.92) or duration of exposure (F2,48 = 1.73, P = 0.19) on shifts of the melatonin offset.
Figure 4.
Magnitude of light-induced phase delays of the (A) dim light melatonin onset (DLMO 50%) and (B) dim light melatonin offset (DLMOff 50%) following exposure to various intensities (2,000, 4,000, or 8,000 lux) and durations (1, 2, or 3 hours) of light exposure (± SD). Phase shifts were calculated as the change in the time of the phase markers on the baseline and post-treatment nights. In order to determine the magnitude of the phase delay attributed to light, the phase change on each subject's control admission (awake for the same periods in dim light) was subtracted from the phase change following light exposure. Analysis by 2-way ANOVA revealed a significant effect of duration (P = 0.01) but not intensity for the magnitude of phase delays of the DLMO 50%; there was no interaction between the 2 factors. The number at the base of the bar denotes the number of subjects for that condition.
Table 1.
Delay in the melatonin rhythm following 1, 2, or 3 hours of light exposure
| Duration of Light (hours) | N | DLMO 50% (hours ± SD)** | DLMOff 50% (hours ± SD) |
|---|---|---|---|
| 1 | 18 | −0.17 ± 1.06 | −0.37 ± 0.92 |
| 2 | 22 | −0.88 ± 1.05 | −0.45 ± 0.85 |
| 3 | 16 | −1.46 ± 1.67 | −0.94 ± 1.41 |
P = 0.01
As illustrated in Figure 4, a higher intensity of exposure tended to increase the magnitude of light-induced phase delays of DLMO 50%, particularly for the 3-h duration, but this did not achieve statistical significance. Table 2 shows results for the intensity of light exposure when averaged across the 3 durations in the 2-way ANOVA, with average phase delays of 41 min at 2000 lux, 55 min at 4,000 lux, and 50 min at 8,000 lux. The intensity of light exposure had no observable effect on the magnitude of light-induced phase delays of the melatonin offset.
Table 2.
Delay in the melatonin rhythm following moderate to high intensity light exposure
| Intensity of Light (lux) | N | DLMO 50% (hours ± SD) | DLMOff 50% (hours ± SD) |
|---|---|---|---|
| 2000 | 16 | −0.68 ± 0.97 | −0.61 ± 1.24 |
| 4000 | 20 | −0.91 ± 1.30 | −0.54 ± 0.99 |
| 8000 | 20 | −0.84 ± 1.67 | −0.56 ± 1.05 |
In order to verify that the midpoint of light exposure was similar in the different conditions, we calculated the beginning, middle, and end of the light exposure periods relative to the midpoint of the melatonin profile. The midpoint of the melatonin profile occurred about 2 h 15 minutes before the CBTmin. As shown in Table 3, the timing of the light exposure occurred at approximately the same circadian time for the 3 durations. The end of the light exposure ranged from 6 min before the melatonin midpoint in the 1-h group to 38 min after the melatonin midpoint in the 3-h group.
Table 3.
Beginning, middle, and end of the light exposure period relative to the midpoint of the melatonin profile
| Duration (hours) | Beginning ± SD | Middle ± SD | End ± SD |
|---|---|---|---|
| 1 | −1.1 ± 1.3 | −0.60 ± 1.3 | −0.10 ± 1.3 |
| 2 | −1.97 ± 1.0 | −0.97 ± 1.0 | 0.03 ± 1.0 |
| 3 | −2.37 ± 1.4 | −0.87 ± 1.4 | 0.63 ± 1.4 |
DISCUSSION
This study examined the magnitude of phase delays with different intensities (2,000-8,000 lux) and different durations (1-3 h) of light exposure in humans entrained to a regular light/dark cycle. In order to evaluate the effects of the light itself (eliminating the effect of the experimental protocol), the change in phase observed during each subject's control GCRC stay (mean delay drift of 11-19 min) was subtracted from the change in phase following exposure to light. Under these conditions, exposure to bright light targeted to have the midpoint of light exposure 3 hours before the CBTmin resulted in a mean 49-minute delay of the melatonin onset. Unexpectedly, we found that varying the duration of light exposure from 1 to 3 hours increased the magnitude of phase delays of the melatonin onset, while varying the intensity of the light exposure between 2000 and 8000 lux had little impact on the timing of the circadian melatonin rhythm. A similar trend was observed for the melatonin offset, although this did not achieve statistical significance.
The mean phase delay observed in the present protocol (1.5 h following 3 h of light exposure) is similar to that reported by Van Cauter et al. following a 4-h light pulse (5000 lux) administered during a brief 38-h constant routine.11 In contrast, Khalsa et al. found phase delays of nearly 3 hours in subjects studied during a constant routine and exposed to 6.7 hours of bright light (4,000 to 10,000 lux) prior to T-min.16 The greater magnitude of phase delays observed by Khalsa and colleagues might be due to the higher intensity and longer duration of light exposure, and/or to differences in the experimental protocol. For example, the study by Khalsa et al. included 2 days with a very large delay of the sleep period which may have augmented the phase delays to light. Both the number of days of dim light prior to light exposure and the sleep/wake schedule during the study have been shown to influence the response of the circadian clock to light.17,18 In addition, the phase change was measured the 2nd day after the light pulse, rather than the first day. Transient changes in phase after light pulses are well established in rodents. If transient changes also occur in humans, the effects on the 2nd day could be larger than on the 1st day. In another study from the Czeisler laboratory, 100 lux light exposure for 6.5 hours (with 3 days of habituation to dim light and a change in sleep timing) was found to result in phase shifts approximately half of the magnitude of those following exposure to 10,000 lux.19 Together, these results suggest that moderate light exposure over a long duration may lead to clinically significant changes in the timing of the circadian clock.
Since the time of light exposure is also a critical factor in the magnitude of phase changes of the circadian clock, we examined whether the different light durations may have fallen at different times in the PRC. Maximal phase delays have been shown to be induced with light exposure about 2-4 hours prior to the core body temperature minimum or 1-3 hours before the midpoint of the melatonin profile in humans, with the crossover point between light-induced phase delays and advances typically observed after the melatonin midpoint or at the time of the CBTmin.11,16,20 In the present study, the middle of the light exposure was about 45 minutes prior to the midpoint of the melatonin profile. Although we cannot exclude the possibility that the 3-hour light pulse, beginning more than 1 hour earlier than the shorter light exposures, may have fallen at a more effective point on the PRC to light (i.e., maximum delay region vs. the transition zone), it is more likely that the circadian system of diurnal animals such as humans utilizes temporal integration, such that the longer the period of light exposure in the delay zone, the greater the response. Although only one portion of the phase delay region was studied here, and the light exposure was administered in the middle of the usual sleep period, we believe that the results obtained in the present study represent a general property of the human circadian system, and would also be observed in other regions of the PRC.
The ability of the photic entrainment pathway to integrate light input over time has previously been demonstrated in nocturnal rodents, although a seminal study by Nelson and Takahashi suggested that temporal integration was reduced for stimulus durations greater than 300 seconds in hamsters.21 A 1999 study by Nelson and Takahashi found temporal integration of light input even when the light pulses were separated in time by up to 1 hour, but no additional response was observed if the initial light exposure was of a saturating intensity (3 × 1015 photons cm/2).22 More recently, Comas et al. examined responses to single light pulses (about 100 lux) ranging from 1 to 18 hours in duration in mice.23 They found that 9-hour light pulses yielded the maximal amplitude PRC, but that the effect of light after the first hour was reduced. Calculation of phase progression curves from the data by Comas et al. also indicated that phase changes induced by light are larger at the beginning of a light pulse in mice, and that the magnitude of the phase change is limited by response saturation.24 Human data were considered insufficient to allow similar computation. The results of the present study suggest that phase changes induced by up to 8,000 lux of light are not reduced after the first hour in the diurnal human.
To our knowledge, this is the first report of an effect of light duration on phase shifts of the circadian clock without a large shift in the timing of sleep; prior studies have reported the effect of light duration in simulated shift work. Thessing et al. found that 4-but not 2-hours of bright light exposure (8,000-10,000 lux) during a simulated night shift improved sleepiness and performance the subsequent night.25 The authors proposed that the most likely explanation for these findings was a phase delay in the circadian rhythm of sleepiness-alertness. Eastman and colleagues compared bright light (5,000 lux) with durations of either 3 or 6 hours during 8 consecutive simulated night-work, day-sleep days.8 Phase estimates using CBTmin were averaged across days. Three hours of light each night resulted in a change of CBTmin of 8.1 hours over the course of the study, while 6 hours nightly of bright light changed CBTmin by an average of 9.4 hours. These endpoints were not significantly different, and it was concluded that increasing duration up to 3 hours increased the magnitude of the phase shift, and that there was little benefit of increasing duration beyond 3 hours. Burgess et al. found that 3 days of 3.5 hours of continuous bright light in the morning phase advanced the DLMO by 2.1 hours, while intermittent bright light (alternating 0.5 h on and 0.5 h off) induced a phase advance of 1.5 h; the difference was not statistically significant.26 In a different experimental paradigm, Gronfier et al. found that intermittent bright light pulses (15 min on, 60 min off over 6.5 h) delayed DLMO by nearly the same amount (2.34 vs. 3.03 h) as when subjects were exposed to 6.5 h of continuous bright light (9,500 lux); subjects who remained awake in dim light for the same duration had a 0.31-hour delay in phase.27 Together, these results suggest that in humans, continuous light exposure may not be as important as the span of light across the PRC, and that there is diminishing benefit as the light exposure reaches saturating levels (e.g., beyond 2 to 3 hours).
Overall, results from the present and prior studies indicate that increasing the duration of light exposure may be more effective at changing the phase of the circadian clock in humans than increasing the intensity of the light exposure. These results have significant implications for clinical practice, given the difficulty in achieving very bright light (8,000 to 10,000 lux) with artificial light sources. Although it is well established that the circadian system is most sensitive to light in the short-wavelength range, questions remain regarding whether the use of blue-green or blue-enriched light is able to improve the tolerability and/or effectiveness of light therapy for CRSDs.9,28,29 In addition, high intensity artificial light tends to be unpleasant and, when combined with the activity restriction and lifestyle modifications required for bright light treatment (e.g., remaining directly in front of the light source for the duration of the exposure), patient compliance can be a significant problem. The results from the present study suggest that a longer duration of exposure, at a more moderate intensity/less restrictive distance, may be both more tolerable and effective for the treatment of CRSDs. Additional studies are needed to evaluate the most effective duration of light exposure in a clinical setting.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Reid has received research support from Ingram Barge Company. Dr. Zee has received research support from Takeda and has consulted for Sanofi-Aventis and Philips/Respironics. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank the research subject volunteers for their participation, the students and staff of the Circadian Rhythm and Sleep Research Laboratory, and the administrators and staff of the General Clinical Research Center and Core Laboratory for their assistance with this project. This work was supported in part by a grant from the National Center on Clinical Research Resources (NCRR-0048), and Public Health Service grants R01 HL67604 and P01 AG11412.
Footnotes
A commentary on this article appears in this issue on page 559.
REFERENCES
- 1.Morgenthaler TI, Lee-Chiong T, Alessi C, et al. Practice parameters for the clinical evaluation and treatment of circadian rhythm sleep disorders. An American Academy of Sleep Medicine report. Sleep. 2007;30:1445–59. doi: 10.1093/sleep/30.11.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sack RL, Auckley D, Auger RR, et al. Circadian rhythm sleep disorders: part I, basic principles, shift work and jet lag disorders. An American Academy of Sleep Medicine review. Sleep. 2007;30:1460–83. doi: 10.1093/sleep/30.11.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cajochen C. Alerting effects of light. Sleep Med Rev. 2007;11:453–64. doi: 10.1016/j.smrv.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 4.Czeisler CA, Kronauer RE, Allan JS, et al. Bright light induction of strong (type 0) resetting of the human circadian pacemaker. Science. 1989;244:1328–33. doi: 10.1126/science.2734611. [DOI] [PubMed] [Google Scholar]
- 5.Daan S, Pittendrigh CS. A functional analysis of circadian pacemakers in nocturnal rodents. II. The variability of phase response curves. J Comp Physiol. 1976;106:253–66. [Google Scholar]
- 6.Minors DS, Waterhouse JM, Wirz-Justice A. A human phase-response curve to light. Neurosci Lett. 1991;133:36–40. doi: 10.1016/0304-3940(91)90051-t. [DOI] [PubMed] [Google Scholar]
- 7.Benloucif S, Burgess HJ, Klerman EB, et al. Measuring melatonin in humans. J Clin Sleep Med. 2008;4:66–9. [PMC free article] [PubMed] [Google Scholar]
- 8.Eastman CI, Liu L, Fogg LF. Circadian rhythm adaptation to simulated night shift work: effect of nocturnal bright-light duration. Sleep. 1995;18:399–407. doi: 10.1093/sleep/18.6.399. [DOI] [PubMed] [Google Scholar]
- 9.Smith MR, Eastman CI. Phase delaying the human circadian clock with blue-enriched polychromatic light. Chronobiol Int. 2009;26:709–25. doi: 10.1080/07420520902927742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benloucif S, Green K, L'Hermite-Baleriaux M, Weintraub S, Wolfe LF, Zee PC. Responsiveness of the aging circadian clock to light. Neurobiol Aging. 2006;27:1870–9. doi: 10.1016/j.neurobiolaging.2005.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Cauter E, Sturis J, Byrne MM, et al. Demonstration of rapid light-induced advances and delays of the human circadian clock using hormonal phase markers. Am J Physiol. 1994;266:E953–63. doi: 10.1152/ajpendo.1994.266.6.E953. [DOI] [PubMed] [Google Scholar]
- 12.Wirz-Justice A, Werth E, Renz C, Muller S, Krauchi K. No evidence for a phase delay in human circadian rhythms after a single morning melatonin administration. J Pineal Res. 2002;32:1–5. doi: 10.1034/j.1600-079x.2002.10808.x. [DOI] [PubMed] [Google Scholar]
- 13.Cleveland WS. Robust locally weighted regression and smoothing scatterplots. J Am Stat Assoc. 1979;74:829–36. [Google Scholar]
- 14.Martin SK, Eastman CI. Medium-intensity light produces circadian rhythm adaptation to simulated night-shift work. Sleep. 1998;21:154–65. [PubMed] [Google Scholar]
- 15.Benloucif S, Guico ML, Reid KJ, Wolfe LF, L'Hermite-Balériaux M, Zee PC. Stability of melatonin and temperature as circadian phase markers and their relation to sleep times in humans. J Biol Rhythms. 2005;20:178–88. doi: 10.1177/0748730404273983. [DOI] [PubMed] [Google Scholar]
- 16.Khalsa SB, Jewett ME, Cajochen C, Czeisler CA. A phase response curve to single bright light pulses in human subjects. J Physiol. 2003;549:945–52. doi: 10.1113/jphysiol.2003.040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hebert M, Martin SK, Lee C, Eastman CI. The effects of prior light history on the suppression of melatonin by light in humans. J Pineal Res. 2002;33:198–203. doi: 10.1034/j.1600-079x.2002.01885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burgess HJ, Eastman CI. Short nights reduce light-induced circadian phase delays in humans. Sleep. 2006;29:25–30. doi: 10.1093/sleep/29.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zeitzer JM, Dijk DJ, Kronauer R, Brown E, Czeisler C. Sensitivity of the human circadian pacemaker to nocturnal light: melatonin phase resetting and suppression. J Physiol. 2000;526(Pt 3):695–702. doi: 10.1111/j.1469-7793.2000.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kripke DF, Elliott JA, Youngstedt SD, Rex KM. Circadian phase response curves to light in older and young women and men. J Circadian Rhythms. 2007;5:4. doi: 10.1186/1740-3391-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nelson DE, Takahashi JS. Sensitivity and integration in a visual pathway for circadian entrainment in the hamster (Mesocricetus auratus) J Physiol. 1991;439:115–45. doi: 10.1113/jphysiol.1991.sp018660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nelson, Takahashi JS. Integration and saturation within the circadian photic entrainment pathway of hamsters. Am J Physiol. 1999;277:R1351–61. doi: 10.1152/ajpregu.1999.277.5.R1351. [DOI] [PubMed] [Google Scholar]
- 23.Comas M, Beersma DG, Spoelstra K, Daan S. Phase and period responses of the circadian system of mice (Mus musculus) to light stimuli of different duration. J Biol Rhythms. 2006;21:362–72. doi: 10.1177/0748730406292446. [DOI] [PubMed] [Google Scholar]
- 24.Beersma DG, Comas M, Hut RA, Gordijn MC, Rueger M, Daan S. The progression of circadian phase during light exposure in animals and humans. J Biol Rhythms. 2009;24:153–60. doi: 10.1177/0748730408330196. [DOI] [PubMed] [Google Scholar]
- 25.Thessing VC, Anch AM, Muehlbach MJ, Schweitzer PK, Walsh JK. Two- and 4-hour bright-light exposures differentially effect sleepiness and performance the subsequent night. Sleep. 1994;17:140–5. doi: 10.1093/sleep/17.2.140. [DOI] [PubMed] [Google Scholar]
- 26.Burgess HJ, Crowley SJ, Gazda CJ, Fogg LF, Eastman CI. Preflight adjustment to eastward travel: 3 days of advancing sleep with and without morning bright light. J Biol Rhythms. 2003;18:318–28. doi: 10.1177/0748730403253585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gronfier C, Wright KP, Jr, Kronauer RE, Jewett ME, Czeisler CA. Efficacy of a single sequence of intermittent bright light pulses for delaying circadian phase in humans. Am J Physiol Endocrinol Metab. 2004;287:E174–81. doi: 10.1152/ajpendo.00385.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lack LC, Wright HR. Clinical management of delayed sleep phase disorder. Behav Sleep Med. 2007;5:57–76. doi: 10.1207/s15402010bsm0501_4. [DOI] [PubMed] [Google Scholar]
- 29.Smith MR, Revell VL, Eastman CI. Phase advancing the human circadian clock with blue-enriched polychromatic light. Sleep Med. 2009;10:287–94. doi: 10.1016/j.sleep.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]