Crystals of the lytic transglycosylase MltE from E. coli were grown using the microbatch method and diffracted to a resolution of 2.1 Å.
Keywords: MltE, lytic transglycosylases, cell-wall recycling, Escherichia coli
Abstract
MltE from Escherichia coli (193 amino acids, 21 380 Da) is a lytic transglycosylase that initiates the first step of cell-wall recycling. This enzyme is responsible for the cleavage of the cell-wall peptidoglycan at the β-1,4-glycosidic bond between the N-acetylglucosamine and N-acetylmuramic acid units. At the end this reaction generates a disaccharide that is internalized and initiates the recycling process. To obtain insights into the biological functions of MltE, crystallization trials were performed and crystals of MltE protein that were suitable for X-ray diffraction analysis were obtained. The MltE protein of E. coli was crystallized using the hanging-drop vapour-diffusion method at 291 K. Crystals grew from a mixture consisting of 28% polyethylene glycol 4000, 0.1 M Tris pH 8.4 and 0.2 M magnesium chloride. Further optimization was performed using the microbatch technique. Single crystals were obtained that belonged to the orthorhombic space group C2221, with unit-cell parameters a = 123.32, b = 183.93, c = 35.29 Å, and diffracted to a resolution of 2.1 Å.
1. Introduction
The bacterial cell wall is comprised of cross-linked strands of peptidoglycan which encase the entire cytoplasm. A healthy cell wall is critical for the survival of bacteria. During homeostatic processes, including growth, the cell wall is simultaneously biosynthesized and degraded. This recycling event is little understood; it is an integral process that takes place during normal bacterial growth but also in response to damage that the cell wall might experience in response to antibiotic action. In Gram-negative bacteria the peptidoglycan is a thin layer that surrounds the cytoplasmic membrane and consists of repeats of N-acetylglucosamine (NAG) and N-acetylmuramic acid (NAM) units. The NAM unit typically has a pentapeptide stem attached to it. The NAG-NAM disaccharide, as introduced by bacterial lipid II, is polymerized by the action of transglycosylases and the pentapeptide stems undergo cross-linking to neighbouring strands in the final steps (Hesek et al., 2009 ▶; Suvorov et al., 2008 ▶).
The recycling process essentially reverses these events using an unusual reaction performed by lytic transglycosylases. The details of the reactions of these enzymes are currently under investigation, but the process is not hydrolytic. It requires entrapment of the C6 hydroxyl of the muramic acid, giving rise to a 1,6-anhydromuramyl moiety in the product. The product of the lytic transglycosylase reaction liberates one disaccharide at a time from the peptidoglycan structure. This reaction product is subsequently internalized into the cytoplasm by the permease AmpG, where it is converted into a series of derivatives en route to recycling into lipid II, which is introduced into the nascent cell wall (Suvorov et al., 2008 ▶). Hence, the process repeats itself.
In Escherichia coli there are seven known lytic transglycoylases (Powell et al., 2006 ▶): six are bound to the outer membrane (MltA, MltB, MltC, MltD, MltE and MltF) and one is soluble (Slt70) and is located in the periplasm. Three families have been defined for these enzymes: family 1 is comprised of Slt70, MltC, MltD, MltE and MltF, and families 2 and 3 are made up of single examples, the enzymes MltA (van Straaten et al., 2007 ▶) and MltB (van Asselt, Dijkstra et al., 1999 ▶), respectively. The specific function of each of these transglycosylases in peptidoglycan recycling is presently unknown, although they all play an active part in degradation of the cell wall.
All of the lytic transglycosylases seem to act as exoenzymes (Madoori & Thunnissen, 2010 ▶), with the sole exception of MltE, which has been shown to be an endoenzyme. The exolytic transglycosylases start their cleavage at one end of the peptidoglycan (this end is still unknown) and degrade it in a processive way, while MltE acts as an endoenzyme and does not need an end to start its function.
Here, we describe the preliminary results obtained for the crystallization of MltE, an endo-acting lytic transglycosylase, and the preliminary diffraction analysis of crystals diffracting to a resolution of 2.1 Å.
2. Experimental procedures
2.1. Expression and purification of MltE
The gene encoding the lytic transglycosylase MltE (amino acids 18–203) was amplified from the Escherichia coli K12 substrain MG1655 chromosome using high-fidelity Pfu Ultra II Fusion HS DNA Polymerase (Stratagene) and the following oligonucleotide primers: mltE_fw_NdeI, 5′-AGATATACATATGAAGCATGACTATACGAAC-3′, and mltE_rv_XhoI, 5′-ATCTCGAGCATCGCGTCCAGTGC-3′. The primer mltE_fw_NdeI introduced an S18M amino-acid substitution. The PCR product was cloned into pET-24a(+) vector from Novagen to give a gene that codes for MltE (amino acids 18–203; S18M) with a C-terminal His6 tag preceded by two additional amino acids (LE). The complete sequence of the peptide tag fused to the C-terminus of MltE was LEHHHHHH. The protein (194 amino acids, 21 385 Da) was expressed in E. coli BL21 (DE3). Cells containing the plasmid were selected on LB–agar supplemented with 50 µg ml−1 kanamycin. The transformants were inoculated overnight in 5 ml Luria–Bertani (LB) medium with 50 µg ml−1 kanamycin. This culture was used to inoculate 500 ml LB medium supplemented with 50 µg ml−1 kanamycin in a 3 l Erlenmeyer flask, which was grown at 310 K and 120 rev min−1. Protein expression was induced at an OD600 of 0.8 with 0.4 mM isopropyl β-d-1-thiogalactopyranoside (IPTG) and incubation was continued at 288 K for 12 h to minimize the presence of inclusion bodies. Cells were harvested by centrifugation for 30 min at 3220g and 277 K and the cell pellet was resuspended in 10 ml 20 mM HEPES buffer pH 7.0 supplemented with 0.5 M NaCl, 10% glycerol, 25 mM imidazole and 0.1% Triton X-100. The protein was released from the cells by sonification on ice (ten cycles of 2 min pulsed sonification with 1 min rest between sonification cycles using a Branson 450 Sonifier). The cell extract was then centrifuged for 45 min at 18 514g and 277 K. The supernatant was loaded onto a 5 ml HiTrap Chelating HP column (GE Healthcare) prepared with NiSO4. The column was washed with 10 ml 20 mM HEPES buffer pH 7.0, 0.5 M NaCl, 10% glycerol, 25 mM imidazole and 0.1% Triton X-100 (buffer A). Elution was performed using a gradient from 0 to 100% buffer B (20 mM HEPES buffer pH 7.0, 0.5 M NaCl, 10% glycerol, 500 mM imidazole and 0.1% Triton X-100). After dialysis against 20 mM HEPES buffer pH 7.0, 0.25 M NaCl and 0.1% Triton X-100, the protein concentration was determined using the Bio-Rad Protein Assay Dye Reagent with a solution of bovine serum albumin (BSA) of known concentration as a standard.
2.2. Crystallization
Initial crystallization trials were performed by the sitting-drop vapour-diffusion method at 291 K in Innovaplate SD-2 microplates (Innovadyne Technologies Inc., California, USA), mixing 250 nl protein solution with 250 nl precipitant solution and equilibrating against 65 µl well solution. Crystallization conditions were assayed by high-throughput techniques using a NanoDrop robot (Innovadyne Technologies Inc.) with native MltE at a concentration of 22 mg ml−1 in 25 mM HEPES pH 7.0, 100 mM NaCl and 0.1% Triton X-100. The PACT Suite and JCSG+ Suite from Qiagen (Düsseldorf, Germany) and Crystal Screen, Crystal Screen 2, Crystal Screen Lite, Index HT and SaltRX from Hampton Research (Aliso Viejo, USA) were tested. Successful initial conditions were optimized using hanging-drop methods by mixing 1 µl protein solution with 1 µl precipitant solution and equilibrating against 500 µl reservoir solution. Further crystallization experiments were performed by the microbatch technique, using 8 ml of Al’s oil from Hampton Research and mixing 1 µl protein solution with 1 µl precipitant solution.
2.3. X-ray data collection and processing
All crystals were soaked for 5 s in 1 µl cryoprotective solution consisting of 15%(v/v) PEG 400 in the crystallization solution and then flash-cooled to 100 K using a cryogenic system. A native data set was collected using synchrotron radiation on beamline ID23-2 at the ESRF (Grenoble, France) using a CCD detector and a fixed wavelength of 0.8726 Å. The images collected were processed and scaled using XDS (Kabsch, 1993 ▶, 2010 ▶), and using iMOSFLM (Leslie, 2006 ▶) and SCALA (Collaborative Computational Project, Number 4, 1994 ▶). The two methods produced the same results.
2.4. Preliminary structure solution
Structure determination was initiated using the catalytic domain of the E. coli Slt70 structure (PDB code 1qsa; van Asselt, Thunnissen et al., 1999 ▶), which shows 34% sequence identity, as an initial model for the molecular-replacement method using MOLREP (Vagin & Teplyakov, 2010 ▶).
3. Results
After performing initial screening experiments, native MltE crystals were obtained from three different conditions: (i) 20%(w/v) PEG 6000, 0.1 M MES pH 6.0 and 0.2 M calcium chloride, (ii) 20%(w/v) MPD, 0.1 M Tris pH 8.5 and (iii) 30%(w/v) PEG 4000, 0.1 M Tris pH 8.5 and 0.2 M magnesium chloride. Only condition (iii) was scaled up for further optimization.
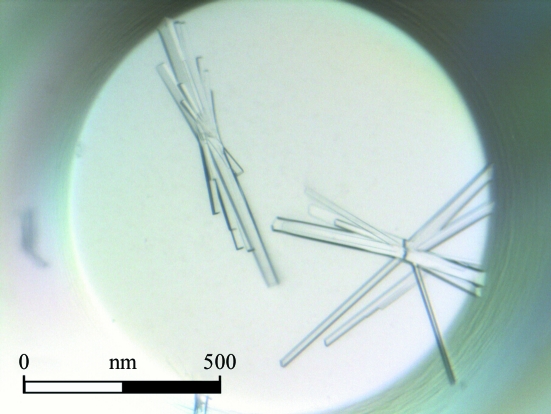
The best crystals, which had maximum dimensions of about 0.20 × 0.10 × 0.05 mm, were obtained using the microbatch technique from precipitant consisting of 28%(w/v) PEG 4000, 0.1 M Tris pH 8.4 and 0.2 M magnesium chloride in one week (Fig. 1 ▶).
Figure 1.
MltE crystals obtained using the microbatch technique with a precipitant consisting of 28% polyethylene glycol 4000, 0.1 M Tris pH 8.4 and 0.2 M magnesium chloride.
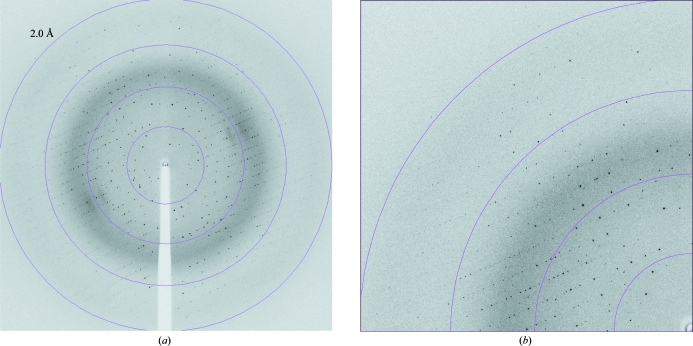
An X-ray data set from a single MltE crystal was collected to 2.1 Å resolution and displayed a well defined diffraction pattern (Fig. 2 ▶). The X-ray diffraction data were processed and showed good data-collection statistics (Table 1 ▶). The crystals belonged to the orthorhombic space group C2221, with unit-cell parameters a = 123.32, b = 183.93, c = 35.29 Å. Specific volume calculations based on the molecular weight of MltE (193 amino acids, 21 380 Da) and the unit-cell parameters indicated the probable presence of two molecules in the asymmetric unit, with 46.45% solvent content and a Matthews coefficient V M of 2.30 Å3 Da−1 (Matthews, 1968 ▶). Structural determination of the MltE protein is currently in progress.
Figure 2.
(a) Diffraction image of an MltE crystal diffracting to 2.1 Å resolution (oscillation range 1.0°); (b) enlargement showing the highest resolution area.
Table 1. Data-collection statistics for MltE.
Values in parentheses are for the highest resolution shell.
| Crystal data | |
| Space group | C2221 |
| Unit-cell parameters | |
| a (Å) | 123.32 |
| b (Å) | 183.93 |
| c (Å) | 35.29 |
| α = β = γ (°) | 90 |
| Data collection | |
| Temperature (K) | 100 |
| Radiation source | Synchrotron |
| Detector | CCD |
| Wavelength (Å) | 0.8726 |
| Resolution range (Å) | 19.26–2.11 (2.25–2.11) |
| No. of images | 180 |
| Rotation range per image (°) | 1.0 |
| Measured reflections | 199120 |
| Unique data | 27678 |
| Multiplicity | 7.2 (7.3) |
| Data completeness (%) | 99.9 (100.0) |
| Average I/σ(I) | 12.1 (3.0) |
| Rmerge† | 0.11 (0.41) |
R
merge = 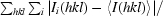
 .
.
Acknowledgments
The authors thank the staff of the ID23-2 beamline at the ESRF for support during synchrotron data collection. This work was supported by grants from the Spanish Ministry of Science and Technology (BFU2008-01711 and the Factoría de Cristalización from CONSOLIDER-INGENIO 2010) and the COMBACT program (S-BIO-0260/2006). The work in the USA was supported by the National Institutes of Health. CA-R is a fellow of the Spanish Ministry of Education and Science (BFU2008-01711/BMC). LIL is a Pew Latin American Fellow in the Biomedical Sciences supported by The Pew Charitable Trusts. The opinions expressed are those of the authors and do not necessarily reflect the views of The Pew Charitable Trusts.
References
- Asselt, E. J. van, Dijkstra, A. J., Kalk, K. H., Takacs, B., Keck, W. & Dijkstra, B. W. (1999). Structure, 7, 1167–1180. [DOI] [PubMed]
- Asselt, E. J. van, Thunnissen, A. M. & Dijkstra, B. W. (1999). J. Mol. Biol. 291, 877–898. [DOI] [PubMed]
- Collaborative Computational Project, Number 4 (1994). Acta Cryst. D50, 760–763.
- Hesek, D., Lee, M., Zhang, W., Noll, B. C. & Mobashery, S. (2009). J. Am. Chem. Soc. 131, 5187–5193. [DOI] [PMC free article] [PubMed]
- Kabsch, W. (1993). J. Appl. Cryst. 26, 795–800.
- Kabsch, W. (2010). Acta Cryst. D66, 125–132. [DOI] [PMC free article] [PubMed]
- Leslie, A. G. W. (2006). Acta Cryst. D62, 48–57. [DOI] [PubMed]
- Madoori, P. K. & Thunnissen, A.-M. W. H. (2010). Acta Cryst. F66, 534–538. [DOI] [PMC free article] [PubMed]
- Matthews, B. W. (1968). J. Mol. Biol. 33, 491–497. [DOI] [PubMed]
- Powell, A. J., Liu, Z.-J., Nicholas, R. A. & Davies, C. (2006). J. Mol. Biol. 359, 122–136. [DOI] [PubMed]
- Straaten, K. E. van, Barends, T. R., Dijkstra, B. W. & Thunnissen, A.-M. (2007). J. Biol. Chem. 282, 21197–21205. [DOI] [PubMed]
- Suvorov, M., Lee, M., Hesek, D., Boggess, B. & Mobashery, S. (2008). J. Am. Chem. Soc. 130, 11878–11879. [DOI] [PMC free article] [PubMed]
- Vagin, A. & Teplyakov, A. (2010). Acta Cryst. D66, 22–25. [DOI] [PubMed]