Abstract
Objective
Sle2 is a lupus susceptibility locus that has been linked toglomerulonephritis in the NZM2410mouse. Byitself, Sle2 does not induce any autoimmune pathology, but results into the accumulation of B1a cells. This study was designed to assess the contribution of Sle2 to autoimmune pathogenesis.
Methods
Sle2 or its sub-congenic intervals (Sle2a, Sle2b and Sle2c) were bred to Fas-deficient B6.lpr mice. Lymphoid phenotypes, focused on T cells, were assessed by flow cytometry, and histopathology was compared between cohorts of B6.Sle2.lpr congenics and B6.lpr mice aged up to 6 mo old.
Results
Sle2 synergized with lpr, resulting in a greatly accelerated lymphadenopathy that largely targeted T cells, and mapped to the Sle2c1 locus. This locus has been identified as the main contributor to B1a cell expansion. Further analyses showed that Sle2c1 expression skewed the differentiation and polarization of Fas-deficient T cells, with a reduction of the CD4+ CD25+ Foxp3+ regulatory T cell subset and an expansion of the TH17 cells. This was associated with a high level of T cell infiltrates that promoted severe nephritis and dermatitis in the B6.Sle2c1.lpr mice.
Conclusion
These results show that Sle2c1 contributes to lupus pathogenesis by affecting T cell differentiation in combination with other susceptibility loci such as lpr. The significance of the co-segregation of this phenotype and B1a cell expansion in Sle2c1-expressing mice for lupus pathogenesis is discussed.
Keywords: lupus, T lymphocytes, genetics, Fas, nephritis
Introduction
Sle2 was identified as a strong locus linked to glomerulonephritis in the NZM2410 lupus-prone mouse (1). A congenic analysis has shown that the main effect of Sle2expression on a C57BL/6 (B6) background was to lower the threshold of the B cell receptor (BCR) activation, which resulted in the accumulation of B1a cells primarily in the peritoneal cavity (2). The addition of Sle2 to the combination of the two other main NZM2410-susceptibility loci, Sle1 and Sle3, significantly enhanced lymphoid activation and end-organ disease, which demonstrated a direct contribution of Sle2 to lupus pathogenesis (3). Furthermore, Sle2expression promoted the breach of tolerance to DNA in B cells expressing the 56R BCR transgene by increasing their selection to the marginal zone and altering receptor editing (4). However, the role of the Sle2-mediated expansion of B1a cells in systemic autoimmunity has not been directly addressed. B1a cells have been proposed to contribute to autoimmunity through the production of cross-reactive low-affinity natural antibodies (Ab) (5). Recent studies have shown that PDL2+B1a cells produce anti-dsDNA Ab(6)and that B1a cells strongly promote T cell proliferation and differentiation into the T helper 1 (TH1) and IL-17 producing T helper (TH17) cell subsets(7).Furthermore, (NZB × NZW)F1 B1a cells are recruited to the sites of inflammationsuch as the kidney(8), where they class-switch and produce anti-dsDNA IgG (9).
We have mapped the accumulation of B1a cells to a ~ 13 Mb NZB-derived segment of Sle2 named Sle2c1 (10). The NZW-derived Sle2a and Sle2b regions also contribute to pathogenesis, but have minor effects on the B1a cell compartment(10). To further assess the contribution of Sle2and B1a cells to autoimmune pathogenesis, we have co-expressed Sle2or its sub-congenic intervals with the lpr mutation that leads to Fas deficiency(11). This strategy was used for Sle1, and B6.Sle1.lpr mice developed an enhanced lymphadenopathy as well as a severe lupus nephritis(12). In this report, we showed that Sle2 also synergizes with lpr, although in a different manner than Sle1, with a greatly accelerated lymphadenopathy that largely targets the B220+ CD4− CD8− double negative (DN) T cells, a population that is expanded by the lpr mutation(11). This massive lymphoid expansion mapped to the Sle2c1 locus. Further analyses showed that Sle2c1 expression skewed the differentiation and polarization of Fas-deficient T cells, with a reduction of the CD4+ CD25+ Foxp3+ regulatory T (Treg) cell subset and an expansion of TH17 cells. This was associated with a high level of T cell infiltrates that promoted severe nephritis in the B6.Sle2c1.lpr mice. These results show that the Sle2c1 locus contributes to lupus pathogenesis by affecting T cell homeostasis in combination with other susceptibility loci such as lpr. The significance of the co-segregation of this phenotype and B1a cell expansion in Sle2c1-expressing mice for lupus pathogenesis is discussed.
Materials and Methods
Mice
The B6.Sle2, B6.Sle2a, B6.Sle2b, and B6.Sle2cl congenic strains have been previously described(10;13). The lpr mutation was bred to homozygosity ontoSle2and its sub-congenic intervals by intercrossing with B6.MRL-Faslpr/J (B6.lpr) mice, which were, as B6 and B6.Cg-Igha.Thy1a.Gpi1a/J mice, originally obtained from the Jackson Laboratories. The B6.Sle1.Sle2.Sle3 (B6.TC) that combines the three major NZM2410 susceptibility loci on a B6 background has also been previously described (3). Both males and females mice were used at 4–6 mo of age, or at the age indicated. All experiments were conducted according to protocols approved by the University of Florida Institutional Animal Care and Use Committee.
Flow cytometry
Lymph node (LN) or splenic single cell suspensions were prepared by lysing RBCs with 0.83% NH4Cl. Cells were blocked with saturating amounts of anti-CD16/CD32 (2.4G2) and then stained with FITC-, PE-, or biotin-conjugated Abs: CD3e (145-2C11), CD4 (RM4-5), CD5 (53-7.3), CD8a (53-6.7), CD19 (1D3), CD25 (PC61), CD90.1 (OX-7), CD90.2 n (53-2.1), B220 (RA3-6B2), and IgMa (IgH6), all purchased from BD Pharmingen. Intracellularstaining for INFγ (XMG1.2), IL-4 (11B11), IL-17A (TC11-18H10), and Foxp3 (FJK-16s, eBioscience) was performed after fixation/permeabilization of cells.Cytokines were detected in cells treated with leukocyte activation cocktail (BD Pharmingen)and with 10 µg/ml Brefeldin A for 5 h. Biotinylated antibodies were revealed by Streptavidin-PerCP-Cy5.5. To evaluate renal infiltrates, kidneys were digested with collagenase D and DNase (Sigma)for 1 h at 37 °C. One million B6.Cg-Igha.Thy1a.Gpi1a splenocytes (or thymocytes for detection of DNT cells) were added to each kidney cell suspension as internal control. The amount of each lymphocyte population was expressed as the ratio of Thy1b/Thy1a or IgMb/IgMa cells. Dead cells and mononuclear cells were gated on the basis of forward and side scatters characteristics. At least 30,000 events were acquired per sample on a FACSCalibur cytometer (BD Biosciences).
T cell suppression assays
In vitro assessment of Treg suppressive function was performed as previously described (14). Briefly, CD4+ CD25− effector T cells (Teff) were magnetic-bead purified from B6 mice and labeled with CFSE. CD4+ CD25+ Treg isolated from B6, B6.lpr, and B6.Sle2c1.lpr spleenswere co-cultured with Teff (105) in the presence of 2 × 104 mitomycin-treated CD11c+ splenic B6 dendritic cells and 1ug/ml anti-CD3 for 4 d. Proliferation was measured by CFSE dilution by flow cytometry. The inhibition ratios indicate Teff proliferation in the presence of Tregs over Teff proliferation alone. To assess the in vivo role of Tregs in the NZM2410 model, we injected intravenously 6 × 106 Tregs or saline in 2 mo old B6.TC female mice and assessed their autoantibody production for 10 weeks. This experiment was conducted twice on 5 mice per group.
AutoAb measurements
The anti-dsDNA IgG ELISA assay was carried out as previously described(3). Test sera were incubated in duplicate at 1:100 dilutions. Relative units were standardized using a positive serum from a B6.TC mouse, arbitrarily setting the reactivity of a 1:100 dilution of this control serum to 100 units.
Histopathology
Proteinuria was determined with Albustix strips (Bayer) using a 0–4 scale. Transverse sections of kidney were processed and stained with hemotoxylin and eosin (H&E) and periodic acid Schiff (PAS). Renal lesions were classified as previously described based on a modified International Society of Nephrology/Renal Pathology Societyclassification (15). Briefly glomerular lesions were classified: negative, mesangial matrix (PAS+) expansion (Mm), mesangial expansion with increased mesangial cellularity (Mc), capillary hyaline (immune complex type) deposits (HD) and proliferative glomerulonephritis (glomerular hypercellularity with capillary loop involvement, which was global [Pg] or segmental [Ps]). Extent of involvement was graded on a 1–4 scale. Glomerular sclerosis was graded separately. Chronic (mononuclear cell) inflammation was classified as perivascular or interstitial (ICI) and graded as for glomerular inflammation. Glomerular macrophages and CD4+ T cells weredetected on re-hydrated 8 µM sections first incubated with 0.6% H2O2 and 0.2% NaN3 to inhibit endogenous peroxidase activity and stained with biotin-conjugated anti-CD68 (Serotec) or anti-CD4 (BD Pharmingen). HRP-streptavidin (Vector) and DAB substrate were added and positive cells developed a brown color. Sections were counterstained with Mayer's hematoxylin solution (Sigma). The numbers of CD68+ or CD4+ cells were averaged from 10 randomly selected glomeruli under high power field for each kidney section. Detection of glomerular immune complex deposits were assessed on frozen sections stained with anti-IgG2a or anti-C3 FITC as previously described(15).Formalin-fixed inter-scapular skin sections were stained with H&E.
Statistics
Statistical analyses were performed using GraphPad Prism 4. Unless specified, graphs show mean values. Phenotypes were compared between strains with Dunnett's Multiple Comparison Tests, or t tests when only two strains were involved, after verification of normality of distribution.
Results
Sle2c1 enhances lpr-mediated lymphadenopathy
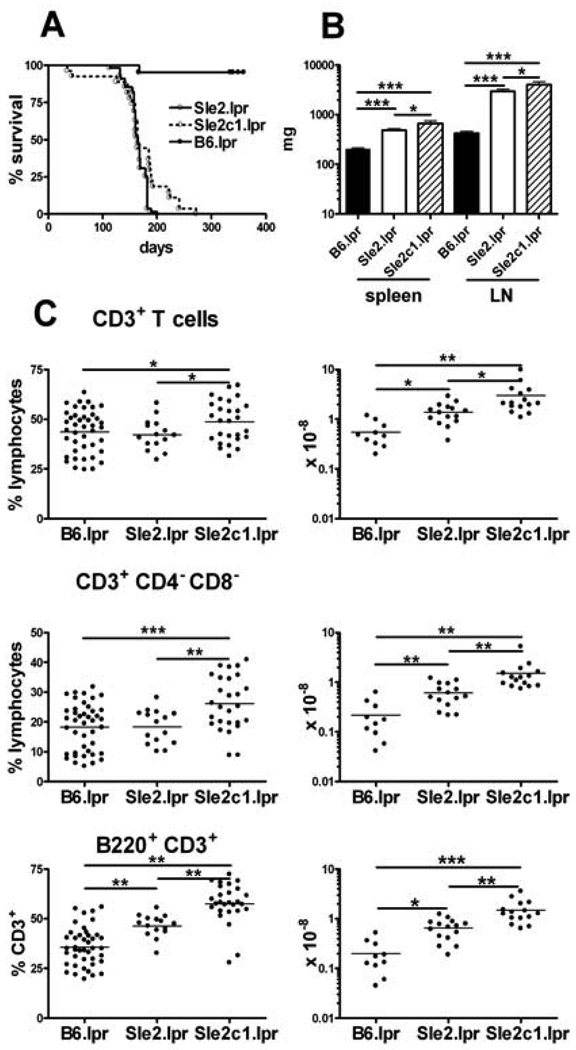
Sle2 by itself does not affect survival or lymphoid expansion(2). Massive lymphoproliferation, especially in the cervical LNs, required euthanasia for the majority of B6.Sle2.lpr mice between 4 and 7 mo of age, resulting in a significantly reduced survival as compared to B6.lpr (Fig. 1A). At sacrifice, the average B6.Sle2.lpr spleen weight doubled as compared to age-matched B6.lpr, and the lymphoid expansion was exaggerated in the LNs whose weight was on average 7 times larger than in B6.lpr (Fig. 1B). Histological examination of the spleens showed both lymphoid and myeloid expansionthat largely invaded the red pulpand caused a severe disruption of the splenic architecture (data not shown). When the effect of the individual Sle2 sub-loci were examined, the decreased survival (Fig. 1A) and lymphoid expansion (Fig. 1B) clearly mapped to Sle2c1, with a 3 and 9 fold average increase in spleen and LN weight, respectively, between B6.Sle2c1.lpr and B6.lpr mice. The life-span and lymphoid organs of B6.Sle2a.lpr and B6.Sle2b.lpr mice were similar to that of B6.lprmice (data not shown). The number of B cells, including IgM+ CD5+ B1a cells, and myeloid cells were significantly higher in B6.Sle2.lpr and B6.Sle2c1.lpr than in B6.lpr mice (data not shown). The lymphoid expansion, however, mostly targeted the T cell population. The percentage and absolute numbers of total T cells, CD3+ CD4− CD8− DNT cells and B220+ T cells,the majority of which were DNT cells (data not shown) were significantly higher in B6.Sle2.lpr and B6.Sle2c1.lpr than in B6.lpr spleens (Fig. 1C) or LN (data not shown).T cell percentages and numbers were similar between B6.lpr and B6.Sle2a.lpror B6.Sle2b.lpr mice(data not shown), and the phenotype of these mice was not examined further.Interestingly, the lymphoid and T cell expansion were consistently greater in B6.Sle2c1.lpr than in B6.Sle2.lpr mice (Fig. 1B and C). This data shows that the expression of the telomeric NZB-derived region of Sle2, Sle2c1, greatly enhances the lpr-induced lymphadenopathy, resulting in a significant expansion of the T cell pool, including DNT and B220+ T cells.
Figure 1.
Sle2c1 expression increases lpr-mediated lymphadenopathy. A. Survival in B6.lpr (n =21), B6.Sle2.lpr (n = 55) and B6.Sle2c1.lpr (n = 27) mice. The survival curves for either of the two latter strains were significantly different from that of B6.lpr (Long rank tests, p < 0.0001). B. Spleen and LN weight (average and SEM, 15–25 mice per strain). C. Percentage and absolute number of splenicCD3+ T cells, CD3+ CD4− CD8− DN T cells, and CD3+ B220+ T cells.*: p< 0.05; **: p < 0.01; ***: p < 0.001.
Sle2c1 alters T cell polarization
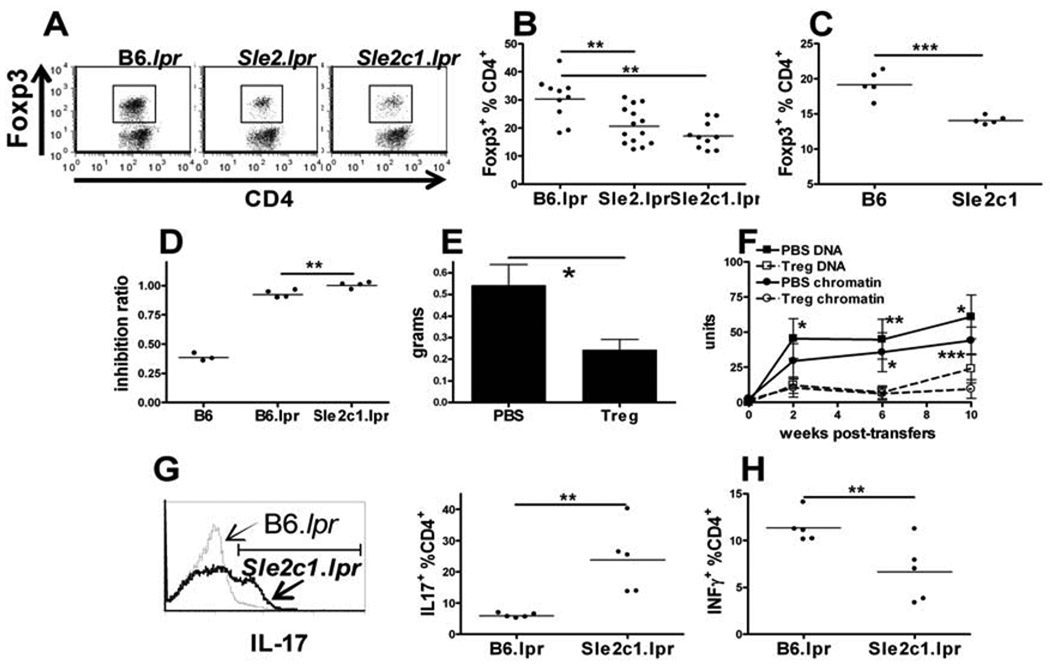
We evaluated the size of the Treg compartment as well as the cytokine polarization in Sle2c1-expressing T cells. Very few CD4+ Foxp3+ cells were detected by immunofluorescence in B6.Sle2c1.lpr spleens (Fig. 2A) and LNs (data not shown) as compared to B6.lpr. The lower relative abundance of Tregs in B6.Sle2.lpr and B6.Sle2c1.lpr mice was confirmed by flow cytometry as early as 2 mo of age (Fig. 3A and B). The absolute Treg numbers did not differ between strains (B6.lpr: 10.59 ± 1.79, B6.Sle2.lpr: 11.66 ± 3.08; B6.Sle2c1.lpr: 11.07 ± 2.38 × 106 Foxp3+ CD4+-gated cells), as lymphoid expansion compensated for lower percentages in the Sle2 strains.The same result was obtained in the LN (B6.lpr: 23.17±5.53, B6.Sle2.lpr: 14.95±0.83; B6.Sle2c1.lpr: 13.59± 0.98 % Foxp3+ CD4+-gated cells). Furthermore, a significantly decreased Foxp3+ Treg population was found in Fas-intact B6.Sle2c1as compared to B6 mice (Fig. 3C). The ability of B6.lpr and B6.Sle2c1.lpr Tregs to suppress the invitro proliferation of B6 effector CD4+ T cells was compared to B6 Tregs. Both lpr strains showed very poor suppressive functions, even at a 1:1 Teff:Treg ratio (Fig. 3D). B6.Sle2c1.lpr Tregs performed significantly worse than B6.lpr Tregs (Fig. 3D), indicating that Sle2c1 expression further impaired the function of B6.lpr Tregs. Therefore, the Sle2c1 locus affected both the percentage and function of Tregs. A protective role of Tregs has been demonstrated in the (NZB × NZW)F1 model (16), but not in the NZM2410 model. To assess whether autoimmune phenotypes could also be prevented by Tregs in this model, B6.Sle1.Sle2.Sle3 (B6.TC) mice were treated with syngeneic Tregs at 2 mo of age and followed for 10 weeks. Treg-treated B6.TC mice showed a significantly reduced lymphoid expansion (Fig. 3E) and production of autoantibodies (Fig. 3F) as compared to controls. This indicated that the defective Treg population in B6.TC mice, to which the Sle2c1 locus contributes, plays a role in this model autoimmune pathology.
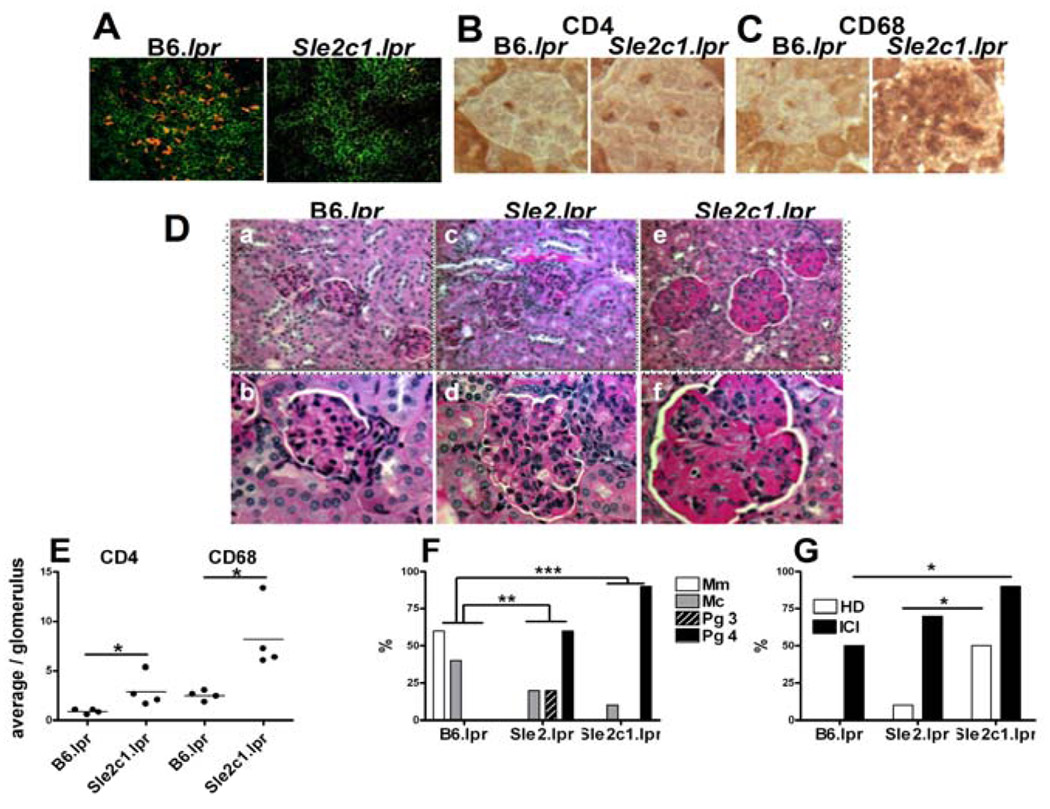
Figure 2.
Sle2c1 expression skews CD4+ T cell polarization and induces kidney pathology. Representative stains performed on 4–6 mo tissue sections. A. CD4-FITC and Foxp3-PE immunofluorescence staining of B6.lpr and B6.Sle2c1.lpr spleens. CD4+ T cell (B) and CD68+ macrophage (C) glomerular infiltrates 1000× original amplification. D. Kidneys with PAS stain at 400× (a, c and e) and 1000× (b, d and f) original magnification. a–b B6.lpr kidney showed none to mild evidence of inflammation and mesangial matrix expansion. c–d. B6.Sle2.lpr kidney showed global glomerular enlargement and hypercellularity involving most of the glomeruli. Focal interstitial inflammation was also present (c, right center).e–f. B6.Sle2c1.lpr kidney showed glomerular hypercellularity with increased glomerular enlargement and large hyaline deposits, as well as interstitial inflammation. E. Average number of CD4+ T cells or CD68+ macrophages per glomerulus. F. Percentage of mice showing Mm, Mc, or Pg 3 and 4 glomerulonephritis scores. G. Percentage of mice showing renal HD and ICI. ForFand G, significance corresponds to chi-square tests calculated on absolute numbers in each category for 10 mice per strain.*: p < 0.05, **: p < 0.01; ***: p < 0.001.
Figure 3.
T cell polarization and renal pathology are enhanced by Sle2c1 expression.CD4+ Foxp3+ staining in CD4+ gated cells withrepresentative FACS plots (A) and quantitation in B6.lpr, B6.Sle2.lpr and B6.Sle2c1.lpr(B), and Fas+ B6 and B6.Sle2c1(C) spleens.D. Inhibition ratios of B6 Teff proliferation by B6, B6.lpr or B6.Sle2c1.lpr Tregs at a 1:1 ratio. The values for B6 Tregs were significantly lower than that of either other two strains (p < 0.001).E. B6.TC average spleen weight 10 wks after injection of syngenic Tregs or saline (PBS). F. Anti-dsDNA and anti-chromatin IgG in B6.TC mice injected with syngenic Tregs or saline. Statistical significance corresponds to t test between Treg and saline treated mice for a given autoantibody and time point. G. Representative IL-17A staining of B6.lpr (thin line) and B6.Sle2c1.lpr (thick line) CD4+ T cells and corresponding quantitation on the right. H. INFγ production in splenic CD4+ T cells from B6.lpr and B6.Sle2c1.lpr mice. Datawere obtained from 2 mo old mice.*: p < 0.05, **: p < 0.01; ***: p < 0.001.
Conversely, more IL-17A+ (Fig. 3G) and less IFNγ+ (Fig. 3H) CD4+ T cellswere found in B6.Sle2c1.lpr than in B6.lpr spleens, resulting a significantly skewedIFNγ/IL-17 ratio (0.38±0.14 vs. 1.94 ± 0.18, respectively, p < 0.0001). The IFNγ/IL-17 ratio was however similar between Fas-intact B6.Sle2c1 and B6 CD4+ T cells (data not shown). These results demonstrate that Sle2c1 expression results in a reduced Treg compartment, and that in combination with lpr, it further skews CD4+ T cell polarization in favor of a TH17 over TH1 phenotype.
Sle2c1 induces autoimmune renal pathology in Fas-deficient mice
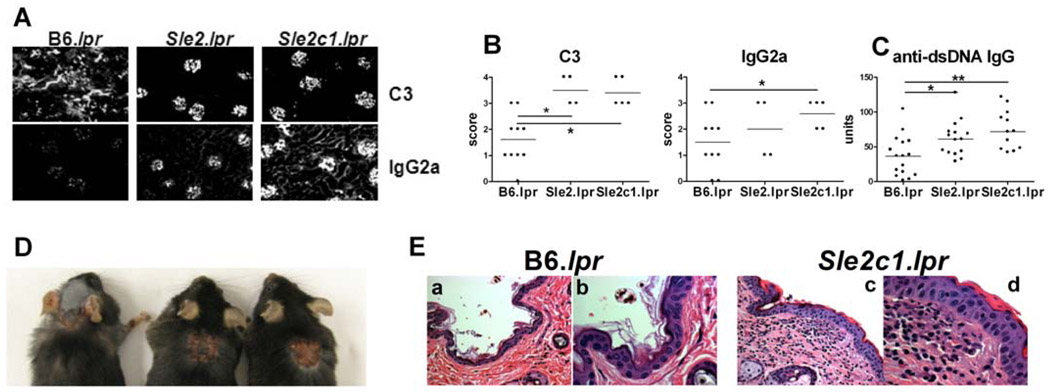
B6.Sle2 mice do not present any autoimmune pathology (2). The expansion of DNT cells (Fig. 1) and imbalance between regulatory and inflammatory T cells (Fig. 3) that resulted from the co-expression of Sle2c1 and lpr prompted us to examine therenal pathology of these mice. In 4–6 mo old mice, proteinuria was relatively modest but significantly higher in B6.Sle2c1.lpr than in B6.lpr mice (score: 1.31 ± 0.48 vs. 0.12 ± 0.13, respectively p < 0.05). No difference was found between B6.Sle2.lpr and B6.lpr.CD4+ T cell and CD68+ macrophage infiltration (Fig. 2B, C, and E) and C3-IgG2a immune complex deposits (Fig. 4A and B) were significantly heavier in B6.Sle2c1.lpr than in B6.lpr glomeruli. B6.Sle2.lpr and B6.Sle2c1.lpr kidneys showed significant levels of proliferative glomerulonephritis as compared to B6.lpr kidneys, in which minimal mesangial changes were observed (Fig. 2D and F). In addition, a significant percentage of B6.Sle2c1.lprkidneys showed hyaline deposits (HD) and insterstitial chronic inflammation (ICI, Fig. 2D and G). Finally, Sle2c1 enhanced significantly, but relatively modestly, the lpr-mediated production of anti-dsDNA IgG (Fig. 4C). Overall, as for lymphoid expansion, the renal pathology was significantly more severe and the production of anti-dsDNA IgG was greater inB6.Sle2c1.lprthan in B6.Sle2.lpr mice, both of which were significantly more affected than B6.lpr mice. Finally, dermatitis reminiscent of the lesions described in MRL/lpr mice (17)was observed in about 20% (13/56) of the B6.Sle2c1.lpr mice, but in none of either B6.lpr or B6.Sle2.lpr mice (Fig. 4D and E, and data not shown). As expected, the B6.lpr skin was grossly and microscopically unremarkable (Fig. 4 Ea and b). In contrast, the skin of affected B6.Sle2c1.lpr mice showed three concentric rings of inflammation and injury. The central zone showed a necrotizing ulcer to the papillary dermis with a crust. This was underlain with acute and chronic inflammation to the subcutis and lateral extension with follicular plugging (not shown). Third, there was a ring composed of interface dermatitis (Fig. 4 Ec and d), lymphocytic epidermotropism, focal spongiosis and vacuolar degeneration of the basal layer. Overall these results showed that the expression of Sle2c1 promotes autoimmune pathology in Fas-deficient mice.
Figure 4.
Sle2c1 promotes glomerular immune complex deposits and skin pathology in Fas-deficient mice. A and B. C3 and IgG2a deposits in B6.lpr and B6.Sle2.lprkidneys. Representative immunofluorescence sections and quantitation. C. Serum anti-dsDNA IgG. D. Representative B6.Sle2c1.lpr mice showing interscapular dermatitis. E. Representative B6.lprskin (a–b)showing normal histology; andB6.Sle2c1.lprskin showing thickening of the epidermis, edema and inflammation of the papillary and reticular dermis (c). A higher magnification (d) shows parakeratosis and lymphocytic epidermotropism (arrow heads) and a mixed infiltrate in the papillary dermis with fibrinoid change along the basement membrane. H&E stains, a–c: 400× and b–d: 1000× original magnification. *: p < 0.05, **: p < 0.01.
Sle2c1 induced renal infiltrates of inflammatory T cells
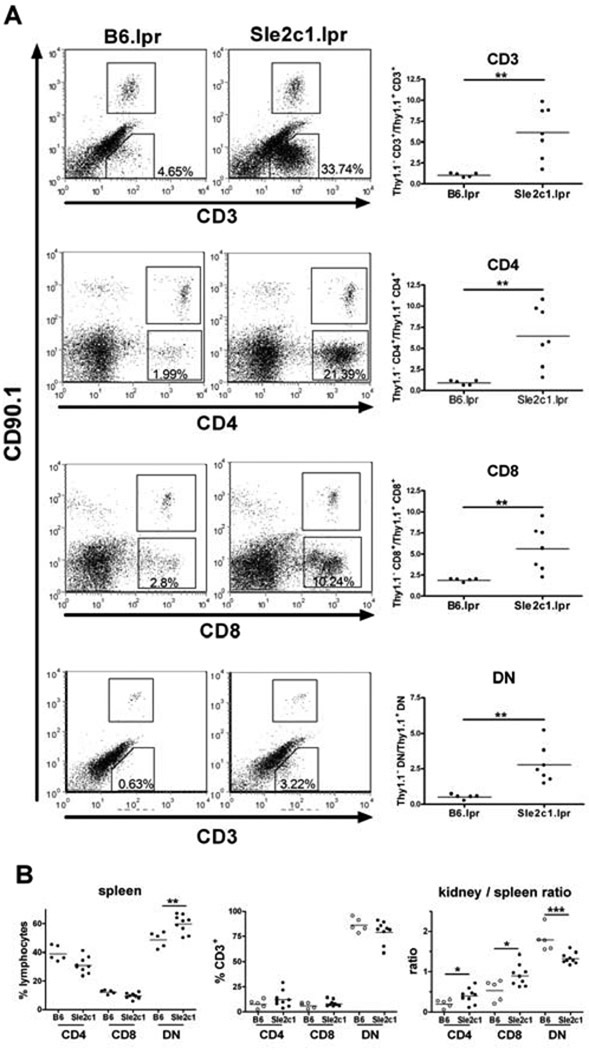
We further investigated the nature of the kidney infiltrating cells by flow cytometry. B6.Sle2c1.lpr kidneys contained about 5 times more T cells than B6.lpr kidneys (Fig. 5A),but no difference was found forthe number of B cells (data not shown). CD4+,CD8+ and DNTCD3+ cellswere all present in significantly higher numbers in B6.Sle2c1.lpr than in B6.lpr kidneys, but thedistribution of these subsets was not different between the kidneys of the two strains (Fig. 5A and B). Since the percentage of DNT cells was higher in the B6.Sle2c1.lprthan B6.lprspleens (Fig. 1C), it resulted in a significantly decreased DNTcell population in the kidneys over the spleens (kidney / spleen ratio) of B6.Sle2c1.lpr as compared to B6.lpr mice. In contrast, the proportion of CD4+ and CD8+ T cells was significantly higher in the B6.Sle2c1.lpr than B6.lpr kidneys relative to their spleens (Fig. 5B). Although the different method of lymphocyte isolation for the two tissues (RBC lysis vs. collagenase digestion) may be a source of variability, this data indicate that Sle2c1 promotes T cell infiltration in the kidneys of Fas-deficient mice. Although the majority of these T cells were DNTs, this T cell subset was not preferentially found in the renal tissue as compared to lymphoid organs.
Figure 5.
T cells infiltrate the B6.Sle2c1.lpr kidneys.A. Representative FACS plots and quantitation of CD3+, CD4+, CD8+, and DN T cells in the kidneys of B6.lpr and B6.Sle2c1.lpr mice. The numbers shown in each FACS plot correspond to the percentages of each endogenous T cell subset in the lymphocyte gate. The absolute numbers of each T cell population was expressed as a ratio to the CD90.1 (Thy1a) T cells contained in the B6.Cg-Igha.Thy1a.Gpi1acells added to each kidney preparation for normalization. B. Percentage of T cell subsets in the kidney expressed as percentages of CD3+ cells, corresponding absolute numbers per kidney, and kidney/spleen T cell ratios. White and black symbols represent B6.lpr and B6.Sle2c1.lpr, respectively. *: p < 0.05; **: p < 0.01; ***: p < 0.001.
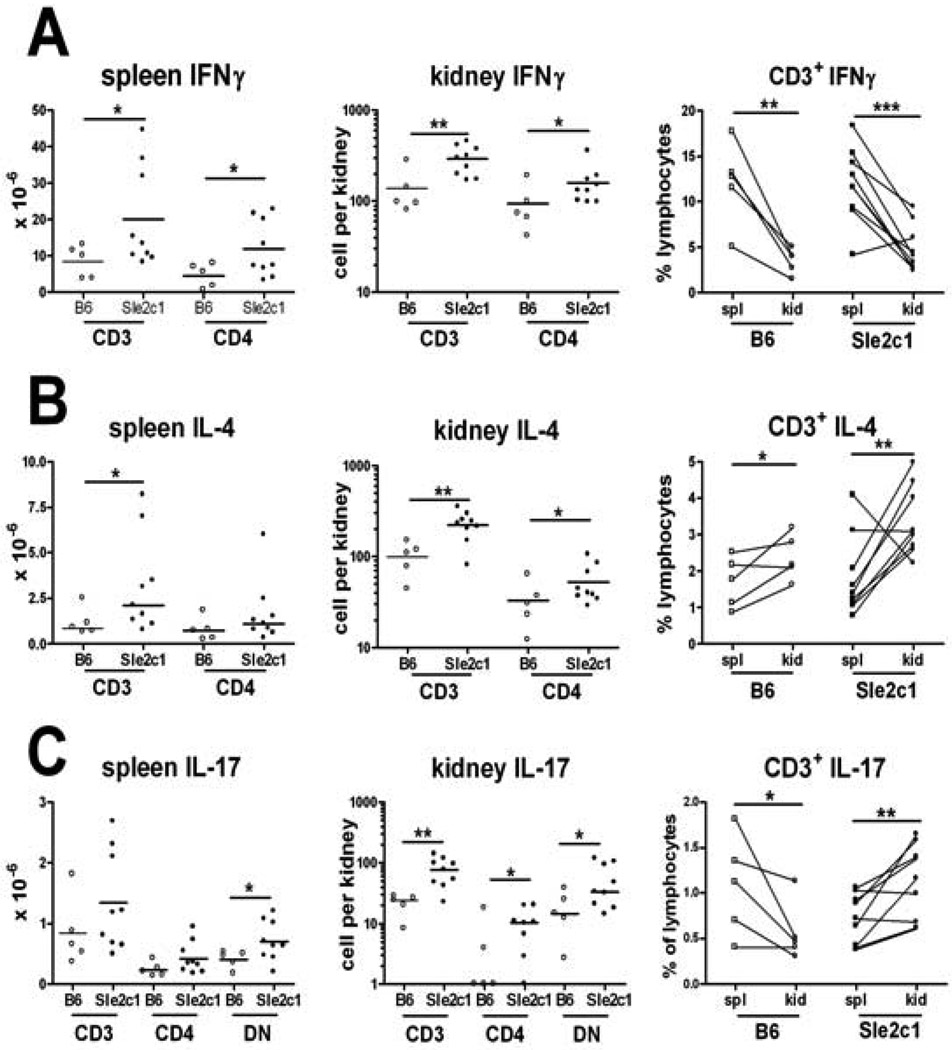
There were more IFNγ+ (Fig. 6A), IL-4+ (Fig. 6B) and IL-17A+ (Fig. 6C) T cells infiltrating the B6.Sle2c1.lpr than the B6.lpr kidneys. The relative distribution of these cells in the renal tissue as compared to the lymphoid organs was however different between cytokines. The number of IFNγ+ T cellswas also higher in the B6.Sle2c1.lpr than the B6.lpr spleens, but these cells were relatively excluded from the kidneys in both strains (Fig. 6A). In spite of this relative exclusion, the large number of INFγ producing T cells in the B6.Sle2c1.lpr kidneysis likely to contribute to renal pathology. On the opposite, IL-4+ T cells were preferentially recruited to both B6.Sle2c1.lpr and B6.lpr kidneys, in which they represent a higher percentage of the lymphocytes than in the spleens (Fig. 6B). Finally, the tissue distribution of IL-17A+ T cells was significantly different between the two strains, with their relative exclusion from the B6.lpr kidneys but their relative enrichment in the B6.Sle2c1.lpr kidneys (Fig. 6C). Although there were more IL-17A+ DNT than IL-17A+CD4+ T cells in the B6.Sle2c1.lpr kidneys (Fig. 6C), the renal enrichment of these two subsets was similar (data not shown), excluding a preferential effect of Sle2c1 on one subset over the other.Overall, these results show that the expression of Sle2c1 inFas-deficient miceleads heavy renal infiltrates with a relative enrichment of TH17 cells.
Figure 6.
Sle2c1 preferentially targets IL-4+ and IL-17+ T cells to the kidney Fas-deficient mice. Absolute numbers of INFγ+ (A), IL-4+ (B), and IL-17A+ (C) T cells in the spleen and kidneys, gated as total CD3+ and CD3+ CD4+ (and DN in panels C) cells. The graphs on the right show the distribution of CD3+ lymphocytes between the spleen and kidney of individual mice. *: p < 0.05; **: p < 0.01; ***: p < 0.001.
Discussion
Lupus pathogenesis results from the sequential expression of genes belonging to three main pathways, loss of tolerance to nuclear antigens, immune regulation, and tissue damage(18). Both Sle2 and lprhave been placed in the second pathway, and their independent expression in B6 mice is not sufficient to induce significant renal pathology (2;11). We show here that the co-expression of these two lupus susceptibility loci resulted in an enhanced lymphoproliferation with a greater impact on T cells than other hematopoietic cells, and in renal pathology associated with heavy T cell infiltrates skewed toward TH17 polarization. Furthermore, we have mapped the synergy between Sle2 and lpr to Sle2c1,and showed that the phenotypes of B6.Sle2c1.lprmice were consistently greater than that of B6.Sle2.lpr mice. This showed that Sle2c1 was the only contributor to the enhanced T cell phenotypes and related pathology observed in B6.Sle2.lpr mice. It also suggested the presence of a suppressor locus within Sle2, which we have located through independent experiments in a region telomeric to Sle2c1 (Xu et al. unpublished).We hypothesize that Sle2c1 enhances lymphadenopathy by functionally inactivating the residual Fas expression (11) in B6.lpr mice, either by increasing cell proliferation or further decreasing apoptosis. Independent experiments suggest that the former mechanism is involved (Xu et al., submitted).
Sle2c1has been characterized as the main contributor to the expanded B1a cell population in B6.Sle2 mice (10). B1a cells have long been associated with autoimmunity, but their specific contribution to autoimmune pathogenesis has been controversial(5). NZM2410 B1a cells have enhanced antigen-presenting functions (19) that may contribute to autoimmunity.Recent studies have indicated a novel link between B1a cells and autoimmune pathogenesis, with antigen presentation by B1a cells favoring TH17 differentiation, contrary to B2 cells favoring Treg differentiation (7). Here we show that Sle2c1 expression was associated with a Treg number reduction, whichwas amplified by Fas-deficiency. Co-expression of lpr and Sle2c1further polarized T cells toward IL-17 production and infiltration in the kidneys. Sle2c1was also associated with large numbers of IFNγ producing T cells in the lymphoid organs and kidneys, and the relative contribution of TH1 and TH17 in the renal pathology of these mice will have to be determined by additional experiments. Overall these combined results suggest a causal relationship between the B1a expansion and the altered T cell homeostasis, with an accumulation of B1a cells progressively skewing T cell differentiation away from the Treg in favor of the TH17 subset. It is possible, however, that B1a cell expansion and TH17 polarization are mediated by different genes within Sle2c1, a question that will be resolved through the on-going characterization of Sle2c1 recombinant strains.
Regardless of the causal link between B1a cells and alterations in T cell homeostasis, our study reports a novel association between an NZB-derived genetic locus and skewed Treg/TH17 subsets. Reduced numbers of Tregs and/or defective Treg functions have been described in lupus patients(20), and Treg transfers offer a protective effect in (NZB × NZW)F1(16), MRL/lpr(21) and B6.TC (this report) mice. Two other NZM2410 loci, Sle1a and Sle1c, are associated with a reduced Treg compartment, coincidental with the production of chromatin-specific autoreactive T cells(22–24). Sle2c1 does not regulate tolerance to nuclear antigens, and our results suggest that its effect on autoimmune pathogenesis reside in its promotion of TH17 polarization. Increasing evidence have emerged that IL-17 promotes disease in SLE patients and as well as in murine models of lupus (25). Particularly relevant to our results, TH17 T cells are found in the kidneys of patients with lupus nephritis (26) and in the kidneys of MRL/lpr mice (27). Moreover, in vitro polarized TH17 T cells induce nephritis in lymphopenic non-autoimmune mice (27;28). Finally, deficiency in IL-23R expression, which is necessary for TH17 polarization, prevented T cell expansion and renal pathology in B6.lpr mice (29). Our results suggest that the expansion of TH17 T cells in the lymphoid organs of B6.Sle2c1.lpr and their disproportionate migration to the kidneys contributed to the renal pathology found in these mice.
Although conventional CD4+T cells were affected, CD4− CD8−DNT cells were proportionally more expanded by the co-expression of Sle2c1 and lpr. DNT cells are the most abundant in Fas-deficient mice, but they are also expanded in the peripheral blood of lupus patients in which they present an activated phenotype(30), produce cytokines including IL-17, and infiltrate the kidneys(26). DNT cells were the most abundant lymphocytes in the B6.Sle2c1.lpr kidneys but their percentage or absolute numbers were similar to that of B6.lpr kidneys. To the contrary, single positive T cells were relatively enriched in B6.Sle2c1.lpr kidneys. These results indicate that Sle2c1 does not play a specific role in DNT cell functions, and its relative contribution to the pathogenicity of DNT vs. CD4+ Fas-deficient T cell subsets will have to be determined by adoptive transfers.
Sle2 synergizes with lpr to enhance lymphadenopathy and renal pathology, but in a different manner than what was previously reported for Sle1 (12). The lymphadenopathy in B6.Sle2.lpr mice primarily targeted T cells, while B6.Sle1.lpr mice suffered from a generalized lymphadenopathy. The renal pathology of B6.Sle2.lpr mice with massive cellular infiltrates composed primarily of T cells and macrophages, differed from the largely immune complex-mediated glomerulonephritis in B6.Sle1.lpr mice. In addition, skin pathology with lymphocytic infiltrates was found in the B6.Sle2c1.lpr but not in B6.Sle1.lpr mice. Within Sle1, the Sle1b sub-locus, which is associated with the production of large amounts of autoAbs, was largely responsible for the synergy with lpr (31). To the contrary, the Sle2c1 sublocusis not by itself associated with the production of autoAbs and its expression only increased modestly the production of anti-dsDNA IgG in B6.lpr mice. The disparity of the B6.Sle1.lpr and B6.Sle2c1.lprmodels is associated with a differentrenal pathology, which supports the hypothesis that there are two or more immunologic and inflammatory mechanisms producing severe human lupus nephritis. The classic and somewhat more prevalent form (WHO Class IV) is characterized by heavy glomerular immune complex deposits and complement activation, whereas an alternant form (WHO Class III severe and ISN Class IV-S) in which cellular immunity plays a greater role has features reminiscent of small vessel vasculitis with more prominent and segmental fibrinoid necrosis, relative paucity of immune complexes and increased frequency of anti-neutrophil cytoplasmic antibodies(32–34). Thus there is the likelihood that different mouse models of lupus nephritis such as the B6.Sle1.lpr and B6.Sle2c1.lpr strains represent valuable systems to examine the varied mechanisms of tissue injury, genetics and more specific therapeutic modalities(35).
Acknowledgements
This work was supported by National Institutes of Health grants RO1 AI068965to LM and K01 AR056725-01 to ZX.We thank Leilani Zeumer for excellent technical help, Xuekun Su and Nathan Weinstock for outstanding animal care, and the members of the Morel lab for stimulating discussions.
Abbreviations used in this paper
- BCR
B cell receptor
- B6
C57BL/6
- Ab
antibody
- TH1
T helper 1
- TH17
IL-17 producing T helper cells
- DN T cells
CD4− CD8− double negative T cells
- Treg
CD4+ CD25+ Foxp3+ regulatory T cells
- LN
lymph node
- H&E
hemotoxylin and eosin
- PAS
periodic acid Schiff
Footnotes
Disclosures
The authors have no financial conflict of interest.
References
- 1.Morel L, Rudofsky UH, Longmate JA, Schiffenbauer J, Wakeland EK. Polygenic control of susceptibility to murine systemic lupus erythematosus. Immunity. 1994;1:219–229. doi: 10.1016/1074-7613(94)90100-7. [DOI] [PubMed] [Google Scholar]
- 2.Mohan C, Morel L, Yang P, Wakeland EK. Genetic dissection of systemic lupus erythematosus pathogenesis - Sle2 on murine chromosome 4 leads to B cell hyperactivity. J Immunol. 1997;159:454–465. [PubMed] [Google Scholar]
- 3.Morel L, Croker BP, Blenman KR, Mohan C, Huang G, Gilkeson G, et al. Genetic reconstitution of systemic lupus erythematosus immunopathology with polycongenic murine strains. Proc Natl Acad Sci U S A. 2000;97:6670–6675. doi: 10.1073/pnas.97.12.6670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu Y, Li L, Kumar KR, Xie C, Lightfoot S, Zhou XJ, et al. Lupus susceptibility genes may breach tolerance to DNA by impairing receptor editing of nuclear antigen-reactive B cells. J Immunol. 2007;179:1340–1352. doi: 10.4049/jimmunol.179.2.1340. [DOI] [PubMed] [Google Scholar]
- 5.Duan B, Morel L. Role of B-1a cells in autoimmunity. Autoimmun Rev. 2006;5:403–408. doi: 10.1016/j.autrev.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 6.Zhong X, Lau S, Bai C, Degauque N, Holodick NE, Steven SJ, et al. A novel subpopulation of B-1 cells is enriched with autoreactivity in normal and lupus-prone mice. Arthritis Rheum. 2009;60:3734–3743. doi: 10.1002/art.25015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhong X, Gao W, Degauque N, Bai C, Lu Y, Kenny J, et al. Reciprocal generation of Th1/Th17 and T(reg) cells by B1 and B2 B cells. Eur J Immunol. 2007;9:2400–2404. doi: 10.1002/eji.200737296. [DOI] [PubMed] [Google Scholar]
- 8.Morshed SR, Mannoor K, Halder RC, Kawamura H, Bannai M, Sekikawa H, et al. Tissue-specific expansion of NKT and CD5+B cells at the onset of autoimmune disease in (NZB×NZW)F1 mice. Eur J Immunol. 2002;32:2551–2561. doi: 10.1002/1521-4141(200209)32:9<2551::AID-IMMU2551>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 9.Enghard P, Humrich JY, Chu VT, Grussie E, Hiepe F, Burmester GR, et al. Class switching and consecutive loss of dsDNA reactive B1a B cells from the peritoneal cavity during murine lupus development. Eur J Immunol. 2010 doi: 10.1002/eji.200940050. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 10.Xu Z, Duan B, Croker BP, Wakeland EK, Morel L. Genetic dissection of the murine lupus susceptibility locus Sle2: contributions to increased peritoneal B-1a cells and lupus nephritis map to different loci. J Immunol. 2005;175:936–943. doi: 10.4049/jimmunol.175.2.936. [DOI] [PubMed] [Google Scholar]
- 11.Cohen PL, Eisenberg RA. The Lpr and Gld genes in systemic autoimmunity - Life and death in the Fas lane. Immunology Today. 1992;13:427–428. doi: 10.1016/0167-5699(92)90066-G. [DOI] [PubMed] [Google Scholar]
- 12.Shi XY, Xie C, Kreska D, Richardson JA, Mohan C. Genetic dissection of SLE: SLE1 and FAS impact alternate pathways leading to lymphoproliferative autoimmunity. J Exp Med. 2002;196:281–292. doi: 10.1084/jem.20010955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morel L, Yu Y, Blenman KR, Caldwell RA, Wakeland EK. Production of congenic mouse strains carrying genomic intervals containing SLE-susceptibility genes derived from the SLE-prone NZM2410 strain. Mammalian Genome. 1996;7:335–339. doi: 10.1007/s003359900098. [DOI] [PubMed] [Google Scholar]
- 14.Fantini MC, Dominitzki S, Rizzo A, Neurath MF, Becker C. In vitro generation of CD4+CD25+ regulatory cells from murine naive T cells. Nat Protocols. 2007;2:1789–1794. doi: 10.1038/nprot.2007.258. [DOI] [PubMed] [Google Scholar]
- 15.Xu Z, Duan B, Croker BP, Morel L. STAT4 deficiency reduces autoantibody production and glomerulonephritis in a mouse model of lupus. Clin Immunol. 2006;120:189–198. doi: 10.1016/j.clim.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 16.Scalapino KJ, Tang Q, Bluestone JA, Bonyhadi ML, Daikh DI. Suppression of disease in New Zealand Black/New Zealand White lupus-prone mice by adoptive transfer of ex vivo expanded regulatory T cells. J Immunol. 2006;177:1451–1459. doi: 10.4049/jimmunol.177.3.1451. [DOI] [PubMed] [Google Scholar]
- 17.Furukawa F, Tanaka H, Sekita K, Nakamura T, Horiguchi Y, Hamashima Y. Dermatopathological studies on skin lesions of MRL mice. Arch Dermatol Res. 1984;276:186–194. doi: 10.1007/BF00414018. [DOI] [PubMed] [Google Scholar]
- 18.Wakeland EK, Wandstrat AE, Liu K, Morel L. Genetic dissection of systemic lupus erythematosus. Curr Opin Immunol. 1999;11:701–707. doi: 10.1016/s0952-7915(99)00039-4. [DOI] [PubMed] [Google Scholar]
- 19.Mohan C, Morel L, Yang P, Wakeland EK. Accumulation of splenic B1a cells with potent antigen-presenting capability in NZM2410 lupus-prone mice. Arthritis Rheum. 1998;41:1652–1662. doi: 10.1002/1529-0131(199809)41:9<1652::AID-ART17>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 20.Kuhn A, Beissert S, Krammer P. CD4+CD25+ regulatory T cells in human lupus erythematosus. Arch Dermatol Res. 2009;301:71–81. doi: 10.1007/s00403-008-0891-9. [DOI] [PubMed] [Google Scholar]
- 21.Yang CH, Tian L, Ling GS, Trendell-Smith NJ, Ma L, Lo CK, et al. Immunological mechanisms and clinical implications of regulatory T cell deficiency in a systemic autoimmune disorder: Roles of IL-2 versus IL-15. Eur J Immunol. 2008;38:1664–1676. doi: 10.1002/eji.200838190. [DOI] [PubMed] [Google Scholar]
- 22.Chen Y, Cuda C, Morel L. Genetic determination of T cell help in loss of tolerance to nuclear antigens. J Immunol. 2005;174:7692–7702. doi: 10.4049/jimmunol.174.12.7692. [DOI] [PubMed] [Google Scholar]
- 23.Cuda CM, Wan S, Sobel ES, Croker BP, Morel L. Murine lupus susceptibility locus Sle1a controls regulatory T cell number and function through multiple mechanisms. J Immunol. 2007;179:7439–7447. doi: 10.4049/jimmunol.179.11.7439. [DOI] [PubMed] [Google Scholar]
- 24.Cuda CM, Zeumer L, Sobel ES, Croker BP, Morel L. Murine lupus susceptibility locus Sle1a requires the expression of two subloci to induce inflammatory T cells. Genes Immun. 2010 doi: 10.1038/gene.2010.23. doi: 10.1038/gene.2010.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nalbandian A, Crispin JC, Tsokos GC. Interleukin-17 and systemic lupus erythematosus: current concepts. Clin Exp Immunol. 2009;157:209–215. doi: 10.1111/j.1365-2249.2009.03944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crispin JC, Oukka M, Bayliss G, Cohen RA, Van Beek CA, Stillman IE, et al. Expanded double negative T cells in patients with systemic lupus erythematosus produce IL-17 and infiltrate the kidneys. J Immunol. 2008;181:8761–8766. doi: 10.4049/jimmunol.181.12.8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Z, Kyttaris VC, Tsokos GC. The role of IL-23/IL-17 axis in lupus nephritis. J Immunol. 2009;183:3160–3169. doi: 10.4049/jimmunol.0900385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Summers SA, Steinmetz OM, Li M, Kausman JY, Semple T, Edgtton KL, et al. Th1 and Th17 cells induce proliferative glomerulonephritis. J Amer Soc Nephrol. 2009;20:2518–2524. doi: 10.1681/ASN.2009030337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kyttaris VC, Zhang Z, Kuchroo VK, Oukka M, Tsokos GC. Cutting Edge: IL-23 receptor deficiency prevents the development of lupus nephritis in C57BL/6-lpr/lpr mice. J Immunol. 2010 doi: 10.4049/jimmunol.0903595. doi:10.4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anand A, Dean GS, Quereshi K, Isenberg DA, Lydyard PM. Characterization of CD3+ CD4− CD8− (double negative) T cells in patients with systemic lupus erythematosus: activation markers. Lupus. 2002;11:493–500. doi: 10.1191/0961203302lu235oa. [DOI] [PubMed] [Google Scholar]
- 31.Croker BP, Gilkeson G, Morel L. Genetic interactions between susceptibility loci reveal epistatic pathogenic networks in murine lupus. Genes Immun. 2003;4:575–585. doi: 10.1038/sj.gene.6364028. [DOI] [PubMed] [Google Scholar]
- 32.Hill GS, Delahousse M, Nochy D, Bariety J. Class IV-S versus class IV-G lupus nephritis: Clinical and morphologic differences suggesting different pathogenesis. Kidney Int. 2005;68:2288–2297. doi: 10.1111/j.1523-1755.2005.00688.x. [DOI] [PubMed] [Google Scholar]
- 33.Schwartz MM, Korbet SM, Lewis EJ for the Collaborative Study Group. The prognosis and pathogenesis of severe lupus glomerulonephritis. Nephrol Dial Transplant. 2008;23:1298–1306. doi: 10.1093/ndt/gfm775. [DOI] [PubMed] [Google Scholar]
- 34.Sen D, Isenberg DA. Antineutrophil cytoplasmic autoantibodies in systemic lupus erythematosus. Lupus. 2003;12:651–658. doi: 10.1191/0961203303lu456rr. [DOI] [PubMed] [Google Scholar]
- 35.Glassock RJ. Multitarget therapy of lupus nephritis: base hit or home run? J Am Soc Nephrol. 2008;19:1842–1844. doi: 10.1681/ASN.2008070779. [DOI] [PubMed] [Google Scholar]