Abstract
Purpose
Reducing dosing demands of medications generally increases adherence, although this relationship has not been demonstrated with the once-monthly oral bisphosphonates (BP). The study aim is to test whether switching from once-weekly BPs to once-monthly BPs improves adherence and fracture risk.
Methods
This is an interrupted times-series analysis of new users of once-weekly BPs in a nationwide administrative health database from 2003–2007. Participants include 1835 individuals who switched to once-monthly BPs and two propensity-matched comparator groups: 1835 individuals who switched to a different once-weekly BP, and 1835 who did not switch. We measured changes in adequate adherence pre- and post-switch as monthly medication possession ratio >0.80, and calculated incidence rate ratios [IRR] of osteoporotic fractures.
Results
All study groups experienced major adherence failure in the first year of therapy: the proportion of adequate adherers was 42% among once-monthly switchers, 47% among once-weekly switchers, and 37% among nonswitchers. However, the once-monthly switch was associated with less adherence failure (4% fewer adherers per month pre-switch vs. 1% fewer adherers per month post-switch, p<.000). There was no statistically significant change in adherence rates for the other groups. We did not detect significantly reduced fracture risk with once-monthly switch: 1 year post-switch, the fracture incidence risk ratios for once-monthly switchers relative to once-weekly switchers were IRR 0.83, 95% CI: 0.50–1.36, and IRR 0.90, 95% CI: 0.54–1.49, relative to nonswitchers).
Conclusions
Reducing the dosing demands of oral bisphosphonates from once-weekly to once-monthly decreased adherence failure but had an uncertain impact on fracture risk.
Keywords: patient compliance, bisphosphonates, osteoporosis, osteoporotic fractures, medication adherence
Introduction
Most patients who initiate a medication for osteoporosis discontinue therapy within 1 year or take too little of the therapy to achieve any benefit.1–3 One strategy for addressing this problem has been to reduce the dosing demands of the oral bisphosphonates, first through a once-weekly regimen and then most recently through a once-monthly regimen, available since April 2005.4 The once-monthly ibandronate sodium is a bisphosphonate, a class of drugs that inhibit bone resorption and are commonly prescribed for the treatment and prevention of osteoporosis in postmenopausal women.5, 6 The efficacy and safety of once-monthly bisphosphonates were demonstrated in a 1-year, double-blind study of postmenopausal women with osteoporosis whose treatment with 150 mg once-monthly ibandronate (n=327) was shown to be noninferior to 2.5 mg daily ibandronate (n=318) in increasing the bone mineral density in the lumbar spine.7, 8
Recent research has generally failed to find any additional adherence benefit with the monthly regimen over the weekly regimens, however selection bias may explain these results.9–11 The once-monthly bisphosphonates may be preferentially channeled to patients with a poorer prognosis for adequate adherence, although the evidence for this bias is not established.10 Given that treatment assignment is not randomly assigned in observational studies, one approach for isolating the impact of once-weekly versus once-monthly regimens is to compare measures of effectiveness in individuals who are exposed to both formulations; this is the case when individuals switch from one dosing regimen to another.
Studying changes in the adherence of patients who switch dosing regimens offers several advantages. First, adherence with alternative dosing regimens is measured in the same individual, thus eliminating the confounding that occurs when comparing outcomes in different individuals who may vary in many health behaviors besides taking medications.12 Second, the relationship between switching and adherence is poorly understood, since switchers are rarely analyzed as a separate group. Instead, switchers are excluded from study samples, their multiple therapies summed as one measure of overall adherence, or their adherence truncated at the switch.13–15 As a result, providers often make assumptions about improving adherence by switching therapy in the absence of clear evidence. In the one study that characterized the adherence of bisphosphonate switchers who changed from the once-daily to a once-weekly regimen, switchers attained a higher adherence rate than new users, but a lower adherence rate than prevalent users.16 There is no information about switching behavior and fracture outcomes.
The objective of this study was to test whether reducing the dose regimen of treatment with oral bisphosphonates increases adherence and decreases the risk of osteoporotic fractures. We hypothesized that there would be no difference in fracture risk after a dose regimen switch if there is no significant improvement in adherence.
Methods
Study population and data sources
This study used 2003–2007 MarketScan Commercial Claims and Encounters and Medicare Supplemental Databases (Medstat: Ann Arbor, MI). This database contains over 500 million claim records per year from individuals with private health care insurance. The data come from approximately 45 large employers who self-insure their employees and dependents. The MarketScan database offers advantages over raw administrative claims because data files undergo validity and editing procedures to ensure high quality and consistency in fields across years.17 The data are evaluated against population norms, previous year summaries, and validated data subsets. Outliers are flagged and reviewed for coding or processing errors. Encounter data are audited at the health plan level and plans submitting incomplete data are excluded. Diagnostic and procedural codes are compared against validity algorithms and set to missing values if inconsistent. The encounter files contain age, sex, geographic residence, and eligibility information. The prescription claims include the national drug codes, date of purchase, quantity dispensed, and days supply. The medical claims contain diagnoses, procedure codes, and type of provider. For this analysis, we merged together annual files to create a dataset of approximately 15 million people with up to 5 years of observation.
The study sample was drawn from a larger cohort study of incident oral bisphosphonate users (ibandronate, alendronate or risedronate; n=61,125) who were aged 50 years or older, had an osteoporosis diagnosis (ICD-9-CM 733.xx) and at least 2 years of observation.10 Incident use was defined as no bisphosphonate therapy for at least 12 months prior to initiating therapy. From this group, we identified 2,178 individuals who switched to a medication with a different regimen within the first year of therapy (termed “regimen switchers”). Individuals switching from once-monthly to once-weekly regimens (n=201), or from or to the once-daily regimens (n=142) were excluded due to small samples size and our assessment that these individuals differed considerably from the weekly to monthly dosing groups.10
We then matched the regimen switchers to two comparator groups, using propensity score methodology. The first comparator group consisted of individuals who remained on the same therapy throughout the study (termed “nonswitchers”). The second comparator group consisted of individuals who changed medications but the dose regimen remained the same, for example weekly alendronate to weekly risedronate (termed “drug-only switchers”). Our procedure for identifying the matched comparator groups was as follows.18 We estimated the probability of switching using a logistic regression with the following variables: age, sex, geographic residence, health plan type, any pre-period bone mineral density testing, serious gastro-intestinal risk19, osteoporotic fractures of the hip, wrist, or humerus, acute care hospitalizations, month and year of initiating bisphosphonate therapy, and a comorbidity risk score from the Diagnostic Cost Group Hierarchical Condition Category (DCG/HCC) classification system (DxCG, Boston, MA).20, 21 Diagnostics of the propensity score models showed adequate ability to distinguish dose regimen switchers from drug-only switchers and nonswitchers, as indicated by c-statistics of 0.7677 and 0.7325, respectively.22 From these models, we generated a propensity of score, which was used to match regimen switchers to both drug-only switchers and nonswitchers using a 1-1 nearest neighbor method without replacement.
Each individual in all three groups was assigned a switching month. For regimen switchers, the switching month was the first month when a new bisphosphonate medication was dispensed with a different regimen from the initial bisphosphonate. For the nonswitcher group (those who remained on the same therapy), we assigned a random switching month from the first 12 months while on therapy since all regimen switchers changed their therapy within that timeframe. For the second comparator group of drug-only switchers (those who changed from one weekly bisphosphonate to another weekly bisphosphonate), the switching month was the first month when a product with a different generic name was dispensed from the initial medication. Observations containing multiple switches (n=2) were truncated at the second switch.
Measures
The main study variable was the type of switch. Dosing regimen and drug-only switches were identified by comparing the generic drug name and the days supply field divided by the metric quantity for each dispensing of the study drugs. Outlier values were manually checked for error (<0.5% of patients), and individuals were assigned into mutually-exclusive dosing schedules based on set thresholds.
The dependent variables were osteoporotic fractures and adherence as measured by a monthly Medication Possession Ratio (MPR). We calculated the MPR based on the sum of the days supply of study medication dispensed during the month divided by the number of days in the month.23 Overlaps in the dispensing days were eliminated and the value of the days supply was truncated if the supply extended beyond the time period of observation. For the month of the switch, leftover supplies from earlier refills were discarded to begin the newer medication (e.g., a change in therapy). Adequate adherence was defined as a MPR ≥80%, although sensitivity analyses were conducted at a MPR ≥60%. Osteoporotic fractures were defined as fractures of the wrist, hip, or proximal humerus, as developed by previous research.24, 25 (The list of ICD-9 and CPT codes is available upon request). We did not count vertebral fractures due to the lack of access to radiographs to confirm an incident or pre-existing vertebral fracture.
Statistical analysis
We conducted descriptive analyses using monthly person-level data, for a total observation period of 156,559 person months (50,908 regimen switcher months; 53,178 drug-only switcher months; and 52,473 nonswitcher months). The monthly occurrence of any osteoporotic fracture and the monthly MPR were arrayed in relationship to whether they occurred before or after the switching month. Incidence rate ratios were calculated as the number of osteoporotic fractures per person-month time for each switching group. Interrupted times series estimation with segmented regression methods and autoregressive correlations of the first order were used for testing changes in the trend (slope and level) of achieving adequate adherence following the switch and adjusting for pre-switch adherence trends.26 The model included a constant summarizing the baseline level, and three terms. The first term estimated monthly changes per person in the pre-switch adherence trend, the second term estimated the average level change per person in the first month after the switch, and the third term was the post-switch trend relative to the pre-switch trend. Models were estimated separately for each group. Time-series analyses were conducted using the time-series commands in STATA 10.0. The institutional review board of the University of Massachusetts Medical School exempted this research from review.
Results
We identified 1835 individuals who switched from once-weekly to once-monthly dosing regimens. Before the propensity score match, dose regimen switchers differed from the two comparator groups (drug-only switchers and nonswitchers) across most characteristics (Table 1). After the propensity score match, the dosing switchers and the two comparator groups showed no statistically significant differences except for the following: comorbidity risk score (0.75 regimen switchers vs. 0.68 nonswitchers, p<.01); baseline hospitalization rates (15.9% regimen switchers vs. 12.9% nonswitchers, p<.02); and residence in the U.S. South (33.5% regimen switchers vs. 30.5% nonswitchers, p<.02).
Table 1.
Baseline Characteristics of Study Groups before and After PS Match
| Before PS Match | After PS Match | |||||
|---|---|---|---|---|---|---|
| Regimen Switchers | Comparator 1 Nonswitchers | Comparat or 2 Drug-only Switchers | Regimen Switchers | Comparator 1 Nonswitchers | Comparator 2 Drug-only Switchers | |
| No. of patients | 1835 | 57,987 | 5862 | 1835 | 1835 | 1835 |
| Age, mean | 63.6 | 64.4* | 64.9* | 63.6 | 63.6 | 64.2 |
| Female sex (%) | 94.3 | 91.0* | 91.5* | 94.3 | 94.4 | 93.2 |
| Comorbidity risk score, mean | 0.75 | 0.73 | 0.74 | 0.75 | 0.68* | 0.77 |
| Diagnoses/medical history | ||||||
| Any bone mineral density testing, (%) | 79.7 | 74.6* | 75.0* | 79.7 | 80.2 | 78.7 |
| Any serious GI risk, (%) | 3.4 | 3.2 | 3.5 | 3.4 | 2.8 | 3.9 |
| Any osteoporotic fracture, (%) | 3.1 | 3.7 | 4.0 | 3.1 | 3.2 | 2.9 |
| Any hospitalization (%) | 15.9 | 14.3 | 14.6 | 15.9 | 12.9* | 15.3 |
| Geographic residence (%) | ||||||
| North East | 9.2 | 9.8 | 10.2* | 9.2 | 8.9 | 8.8* |
| North Central | 40.3 | 37.7 | 39.4 | 40.3 | 41.1 | 43.5 |
| South | 34.6 | 34.1 | 27.9 | 34.6 | 33.5 | 30.5 |
| West | 15.8 | 18.3 | 22.4 | 15.8 | 16.5 | 16.9 |
| Type of health plan (%) | ||||||
| Comprehensive | 48.0 | 44.5 | 50.7* | 48.0 | 47.9 | 51.5 |
| Health maintenance organization | 8.1 | 9.3 | 7.5 | 8.1 | 8.1 | 8.6 |
| Point-of-service | 9.1 | 9.3 | 9.7 | 9.1 | 8.1 | 8.1 |
| Preferred provider organization | 34.7 | 36.4 | 31.9 | 34.7 | 35.8 | 31.7 |
| Year of Initiation | ||||||
| 2004 | 14.2 | 47.5* | 50.4* | 14.2 | 13.8 | 13.1 |
| 2005 | 85.8 | 52.2 | 49.6 | 85.8 | 86.2 | 86.9 |
| Month of Initiation | ||||||
| Jan | 5.1 | 9.0* | 9.8* | 5.1 | 5.5 | 5.3 |
| Feb | 5.9 | 8.1 | 8.9 | 5.9 | 5.3 | 5.4 |
| Mar | 7.0 | 9.3 | 10.4 | 7.0 | 6.9 | 6.9 |
| Apr | 6.9 | 8.5 | 9.4 | 6.9 | 7.6 | 7.3 |
| May | 7.9 | 8.5 | 8.9 | 7.9 | 7.8 | 7.4 |
| Jun | 8.7 | 8.3 | 8.0 | 8.7 | 8.2 | 8.2 |
| Jul | 9.3 | 8.0 | 7.0 | 9.3 | 8.6 | 8.9 |
| Aug | 9.1 | 8.7 | 8.2 | 9.1 | 8.9 | 9.4 |
| Sep | 9.8 | 7.9 | 7.6 | 9.8 | 9.7 | 10.0 |
| Oct | 9.8 | 8.0 | 7.5 | 9.8 | 10.5 | 9.9 |
| Nov | 8.8 | 8.1 | 7.3 | 8.8 | 9.6 | 9.9 |
| Dec | 11.8 | 7.8 | 7.0 | 11.8 | 11.6 | 11.6 |
p=<.05 relative to regimen switcher
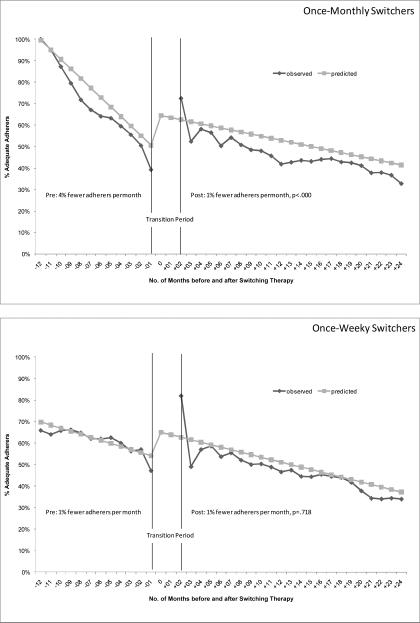
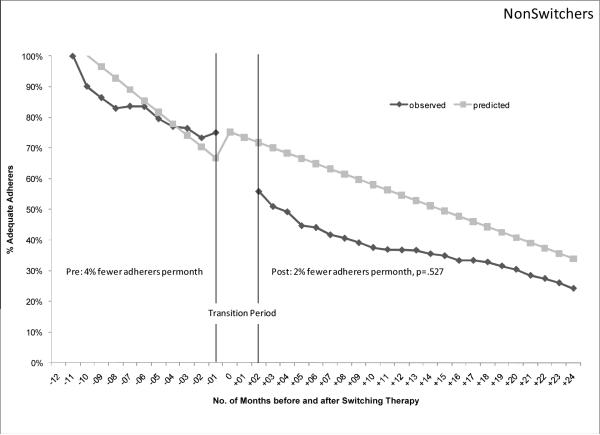
Figure 1 shows the observed and predicted proportion of patients achieving adequate adherence each month, 12 months before and 24 months after the therapy switch. In the top panel, the proportion of once-monthly switchers achieving adequate adherence decreased by 4 percentage points each month in the pre-switch period (95% confidence interval [CI], −0.06 – −0.03). After the regimen switch, the proportion of switchers achieving adequate adherence still declined each month but the failure rate was slower than before the switch (1% fewer adherers per month post-switch; 95% CI, −0.01 – −0.01; p<.000). One year post-switch, 42% of once-monthly switchers achieved adequate adherence. As a comparison in the second panel, the once-weekly switchers also exhibited a steady decline in adherence before the switch (1% fewer adequate adherers per month pre-switch, 95% CI, −0.03 – 0.00). However, after the switch, the rate of adherence failure remained the same as the pre-switch rate (1% fewer adequate adherers per month post-switch, 95% CI, −0.01 – −0.01;p=.718). One year post-switch, 47% of the once-weekly switchers achieved adequate adherence. The last comparison is among nonswitchers with a randomly assigned switch data. In the last panel, the adherence of nonswitchers also declined over time although the decline was not associated with the statistically-generated switch date (4% fewer adequate adherers per month, 95% CI, −0.03 – 0.00; p=.148). After the “switch”, the rate of adherence failure remained the same as the pre-switch rate (2% fewer adequate adheres per month, 95% CI, −0.04–.08; p=.527). Sensitivity analyses with adequate adherence set at MPR>=60% showed similar results.
Figure 1.
Proportion of Patients Achieving Adequate Adherence by Switching Behavior
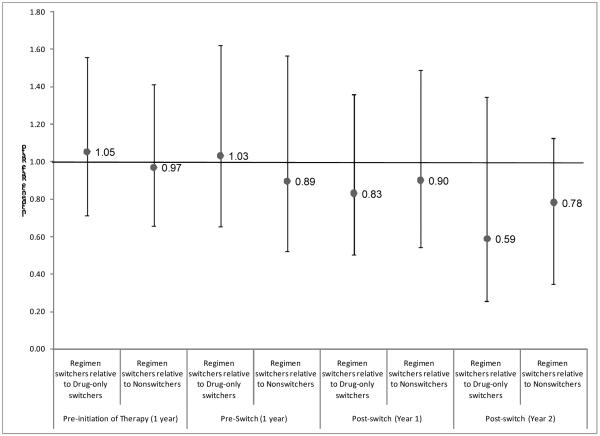
Overall, the study population experienced 425 osteoporotic fractures while observed: 142 in regimen switchers, 150 in drug-only switchers, and 133 in nonswitchers. Figure 2 shows the incidence rate ratios [IRR] of osteoporotic fractures by switching behavior. Before initiating therapy, regimen switchers had a similar risk of osteoporotic fractures as drug-only switchers (IRR 1.05, 95% CI 0.71–1.56) and nonswitchers (IRR 0.97, 95% CI 0.66–1.41). The similarity in risk of osteoporotic fractures for regimen switchers and the comparator groups persisted into the pre-switch period (IRR 1.03, 95% CI 0.66–1.62, relative to drug-only switchers; and IRR 0.89, 95% CI 0.52–1.57, relative to nonswitchers). The relative risk of osteoporotic fractures also did not change in the post-switch period, neither in the first year (IRR 0.83, 95% CI 0.50–1.36, relative to drug-only switchers; and IRR 0.90, 95% CI 0.54–1.49, relative to nonswitchers), nor in the second year (IRR 0.59, 95% CI 0.25–1.35, relative to drug-only switchers; and IRR 0.78, 95% CI 0.35–1.74, relative to nonswitchers).
Figure 2.
Relative Risk of Osteoporotic Fractures by Switching Behavior
Discussion
In this study of 1835 individuals who initiated once-weekly oral bisphosphonate therapy and then switched to a once-monthly dosing regimen, we found a statistically significant but clinically modest deceleration in the failure rates of adherence related to the therapy switch: the monthly rate of individuals achieving adequate adherence decreased from 4% fewer adherers per month pre-switch to 1% fewer adherers per month post-switch, p<.000). This deceleration in the adherence failure rate after a therapy switch did not occur in the drug-only switchers or nonswitchers. However, the benefits of a slower adherence failure rate are unclear since at 12 months after the switch date, only 41% of the regimen switchers achieved adequate adherence compared to 47% of drug-only switchers, and 37% of nonswitchers.
We detected no significant difference in the risk for osteoporotic fractures associated with the regimen switch. Relative to the risk of the comparator groups, the risk of osteoporotic fractures did not change for the regimen switchers in the first year or second year after the switch in therapy. This suggests that decelerating the adherence failure rate by switching the dosing regimen may not have translated to any improvement in fracture outcomes. This finding is in accordance with research showing compliance levels of less than 66% with osteoporosis therapy result in suboptimal improvement in bone density.3
Our adherence findings are somewhat inconsistent with the body of research showing that adherence is inversely proportional to the frequency of the dose.27 However, these studies are mainly drawn from studies comparing once-daily dosing to multiple-daily dosings. Once-weekly oral bisphosphonates have also been associated with higher adherence over the once-daily regimens, although overall adherence has remained suboptimal in that drug class.16, 28, 29 Between 52% to 87% of patients starting daily or weekly oral bisphosphonates discontinue the therapy within 1 year or do not fill enough prescriptions to cover 80% of a year of therapy.2, 16 Even early reports of patient compliance with the once-monthly bisphosphonate show only 57% remained on therapy after 6 months of therapy.30 Our study cannot explain the lackluster adherence benefits with the once-monthly regimen; however, the growing body of evidence shows that gastrointestinal adverse effects and patient beliefs rather than dosing complexity are the main reasons for nonadherence with this drug class.28, 31
Our study has several important limitations. First, we cannot assess the reasons for the switch in therapy or discontinued therapy, so some of our classification of noncompliance may have been in accordance with the advice of physicians. We also did not evaluate the use of estrogen therapy, etidronate, nasal calcitonin or intravenous bisphosphonates, so patients who switched to these therapies would have been considered noncompliant. Secondly, we do not know if patients actually took the medication, only that they acquired the medication. Thirdly, the adherence patterns of the once-monthly regimen come from the first year of market availability of these agents and these patterns may change over time. The estimates related to fracture risk have wide confidence intervals that suggest imprecision. Lastly, the generalizability of our study is limited to insured patients who face fewer cost barriers to medications.
The strength of this study lies in the longitudinal design, the use of incidence drug users so that initial therapy exposure is uniformly observed, and the novel approach to examining the impact of switching behavior. Since we compare the effectiveness of the weekly and monthly dosing regimens in the same individuals, the results are not confounded by unmeasured factors that did not change before and after the therapy switch (e.g. a tendency to employ healthy behaviors). Potential confounding related to changeable factors was addressed with the use of two contemporary comparator groups. Changeable factors could bias the results of this study only if they occurred at the same time as the regimen switch and occurred more in the regimen switchers than in the other comparator groups.
The low adherence observed among all study groups suggests that switching patients to a different oral bisphosphonate, even one with a reduced dosing regimen, may not be enough to improve fracture risk. Furthermore, the increased costs of the once-monthly bisphosphonate may not be justified, especially given that generic forms of the weekly oral bisphosphonates have been available since early 2008.
Key Points.
Previously it was known that adherence with oral bisphosphonates is suboptimal but improved with the once-weekly dose regimens over the once-daily regimens.
This study found patients who were switched to a once-monthly bisphosphonate demonstrated a modest reduction in nonadherence over the once-weekly regimen.
However, we did not detect significant improvement in fracture risk with the adherence improvement.
Acknowledgments
Funding Source: This study was funded in part by an unrestricted research grant from Novartis Pharmaceuticals Corporation, East Hanover, NJ. Dr. Harrold was supported by Grant Number K23AR053856 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Briesacher was supported by Award Number K01AG031836 from the National Institute on Aging. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute On Aging or the National Institutes of Health.
Conflict of Interest: In the past 3 years, the author and co-authors have received unrestricted research support, including for this study, from Novartis Pharmaceutical Corporation, which manufactures products for osteoporosis. In addition, Dr. Briesacher has served as a consultant for Novartis Pharmaceutical Corporation. The funding source played no role in the design and conduct of the study, collection, analysis, and interpretation of the data, or in the preparation of this manuscript.
Bibliography
- 1.Briesacher BA, Andrade SE, Yood RA, et al. Consequences of poor compliance with bisphosphonates. Bone. 2007;41:882–887. doi: 10.1016/j.bone.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 2.Solomon DH, Avorn J, Katz JN, et al. Compliance with osteoporosis medications. Arch Intern Med. 2005;165:2414–2419. doi: 10.1001/archinte.165.20.2414. [DOI] [PubMed] [Google Scholar]
- 3.Yood RA, Emani S, Reed JI, et al. Compliance with pharmacologic therapy for osteoporosis. Osteoporos Int. 2003;14:965–968. doi: 10.1007/s00198-003-1502-4. [DOI] [PubMed] [Google Scholar]
- 4.Kripalani S, Yao X, Haynes RB. Interventions to enhance medication adherence in chronic medical conditions: a systematic review. Arch Intern Med. 2007;167:540–550. doi: 10.1001/archinte.167.6.540. [DOI] [PubMed] [Google Scholar]
- 5.Chesnut IC, Skag A, Christiansen C, et al. Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res. 2004;19:1241–1249. doi: 10.1359/JBMR.040325. [DOI] [PubMed] [Google Scholar]
- 6.Delmas PD, Recker RR, Chesnut CH, 3rd, et al. Daily and intermittent oral ibandronate normalize bone turnover and provide significant reduction in vertebral fracture risk: results from the BONE study. Osteoporos Int. 2004;15:792–798. doi: 10.1007/s00198-004-1602-9. [DOI] [PubMed] [Google Scholar]
- 7.Boniva Product Labelling. v3.13 vol. Mar, 2005. [Google Scholar]
- 8.Miller PD, McClung MR, Macovei L, et al. Monthly oral ibandronate therapy in postmenopausal osteoporosis: 1-year results from the MOBILE study. J Bone Miner Res. 2005;20:1315–1322. doi: 10.1359/JBMR.050313. [DOI] [PubMed] [Google Scholar]
- 9.Weiss TW, Henderson SC, McHorney CA, et al. Persistence across weekly and monthly bisphosphonates: analysis of US retail pharmacy prescription refills. Curr Med Res Opin. 2007;23:2193–2203. doi: 10.1185/030079907X226069. [DOI] [PubMed] [Google Scholar]
- 10.Briesacher BA, Andrade SE, Harrold LH, Fouayzi H, Yood RA. Adoption of Once-monthly Oral Bisphosphonates and the Impact on Adherence. Am J Med. doi: 10.1016/j.amjmed.2009.05.017. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gold DT, Safi W, Trinh H. Patient preference and adherence: comparative US studies between two bisphosphonates, weekly risedronate and monthly ibandronate. Curr Med Res Opin. 2006;22:2383–2391. doi: 10.1185/030079906X154042. [DOI] [PubMed] [Google Scholar]
- 12.Brookhart MA, Patrick AR, Dormuth C, et al. Adherence to lipid-lowering therapy and the use of preventive health services: an investigation of the healthy user effect. Am J Epidemiol. 2007;166:348–354. doi: 10.1093/aje/kwm070. [DOI] [PubMed] [Google Scholar]
- 13.Blouin J, Dragomir A, Ste-Marie LG, et al. Discontinuation of antiresorptive therapies: a comparison between 1998–2001 and 2002–2004 among osteoporotic women. J Clin Endocrinol Metab. 2007;92:887–894. doi: 10.1210/jc.2006-1856. [DOI] [PubMed] [Google Scholar]
- 14.Lo JC, Pressman AR, Omar MA, et al. Persistence with weekly alendronate therapy among postmenopausal women. Osteoporos Int. 2006;17:922–928. doi: 10.1007/s00198-006-0085-2. [DOI] [PubMed] [Google Scholar]
- 15.Penning-van Beest FJ, Goettsch WG, Erkens JA, et al. Determinants of persistence with bisphosphonates: a study in women with postmenopausal osteoporosis. Clin Ther. 2006;28:236–242. doi: 10.1016/j.clinthera.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 16.Recker RR, Gallagher R, MacCosbe PE. Effect of dosing frequency on bisphosphonate medication adherence in a large longitudinal cohort of women. Mayo Clin Proc. 2005;80:856–861. doi: 10.4065/80.7.856. [DOI] [PubMed] [Google Scholar]
- 17.MarketScan Research Databases User Guide and Database Dictionary. Thomson Medstat; Ann Arbor, MI: 2006. [Google Scholar]
- 18.Sturmer T, Joshi M, Glynn RJ, et al. A review of the application of propensity score methods yielded increasing use, advantages in specific settings, but not substantially different estimates compared with conventional multivariable methods. J Clin Epidemiol. 2006;59:437–447. doi: 10.1016/j.jclinepi.2005.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andrade SE, Gurwitz JH, Chan KA, et al. Validation of diagnoses of peptic ulcers and bleeding from administrative databases: a multi-health maintenance organization study. J Clin Epidemiol. 2002;55:310–313. doi: 10.1016/s0895-4356(01)00480-2. [DOI] [PubMed] [Google Scholar]
- 20.Ash AS, Ellis RP, Pope GC, et al. Using diagnoses to describe populations and predict costs. Health Care Financ Rev. 2000;21:7–28. [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao Y, Ellis RP, Ash AS, et al. Measuring population health risks using inpatient diagnoses and outpatient pharmacy data. Health Serv Res. 2001;36:180–193. [PMC free article] [PubMed] [Google Scholar]
- 22.Harrell FE, Jr., Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 23.Gibson TB, Mark TL, McGuigan KA, et al. The effects of prescription drug copayments on statin adherence. Am J Manag Care. 2006;12:509–517. [PubMed] [Google Scholar]
- 24.Patel S, Kwan JT, McCloskey E, et al. Prevalence and causes of low bone density and fractures in kidney transplant patients. J Bone Miner Res. 2001;16:1863–1870. doi: 10.1359/jbmr.2001.16.10.1863. [DOI] [PubMed] [Google Scholar]
- 25.Ray WA, Daugherty JR, Griffin MR. Lipid-lowering agents and the risk of hip fracture in a Medicaid population. Inj Prev. 2002;8:276–279. doi: 10.1136/ip.8.4.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shadish WR, Cook TD, Campbell DT. Experimental and Quasi-Experimental Designs for Generalized Causal Inference. Houghton Mifflin Company; Boston: 2002. [Google Scholar]
- 27.Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther. 2001;23:1296–1310. doi: 10.1016/s0149-2918(01)80109-0. [DOI] [PubMed] [Google Scholar]
- 28.Cramer JA, Silverman S. Persistence with bisphosphonate treatment for osteoporosis: finding the root of the problem. Am J Med. 2006;119:S12–S17. doi: 10.1016/j.amjmed.2005.12.018. [DOI] [PubMed] [Google Scholar]
- 29.Cramer JA, Amonkar MM, Hebborn A, et al. Compliance and persistence with bisphosphonate dosing regimens among women with postmenopausal osteoporosis. Curr Med Res Opin. 2005;21:1453–1460. doi: 10.1185/030079905X61875. [DOI] [PubMed] [Google Scholar]
- 30.Cooper A, Drake J, Brankin E. Treatment persistence with once-monthly ibandronate and patient support vs. once-weekly alendronate: results from the PERSIST study. Int J Clin Pract. 2006;60:896–905. doi: 10.1111/j.1742-1241.2006.01059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yood RA, Mazor KM, Andrade SE, et al. Patient decision to initiate therapy for osteoporosis: the influence of knowledge and beliefs. J Gen Intern Med. 2008;23:1815–1821. doi: 10.1007/s11606-008-0772-0. [DOI] [PMC free article] [PubMed] [Google Scholar]