Abstract
Study Objectives:
Disturbances of the internal biological clock manifest as fatigue, poor concentration, and sleep disturbances—symptoms reminiscent of chronic fatigue syndrome (CFS) and suggestive of a role for circadian rhythm disturbance in CFS. We examined circadian patterns of activity, sleep, and cortisol secretion in patients with CFS.
Design:
Case-control study, 5-day behavioral observation.
Setting:
Natural setting/home environment
Participants:
15 patients with CFS and 15 healthy subjects of similar age, sex, body mass index (BMI), and activity levels.
Interventions:
N/A
Measurements:
Self-report questionnaires were used to obtain medical history and demographic information and to assess health behaviors, somatic and psychological symptoms, and sleep quality. An actiwatch accelerometer recorded activity and sleep patterns over 5 days with concurrent activity and symptom logs. Diurnal salivary cortisol secretion was measured. Additionally, overnight heart rate monitoring and pain sensitivity assessment was undertaken.
Results:
Ratings of symptoms, disability, sleep disturbance, and pain sensitivity were greater in patients with CFS. No between-group differences were found in the pattern or amount of sleep, activity, or cortisol secretion. Afternoon activity levels significantly increased evening fatigue in patients but not control subjects. Low nocturnal heart rate variability was identified as a biological correlate of unrefreshing sleep.
Conclusions:
We found no evidence of circadian rhythm disturbance in CFS. However, the role of autonomic activity in the experience of unrefreshing sleep warrants further assessment. The activity symptom-relationship modelled here is of clinical significance in the approach to activity and symptom management in the treatment of CFS.
Citation:
Rahman K; Burton A; Galbraith S; Lloyd A; Vollmer-Conna U. Sleep-wake behavior in chronic fatigue syndrome. SLEEP 2011;34(5):671-678.
Keywords: Circadian rhythms, unrefreshing sleep, chronic fatigue syndrome, heart rate variability, actigraphy
INTRODUCTION
Chronic fatigue syndrome (CFS) is a severe, disabling disorder that poses a significant personal and economic burden for sufferers, their families, and the community. The etiology and pathophysiology of this enigmatic clinical disorder remains obscure, and curative therapies are not available.
CFS is diagnosed by consensus-derived, criterion-based case definitions, requiring persistent, medically unexplained, fatigue for ≥ 6 months in the presence of characteristic constitutional and neuropsychiatric symptoms.1 The profound experience of unrefreshing sleep and complaints of disturbed or restless sleep are prominent in subjective reports of symptoms in CFS.2,3
While classical sleep studies employing polysomnographic techniques have shown varied, nonspecific changes in sleep structure and efficiency in a subgroup of patients with CFS, these studies have not revealed any substantive evidence indicative of a primary sleep disorder.4,5 Pertinent to this, studies of monozygotic twins discordant for CFS6,7 have not revealed any significant alterations in sleep architecture. Nevertheless, patients with CFS consistently report a profound reduction in sleep quality.4,6,7 To date, little is known about the mechanisms responsible for the experience of poor quality sleep in CFS.
Human sleep is part of an oscillating sleep-wake pattern following a circadian rhythm.8 These cyclical rhythms are directed by the brain's biological “clock” located in the suprachiasmatic nucleus and cause fluctuation of body temperature, hormone levels, and sleep over a 24-h period. These behavioral and physiological rhythms are synchronized to external physical environmental and social/work schedules. The strongest synchronizing agent in humans is changes in light and darkness which “set” the biological clock and help determine the need to wake up or go to sleep. The circadian clock not only provides temporal synchronization between these various rhythms, but it also promotes wakefulness, and coordinates the timing of sleep-wake behavior, which are involved in aspects of physiological and neurocognitive functioning.8
Recent evidence has underscored the importance of circadian rhythms in maintaining good health with alterations in the normal circadian clock linked to a number of conditions including mood disorders.9 Asynchrony between the internal clock and sleep-wake behavior also frequently accompanies shift work, or international travel (i.e., jet lag), leading to symptoms of fatigue, poor concentration, and sleep abnormalities.10,11 The similarity between these symptoms and those associated with CFS has stimulated research interest in disturbances in the endogenous circadian rhythm as a pathophysiological mechanism in CFS.12,13 To date, a limited number of studies assessing specific parameters of circadian rhythmicity such as sleep/wake times, activity cycles,14 melatonin excretion patterns,15 and core body temperature16 have been largely inconclusive in their outcomes. Alternations in the circadian rhythm of cortisol secretion, as well as relative hypocorticalism, are among the better replicated findings in case-control studies of CFS.17,18
Changes in sleep patterns and activity levels are thought to play a significant role in maintaining the symptoms in CFS,19 with post-exertional exacerbation of fatigue being a characteristic feature of the illness. This post-exertional exacerbation is also characterized by an abnormally low “threshold”, that is, activities previously achieved with ease (e.g., a short walk) become associated with a severe and prolonged exacerbation of symptoms following the onset of CFS. The only evidence-based treatment for CFS—cognitive-behavioral therapy, includes graded physical exercise as a key component to improve tolerance to physical activity and to allow maintenance of sustainable activity levels.19–21 Given that disturbances in sleep quality and sleep/wake regulation also have the potential to affect daytime fatigue, it is important to identify abnormal activity and sleep behavior in a valid and reliable way.
Polysomnography studies are often criticized for limiting investigations to one or two nights, in artificial laboratory conditions (sleep laboratory) which do not reflect normal sleep at home. With the availability of highly sensitive accelerometers such as actigraphs, improved evaluation of continuous sleep/wake and activity patterns in the normal environment is now possible. Notably, with wrist placement and 30-second collection intervals actigraphy data have correlated well with parameters obtained from polysomnography, such as sleep efficiency and fragmentation.22,23
A number of studies have used actigraphy to monitor diurnal patterns in ambulatory physical activity in CFS. Some of these studies have documented significant reductions in daytime activity levels14,24; however others found no differences in activity levels between patients with CFS and healthy controls subjects.24 Additionally, the latter study reported that physical disability and symptoms such as pain and fatigue were associated with lower concurrent and subsequent activity levels. Although patients generally attribute exacerbations of symptoms to a wide range of factors including exercise, in the study by Kop et al.,25 activity levels were not predictive of a subsequent exacerbation of symptoms.
Inconsistencies in these findings may be explained by the well-recognized heterogeneity in cross-sectional samples of patients fulfilling diagnostic criteria for CFS.3,26 However, poor matching of cases to healthy subjects, particularly in terms of restricted activity and exercise levels may also be a contributing factor. Substantiation of patients' report of activity levels with objective measures, and a better understanding of the dynamic relationship(s) between activity levels and symptom manifestations would have important implications for both research and clinical practice. The combination of actigraphy with concurrent symptom measurements (as opposed to simply baseline and end of monitoring period evaluations) would allow almost real-time evaluation of the contingent relationships between activity and symptoms.27 This approach has yet to be applied to CFS in a natural setting.
Most studies to date have evaluated alterations in the circadian patterns of activity and sleep independently of cofactors, including disturbed mood, pain, and autonomic hyperarousal, which are known to be associated with fatigue and the experience of unrefreshing sleep, and capable of modulating circadian rhythmicity in their own right.3 For example, the presence of elevated heart rate and lower heart rate variability (HRV), have been demonstrated during sleep in patients with CFS who also reported high levels of sleep problems.28,29
To provide a more comprehensive account of the potential role of altered circadian rhythms and their relationship to symptom manifestations in CFS, the current study included several indices of circadian variation (diaries of sleep/wake behavior and activity/rest patterns, actigraphy, and diurnal cortisol secretion). Specifically, the study aimed to assess the hypothesis that a disturbance in circadian rhythms underlies the key symptoms of debilitating fatigue and unrefreshing sleep in CFS. The impact of covariates, including concurrent mood disturbance, pain, and autonomic hyperarousal during sleep was also assessed. A second aim was to evaluate the contingent relationship between activity levels and the experience of symptoms in CFS.
METHODS
Participants
Fifteen patients fulfilling international diagnostic criteria for CFS1 were recruited from a tertiary referral clinic associated with a university teaching hospital, which provides a graded-activity oriented cognitive-behavioral therapy program for patients with fatigue syndromes. Everyone who passed the inclusion/exclusion criteria between March 2009 and Sept 2009 was invited to participate in the study at their second (of a total of 12) appointment in the clinic; thus participants were in the initial stages of treatment when recruited. Those who fulfilled the inclusion criteria were handed an information brochure and agreement to be contacted by the researcher was obtained by the clinic staff. These potential participants were then contacted by the researchers. All but one of those invited (15/16 [94%]) agreed to participate. One male (40 y) declined to participate due to other commitments; he was not different in terms of patient characteristics from those patients who did participate.
Fifteen healthy control subjects of similar age, sex, exercise, and body mass index (BMI) participated in this study. Community participants within the required age range were recruited via fliers and notices from the neighborhood of the clinic. Participation was on an “opt-in” basis.
Exclusion criteria for the study were: pregnancy, primary sleep disorder (obstructive sleep apnea or narcolepsy); endocrine (untreated diabetes, uncontrolled thyroid disease) or neurological (uncontrolled epilepsy, stroke, dementia, autonomic neuropathy) comorbidities; uncontrolled/untreated cardiovascular disease (hypertension, heart failure) or a pacemaker; autoimmune disease (e.g., Sjögren's syndrome, rheumatoid arthritis); and major depressive disorder, psychosis, or substance abuse disorders. Medications known to affect autonomic functioning, including β-blockers, benzodiazepines, corticosteroids (e.g., prednisone, cortisone acetate, fludrocortisone), other centrally active drugs (e.g., methylphenidate, dexamphetamine, tricyclic, and SSRI antidepressants) were also exclusionary. Menstrual cycle was not controlled in this study.
The subjects who participated in the overnight HRV recordings constitute a subset of a larger and more detailed study of nocturnal HRV in CFS previously reported by our group.29
Written informed consent was obtained from all subjects prior to participation in the study. The relevant human research ethics committees approved the study protocol.
Measures
Self-report questionnaires
Participants completed questionnaires to provide relevant demographic, medical history, and general health information, and to assess physical symptoms, psychological variables, and functional impairment. Specifically, the 34-item Somatic and Psychological Health Report (SPHERE30) was used to assess a wide range of physical and psychological symptoms. An empirically derived subscale (the SOMA) identified the key clinical features of prolonged fatigue states. The Brief Disability Questionnaire (BDQ31) measured functional impairment; notably the “days out of role” quantified the days over the past month during which the respondent was unable to carry out usual daily activities fully. The Pittsburgh Sleep Quality Index (PSQI32) recorded the quality and pattern of sleep. The Perceived Stress Questionnaire (PSQ33) was used to assess perceived levels of current stress.
Pain sensitivity testing
A Pain Test Algometer with a 1-cm2 flat rubber tip (Force Ten FDX Force Gage, Wagner Instruments Greenwich, CT) was used to quantify subjects' pressure pain threshold—the minimum amount of pressure that triggers pain. Two sites on each hand were tested: the muscle belly of the first dorsal interosseus muscle and the middle phalanx of the middle digit. “Neutral” regions, such as the thumb accurately reflect overall pressure-pain sensitivity even in individuals where pain sensitivity is part of the clinical presentation (e.g., fibromyalgia34). Each site was tested 3 times in a counterbalanced manner. To avoid habituation or sensitization at the level of peripheral nociception, the 3 measurements were taken at separate times interspersed with other assessment tasks.
Five-day monitoring of activity-rest-sleep patterns
Actiwatch:
Ambulatory activity counts were recorded for 5 continuous days (including a weekend) using an actigraph accelerometer (Actiwatch 64, Mini Mitter, USA), worn on the non-dominant wrist, set to a sampling epoch of 30 sec and a medium threshold value. This device is watch-sized (29 × 37 × 12 mm), lightweight (16 g), and has been validated for objective measurement of physical activity and sleep/wake cycles. The actiwatch reacts to omnidirectional changes in acceleration which generate a voltage via a piezoelectric sensor. This device is sensitive to 0.05 g-force (1-G-force ≈ 251 activity counts) with bandwidths of 3–11 Hz. The signal is amplified, digitized, and stored in memory as activity counts. Transfer of recorded data was achieved via an ActiReader (Mini Mitter, USA).
Total activity (the sum of all activity counts for the given interval), average activity, and peak activity (the highest artifact-free physical count for the given interval) levels, as well as sleep fragmentation (a score of mobility during sleep) and efficiency (total actual sleep time, as % of total sleep period) were calculated and extracted using the algorithms provided in the Actiwatch Analysis Software (version 5.57.006, Mini-Mitter, USA). Outlying sleep efficiency scores > 2 standard deviations from group sleep efficiency means were excluded from analysis.
The actiwatch data were cleaned and scored using a standard protocol25 to derive night, morning, afternoon, and evening recording intervals that matched those used for symptom recording in the diary (i.e., sleep, wake-12 noon, 12 noon-7 pm, and 7 pm till sleep, respectively—see below). The morning and evening interval start times were selected from the first epoch scored as awake after the sleep interval, and the last epoch prior to sleep.
Activity and symptom diary
To aid interpretation of the activity data, subjects kept a structured diary that allowed monitoring of activities and the experience of symptoms over the 5 days. Daily activities were recorded in addition to the subject's assessment of the associated level of perceived cognitive or physical intensity using the perceived exertion scale.35 The intensity of symptoms of fatigue, mood, and pain was recorded in the morning, afternoon, and evening on a scale ranging from 0–10. Questions regarding overnight sleep timing and quality were answered once each morning.
Salivary cortisol production
Subjects were provided with a test kit (Analytical Reference Laboratories, ARL Pathology, Melbourne), which required 4 saliva specimen collections (06:00–08:00 [30 min after awakening], 12:00, 18:00, and 22:00] on the same day. Detailed explanations and written instructions were provided. The tubes were kept in a −20°C freezer until shipped to a specialised laboratory for analysis by electrochemiluminescence immunoassay (ECLIA).
Heart rate monitoring during nocturnal sleep
An ambulatory Polar Heart Rate Monitor (Model S810i) with a Polar Wearlink W.I.N.D. HR chest band transmitter (Polar Electro, Finland) was used to permit continuous monitoring of heart rate (HR) (as R-R intervals) for a maximal duration of 8 h. Subjects wore the device for the first night of the study, following detailed instructions to activate the watch prior to sleep. HRV results were obtained using Polar Protrainer v5.35.161 software. Analysis algorithms consisted of both time domain variables (root mean square successive differences - rMSSD) and spectral analysis. Power spectral density calculated using autoregressive modelling to derive HRV parameters included a high frequency component (HF [0.15–0.40 Hz]), which reflects vagal drive to the heart, and low frequency component (LF [0.04-0.15 Hz]), which comprises both vagal and sympathetic influences.36 LF:HF ratio (an index of cardiac autonomic balance) was derived from normalized units of LF and HF components. The first hour of recording was discarded and transient noise and movement artifact were manually removed according to previously described methods.29
Statistical Analyses
Statistical analyses were performed using SPSS for Windows version 17 (SPSS Inc., Chicago, IL, USA) and included simple group comparisons using t-tests, correlational (Pearson) analyses, and Fisher exact test to assess independence of categorical data. Multiple regression was used to explore the contribution of HRV parameters to subjective reports of disturbed sleep in the whole sample. Between-group differences for variables recorded repeatedly were analyzed using repeated-measures ANOVA in a general linear model (GLM).
The effects of activity on the subsequent experience of fatigue was explored using linear mixed-effects models, which take into account the correlation between repeated daily measurements of activity and fatigue on the same subject. These analyses were performed using SAS PROC MIXED (SAS version 9.1.3, SAS Institute Inc., Cary, NC, USA) and R (R Development Core Team), with the models fitted using residual maximum likelihood (REML). P < 0.05 was taken as the level of significance for all statistical analyses.
RESULTS
Subject Characteristics
The characteristics of the sample are summarized in Table 1. There were no between-groups differences in age, sex, BMI, or levels of self-reported exercise. The sample was predominantly female (87%), consistent with the CFS literature. Fatigue-related symptom scores (SOMA) were significantly higher in patients than control subjects. Patients also reported greater impairment in performing everyday activities as indicated by the significantly higher scores on overall disability (BDQ) and “days out of role.” Significantly higher PPT scores were evident in the patient group, suggesting increased pain sensitivity in CFS. There were no between-group differences in current levels of perceived stress.
Table 1.
Demographic and clinical characteristics of participants
| Variable | CFS (n = 15) | Control (n =1 5) | P-value |
|---|---|---|---|
| Age | 32.5 (11.1) | 35.6 (13.9) | 0.50 |
| Sex (Males: Females) | 2:13 | 5:10 | 0.39 |
| BMI | 23.6 (2.7) | 24.5 (3.9) | 0.54 |
| Exercise (h/week) | 4.9 (6.3) | 6.9 (3.7) | 0.31 |
| SOMA | 4.3 (3.7) | 0.3 (0.7) | 0.00 |
| BDQ | 12.9 (5.0) | 1.5 (2.4) | 0.00 |
| Days out of role | 12.5 (11.9) | 2.9 (8.1) | 0.02 |
| PPT | 1.7 (0.8) | 2.4 (0.9) | 0.04 |
| PSQ | 68.2 (12.5) | 65. 8 (6.8) | 0.53 |
Values are group means (standard deviations). BMI, body mass index; SOMA, somatic subscale of Somatic and Psychological Health Report; BDQ, Brief Disability Questionnaire; PPT, Pain Pressure Threshold; PSQ, Perceived Stress Questionnaire.
Sleep Parameters
The objective assessment of sleep duration and quality obtained via actiwatch monitoring (Table 2) did not reveal significant between-group differences in either the duration, efficiency, or restlessness (i.e., fragmentation) during sleep. Patients on average went to bed somewhat earlier (at approximately 23:10) than control subjects (23:42), with comparable wake up times for CFS (07:46) and control subjects (07:53). In contrast, patients with CFS rated their quality of sleep as significantly poorer than the healthy control subjects.
Table 2.
Sleep variables from the actiwatch and self-report
| Variable | CFS (n = 15) | Control (n = 15) | P-value |
|---|---|---|---|
| Actiwatch | |||
| Duration (h) | 8.8 (0.8) | 8.1 (0.9) | 0.07 |
| Sleep Efficiency (%) | 79.9 (8.9) | 83.6 (4.3) | 0.21 |
| Sleep Fragmentation (%) | 19.7 (9.5) | 18.6 (4.1) | 0.72 |
| Self-reported sleep quality | |||
| PSQI | 7.7 (3.9) | 3.5 (2.2) | 0.002 |
| Activity patterns | |||
| Total AC/day over 5 days | 307613 (78621) | 317414 (51220) | 0.70 |
| Average AC/min over 5 days | 342 (94) | 340 (48) | 0.95 |
| Peak AC | 2360 (1349) | 1881 (443) | 0.21 |
Values are group means (standard deviations). PSQI, Pittsburg Sleep Quality Index; AC, activity counts.
Overnight Heart Rate Monitoring
The analysis of data obtained from overnight HR monitoring (CFS: N = 11; Healthy: N = 12) showed no between-group differences in HR during sleep; however, significant differences emerged from HRV analysis (Table 3). rMSSD was significantly lower in patients than healthy control subjects. The significant reduction of the proportion of power in the high frequency band (as HF%) further confirmed a pattern of reduced vagal modulation of heart rate in CFS. The low frequency component (LF%) was not significantly different between the groups. Accordingly, the LF:HF ratio was markedly higher in the patients with CFS, generally portraying an imbalance in efferent autonomic cardiac outflow favoring sympathetic drive.
Table 3.
Overnight heart rate and heart rate variability parameters
| Variable | CFS (n = 11) | Healthy (n = 12) | P-value |
|---|---|---|---|
| HR (bpm) | 66 (10) | 66 (7) | 0.97 |
| RMSSD | 34.1 (16.5) | 68.4 (46.5) | 0.03 |
| LF (%) | 19.6 (5.9) | 21.3 (6.4) | 0.50 |
| HF (%) | 10.3 (7.3) | 35.7 (19.2) | 0.002 |
| LF/HF ratio | 2.44 (1.07) | 0.84 (0.58) | 0.002 |
Values are group means (standard deviations). HR, heart rate; RMSSD, root mean squared of successive RR intervals; LF, low frequency (0.04–0.15 Hz); HF, high frequency (0.15–0.40 Hz).
We then employed multiple regression analysis to examine whether these changes in nocturnal autonomic activation were linked to poor sleep quality. As the rMSSD variable was not normally distributed, we applied log transformation to this variable prior to entering in the analysis. In addition to the HRV variables, we entered relevant covariates, including age, sex, pain sensitivity (as PPT scores), and current levels of perceived stress (PSQ) into the equation. The identical best solution was obtained from both forward and backward selection methods. The final solution explained 57% of the variance in the PSQI scores (R2 = 0.57, P = 0.003). The identified strongest predictor of sleep quality was the HF component (β = −0.89, P = 0.001); thus a reduction in HF of 1 SD was linked to a 0.89 SD decrease in rated sleep quality. The only other significant independent predictor was young age (β = −0.68, P = 0.006). In our sample, younger patients with CFS were more severely ill as reflected by the significant correlation between age and BDQ scores: r = −0.55, P = 0.02. This may account for the obtained inverse relationship between age and poor sleep quality.
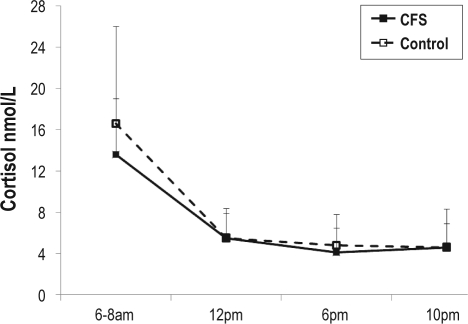
Salivary Cortisol
The diurnal pattern and overall production of cortisol did not differ between groups (Figure 1). As sex was previously found to be a critical covariate to reveal significant group differences in similar studies of CFS,37 we added sex as a covariate in the analysis. We found no significant sex-dependent effects on overall salivary cortisol production (P = 0.74). In addition, the inclusion of sex as a covariate did not result in significant between-group differences on this measure (P = 0.39), nor did it produce any differentially impact on the diurnal trajectory in the cortisol production over the day (P = 0.81). The values obtained for both groups were within the normal reference range provided by the Australian reference laboratory (mean values and SD for CFS versus healthy subjects at 6:00: 13.7 [5.2] vs 16.5 [9.0]; 12:00: 5.5 [2.3] vs 5.5 [2.7]; 18:00: 4.0 [3.5] vs 4.8 [2.9]; 22:00: 4.6 [3.6] vs 4.5 [2.2]).
Figure 1.
Diurnal salivary cortisol secretion in patients with CFS and healthy control subjects. Measures were taken 30 minutes after awakening (denoted 6–8 am), and at 12 pm, 6 pm and 10 pm of the same day. Data are shown as group means with standard deviations as error bars.
Activity and Symptoms
No significant between-group differences were evident from the actiwatch-derived data relating to total activity; average activity per minute or peak activity over the morning, afternoon, or evening periods; or over each of the 5 days of monitoring. Neither was there evidence of a weekend/weekday effect. Diary-reported severity scores for fatigue and pain were significantly higher in patients with CFS than healthy control subjects overall, and in the morning, afternoon, and evening recording periods, on both weekdays and weekends (all P < 0.004 for pain and P < 0.001for fatigue). The differences in mood were at borderline significance levels (P = 0.05). Between-group differences in subjective physical or cognitive intensity (of activity) ratings also were not found to be significant (all P > 0.05).
Predictors of Evening Fatigue Levels
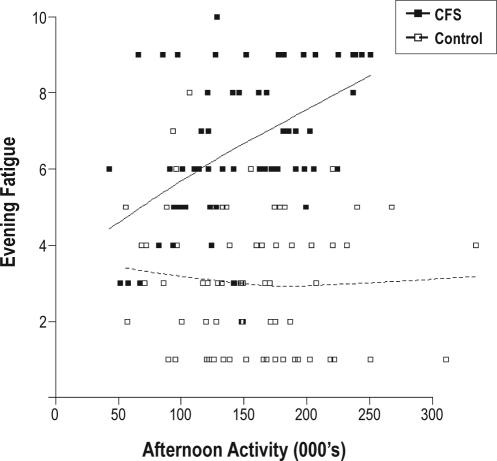
Explanatory variables (covariates) considered for inclusion in the mixed-effect modelling of the effects of activity earlier in the day on levels of evening fatigue (see Figure 2) were case/control status, morning and afternoon total activity, age, sex, previous night's sleep parameters, a weekend effect, and morning and afternoon fatigue. To account for potentially different effects of activity and fatigue for cases compared to controls, interaction terms for group (case/control) × activity and group × fatigue were also considered.
Figure 2.
The effect of afternoon activity on evening fatigue. The boxes on the graph are individual data points (see legend). The unbroken line represents the overall relationship for CFS and broken line for controls. Afternoon activity is in thousands of activity counts. Evening fatigue refers to ratings on a 10-point scale.
Afternoon and morning fatigue were found to be the most significant single predictive variables (P < 0.001), followed by sex (P = 0.034) and sleep fragmentation (P = 0.156). The final model chosen included morning and afternoon fatigue, afternoon activity, as well as group and its interaction with afternoon activity and afternoon fatigue.
Compared to healthy control subjects, patients with CFS had higher average levels of fatigue overall (6.5, SD 1.5 versus 2.6, SD 1.0; P < 0.001). Additionally, the model revealed that afternoon activity did not significantly affect levels of evening fatigue in control subjects; however, for patients with CFS, an increase of 100,000 counts in afternoon activity (e.g., 1 h of brisk walking or tennis) produced an increase of 1 unit on the fatigue score (on the 0–10 scale). This effect was highly significant (P = 0.002).
Predictors of Average Daily Fatigue (Across Morning, Afternoon And Evening Fatigue Scores)
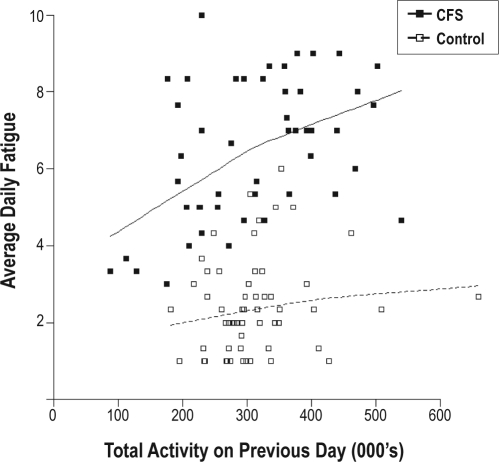
In modelling the effects of daily activity on fatigue levels the following day the same explanatory variables as used for evening fatigue were initially included. However, in the best-fitted model only case/control status, average fatigue and total activity on the previous day were retained (Figure 3).
Figure 3.
Preceding day total activity and daily fatigue. The boxes on the graph are individual data points (see legend). The unbroken line represents the overall relationship for CFS and broken line for controls. Total activity is in thousands of activity counts. Average daily fatigue refers to fatigue ratings on a 10-point scale.
In this day-to-day fatigue model, no interaction terms were significant, thus the fatigue levels of all participants on a given day were similarly affected by activity (P = 0.02) and fatigue (P < 0.001) on the previous day.
DISCUSSION
This study examined circadian patterns of sleep/wake and activity in patients with CFS and their ability to predict future symptoms. No differences in circadian rhythm were evident from the recordings of the pattern or amount of sleep, activity, or cortisol secretion between patients with CFS and control subjects. However, a subjective sleep complaint was apparent in patients in conjunction with evidence of a loss of vagal modulation of heart rate as indicated by significantly lower HRV in the CFS group, but not in control subjects. Modelling of the activity, sleep, and symptom patterns revealed that in patients but not control subjects; afternoon activity was the strongest predictor of evening fatigue—the first objective recording of the typical patient report of a post-exertional exacerbation of the key symptom of fatigue.
The lack of between-group differences in sleep timing, duration, and quality, as well as in the overall sleep-activity patterns do not support a conclusion of circadian rhythm disruption in CFS. This conclusion is strengthened by the results indicating normal diurnal cortisol secretion in CFS. Although hypocorticolism16 and/or an attenuated diurnal production of cortisol have been reported repeatedly,17,18 close inspection of the body of research into diurnal cortisol assessment in urine,38 blood,39 and saliva40 revealed frequent failures to confirm a substantive differences between healthy subjects and patients with CFS. Moreover, an essentially normal pattern of diurnal cortisol secretion in CFS is consistent with the findings from treatment studies in which corticosteroid supplementation produced limited relief of symptoms of CFS.41,42
While the current results do not support impairment in circadian rhythms in CFS, studies observing changes in melatonin levels15 and a reduction in fatigue symptoms upon treatment with melatonin43 reflect a need for further research into this area. Therefore more sensitive measures of circadian phase such as the dim light melatonin onset (DLMO) test - considered to be the “gold standard” for measuring melatonin and circadian rhythm disorders44 should be employed in future studies evaluating this hypothesis in CFS.
The current actigraphy data did not substantiate previous reports of objective sleep disturbances (low sleep efficiency or sleep fragmentation) or poor sleep hygiene (e.g., daytime napping).4 Enrolment in a behavioral intervention program that explicitly instructed patients not to engage in daytime napping may in part explain this finding. However, recruitment coincided with the second week of treatment, thus drastic improvements in sleep patterns were unlikely at this early stage of intervention. Despite this lack of objective evidence of sleep disturbance in our sample, consistent with the literature, patients uniformly reported poor sleep quality.3 These findings are also consistent with investigation of other objective parameters in CFS such as assessment of neuromuscular or neurocognitive performance, in which little objective deficit has been recorded45,46 despite prominent subjective complaints in these domains.
This led us to examine other possible causes of poor sleep quality including autonomic hyperarousal, perceived stress, and pain. Analysis of HR recordings during sleep confirmed previous reports of decreased HRV, with an attendant increase in the HF:LF ratio during sleep28,29 in CFS. Such a profile indicates a loss of vagal modulation of heart rate, which engenders a hyper-vigilant, inflexible physiological state.47,48 Resting and sleep are normally accompanied by an increase in vagal tone, which serves a restorative function.48,49 Therefore, the significant reduction in vagal cardiac modulation, as demonstrated here, constitutes a plausible biological correlate of poor sleep.
Linear regression modelling supported this conclusion, as the model that best accounted for the variance in sleep quality ratings identified a reduction in the HF component as a highly significant predictor of poor sleep quality scores on the PSQI. Although perceived stress and pain parameters were included in the analyses, these did not significantly impact on sleep quality. These findings are consistent with our earlier report in an overlapping subject group29 and indicate the diversity of factors that can provide possible explanations for the experience of disturbed sleep documented in CFS.
Activity levels throughout the day did not differ significantly between patients with CFS and healthy controls—an observation that is inconsistent with other studies that have utilized accelerometers.24 However, control subjects were not matched for exercise levels in the majority of these studies, which is imperative, as high levels of routine exercise will confound activity results. It needs to be kept in mind, however, that the current sample was enrolled in an intervention program, which raises the possibility that these participants were well motivated to change maladaptive activity-rest patterns.
Kop and colleagues reported lower peak activity levels in patients with CFS and fibromyalgia than healthy subjects.25 Inspection of the data representing peak activity in our study, however, revealed high singular peaks during periods of comparatively low activity, which questions the usefulness of this measure as an accurate reflection of maximal activity. Considering the difficulties introduced by individual differences within patient samples, longitudinal designs of within-subject time point comparisons at different stages of the illness are recommended for future studies.
The experience of profound exacerbation of fatigue after activity constitutes another key characteristic of CFS.1 The relationship between activity and fatigue exemplified clinically by post-exertional exacerbations was therefore modelled. Afternoon activity was found to be the best predictor of evening fatigue on the same day in CFS, with preexisting levels of fatigue further compounding the effect of preceding activity. In contrast, comparable increases in activity did not cause the same increase in evening fatigue in control subjects. This finding provides the first objective recording of post-exertional exacerbation in CFS and highlights the extent of the disability that can be experienced by patients on an everyday basis.
Modelling of day-to-day fatigue revealed significant effects of both preceding activity and fatigue on the subsequent fatigue levels the next day. Although this effect was not substantively different between the groups, in view of the high baseline levels of fatigue in CFS and the demonstrated significant within-day effect of post-activity exacerbation of symptoms, this more general effect may function to potentiate the effects of exertion on a sense of overwhelming fatigue in CFS. These findings provide support for the potential benefits of gradually increasing activity levels, which is the key element of graded exercise therapy for CFS.19
The time course of the activity-fatigue relationship was previously explored using a maximal aerobic exercise test as the activity which resulted in a worsening of physical, but not psychological or cognitive symptoms27 five days following the original stressor. As patients with CFS are unlikely to engage in high intensity activity,50 the studies using vigorous activity to model symptom exacerbations may not capture ideally the typical activity-fatigue contingencies that operate in the everyday life of patients with CFS. In essence, the results of the current study may be more informative to clinicians treating patients within a naturalistic setting.
Limitations and Future Directions
This study used a cross-sectional case-control study design with the patients in the initial stages of treatment. Thus, the current sample may not be representative of CFS patients in general, as the participants reported here may have higher levels of motivation as well as more knowledge of behavioral patterns conducive to a reduction in symptoms.
Although the sample was well characterized and repeated measures were obtained for each participant, the numbers were relatively small, which could have affected statistical power. The results therefore need to be replicated in a larger sample. To further explore the potential importance of a reduction in vagal cardiac drive in poor sleep quality and the maintenance of symptoms in CFS, ambulatory monitoring of autonomic responses for extended time periods are warranted.
A further potential limitation of this study is that polysomnography was not included to monitor sleep stages. Therefore, it is difficult to elucidate actual sleep versus inactivity (lying still in bed, but awake) with actigraphy alone. Again, the absence of polysomnographic equipment did not allow us to observe the relationship between HRV and different sleep stages, but only to nocturnal sleep per se. Importantly, as the literature does not support altered sleep architecture in subjects with CFS compared with healthy subjects, it is unlikely that the differences in heart rate variability relate specifically to differences in sleep stages in patients with CFS compared to healthy control subjects.
CONCLUSIONS
The findings reported here do not provide any evidence of disturbed circadian rhythms in CFS. However, the data are consistent with the notion that reduced vagal modulation of heart rate represents a biological correlate of the profound experience of poor quality sleep in CFS. In addition, the study has documented, in a naturalistic setting, the existence of activity-induced exacerbation of fatigue. The activity symptom-relationship modelled here is of clinical significance as it can inform the approach to activity pacing and symptom management in cognitive behavioral therapy in CFS.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The support of the staff at the Lifestyle Clinic, UNSW, as well as the enduring cooperation of the participants in this research is gratefully acknowledged.
This work was supported by a grant from the Mason Foundation (CT8924), Australia
REFERENCES
- 1.Fukuda K, Straus SE, Hickie I, et al. The chronic fatigue syndrome: a comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study Group. Ann Intern Med. 1994;121:953–9. doi: 10.7326/0003-4819-121-12-199412150-00009. [DOI] [PubMed] [Google Scholar]
- 2.Komaroff AL, Buchwald D. Symptoms and signs of chronic fatigue syndrome. Rev Infect Dis. 1991;13(Suppl 1):S8–11. doi: 10.1093/clinids/13.supplement_1.s8. [DOI] [PubMed] [Google Scholar]
- 3.Vollmer-Conna U, Aslakson E, White PD. An empirical delineation of the heterogeneity of chronic unexplained fatigue in women. Pharmacogenomics. 2006;7:355–64. doi: 10.2217/14622416.7.3.355. [DOI] [PubMed] [Google Scholar]
- 4.Neu D, Cappeliez B, Hoffmann G, et al. High slow-wave sleep and low-light sleep: chronic fatigue syndrome is not likely to be a primary sleep disorder. J Clin Neurophysiol. 2009;26:207–12. doi: 10.1097/WNP.0b013e3181a1841b. [DOI] [PubMed] [Google Scholar]
- 5.Reeves WC, Heim C, Maloney EM, et al. Sleep characteristics of persons with chronic fatigue syndrome and non-fatigued controls: results from a population-based study. BMC Neurol. 2006;6:41. doi: 10.1186/1471-2377-6-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ball N, Buchwald DS, Schmidt D, et al. Monozygotic twins discordant for chronic fatigue syndrome: objective measures of sleep. J Psychosom Res. 2004;56:207–12. doi: 10.1016/S0022-3999(03)00598-1. [DOI] [PubMed] [Google Scholar]
- 7.Armitage R, Landis C, Hoffmann R, et al. The impact of a 4-hour sleep delay on slow wave activity in twins discordant for chronic fatigue syndrome. Sleep. 2007;30:657–62. doi: 10.1093/sleep/30.5.657. [DOI] [PubMed] [Google Scholar]
- 8.Toh KL. Basic science review on circadian rhythm biology and circadian sleep disorders. Ann Acad Med Singap. 2008;37:662–68. [PubMed] [Google Scholar]
- 9.Murray G. Diurnal mood variation in depression: a signal of disturbed circadian function? J Affect Disord. 2007;102:47–53. doi: 10.1016/j.jad.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 10.Burch JB, Tom J, Zhai Y, et al. Shiftwork impacts and adaptation among health care workers. Occup Med (Lond) 2009;59:159–66. doi: 10.1093/occmed/kqp015. [DOI] [PubMed] [Google Scholar]
- 11.Van Someren EJW, Riemersma-Van Der Lek RF. Live to the rhythm, slave to the rhythm. Sleep Med Rev. 2007;11:465–84. doi: 10.1016/j.smrv.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 12.Di Giorgio A, Hudson M, Jerjes, Cleare AJ. 24-hour pituitary and adrenal hormone profiles in chronic fatigue syndrome. Psychosom Med. 2005;67:433–40. doi: 10.1097/01.psy.0000161206.55324.8a. [DOI] [PubMed] [Google Scholar]
- 13.Moldofsky H. Sleep, neuroimmune and neuroendocrine functions in fibromyalgia and chronic fatigue syndrome. Adv Neuroimmunol. 1995;5:39–56. doi: 10.1016/0960-5428(94)00048-s. [DOI] [PubMed] [Google Scholar]
- 14.Tryon WW, Jason L, Frankenberry E. Torres-Harding S Chronic fatigue syndrome impairs circadian rhythm of activity level. Physiol Behav. 2004;82:849–53. doi: 10.1016/j.physbeh.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 15.Knook L, Kavelaars A, Sinnema G, Kuis W, Heijnen CJ. High nocturnal melatonin in adolescents with chronic fatigue syndrome. J Clin Endocrinol Metab. 2000;85:3690–92. doi: 10.1210/jcem.85.10.6857. [DOI] [PubMed] [Google Scholar]
- 16.Hamilos DL, Nutter D, Gershtenson J, et al. Circadian rhythm of core body temperature in subjects with chronic fatigue syndrome. Clin Physiol. 2001;21:184–95. doi: 10.1046/j.1365-2281.2001.00321.x. [DOI] [PubMed] [Google Scholar]
- 17.Nater UM, Youngblood LS, Jones JF, et al. Alterations in diurnal salivary cortisol rhythm in a population-based sample of cases with chronic fatigue syndrome. Psychosom Med. 2008;70:298–305. doi: 10.1097/PSY.0b013e3181651025. [DOI] [PubMed] [Google Scholar]
- 18.Cleare AJ. The neuroendocrinology of chronic fatigue syndrome. Endocr Rev. 2003;24:236–52. doi: 10.1210/er.2002-0014. [DOI] [PubMed] [Google Scholar]
- 19.White PD, Sharpe MC, Chalder T, DeCesare JC, Walwyn R. Protocol for the PACE trial: a randomised controlled trial of adaptive pacing, cognitive behaviour therapy, and graded exercise, as supplements to standardised specialist medical care versus standardised specialist medical care alone for patients with the chronic fatigue syndrome/myalgic encephalomyelitis or encephalopathy. BMC Neurol. 2007;7:6. doi: 10.1186/1471-2377-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Price JR, Mitchell E, Tidy E, Hunot V. Cognitive behaviour therapy for chronic fatigue syndrome in adults. Cochrane Database Syst Rev. 2008:CD001027. doi: 10.1002/14651858.CD001027.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moss-Morris R, Sharon C, Tobin R, Baldi JC. A randomized controlled graded exercise trial for chronic fatigue syndrome: outcomes and mechanisms of change. J Health Psychol. 2005;10:245–59. doi: 10.1177/1359105305049774. [DOI] [PubMed] [Google Scholar]
- 22.Sivertsen B, Omvik S, Havik OE, et al. A comparison of actigraphy and polysomnography in older adults treated for chronic primary insomnia. Sleep. 2006;29:1353–58. doi: 10.1093/sleep/29.10.1353. [DOI] [PubMed] [Google Scholar]
- 23.Blackwell T, Redline S, Ancoli-Israel S, et al. Comparison of sleep parameters from actigraphy and polysomnography in older women: the SOF study. Sleep. 2008;31:283–91. doi: 10.1093/sleep/31.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Werf SP, Prins JB, Vercoulen JH, van der Meer JW, Bleijenberg G. Identifying physical activity patterns in chronic fatigue syndrome using actigraphic assessment. J Psychosom Res. 2000;49:373–79. doi: 10.1016/s0022-3999(00)00197-5. [DOI] [PubMed] [Google Scholar]
- 25.Kop WJ, Lyden A, Berlin AA, et al. Ambulatory monitoring of physical activity and symptoms in fibromyalgia and chronic fatigue syndrome. Arthritis Rheum. 2005;52:296–303. doi: 10.1002/art.20779. [DOI] [PubMed] [Google Scholar]
- 26.Aslakson E, Vollmer-Conna U, Reeves WC, White PD. Replication of an empirical approach to delineate the heterogeneity of chronic unexplained fatigue. Popul Health Metr. 2009;7:17. doi: 10.1186/1478-7954-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoshiuchi K, Cook DB, Ohashi K, et al. A real-time assessment of the effect of exercise in chronic fatigue syndrome. Physiol Behav. 2007;92:963–68. doi: 10.1016/j.physbeh.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boneva RS, Decker MJ, Maloney EM, et al. Higher heart rate and reduced heart rate variability persist during sleep in chronic fatigue syndrome: a population-based study. Auton Neurosci. 2007;137:94–101. doi: 10.1016/j.autneu.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 29.Burton A, Rahman K, Kadota Y, Lloyd A, Vollmer-Conna U. Reduced heart rate variability predicts poor sleep quality in a case control study of chronic fatigue syndrome. Exp Brain Res. 2010;204:71–78. doi: 10.1007/s00221-010-2296-1. [DOI] [PubMed] [Google Scholar]
- 30.Hickie IB, Davenport TA, Hadzi-Pavlovic D, et al. Development of a simple screening tool for common mental disorders in general practice. Med J Aust. 2001;175(Suppl):S10–17. doi: 10.5694/j.1326-5377.2001.tb143784.x. [DOI] [PubMed] [Google Scholar]
- 31.Von Korff M, Ustun TB, Ormel J, Kaplan I, Simon GE. Self-report disability in an international primary care study of psychological illness. J Clin Epidemiol. 1996;49:297–303. doi: 10.1016/0895-4356(95)00512-9. [DOI] [PubMed] [Google Scholar]
- 32.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 33.Levenstein S, Prantera C, Varvo V, et al. Development of the Perceived Stress Questionnaire: a new tool for psychosomatic research. J Psychosom Res. 1993;37:19–32. doi: 10.1016/0022-3999(93)90120-5. [DOI] [PubMed] [Google Scholar]
- 34.Petzke F, Khine A, Williams D, et al. Dolorimetry performed at 3 paired tender points highly predicts overall tenderness. J Rheumatol. 2001;28:2568–9. [PubMed] [Google Scholar]
- 35.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14:377–81. [PubMed] [Google Scholar]
- 36.Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93:1043–65. [PubMed] [Google Scholar]
- 37.Nater U, Maloney E, Boneva R, et al. Attenuated morning salivary cortisol concentrations in a population-based study of persons with chronic fatigue syndrome and well controls. J Clin Endocrinol Metab. 2008;93:703–9. doi: 10.1210/jc.2007-1747. [DOI] [PubMed] [Google Scholar]
- 38.Jerjes WK, Taylor NF, Peters TJ, Wessely S, Cleare AJ. Urinary cortisol and cortisol metabolite excretion in chronic fatigue syndrome. Psychosom Med. 2006;68:578–82. doi: 10.1097/01.psy.0000222358.01096.54. [DOI] [PubMed] [Google Scholar]
- 39.MacHale SM, Cavanagh JT, Bennie J, et al. Diurnal variation of adrenocortical activity in chronic fatigue syndrome. Neuropsychobiology. 1998;38:213–17. doi: 10.1159/000026543. [DOI] [PubMed] [Google Scholar]
- 40.Gaab J, Hüster D, Peisen R, et al. Low-dose dexamethasone suppression test in chronic fatigue syndrome and health. Psychosom Med. 2002;64:311–18. doi: 10.1097/00006842-200203000-00015. [DOI] [PubMed] [Google Scholar]
- 41.Blockmans D, Persoons P, Van Houdenhove B, Lejeune M, Bobbaers H. Combination therapy with hydrocortisone and fludrocortisone does not improve symptoms in chronic fatigue syndrome: a randomized, placebo-controlled, double-blind, crossover study. Am J Med. 2003;114:736–41. doi: 10.1016/s0002-9343(03)00182-7. [DOI] [PubMed] [Google Scholar]
- 42.McKenzie R, O'Fallon A, Dale J, et al. Low-dose hydrocortisone for treatment of chronic fatigue syndrome: a randomized controlled trial. JAMA. 1998;280:1061–6. doi: 10.1001/jama.280.12.1061. [DOI] [PubMed] [Google Scholar]
- 43.van Heukelom RO, Prins JB, Smits MG, Bleijenberg G. Influence of melatonin on fatigue severity in patients with chronic fatigue syndrome and late melatonin secretion. Eur J Neurol. 2006;13:55–60. doi: 10.1111/j.1468-1331.2006.01132.x. [DOI] [PubMed] [Google Scholar]
- 44.Pandi-Perumal SR, Smits M, Spence W, et al. Dim light melatonin onset (DLMO): a tool for the analysis of circadian phase in human sleep and chronobiological disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1–11. doi: 10.1016/j.pnpbp.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 45.McCully KK, Smith S, Rajaei S, Leigh JS, Natelson BH. Blood flow and muscle metabolism in chronic fatigue syndrome. Clin Sci. 2003;104:641–7. doi: 10.1042/CS20020279. [DOI] [PubMed] [Google Scholar]
- 46.Schmaling KB, Lewis DH, Fiedelak JI, Mahurin R, Buchwald DS. Single-photon emission computerized tomography and neurocognitive function in patients with chronic fatigue syndrome. Psychosom Med. 2003;65:129–36. doi: 10.1097/01.psy.0000038942.33335.9b. [DOI] [PubMed] [Google Scholar]
- 47.Thayer JF, Brosschot JF. Psychosomatics and psychopathology: looking up and down from the brain. Psychoneuroendocrinology. 2005;30:1050–8. doi: 10.1016/j.psyneuen.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 48.Thayer JF, Sternberg E. Beyond heart rate variability: vagal regulation of allostatic systems. Ann NY Acad Sci. 2006;1088:361–72. doi: 10.1196/annals.1366.014. [DOI] [PubMed] [Google Scholar]
- 49.Coote JH. Respiratory and circulatory control during sleep. J Exp Biol. 1982;100:223–44. doi: 10.1242/jeb.100.1.223. [DOI] [PubMed] [Google Scholar]
- 50.Fulcher KY, White PD. Strength and physiological response to exercise in patients with chronic fatigue syndrome. J Neurol Neurosurg Psychiatr. 2000;69:302–7. doi: 10.1136/jnnp.69.3.302. [DOI] [PMC free article] [PubMed] [Google Scholar]