Abstract
Study Objectives:
The mechanisms underlying infant sleep irregularity are unknown. This study tests the hypothesis that sleep and episodic (saltatory) growth in infant length are temporally coupled processes.
Study design:
Daily parental diaries continuously recorded sleep onset and awakening for 23 infants (14 females) over 4-17 months (n = 5798 daily records). Multiple model-independent methods compared day-to-day sleep patterns and saltatory length growth.
Measurements and Results:
Approximate entropy (ApEn) quantified temporal irregularity in infant sleep patterns; breastfeeding and infant sex explained 44% of inter-individual variance (P = 0.001). Random effects mixed-model regression identified that saltatory length growth was associated with increased total daily sleep hours (P < 0.001) and number of sleep bouts (P = 0.001), with breastfeeding, infant sex, and age as covariates. Infant size and illness onset were non-contributory. CLUSTER analysis identified peaks in individual sleep of 4.5 more h and/or 3 more naps per day, compared to intervening intervals, that were non-randomly concordant with saltatory length growth for all individuals (P < 0.05), with a time lag of 0-4 days. Subject-specific probabilities of a growth saltation associated with sleep included a median odds ratio of 1.20 for each additional hour (n = 8, 95% CI 1.15 to 1.29) and 1.43 for each additional sleep bout (n = 12, 95% CI 1.21-2.03). Increased sleep bout duration predicted weight (P < 0.001) and abdominal skinfold accrual (P = 0.05) contingent on length growth, and truncal adiposity independent of growth (P < 0.001).
Conclusions:
Sleeping and length growth are temporally related biological processes, suggesting an integrated anabolic system. Infant behavioral state changes may reflect biological mechanisms underlying the timing and control of human growth.
Citation:
Lampl M; Johnson ML. Infant growth in length follows prolonged sleep and increased naps. SLEEP 2011;34(5):641-650.
Keywords: Sleep regulation, biological rhythms, saltatory growth, saltations
INTRODUCTION
The variability and unpredictability in infant sleeping patterns are primary concerns to parents, as their own sleep patterns follow their child's.1 Previous research has established that cyclic patterns of active and quiet sleep, already present prenatally,2,3 are organized during the first weeks of life,4,5 as are circadian and ultradian rhythms.6–10 The maturation of the sleep/wake cycle continues to about 6 months11–13 with significant inter-individual variation,14 and day/night effects may continue for the first two years.15
Less is known about variability in the patterns of day-to-day infant sleeping. This study aimed to investigate sleep patterns of infants in their natural environment through continuous sleep records. Previous studies have noted that actigraphy is a better research method than parental recall.16 The present study did not rely on recall. Parental records were kept in real-time registration and were chosen as actigraphy was not practical for a longitudinal study duration of up to 500 continuous days because of externally induced motion at young ages and infant interference with the device at older ages. This study did not aim to investigate electrophysiological aspects of sleep cycles or quantify sleep states.
The infants in this study were participants in a longitudinal growth study. Their growth in body length has been previously reported as occurring in aperiodic episodic saltations punctuating intervals of no significant growth.17 A saltatory pattern of intermittent growth spurts has been observed to be the normal process by which individuals grow when carefully measured at time-intensive intervals across developmental ages and species, in both endochondral and membranous bone.18,19
The well-known associations between growth hormone (GH) and sleep20 led to the hypothesis that sleep patterns may be associated with saltatory growth spurts. Previous reports have postulated that ultradian GH physiology may be related to discontinuous, saltatory growth patterns in children,21 but the association between GH, sleep, and growth remains circumstantial. The precise sequence of biological events that clock when normal children grow—and how much they grow—remains unknown. This study aimed to objectively quantify patterns in day-to-day infant sleep and to investigate if changes in infant sleep were temporally concurrent with their growth spurts in total body length.
METHODS
Subjects and Data Protocol
After written parental informed consent of an institutionally approved human subjects' protocol, infants participated in a longitudinal growth study during the first year of life with growth measurement protocols of weekly, semi-weekly, or daily assessments. Parents were asked to keep daily records of sleep, indicated as continuous lines from onset to waking to the quarter hour on linear graphical charts of the 24-h day, similar to sleep charts used by other groups.22 Parents were also asked to note each day whether they were breastfeeding and/or formula-feeding, and to record whether their infant experienced an illness (vomiting, diarrhea, fever, rash, congestion, or other medically diagnosed condition). The sleep records are the focus of this report.
The study subjects were a non-random convenience sample of middle-class American families selected for their willingness to participate in the protocol, and was unintentionally weighted to educators and medical personnel. Growth in total body length was assessed using the maximum stretch technique,23 and weight and subcutaneous skinfolds were measured24 for durations ranging from 4 to 17 months. Ninety percent of the subjects' measurements were taken at the same time of day (± 1 h).17
The specific aims of this study were to describe temporal characteristics of infant sleep and to investigate the timing relationship between infant sleep and growth in total body length. Three daily sleep variables were investigated: number of sleep bouts, number of hours per bout (HPB), and total sleep hours. A sleep bout was defined as bounded by parent-recorded sleep onset and waking. The number and duration of daily sleep bouts were calculated from the sleep records. The sum of the durations of all sleep bouts in 24 h was the total daily sleep hours. For each individual, the 24-h sleep clock was calibrated for the analysis to the time of growth measurement to permit a comparison between growth and sleep across coincident intervals.
The challenges in the present analysis included identifying an analytic method to quantify the temporal patterns in sleep that did not impose assumptions regarding the time-series nature of the data. That is, in lieu of a periodic analysis, for example, that assumes a sine wave, a model-independent method was needed to permit an objective assessment of the biological timing patterns in the data, and to permit investigation of temporal correspondence, if any, with growth. In order to isolate the nature of the relationship between sleeping and growth, the first steps in this investigation involved an exploration of other potential influences on sleeping patterns, including breastfeeding,25,26 illness,27 and infant sex28 as potentially significant covariates.
All variables were investigated for distributional characteristics by Shapiro-Francia and Kolmogorov-Smirnov procedures, and comparisons among means and medians employed T-test, Kruskal-Wallis, and K-sample median tests.29
Quantifying Infant Sleep Patterns
Approximate entropy (ApEn), developed to statistically discriminate patterns in time series data by quantifying the irregularity in data series such as electrocardiographic sequences,30 was used in the first step in this analysis. Specifically, ApEn measures the logarithmic likelihood that patterns in a time series for m consecutive observations remain similar when considered at m+1 serial observations. A higher probability of remaining similar, or “close” (less irregularity) gives smaller ApEn values, and greater independence in sequential values results in larger ApEn values. Calculation of ApEn requires prior definition of m, the length of the series to be compared, and r, the magnitude of “closeness” that defines irregularity.
In the present analysis, ApEn was used to quantify the probability that sleep patterns of hours and bouts on sequential days were similar within errors of measurement. ApEn was applied to both raw and detrended data (first differences, or day-to-day changes, to remove effects of developmental trends in sleeping behaviors), and calculated with m = 1 (one day in the present study) and r = 0.20 times the standard deviation of the data (the error estimate appropriate for shorter time series according to experimental simulations).30 Day-to-day changes in sleep were assessed for their relative temporal irregularity compared to randomness by comparing the observed ApEn values to random ApEn values, generated by shuffling each original data series by Monte Carlo procedures 1000 times. A normalized ratio of the observed-to-random ApEn is the result. A ratio of 1 identifies randomness in the sleep patterns of each child, with increasing ApEn values identifying greater disorderliness, and decreasing values representing less irregularity. Regression analysis with ApEn value as the dependent variable then investigated the potential influences on sleep irregularity of feeding mode (breastfeeding vs non- breastfeeding), infant sex, illness (presence/absence), and length growth.
Identifying Influences on Infant Sleep Patterns
The data were further explored for covariate effects in random effects multi-level regression for repeated measures, in separate models for total daily hours slept, number of daily sleep bouts, and hours per bout (HPB) as the continuous outcome variables, and individual as the random effect (xtreg for hours and HPB, xtpoisson for number of bouts, STATA 11.29) The potential systematic effect of sex was assessed, together with influences of feeding style (breastfeeding, non-breastfeeding), and illness (presence, absence).
The effect of length growth was then investigated with infant sex, feeding style, and age as covariates, and an interaction term between infant sex and saltatory length growth investigated potential sex differences in sleep changes associated with growing.
In addition to saltatory length growth, infant size was investigated: anthropometric variables included recumbent length, weight, and the weight-for-length ratio, as well as 3 skinfold variables estimating limb (upper arm, thigh, and calf), trunk (subscapular and abdominal), and abdominal (abdominal and suprailiac) subcutaneous adiposity. The mixed-model approach with individual as a random covariate permits consideration of unmeasured individual-level confounding effects, such as genetically based metabolic differences, maternal prenatal milieu, or other unknown confounders that may contribute to inter-individual variability in sleep.31
Sleep and Growth Timing
This analysis was conducted at the level of the individual, with each subject serving as his/her own control. The investigation of whether time-specific changes in sleep were concordant with discrete growth saltations involved 2 steps. In the first step, the sleep data were subjected to an objective quantitative analysis designed to identify statistically significant increases and decreases in a time series data set. The CLUSTER pulse identification program uses a sliding T-test to investigate significant increases and decreases between groups of serial data, employing a 5% false positive criterion.32 This method defines “peaks”, “nadirs”, and inter-peak sequences, if present, in serial data, relative to measurement error. As employed here, a 0.5-h measurement error of recording was used (sleep records were recorded to the nearest quarter h), with a minimum time interval of one day defining either a peak or a valley. Due to the significant trends with age in the raw data, the data were transformed by first differences before analysis by the CLUSTER 8 software (PULSE_XP).33 The second step assessed the concordance between bursts in sleeping hours or bouts, and growth saltations as previously identified.17 The present investigation examined the null hypothesis that significant changes in sleep co-occurred with growth saltations due to random chance.34 This algorithm uses computer simulations to calculate the probability distributions for purely random concordance between ≥ 2 independently regulated processes in repeated measures. The null hypothesis was rejected when it was less than 5% likely that the number of concordances was due to chance alone, permitting temporal coupling between the 2 processes (sleep and length growth) to be inferred.
In order to address the question of whether there is a predictive relationship between sleeping and growth, the data were investigated by subject-specific logistic regression. Random effects mixed model regression investigated the potential effects of sleep on infant weight and skinfolds, both contingent on and independent of length growth.
RESULTS
Twenty-three parents (14 girls, 9 boys) maintained daily records that consistently recorded sleep bouts to the quarter hour. The total number of daily sleep bouts and hours were determined from these parental records (n = 5798 daily records). All parents slept within audible range of their infants. One infant co-slept with her parents for the duration of the study. The infants in this study were clinically healthy at birth, delivered from uncomplicated pregnancies after a mean 39.5 gestational weeks of age, birth weight of 3500 grams, birth length of 50.3 cm, and ponderal index (weight/length3 × 100) of 2.33 gm/cm3. All were free of colic and medical complications during their first year. Sixteen infants were predominantly breastfed to 4 months of age (10 girls, 6 boys), and 7 received formula. Eighteen infants were measured semi-weekly, 3 infants were measured daily, and 2 were measured weekly for durations ranging from 4 to 17 continuous months. The median age at study onset was 12 days (interquartile [IQR] 8-18 days), with a range of 4 to 316 days.
Daily Sleep
For the sample, the distributions of total hours slept, number of sleep bouts, and HPB were non-Gaussian. With increasing age, there were significant negative trends for total hours and bouts, and a positive trend for hours per bout (P < 0.001 for each). Across the first year, there was a decline in median total daily sleep of 3 h (14.5 h/day at one month [IQR 12-15.5 h], 12.5 h at 6 months [IQR, 11-14 h], and 11.5 h at one year [IQR, 10.5-12.5 h]) and 2 sleep bouts (median 6 bouts at one month [IQR 5-7], 4 bouts at both 6 and 12 months of age [IQR, 3-5]). The day-to-day changes in total hours, bouts, and HPB were also non-Gaussian. For the sample, the medians for day-to-day changes were zero for all parameters, with IQRs of ± 1.5 h, ± 1 bout, and ± 0.75 HPB, with 1st to 99th percentile ranges of −7 to +7.5 day-to-day changes in total hours slept, ± 4 daily bouts, and −5.25 to +4.5 HPB.
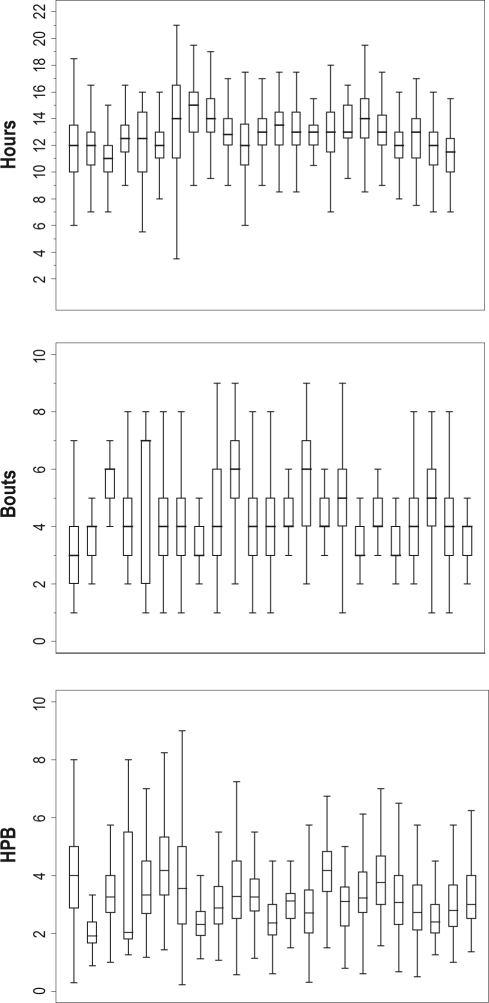
Significant within- and between-individual variability in sleep hours, bouts, and HPB were identified (Figure 1), and individuals did not share a common population median (P < 0.001). Identifying significant variability within- and between-individual subjects in sleep parameters implies individual differences in sleeping patterns over time. The ability to quantitatively compare patterns permits greater insight into this aspect of individual biology.
Figure 1.
Subject-specific sleep with total daily hours (top panel), number of daily bouts (middle panel), and hours per bout (HPB, lower panel). Box plots represent each individual's data. Central lines are the medians, and the whiskers are the upper and lower adjacent values [ ± 1.5 × IQR] for each individual subject (STATA 1129). Inter-individual differences in medians for hours, bouts, and HBP were significant (P < 0.001).
Irregularity in Daily Infant Sleep: Quantifying Global Patterns
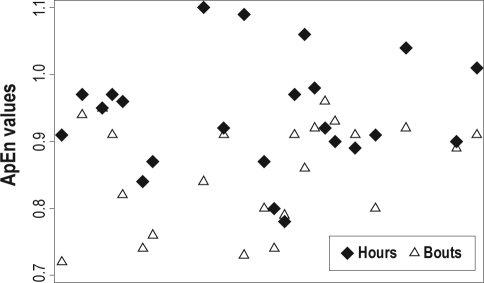
Approximate entropy statistics identified that individual study subjects differed significantly in the temporal irregularity of their sleep patterns (K-sample equality of medians test; P < 0.0001, Figure 2), with greater irregularity in the patterns of day-to-day total sleep hours, compared to sleep bouts (P = 0.004, Kruskal-Wallis).
Figure 2.
Subject-specific approximate entropy (ApEn) values. Individuals differed significantly in the temporal irregularity of their sleep hours (diamonds) and bouts (triangles) (P < 0.001). There was greater irregularity in sleep hours compared to sleep bouts (P = 0.004).
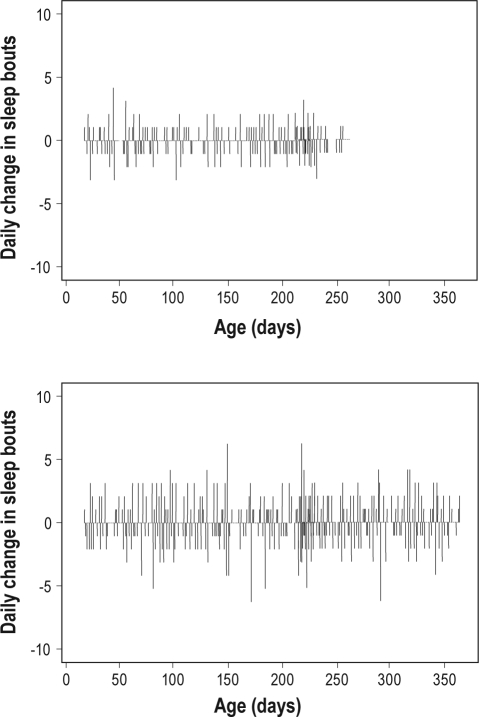
Regression of ApEn values on potential covariates identified that breastfeeding vs formula-feeding accounted for 39% of the variance in daily sleep bout irregularity (P = 0.001, Figure 3). While infant sex did not reach significance as an independent predictor, breastfeeding and sex accounted for 44% of the variance in sleeping bout irregularity among the infants (P = 0.001). No significant effects were identified for irregularity in total hours slept for either feeding style or infant sex. No significant independent effects of study duration, total number of sick days, total growth in length, or number of growth saltations were identified. Further investigation of influences operating at the level of global sleep patterns was limited by sample size. Multi-level models using the power of the serial data permitted more thorough examination into associations between day-to-day sleep and potential sources of variability.
Figure 3.
Breastfeeding increased sleep bout irregularity. Day-to-day changes in the number of sleep bouts (y-axis) by age (x-axis) illustrate the greater sleep bout irregularity (P = 0.001) of the breastfed infant (bottom panel, ApEn score of 0.95) by comparison with the formula-fed infant (top panel, ApEn score of 0.72).
Sleep Effects from Infant Sex, Breastfeeding and Length Growth
Regressing sleep hours, HPB, and number of daily sleep bouts for the pooled sample data identified that neither infant sex nor feeding style had significant independent effects on total daily sleep hours (infant age was a covariate).
Boys had more sleep bouts (P = 0.03) and shorter sleep bout duration compared to girls (P < 0.001), and breastfeeding was associated with more sleep bouts (P < 0.001) of shorter duration than formula-feeding (P < 0.001; Table 1, Model 1).
Table 1.
Multi-level mixed effects regression
| 1Hours | 1Hours per bout | 3Bouts | |
|---|---|---|---|
| Model 1 | Coefficient (P-value) | Coefficient (P-value) | 4IRR (P-value) |
| Boys | ns | −1.18 (< 0.001) | 1.27 (0.03) |
| Breastfeeding | ns | −0.93 (< 0.001) | 1.27 (< 0.001) |
| 2R2 | 0.02, 0.32, 0.05 | 0.07, 0.21, 0.11 | |
| Model 2 | |||
| Length growth | 0.26 (< 0.001) | ns | 1.05 (0.001) |
| Boys | ns | 1.18 (0.001) | 1.28 (0.03) |
| Breastfeeding | ns | −0.93 (< 0.001) | 1.26 (< 0.001) |
| 2R2 | 0.02, 0.31, 0.05 | 0.07, 0.21, 0.11 | |
| Model 3 | |||
| Length growth | ns | 0.15 (0.05) | ns |
| Boys | ns | −1.29 (< 0.001) | 0.72 (0.02) |
| Breastfeeding | ns | −0.91 (< 0.001) | 1.33 (< 0.001) |
| Sex*Growth | Girls 0.21 (0.15) | Boys 0.27 (0.003) | Girls 0.92 (0.01) |
| 2R2 | 0.02, 0.31, 0.05 | 0.07, 0.20, 0.11 | |
xtreg;
R2 values within-individual, between-individual, and overall assessed by xtreg;
xtpoisson;
relative risk (STATA 11). Regressing daily sleep hours, hours per bout, and number of bouts on sex, breastfeeding, and saltatory length growth.
When saltatory length growth was added to the models, saltatory growth in length was significantly associated with increased total hours (P < 0.001) and number of sleep bouts (P = 0.001; Table 1, Model 2). Investigating sex-specific effects of growth on sleep by adding an interaction term identified that length saltations were associated with increased sleep bout duration among boys compared to girls (P = 0.003), and increased sleep bouts among girls compared to boys (P = 0.01; Table 1, Model 3).
Sleep Effects from Infant Size, Growth in Weight and Subcutaneous Fat
Regressing sleep hours, HPB, and number of daily bouts for the pooled sample data identified that there were no significant effects on sleep from infant size (in terms of length, weight, or weight-for-length ratios), adiposity (limb, trunk, or abdominal skinfolds), weight gain, or changes in subcutaneous skinfolds (data not shown).
In summary, linear regression analyses on the pooled sample data identified significant associations between sleep and saltatory length growth with sex-specific effects: Boys increased their HPB compared to girls, and girls increased their sleep bouts compared to boys. While statistically significant, these effects were relatively modest in terms of real-time changes, ranging from 10 min to 1.5 h, and one sleep bout. Considering the significant intra- and inter-individual variability in sleep (Figure 1), these results suggested that investigation of more specific temporal relationships between sleep and length growth at the individual-level would be useful in generating hypotheses about the biological relationships between length growth and sleep.
Timing of Significant Changes in Sleep Behaviors
Peaks in individual sleeping patterns
The CLUSTER algorithm identified a pattern of statistically significant episodic peaks and nadirs in total daily sleep h for each infant (Table 2). The average duration of peaks in total daily sleep h was 2 days, at an average increase of 4.5 h/day (SEM 0.2, 95% CI 3.9 to 4.9). Nine infants also had significant peaks and nadirs in the number of daily sleep bouts, with an average of 3 extra sleep bouts per day during 2 consecutive days at the peak. Between peaks, the median day-to-day difference in total sleep hours was < 30 min, and less than one sleep bout.
Table 2.
Temporal concordance between sleep and saltatory length growth
| Number of Peaks In Hours | Number of Length saltations | Concordance | Number of Peaks In Bouts | Concordance |
|---|---|---|---|---|
| 23 | 21 | 12 (0.009) | ||
| 19 | 23 | 13 (0.006) | 19 | 13 (0.006) |
| 30 | 33 | 15 (0.001) | 39 | 17 (0.003) |
| 42 | 26 | 21 (0.0001) | ||
| 22 | 20 | 12 (0.003) | ||
| 25 | 30 | 14 (0.025) | ||
| 22 | 27 | 12 (0.02) | 9 | 6 (0.04) |
| 25 | 42 | 16 (0.003) | 25 | 18 (0.00013) |
| 9 | 7 | 6 (6.5×10−7) | ||
| 23 | 25 | 18 (0.004) | ||
| 19 | 17 | 11 (0.03) | ||
| 14 | 13 | 11 (1.8×10−6) | ||
| 25 | 23 | 17 (0.01) | ||
| 11 | 10 | 10 (0.01) | ||
| 26 | 31 | 17 (0.001) | 11 | 8 (0.02) |
| 13 | 13 | 13 (8.7×10−7) | 4 | 4 (0.003) |
| 17 | 13 | 13 (3.5×10−5) | ||
| 19 | 20 | 9 (0.04) | 7 | 6 (0.003) |
| 11 | 15 | 10 (0.01) | ||
| 38 | 26 | 20 (3.2×10−5) | 11 | 5 (0.001) |
| 10 | 9 | 7 (4.9×10−7) | 4 | 2 (0.045) |
Temporal Concordance Between Sleep and Growth Saltations
The comparison of sleep and growth was restricted to infants measured semi-weekly and daily (n = 21 infants, 4790 records). A co-occurrence between body length growth saltations and peaks in total daily sleep h and/or sleep bouts was evident more often than would be expected by chance alone (P < 0.05) (Table 2).
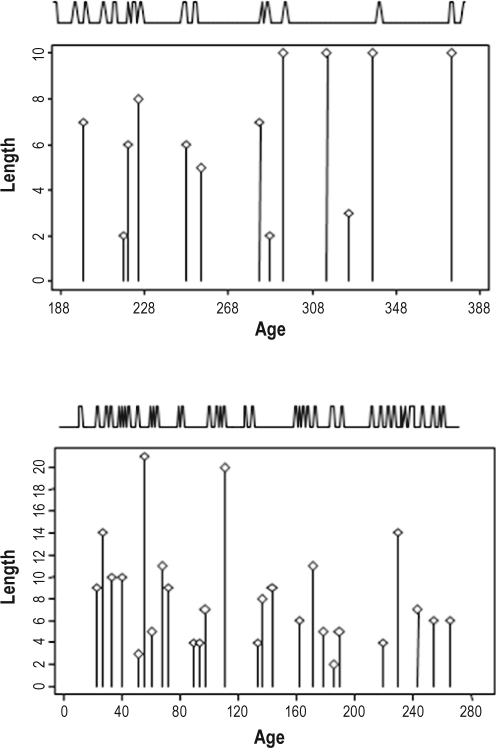
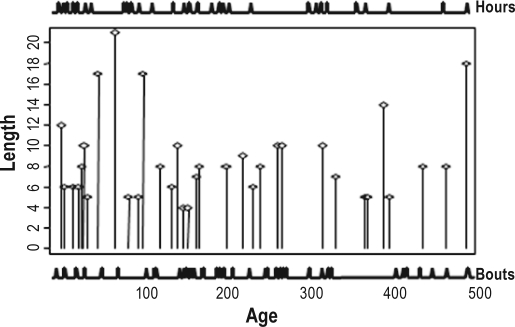
Figure 4 illustrates the temporal correspondence between peaks in total daily sleep hours and length growth saltations for infants measured twice weekly (alternating 3- and 4-day intervals between measurements). The female infant was measured for 200 days from 188 days of age. She experienced 13 length saltations and 14 peaks in daily sleep h, with 11 concordant peaks between growing and sleep. This has a probability of occurring due to random chance of less than 1.8 × 10-6.
Figure 4.
Concordance between peaks in total daily sleep hours and saltatory length growth for a female (top panel) and male (bottom panel) infant measured twice weekly (alternating 3- and 4-day intervals). The locations of the sleep hour peaks (illustrated by the spikes across the top of the graphs) were identified by the CLUSTER algorithm31 and the length saltations (the body of the graphs) by the saltatory algorithm.17 The female (top panel) experienced 14 peaks in daily sleep hours and 13 length saltations; 11 of the 13 length saltations were concordant with sleep peaks. This has a probability of less than 1.8 × 10-6 of occuring by random chance. The male experienced 38 sleep peaks and 26 length saltations; 20 of the 26 length saltations were coincident with sleep. This has a probability of less than 3.2 × 10-5 of occuring by random chance.
Figure 5 illustrates the temporal concordance between peaks in both total daily hours and total daily sleep bouts for an infant girl measured twice weekly (alternating 3- and 4-day intervals between measurements) from 3 to 517 days of age. In her first 17 months, this infant experienced 30 peaks in total daily sleep h and 39 peaks in daily sleep bouts. Her total body length growth of 32.0 cm during this time period was accrued during 33 length growth saltations. Fifteen length saltations were concordant with peaks in sleep h, and 17 were concordant with peaks in sleep bouts. The likelihood that these events were due to random chance were < 0.001 and < 0.003, respectively. These data are the longest continuous individual record in the sample. Developmental effects are evident: Significant changes in the number of sleep bouts (increased naps) have greater correspondence with growth at older ages. The presence of unmatched peaks in both sleep parameters and length growth saltations is also notable. Indeed, while all subjects experienced statistically significant non-random concordance between sleep alterations and length growth saltations, this was not a 100% correspondence, as illustrated for these individuals.
Figure 5.
Concordance between saltatory length growth (the body of the graph) and peaks in total daily sleep hours (spikes located across the top of the frame) and peaks in daily sleep bouts (spikes located across the bottom of the frame). Measured twice weekly (3-day and 4-day intervals) from 3 days of age, in her first 17 months this infant experienced 30 peaks in total daily sleeping hours and 39 peaks in daily sleep bouts. Her total body length growth of 32.0 cm during was accrued during 33 length growth saltations. Fifteen length saltations were concordant with peaks in sleeping hours, and 17 were concordant with peaks in sleeping bouts. The likelihood that these events were due to random chance was less than 0.001 and 0.003, respectively.
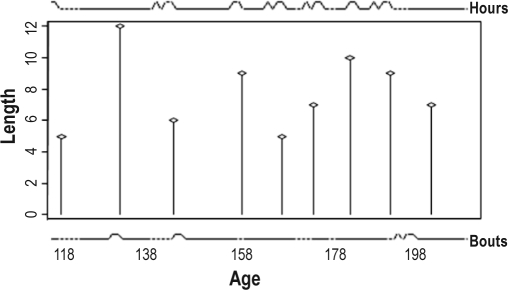
Daily growth data afford a closer resolution for investigating temporal correspondence between sleep and growth. Three infants provided a total of 217 days on which both sleep records and length growth measurements were reported. Among a total of 23 length growth saltations, 12 occurred concurrent with increased sleep in the previous 24 h, and 8 followed increased sleep within 2 days. Figure 6 illustrates a male followed daily for 93 days from 11 days of age, during which time he grew 6.8 cm in 9 saltatory length growth spurts. During this time he experienced 10 peaks in total daily sleep hours, with an average of 14 (SD ± 0.75) h/day. These peaks were preceded by an average nadir of 10 (± 1.3) h, and were separated by 1-20 days of 11.9 (± 0.75, SD) daily sleep hours. In addition, he experienced 4 days of peaks in sleep bouts, when he slept an average of 3 extra bouts; 2 of these co-occurred with peaks in total daily sleep hours. Seven of the 9 length saltations were coincident with a peak in total daily sleep hours, and 2 were associated with peaks in sleep bouts. The probability that this was due to random chance is less than 4.9 × 10-7 (hours) and 0.045 (bouts).
Figure 6.
A comparison between time of peaks in total daily sleep hours (top panel) and bouts (lower panel) as derived from the CLUSTER peak analysis, and body length growth spurts as previously identified (17) (middle panel) during 93 days from 117 days of age in a male infant. He experienced 10 significant peaks in total daily sleeping hours, 4 sleep bouts and grew 6.8 cm in 9 growth spurts. Seven length growth saltations were non-randomly linked to sleep hour peaks (P = 4.96 × 10-7) and 2 to sleep bout peaks (P = 0.045). The first growth saltation also seems to occur at the time of increased sleep, but the time frame was too short to permit identification of an upstroke, if present, and this apparent sleepy day is not identified as a peak by algorithm definition.
Quantitative Predictability in Sleeping and Growth
Subject-specific logistic regression further identified significant individual variability in the relationship between sleep and growth. Among 12 infants, each additional sleep bout increased the probability of a length growth saltation by a median of 43% (the median OR of 1.43, 95% CI = 1.21-2.03), with individual variability ranging from ORs of 1.21 (95% CI 1.09-1.33) to 2.02 (95% CI 1.23-3.35). Among 8 individuals, the probability of a length growth saltation was increased by a median of 20% for each additional h of sleep (median OR 1.20, 95% CI, 1.15-1.29), with individual ORs ranging from 1.16 (CI 1.04-1.29) to 1.41 (CI 1.13-1.77).
In addition, for 2 infants the combination of fewer daily sleep bouts (OR = 0.83 and 0.81, 95% CI 0.68-0.96) of longer duration (OR = 1.43 and 1.35, 95% CI range, 1.03 to 1.68) predicted length growth saltations. One of these individuals was a breastfed male, among the youngest infants in the study (followed from 7 to 120 days of age). The second was a female who was unique in co-sleeping with her parents, and specifically experienced a decrease in night time arousals with saltatory length growth.
In addition, individual relationships changed with developmental age. For example, the strongest predictor of a length saltation during the first 3 months of life for the infant whose data are illustrated in Figure 5 was HPB, such that for each increased hour slept at a bout, the OR was 3.9 of concomitant length growth (95% CI = 1.27-12.0, P = 0.02). She was predominantly breastfed during this time. Subsequently, the OR for a length saltation associated with HPB was 1.26 (95% CI = 1.02-1.55, P = 0.03), similar to the effects of both total daily sleep hours (OR = 1.25, 95% CI = 1.02-1.54, P = 0.03) and number of sleep bouts (OR = 1.28, 95% CI = 1.01-1.62, P = 0.04), from 300 days. Thus, increased sleep preceding and concordant with length growth was accumulated through a variety of altered sleep strategies.
Implications of Sleep and Growth Relationships
While neither infant size nor adiposity influenced sleep, increased sleep had implications for patterns of infant weight gain and subcutaneous fat acquisition. Increasing HPB was related to both greater weekly infant weight gain and greater weekly accrual of abdominal skinfolds (Table 3). As these variables are known to interact with length growth,24 stratified analyses based on intervals during which a length growth saltation occurred vs intervals when no length growth occurred further explored these relationships. Only at times of growth in length were weight gain and abdominal adiposity accrual associated with increased sleep (P < 0.001, P = 0.05, respectively). There were no significant systematic effects of infant sex.
Table 3.
Increased sleep bout duration and infant weight gain and subcutaneous skinfolds
| 2Weight gain | 2Abdominal skinfolds | 3Trunk skinfolds | |
|---|---|---|---|
| Coefficient | 0.36 | 0.07 | 1.20 |
| P-value | < 0.001 | 0.05 | < 0.001 |
| Model P-value | < 0.001 | 0.003 | < 0.001 |
| 1R2 | (0.15, 0.32, 0.17) | (0.06, 0.02, 0.06) | (0.46, 0.50, 0.43) |
Age was a covariate;
R2 values for within individual, between individual and overall model (xtreg, STATA 11);
effects concurrent with length growth;
infant sex, weight, and concomitant length growth as covariates.
Sleeping was also related to subcutaneous skinfold measurements independently of concomitant length growth. Longer sleep at each bout (HPB) was associated with greater truncal adiposity (P < 0.001), with no systematic effects of either infant sex or growth in length.
Summary of Results
Individual variability in day-to-day sleep is quantifiable, with significantly more irregularity in daily infant sleep hours than sleep bouts. No significant effects of either infant sex or feeding style contributed to the irregularity in daily sleep hours, but breastfeeding and infant sex were associated with 44% of variance in sleep bouts.
Multiple regression for the pooled sample clarified that breastfeeding was associated with more frequent, short-duration sleep bouts, and boys experienced more frequent, short-duration daily sleep bouts compared to girls. With infant sex and breastfeeding as covariates, saltatory length growth significantly predicted an increase in both total daily sleep hours and sleep bouts. In the pooled sample, males and females altered their sleep differently during saltatory length growth. Growing boys experienced longer sleep bouts, and growing girls had more sleep bouts.
Individual-level analyses identified that saltatory growth in body length was significantly associated with increases in both total daily sleep hours as well as the number of sleep bouts. This was a nonlinear relationship among individuals, with episodic, aperiodic pulsatile increases in sleep non-randomly concordant with saltatory length growth within 2 days.
DISCUSSION
This report provides the first documentation that infant growth in length is concordant with changes in sleep. Previous research has provided circumstantial evidence for a relationship between sleep and growth in length/height. Secretory growth hormone bursts are known to rise after sleep onset during slow wave sleep (sleep stages 3 and 4)35,36 with linear relationships between the amount of slow wave sleep and concomitant GH secretion identifying an important correspondence between sleep state and growth hormone secretory patterns.37 Decreased time in slow wave sleep has been found among children with psychosocial dwarfism38 and young adults with isolated growth hormone deficiency.39
In the present study, not only did length growth saltations follow increased napping and longer sleep, but the co-sleeping infant experienced less sleep fragmentation,40 common in co-sleeping infants and specifically associated with reduced stages 3-4 sleep.41 Thus, the co-sleeping infant's decreased arousals suggest increased time in sleep stages 3-4 prior to growth spurts. A previous study reported ultradian patterns of growth hormone secretory irregularity and weekly height increments in children.21 The present data triangulate these observations and document for the first time that episodic, saltatory length growth spurts are temporally coupled to sleep.
The exact nature of the relationship between sleep biology and bone growth is unknown. One proposition is that augmented slow wave sleep onset (via either altered sleep patterns and/or duration), with its concomitant release of hormonal signals, translates in short time intervals to bone growth spurts, or cell level saltations as hypertrophic cells expand,42 and matrix deposition follows. An animal study employing implants across the tibial growth plate identified discrete bone elongation pulses occurring during sleep/recumbency in growing lambs.19 Taken together, these observations provide an evidentiary base for the reality of growing pains, the aching limbs that waken children in the night.43,44
The Importance of Studying Growth, Not Size
Several previous studies have failed to find associations between sleep and size among groups of infants, children,45,46 and adolescents.47 Significant differences in data and analytic methods distinguish these reports.
The unique findings in the present study reflect the fact that this is the first study to investigate sleep and growth, permitting a specific test of the temporal concordance between the two processes. The ability to specifically identify the presence of growth is related to sampling frequency,48 data acquisition methods, and analytic approach. Saltatory increments occur within daily timeframes, and growth proceeds intermittently, punctuating intervals of days to weeks of no measurable growth.17 Moreover, growing in length is an individual-level biological process. As individuals vary in the timing of growth saltations, pooled data lose the individual growth signals and fail to identify the unique patterns of growing individuals. The challenge in identifying influences on growth parallels endocrinological investigations: knowledge of where the comparisons are being undertaken, relative to the underlying pulsatile pattern, is essential.
Individual Sleep Pattern Variability
The present study also identifies the importance of investigating subject-specific effects in lieu of population-averaged relationships. The documentation of both inter- and intra-individual variability in sleep hours and bouts associated with growth clarify the poor resolution attendant to sample-level investigations.
The novel observations linking specific features of sleep to real-time infant growth also reflect the use of methods with no a priori assumptions regarding the temporal nature of sleep across time. The lack of resolution between growth and sleep based on linear regression models was illustrated. In lieu of nonlinear models with temporal assumptions, such as periodic functions, this analysis investigated significant differences in day-to-day sleep by two model-independent methods (ApEn and CLUSTER), and focused on individual-level analyses. This permitted identification of individual variability in the irregularity of day-to-day sleep that was further characterized as specific differences in pulsatility characteristics of sleep bouts and duration. This investigation was coupled with the specific intent of investigating temporal correspondence between unique sleep and growth patterns within individuals. The precise nature of this relationship was variable between individuals, as well as within individuals, changing with age.
There may or may not be a direct causal relationship between sleep and bone biology. Instead, some common or synergistic mechanisms may underlie growth, expressed as episodic sleep changes. This is suggested by the observation that in spite of the significant concordance, sleep alterations occurred without concomitant length spurts, and not every growth spurt was preceded by sleep prolongation. We hypothesize that the former may be related to concomitant growth in other body dimensions, most specifically head circumference.49 As for the latter, it is likely that the ability to resolve the specific relationships requires more sensitive methods. The lack of resolution does not exclude a connection. It is likely that the association between sleep and growth biology may involve a larger system, including effects from disinhibition,50 that may be involved in physiological mechanisms of the saltatory growth cascade.
Is Sleep an Anabolic Interface?
The observations that sleep is related to incremental length growth, as well as weight gain and abdominal skinfold accrual, are important contributions to the hypothesis that an integrated physiological system underlies growth timing, involving metabolic regulatory pathways that cross-talk with sleep regulation. A previous study, associating four days of night-time actigraphy with one assessment of infant size at 6 months of age among 96 infants,51 found that longer and relatively thinner six-month-old infants slept for a greater percentage of the night than their peers. This was interpreted by the authors as evidence that shorter nighttime sleep may contribute to infant weight gain and adiposity. The present study found no relationship between sleep and infant size, while documenting increased subcutaneous adiposity with increased sleep. The adiposity relationships were site-specific, with truncal adiposity increasing independently of concomitant length growth, and abdominal adiposity associated with saltatory length growth. In view of the considerable controversy surrounding sleep and obesity,46,52,53 further exploration of these relationships is important. We have previously reported increases in weight and abdominal skinfold changes concordant with length saltations in this sample.24 The present data suggest that sleep is integrating multiple anabolic processes in normal growth and development. This raises questions regarding anabolic pathways that may be driven during sleep more generally.
Previous research has suggested that sleep episode duration is associated with size and metabolic rate per unit body mass,54 with relationships that change during development.55 The present data contribute the perspective that sleep may interface metabolic state and growth of the organism at the whole body level. The significant differences found in this study by infant sex and feeding style identify the importance of further study of these relationships. This is particularly important in view of the multiple potential consequences of infant sleep in development.56
Reflections on the Current Study and Future Research
The strengths of the present investigation include sampling protocol and analytic methods. It is possible that actigraphy would have been a useful adjunct. Actigraphy has been shown to be a useful, relatively noninvasive approach to infant sleep assessment in the home environment.57 However, this longitudinal study, comparing parental sleep records and actigraphy for three days a month across the first year, found no significant differences in the total percentage of sleep across the day, or during waking hours. At night, the two methods differed across developmental age, ranging from about 2 hours at one month of age to one hour subsequently.57 Other studies have found differences in the two methods on the order of 14 minutes.58 From this evidentiary perspective, it is not likely that a potential bias in night-time parental record keeping is a significant confounder in the present study results. The CLUSTER analytics considered error ranges that exceeded these distinctions in the identification of significant day-to-day differences, and the random effects mixed-model regression approach theoretically accommodates unmeasured individual-level confounding effects.
Additional considerations regarding actigraphy concern the impact of external motion influences on actigraphic data, such as commonly used baby swings and rockers, designed to encourage infant sleep.59 The choice to rely on parental records for sleep in the present study was favored by the desire to remain as nonintrusive as possible under the demands of a daily longitudinal study that continued for 120 to more than 500 days. While noninvasive, actigraphy is not unintrusive. In the present study, predominant breastfeeding kept mothers and infants in close communication throughout the night in three-quarters of this sample.
Likewise, it could be suggested that sporadic actigraphic recordings would have improved the present study in terms of methodological rigor, and this should be considered in future studies. It is difficult to rule out how the knowledge that actigraphy was in progress would contribute to the quality of the sleep records. In the present study sample, it is likely to have induced a hypervigilance on the part of parents, thus making these comparative efforts of questionable validity. There is no question that parents who successfully completed meticulous daily diaries of infant sleep in the present study were a self-selected, non-random group.
There is no evidence of systematic bias in the present sample affecting the reported results: The median hours slept per day among infants are similar to those previously reported for their age-matched peers published in some studies,60 and modestly lower than others,61,62 reflecting the present study methods of recording sleep duration in real time, in lieu of intermittent parental questionnaires coding time spent in the bed.
The continuous parental diaries were important in addressing potential influences in daily behaviors that are not endogenous to the infant, including parental scheduling and adoption of societal expectations. The carefully recorded, time-intensive data from this sample of infants provided evidence that growth was a significant effect, after considering infant sex, feeding mode, and illness. Male infants have been previously found to develop sleep rhythms later and to sleep for shorter periods with greater variability in sleep structure.28 These may or may not be related to the specific effects of growth-related sleep changes found in this sample.
This study adds growth biology as a response to the enigmatic question of “why do we sleep?” The present results suggest that daily sleep behavior and growth in infant body length are a temporally coupled biological process. Significantly different patterns of sleep alteration were found according to infant sex, influenced by feeding strategy. The daily data collection protocol establishes the hypothesis that increased sleep both precedes, and is concomitant with, measurable total body length increment by 24-48 hours. The biological basis of this relationship remains to be identified.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
Data collection was supported by the Developmental Psychobiology Research Group and the Wenner-Gren Foundation.
The data were collected by Lampl, the data were analyzed collaboratively by Lampl and Johnson, and the manuscript was written collaboratively by Lampl and Johnson.
ABBREVIATIONS
- ApEn
approximate entropy
- HPB
hours per bout
- GH
growth hormone
- OR
odds ratio
- CI
confidence interval
- SEM
standard error of the mean
- SD
standard deviation
- IQR
interquartile range
REFERENCES
- 1.Gay CL, Lee KA, Lee SY. Sleep patterns and fatigue in new mothers and fathers. Biol Res Nurs. 2004;5:311–8. doi: 10.1177/1099800403262142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peirano P, Algarin C, Uauy R. Sleep-wake states and their regulatory mechanisms throughout early human development. J Pediatr. 2003;143(4Suppl):S70–9. doi: 10.1067/s0022-3476(03)00404-9. [DOI] [PubMed] [Google Scholar]
- 3.Mirmiran M, Maas YJ, Ariagno RL. Development of fetal and neonatal sleep and circadian rhythms. Sleep Med Rev. 2003;7:321–34. doi: 10.1053/smrv.2002.0243. [DOI] [PubMed] [Google Scholar]
- 4.Parmelee AH, Jr, Wenner WH, Schulz HR. Infant sleep patterns: From birth to 16 weeks of age. J Pediatr. 1964;65:576–82. doi: 10.1016/s0022-3476(64)80291-2. [DOI] [PubMed] [Google Scholar]
- 5.Thoman EB, McDowell K. Sleep cyclicity in infants during the earliest postnatal weeks. Physiol Behav. 1989;45:517–22. doi: 10.1016/0031-9384(89)90067-x. [DOI] [PubMed] [Google Scholar]
- 6.Kleitman N, Englemann G. The development of the diurnal (24-hour) sleep wakefulness rhythm in the infant. Acta Med Scand. 1955;152(Suppl 307):106. doi: 10.1111/j.0954-6820.1955.tb16319.x. [DOI] [PubMed] [Google Scholar]
- 7.Meier-Koll A, Hall U, Hellwig U, Kott G, Meier-Koll V. A biological oscillator system and the development of sleep-waking behavior during early infancy. Chronobiologia. 1978;5:425–40. [PubMed] [Google Scholar]
- 8.Freudigman K, Thoman EB. Ultradian and diurnal cyclicity in the sleep states of newborn infants during the first two postnatal days. Early Hum Dev. 1994;38:67–80. doi: 10.1016/0378-3782(94)90218-6. [DOI] [PubMed] [Google Scholar]
- 9.McGraw K, Hoffmann R, Harker C, Herman JH. The development of circadian rhythms in a human infant. Sleep. 1999;22:303–10. doi: 10.1093/sleep/22.3.303. [DOI] [PubMed] [Google Scholar]
- 10.Rivkees SA. Developing circadian rhythmicity in infants. Pediatrics. 2003;112:373–81. doi: 10.1542/peds.112.2.373. [DOI] [PubMed] [Google Scholar]
- 11.Coons S, Guilleminault C. Development of sleep-wake patterns and non-rapid eye movement sleep stages during the first six months of life in normal infants. Pediatrics. 1982;69:793–8. [PubMed] [Google Scholar]
- 12.Salzarulo P, Fagioli I. Post-natal development of sleep organization in man: speculations on the emergence of the ‘S process.’. Neurophysiol Clin. 1992;22:107–15. doi: 10.1016/s0987-7053(05)80748-8. [DOI] [PubMed] [Google Scholar]
- 13.Schechtman VL, Harper RK, Harper RM. Distribution of slow-wave EEG activity across the night in developing infants. Sleep. 1994;17:316–22. doi: 10.1093/sleep/17.4.316. [DOI] [PubMed] [Google Scholar]
- 14.Lohr B, Sigmund R. Ultradian and circadian rhythms of sleep-wake and food-intake behavior during early infancy. Chronobiol Int. 1999;16:129–48. doi: 10.3109/07420529909019081. [DOI] [PubMed] [Google Scholar]
- 15.Louis J, Cannard C, Bastuji H, Challamel MJ. Sleep ontogenesis revisited: a longitudinal 24-hour home polygraphic study on 15 normal infants during the first two years of life. Sleep. 1997;20:323–33. doi: 10.1093/sleep/20.5.323. [DOI] [PubMed] [Google Scholar]
- 16.Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26:342–92. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- 17.Lampl M, Veldhuis JD, Johnson ML. Saltation and stasis: A model of human growth. Science. 1992;258:801–3. doi: 10.1126/science.1439787. [DOI] [PubMed] [Google Scholar]
- 18.Goldsmith MI, Fisher S, Waterman R, Johnson SL. Saltatory control of isometric growth in the zebrafish caudal fin is disrupted in long fin and rapunzel mutants. Dev Biol. 2003;259:303–17. doi: 10.1016/s0012-1606(03)00186-6. [DOI] [PubMed] [Google Scholar]
- 19.Noonan KJ, Farnum CE, Leiferman EM, Lampl M, Markel MD, Wilsman NJ. Growing pains: are they due to increased growth during recumbency as documented in a lamb model? J Pediatr Orthop. 2004;24:726–31. doi: 10.1097/00004694-200411000-00024. [DOI] [PubMed] [Google Scholar]
- 20.Finkelstein JW, Anders TF, Sacher EJ, Roffwarg HP, Hellman LD. Behavioral state, sleep stage and growth hormone levels in human infants. J Clin Endocrinol Metab. 1971;32:368–71. doi: 10.1210/jcem-32-3-368. [DOI] [PubMed] [Google Scholar]
- 21.Gill MS, Tillmann V, Veldhuis JD, Clayton PE. Patterns of GH output and their synchrony with short-term height increments influence stature and growth performance in normal children. J Clin Endocrinol Metab. 2001;86:5860–3. doi: 10.1210/jcem.86.12.8116. [DOI] [PubMed] [Google Scholar]
- 22.Sarfi M, Martinsen H, Bakstad B, Røislien J, Waal H. Patterns in sleep-wakefulness in three-month old infants exposed to methadone or buprenorphine. Early Hum Dev. 2009;85:773–8. doi: 10.1016/j.earlhumdev.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 23.Lampl M, Birch L, Picciano MF, Johnson ML, Frongillo EA. Child factor in measurement dependability. Am J Hum Biol. 2001;13:548–57. doi: 10.1002/ajhb.1087. [DOI] [PubMed] [Google Scholar]
- 24.Lampl M, Thompson AL, Frongillo EA. Sex differences in the relationships among weight gain, subcutaneous skinfold tissue and saltatory length growth spurts in infancy. Pediatr Res. 2005;58:1238–42. doi: 10.1203/01.pdr.0000184327.65102.a6. [DOI] [PubMed] [Google Scholar]
- 25.Elias MF, Nicolson NA, Bora C, Johnston J. Sleep/wake patterns of breast-fed infants in the first 2 years of life. Pediatrics. 1986;77:322–9. [PubMed] [Google Scholar]
- 26.Eaton-Evans J, Dugdale AE. Sleep patterns of infants in the first year of life. Arch Dis Child. 1988;63:647–9. doi: 10.1136/adc.63.6.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tirosh E, Scher A, Sadeh A, Jaffe M, Rubin A, Lavie P. The effects of illness on sleep behaviour in infants. Eur J Pediatr. 1993;152:15–7. doi: 10.1007/BF02072509. [DOI] [PubMed] [Google Scholar]
- 28.Nagy E, Loveland KA, Orvos H, Molnar P. Gender-related physiologic differences in human neonates and the greater vulnerability of males to developmental brain disorders. J Gend Specif Med. 2001;4:41–9. [PubMed] [Google Scholar]
- 29.StataCorp. College Station, TX: StataCorp LP; 2009. Stata Statistical Software: Release 8.0. [Google Scholar]
- 30.Pincus SM. Approximate entropy as a measure of system complexity. PNAS. 1991;88:2297–301. doi: 10.1073/pnas.88.6.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sadeh A, Dark I, Vohr BR. Newborns' sleep-wake patterns: the role of maternal, delivery and infant factors. Early Hum Dev. 1996;44:113–26. doi: 10.1016/0378-3782(95)01698-8. [DOI] [PubMed] [Google Scholar]
- 32.Robeva RS, Kirkwood JR, Davies RL, et al. San Diego: Academic Press; 2008. An invitation to biomathematics. [Google Scholar]
- 33.Veldhuis JD, Johnson ML. Cluster analysis: a simple, versatile and robust algorithm for endocrine pulse detection. Am J Physiol. 1986;250:e486–93. doi: 10.1152/ajpendo.1986.250.4.E486. [DOI] [PubMed] [Google Scholar]
- 34.Veldhuis JD, Johnson ML, Seneta E. Analysis of the copulsatility of anterior pituitary hormones. J Clin Endocrinol Metab. 1991;73:569–76. doi: 10.1210/jcem-73-3-569. [DOI] [PubMed] [Google Scholar]
- 35.Holl RW, Hartman ML, Veldhuis JD, Taylor WM, Thorner MO. Thirty-second sampling of plasma growth hormone in man: correlation with sleep stages. J Clin Endocrinol Metab. 1991;72:854–61. doi: 10.1210/jcem-72-4-854. [DOI] [PubMed] [Google Scholar]
- 36.Radomski MW, Buguet A, Doua F, Bogui P, Tapie P. Relationship of plasma growth hormone to slow-wave sleep in African sleeping sickness. Neuroendocrinology. 1996;63:393–6. doi: 10.1159/000126980. [DOI] [PubMed] [Google Scholar]
- 37.Van Cauter E, Plat L. Physiology of hormone secretion during sleep. J Pediatr. 1996;128:S32–7. doi: 10.1016/s0022-3476(96)70008-2. [DOI] [PubMed] [Google Scholar]
- 38.Guilhaume A, Benoit O, Goumelen M, Richardet JM. Relationship between sleep stage IV deficit and reversible HGH deficiency in psychosocial dwarfism. Pediatr Res. 1982;16:299–303. doi: 10.1203/00006450-198204000-00011. [DOI] [PubMed] [Google Scholar]
- 39.Astrom C, and Lindholm J. Growth hormone-deficient young adults have decreased deep sleep. Neuroendocrinology. 1990;51:82–4. doi: 10.1159/000125320. [DOI] [PubMed] [Google Scholar]
- 40.Hunsley M, Thoman E. The sleep of co-sleeping infants when they are not co-sleeping: evidence that co-sleeping is stressful. Dev Psychobiol. 2002;40:14–22. doi: 10.1002/dev.10009. [DOI] [PubMed] [Google Scholar]
- 41.Mosko S, Richard C, McKenna J, Drummond S. Infant sleep architecture during bedsharing and possible implications for SIDS. Sleep. 1996;19:677–84. doi: 10.1093/sleep/19.9.677. [DOI] [PubMed] [Google Scholar]
- 42.Wilsman NJ, Farnum CE, Leiferman EM, Lampl M. Growth plate biology in the context of growth by saltations and stasis. In: Lampl M, editor. Saltation stasis and human growth and development: evidence, methods and theory. Philadelphia: Smith-Gordon; 1999. [Google Scholar]
- 43.Manners P. Are growing pains a myth? Aust Fam Physician. 1999;28:124–7. [PubMed] [Google Scholar]
- 44.Evans AM, Scutter SC. Prevalence of “growing pains” in young children. J Pediatr. 2004;145:255–8. doi: 10.1016/j.jpeds.2004.04.045. [DOI] [PubMed] [Google Scholar]
- 45.Gulliford M, Price C, Rona R, Chinn S. Sleep habits and height at ages 5 to 11. Arch Dis Child. 1990;65:119–22. doi: 10.1136/adc.65.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bayer O, Rosario AS, Wabitsch M, von Kries R. Sleep duration and obesity in children: Is the association dependent on age and choice of the outcome parameter? Sleep. 2009;32:1183–9. doi: 10.1093/sleep/32.9.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Knutson KL. The association between pubertal status and sleep duration and quality among a nationally representative sample of U.S. adolescents. Am J Hum Biol. 2005;17:418–24. doi: 10.1002/ajhb.20405. [DOI] [PubMed] [Google Scholar]
- 48.Lampl M, Johnson ML. Identifying saltatory growth patterns in infancy: A comparison of results based on measurement protocol. Am J Hum Biol. 1997;9:343–56. doi: 10.1002/(SICI)1520-6300(1997)9:3<343::AID-AJHB7>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 49.Lampl M, Johnson ML. Infant head circumference growth is saltatory and coupled to length growth. Early Hum Dev. 2011 doi: 10.1016/j.earlhumdev.2011.02.001. doi: 10.1016. [DOI] [PubMed] [Google Scholar]
- 50.Fox CR, Farhy LS, Evans WS, Johnson ML. Measuring the coupling of hormone concentration time series using polynomial transfer functions. Methods Enzymol. 2004;384:82–94. doi: 10.1016/S0076-6879(04)84006-0. [DOI] [PubMed] [Google Scholar]
- 51.Tikotzky L, de Marcas G, Har-Toov J, Dollberg S, Bar-Haim Y, Sadeh A. Sleep and physical growth in infants during the first 6 months. J Sleep Res. 2010;19:103–10. doi: 10.1111/j.1365-2869.2009.00772.x. [DOI] [PubMed] [Google Scholar]
- 52.Patel SR, Hu FB. Short sleep duration and weight gain: a systematic review. Obesity. 2008;16:643–53. doi: 10.1038/oby.2007.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Taveras EM, Rifas-Shiman SL, Oken E, Gunderson EP, Gillman M. Short sleep duration in infancy and risk of childhood overweight. Arch Pediatr Adolesc Med. 2008;162:305–11. doi: 10.1001/archpedi.162.4.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lo C-C, Chou T, Penzel T, Scammell TE, Strecker RE, Stanley HE, Ivanov PC. Common scale-invariant patterns of sleep-wake transitions across mammalian species. PNAS. 2004;101:17545–8. doi: 10.1073/pnas.0408242101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Blumberg MS, Seelke AMH, Lowen SB, Karlsson AEK. Dynamics of sleep-wake cyclicity in developing rats. PNAS. 2005;102:14860–4. doi: 10.1073/pnas.0506340102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ednick M, Cohen AP, McPhail GL, Beebe D, Simakajornboon N, Amin RS. A review of the effects of sleep during the first year of life on cognitive, psychomotor, and temperament development. Sleep. 2009;32:1449–58. doi: 10.1093/sleep/32.11.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.So K, Adamson TM, Horne RS. The use of actigraphy for assessment of the development of sleep/wake patterns in infants during the first 12 months of life. J Sleep Res. 2007;16:181–7. doi: 10.1111/j.1365-2869.2007.00582.x. [DOI] [PubMed] [Google Scholar]
- 58.Sadeh A. Evaluating night wakings in sleep-disturbed infants: a methodological study of parental reports and actigraphy. Sleep. 1996;19:757–62. doi: 10.1093/sleep/19.10.757. [DOI] [PubMed] [Google Scholar]
- 59.Tsai S, Burr RL, Thomas KA. Effect of external motion on correspondence between infant actigraphy and maternal diary. Infant Behav Dev. 2009;32:340–3. doi: 10.1016/j.infbeh.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Quillin SI Infant and mother sleep patterns during 4th postpartum week. Issues Compr Pediatr Nurs. 1997;20:115–23. doi: 10.3109/01460869709026882. [DOI] [PubMed] [Google Scholar]
- 61.Iglowstein I, Jenni OG, Molinari L, Largo RH. Sleep duration from infancy to adolescence: reference values and generational trends. Pediatrics. 2003;111:302–7. doi: 10.1542/peds.111.2.302. [DOI] [PubMed] [Google Scholar]
- 62.Jenni OG, Molinari L, Caflisch JA, Largo RH. Sleep duration from ages 1 to 10 years: variability and stability in comparison with growth. Pediatrics. 2007;120:769–76. doi: 10.1542/peds.2006-3300. [DOI] [PubMed] [Google Scholar]