Abstract
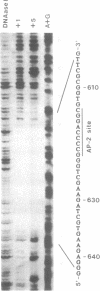
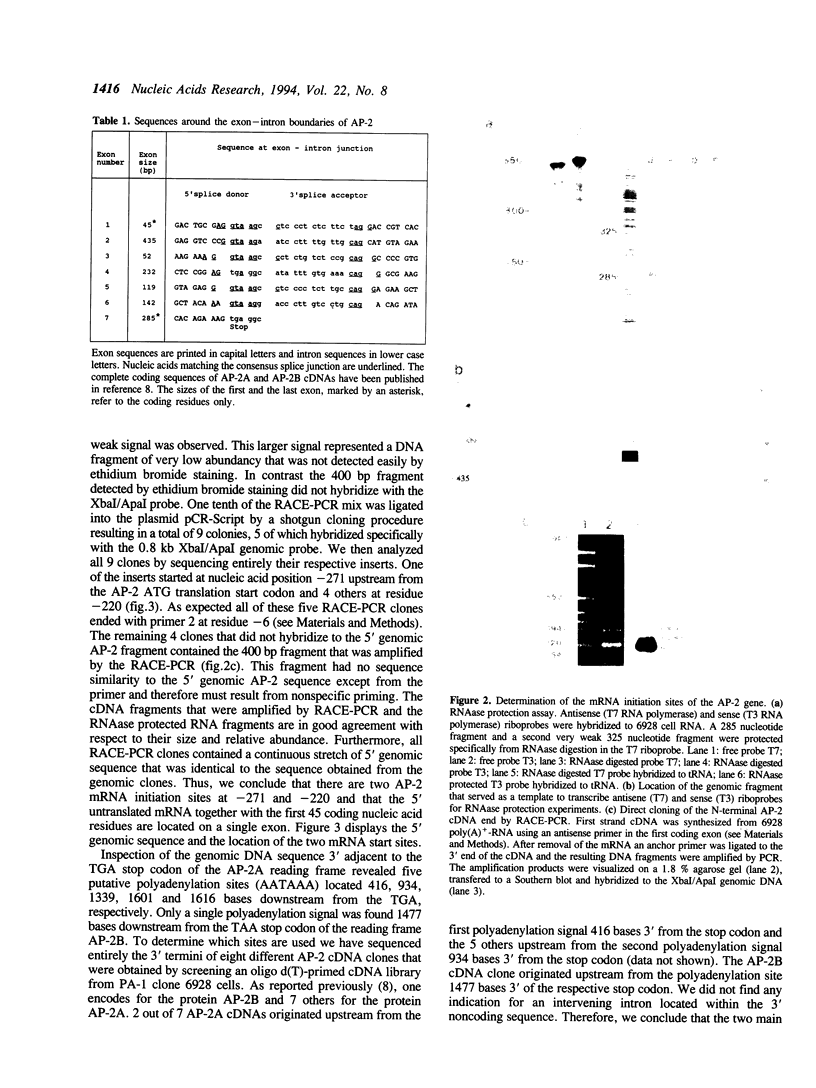
The transcription factor AP-2 is encoded by a gene located on chromosome 6 near the HLA locus. Here we describe the genomic organization of the AP-2 gene including an initial characterization of the promoter. We have mapped two mRNA initiation sites, the entire exon-intron structure and located two polyadenylation sites. The mature AP-2 mRNA is spliced from 7 exons distributed over a region of 18 kb genomic DNA. A recently cloned inhibitory AP-2 protein is generated by alternative usage of a C-terminal exon. The proline-rich transactivation motif is encoded by a single exon within the N-terminal region in contrast to the complex DNA binding and dimerization motif which involves amino acid residues located on four different exons. The sites of mRNA initiation are located 220 and 271 bases upstream from the ATG translation start site. Although the promoter contains no canonical sequence motifs for basal transcription factors, such as TATA-, CCAAT- or SP-1 boxes, it mediates cell-type-specific expression of a CAT reporter gene in PA-1 human teratocarcinoma cells and is inactive in murine F9 teratocarcinoma cells. We demonstrate that the promoter of the AP-2 gene is subject to positive autoregulation by its own gene product. A consensus AP-2 binding site is located at position -622 with respect to the ATG. This site binds specifically to bacterially expressed AP-2 as well as to multiple proteins, including AP-2, present in PA-1 and HeLa cell nuclear extracts. A partial AP-2 promoter fragment including the AP-2 consensus binding site is approximately 5-fold transactivated by cotransfection of an AP-2 expression plasmid.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angel P., Hattori K., Smeal T., Karin M. The jun proto-oncogene is positively autoregulated by its product, Jun/AP-1. Cell. 1988 Dec 2;55(5):875–885. doi: 10.1016/0092-8674(88)90143-2. [DOI] [PubMed] [Google Scholar]
- Behrens J., Löwrick O., Klein-Hitpass L., Birchmeier W. The E-cadherin promoter: functional analysis of a G.C-rich region and an epithelial cell-specific palindromic regulatory element. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11495–11499. doi: 10.1073/pnas.88.24.11495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buettner R., Kannan P., Imhof A., Bauer R., Yim S. O., Glockshuber R., Van Dyke M. W., Tainsky M. A. An alternatively spliced mRNA from the AP-2 gene encodes a negative regulator of transcriptional activation by AP-2. Mol Cell Biol. 1993 Jul;13(7):4174–4185. doi: 10.1128/mcb.13.7.4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buettner R., Yim S. O., Hong Y. S., Boncinelli E., Tainsky M. A. Alteration of homeobox gene expression by N-ras transformation of PA-1 human teratocarcinoma cells. Mol Cell Biol. 1991 Jul;11(7):3573–3583. doi: 10.1128/mcb.11.7.3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards J. B., Delort J., Mallet J. Oligodeoxyribonucleotide ligation to single-stranded cDNAs: a new tool for cloning 5' ends of mRNAs and for constructing cDNA libraries by in vitro amplification. Nucleic Acids Res. 1991 Oct 11;19(19):5227–5232. doi: 10.1093/nar/19.19.5227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaynor R. B., Muchardt C., Xia Y. R., Klisak I., Mohandas T., Sparkes R. S., Lusis A. J. Localization of the gene for the DNA-binding protein AP-2 to human chromosome 6p22.3-pter. Genomics. 1991 Aug;10(4):1100–1102. doi: 10.1016/0888-7543(91)90209-w. [DOI] [PubMed] [Google Scholar]
- Gerster T., Balmaceda C. G., Roeder R. G. The cell type-specific octamer transcription factor OTF-2 has two domains required for the activation of transcription. EMBO J. 1990 May;9(5):1635–1643. doi: 10.1002/j.1460-2075.1990.tb08283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Imagawa M., Chiu R., Karin M. Transcription factor AP-2 mediates induction by two different signal-transduction pathways: protein kinase C and cAMP. Cell. 1987 Oct 23;51(2):251–260. doi: 10.1016/0092-8674(87)90152-8. [DOI] [PubMed] [Google Scholar]
- Johnson A. C., Kageyama R., Popescu N. C., Pastan I. Expression and chromosomal localization of the gene for the human transcriptional repressor GCF. J Biol Chem. 1992 Jan 25;267(3):1689–1694. [PubMed] [Google Scholar]
- Kageyama R., Pastan I. Molecular cloning and characterization of a human DNA binding factor that represses transcription. Cell. 1989 Dec 1;59(5):815–825. doi: 10.1016/0092-8674(89)90605-3. [DOI] [PubMed] [Google Scholar]
- Kopp H., Masters J. R., Wieland W., Hofstaedter F., Buettner R. Differentielle Regulation des Androgenrezeptor-Promoters in hormonsensitiven und -resistenten Prostatakarzinomzellen. Verh Dtsch Ges Pathol. 1993;77:129–132. [PubMed] [Google Scholar]
- Lafyatis R., Denhez F., Williams T., Sporn M., Roberts A. Sequence specific protein binding to and activation of the TGF-beta 3 promoter through a repeated TCCC motif. Nucleic Acids Res. 1991 Dec 11;19(23):6419–6425. doi: 10.1093/nar/19.23.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leask A., Byrne C., Fuchs E. Transcription factor AP2 and its role in epidermal-specific gene expression. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):7948–7952. doi: 10.1073/pnas.88.18.7948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckow B., Schütz G. CAT constructions with multiple unique restriction sites for the functional analysis of eukaryotic promoters and regulatory elements. Nucleic Acids Res. 1987 Jul 10;15(13):5490–5490. doi: 10.1093/nar/15.13.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüscher B., Mitchell P. J., Williams T., Tjian R. Regulation of transcription factor AP-2 by the morphogen retinoic acid and by second messengers. Genes Dev. 1989 Oct;3(10):1507–1517. doi: 10.1101/gad.3.10.1507. [DOI] [PubMed] [Google Scholar]
- Mermod N., O'Neill E. A., Kelly T. J., Tjian R. The proline-rich transcriptional activator of CTF/NF-I is distinct from the replication and DNA binding domain. Cell. 1989 Aug 25;58(4):741–753. doi: 10.1016/0092-8674(89)90108-6. [DOI] [PubMed] [Google Scholar]
- Mitchell P. J., Timmons P. M., Hébert J. M., Rigby P. W., Tjian R. Transcription factor AP-2 is expressed in neural crest cell lineages during mouse embryogenesis. Genes Dev. 1991 Jan;5(1):105–119. doi: 10.1101/gad.5.1.105. [DOI] [PubMed] [Google Scholar]
- Mitchell P. J., Wang C., Tjian R. Positive and negative regulation of transcription in vitro: enhancer-binding protein AP-2 is inhibited by SV40 T antigen. Cell. 1987 Sep 11;50(6):847–861. doi: 10.1016/0092-8674(87)90512-5. [DOI] [PubMed] [Google Scholar]
- Moser M., Pscherer A., Bauer R., Imhof A., Seegers S., Kerscher M., Buettner R. The complete murine cDNA sequence of the transcription factor AP-2. Nucleic Acids Res. 1993 Oct 11;21(20):4844–4844. doi: 10.1093/nar/21.20.4844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snape A. M., Winning R. S., Sargent T. D. Transcription factor AP-2 is tissue-specific in Xenopus and is closely related or identical to keratin transcription factor 1 (KTF-1). Development. 1991 Sep;113(1):283–293. doi: 10.1242/dev.113.1.283. [DOI] [PubMed] [Google Scholar]
- Tainsky M. A., Cooper C. S., Giovanella B. C., Vande Woude G. F. An activated rasN gene: detected in late but not early passage human PA1 teratocarcinoma cells. Science. 1984 Aug 10;225(4662):643–645. doi: 10.1126/science.6740333. [DOI] [PubMed] [Google Scholar]
- Tainsky M. A., Krizman D. B., Chiao P. J., Yim S. O., Giovanella B. C. PA-1, a human cell model for multistage carcinogenesis: oncogenes and other factors. Anticancer Res. 1988 Sep-Oct;8(5A):899–913. [PubMed] [Google Scholar]
- Tainsky M. A., Yim S. O., Krizman D. B., Kannan P., Chiao P. J., Mukhopadhyay T., Buettner R. Modulation of differentiation in PA-1 human teratocarcinoma cells after N-ras oncogene-induced tumorigenicity. Oncogene. 1991 Sep;6(9):1575–1582. [PubMed] [Google Scholar]
- Umesono K., Murakami K. K., Thompson C. C., Evans R. M. Direct repeats as selective response elements for the thyroid hormone, retinoic acid, and vitamin D3 receptors. Cell. 1991 Jun 28;65(7):1255–1266. doi: 10.1016/0092-8674(91)90020-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams T., Admon A., Lüscher B., Tjian R. Cloning and expression of AP-2, a cell-type-specific transcription factor that activates inducible enhancer elements. Genes Dev. 1988 Dec;2(12A):1557–1569. doi: 10.1101/gad.2.12a.1557. [DOI] [PubMed] [Google Scholar]
- Williams T., Tjian R. Analysis of the DNA-binding and activation properties of the human transcription factor AP-2. Genes Dev. 1991 Apr;5(4):670–682. doi: 10.1101/gad.5.4.670. [DOI] [PubMed] [Google Scholar]
- Williams T., Tjian R. Characterization of a dimerization motif in AP-2 and its function in heterologous DNA-binding proteins. Science. 1991 Mar 1;251(4997):1067–1071. doi: 10.1126/science.1998122. [DOI] [PubMed] [Google Scholar]