Abstract
Sihler's staining allows visualization of the nerve distribution within soft tissues without extensive dissection and does not require slide preparation, unlike traditional approaches. This technique can be applied to the mucosa, muscle, and organs that contain myelinated nerve fibers. In particular, Sihler's technique may be considered the best tool for observing nerve distribution within skeletal muscles. The intramuscular distribution pattern of nerves is difficult to observe through manual manipulation due to the gradual tapering of nerves toward the terminal end of muscles, so it should be accompanied by histological studies to establish the finer branches therein. This method provides useful information not only for anatomists but also for physiologists and clinicians. Advanced knowledge of the nerve distribution patterns will be useful for developing guidelines for clinicians who perform operations such as muscle resection, tendon transplantation, and botulinum toxin injection. Furthermore, it is a useful technique to develop neurosurgical techniques and perform electrophysiological experiments. In this review, Sihler's staining technique is described in detail, covering its history, staining protocol, advantages, disadvantages, and possible applications. The application of this technique for determining the arterial distribution pattern is also described additionally in this study.
Keywords: Review, Sihler's staining, Whole mount nerve staining, Nerve, Artery
History
Sihler's staining was first introduced by Dr. Charles Sihler to elucidate the distribution of snake nerve endings [1] and frog end-organs [2], but has since been largely forgotten by anatomists. However, this method was modified by Wharton (kidney, uterus, and ovary in humans) [3], Williams (foot and leg of the human fetus) [4], and Freihofer (fish) [5] to investigate the innervation of entire specimens. More recently, some manners of Sihler's staining procedures were modified in the last half of the 20th century by Liem and Douwe van Willigen [6]; their staining protocol is implemented in most current research on nerve distribution patterns.
Since 1990, the modified Sihler's staining protocol has been used increasingly to elucidate the nerve innervation patterns of mucosa, organs, and muscles [7-12]. Prior to 1990, such studies involved mainly animals, but, since then, human tissues have been increasingly subjected to this protocol to determine nerve innervation patterns.
Staining Protocol
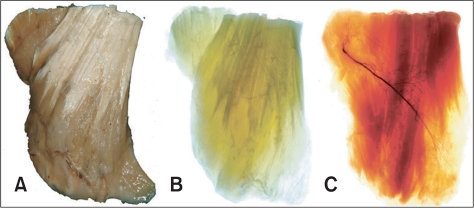
Sihler's staining comprises seven steps, which may take 3-4 months to complete, depending upon the thickness and size of the specimen. Furthermore, the timing of solution replacement requires precision, a quality that is determined by experience (Fig. 1) [13].
Fig. 1.
The appearance of the muscle which is changed through Sihler's staining described every stage. A: Fixed specimen, B: Macerated specimen, C: Stained specimen (intramuscular nerve distribution of the masseter muscle for botulinum toxin injection. Reproduced from J Craniofac Surg 2010;21:588-91, with permission) [13].
Fixation
Harvested specimens are fixed in 10% unneutralized formalin as soon as possible after death of the subject. The post-mortem must be expedited to avoid defective staining.
The specimens are fixed in formalin solution for up to 1 month, but the duration of the fixation varies depending on specimen size. The fixative (formalin) must be replaced freshly whenever it turns cloudy.
Maceration and depigmentation
Fixed specimens are washed in running water for about 1 hour and placed in maceration solution (3% aqueous potassium hydroxide solution containing 0.2 ml 3% hydrogen peroxide per 100 ml) for 4 weeks. This solution should be changed every 1-2 days until the specimen becomes well bleached and translucent; this process can take 3-4 weeks.
Decalcification
Macerated specimens are shifted to Sihler's solution I (1 : 1 : 6=glacial acetic acid : glycerin : 1% aqueous chloral hydrate), which should be refreshed every week for 4 weeks.
Staining
Adequately decalcified specimens are stained with Sihler's solution II (1 : 1 : 6=Ehrlich's hematoxylin : glycerin : 1% aqueous chloral hydrate). The staining process takes approximately 3-4 weeks, depending upon sample size and tissue density. The staining condition, which is the criterion that determines progress to the next step, can be evaluated by observing the degree of penetration by Ehrlich's hematoxylin on a viewing box with sufficient light transmission.
Destaining
Stained specimens are placed into Sihler's solution I, which was also used in the decalcification step, and stirred lightly. This process is stopped when stained nerve twigs are revealed; the timing of which usually depends on specimen size and on the experience of the technician. At this stage, researchers should frequently check the specimen on a viewing box that transmits sufficient light to observe the condition of the specimen. The remaining tissue (i.e., connective and muscle tissue) may not completely bleach at this step.
Neutralization
The specimen, which at this stage is in an acidic state, needs to be neutralized by soaking in 0.05% lithium carbonate solution for approximately 1 hour and then washed under running tap water for 1 hour.
Clearing
The specimens are then cleared with increasing concentrations of glycerin (40%, 60%, 80%, and 100%) for 1 day until any overstained regions are washed out. This process should be terminated when the nerve twigs can be seen clearly on a viewing box. Specimens are then stored in pure glycerin to which a few thymol crystals have been added.
Comparing the Strengths and Limitations of the Procedure
Sihler's staining technique may supplement and complement the limitations of other methods used to visualize the innervation patterns of organs, muscles, and mucosae. The strengths and limitation of Sihler's staining technique are described in detail below.
Strengths
First, Sihler's technique for whole-mount nerve staining makes it possible to observe the finer nerve twigs without a manual dissection. Second, it enables those regions that are generally difficult to visualize (and the communicating pattern of neighboring nerves) to be observed with the unaided eye. Third, this whole-mount technique does not require slide preparation; thus, making it possible to clarify the relationship between adjacent structures. Fourth, both the nerve innervation pattern and the arterial distribution can be observed using this staining technique.
Limitations
First, this staining protocol does not enable the distinction between sensory and motor neurons in the specimen because it selectively stains the myelin sheath that covers the axon. Nerve composition can only be determined by tracking it back to its main branch. Second, compared to other experimental procedures, this staining method is time consuming and its success is very much dependent upon the researcher's experience. Third, as it is a whole-mount staining method, Sihler's staining may result in differences in staining quality depending on the thickness and size of the specimen, and consequent to inaccurate solution changes during the decalcification and clearing steps.
Possible Applications of Sihler's Staining
Sihler's staining has been applied by some researchers to verify the innervation of organs, skeletal muscles, and other tissues in humans and other animals. Similarly, it has been used in our laboratory not only to clarify innervation patterns but also to describe arterial distributions. In the latter case, heavy arteries need to be injected with latex containing a color agent before beginning the dissection; thus, clearly distinguishing their anatomical structure from the surrounding tissues. This staining method is followed only until step 2 (maceration and depigmentation), at which point the specimen becomes translucent. Once clearing is complete, definite arterial branches can be observed within the specimen with the unaided eye. Possible applications of Sihler's staining are described by region in the following.
Nerve distribution pattern of the oral mucosa
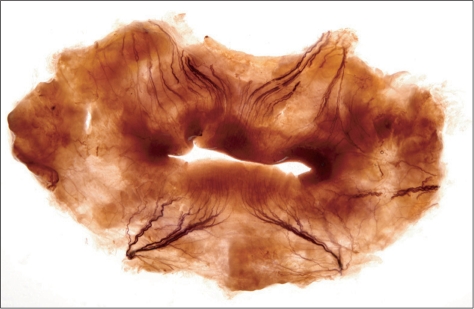
The buccal nerve (BN) runs between the two heads of the lateral pterygoid muscle underneath the tendon of the temporalis muscle, occasionally piercing the temporalis muscle [14]. The BN is a branch of the mandibular nerve, which is derived from the trigeminal nerve and transmits sensory information to the skin on the anterior area of the buccinator muscle, buccal mucosa, and posterior area of the buccal gingivae to the molar teeth. The BN supplies sensation to the buccal area of the face, the so-called cheek, together with adjacent nerves, such as the infraorbital nerve (ION) and the mental nerve (MN), which branch from the mandibular nerve. The distribution areas of both the main trunk and tiny twigs of these nerves should be considered for a diagnosis and guidance of surgical intervention. However, the manipulation of nerves when dissecting the oral mucosa could alter its course and distribution patterns [15]. Sihler's staining is capable of providing precise information about the course and distribution areas of the BN, ION, and MN, and, as it is a whole-mount nerve stain, obviates the need for manual dissection. We used Sihler's stain to investigate the distribution of the BN, ION, and MN and to determine a diagnostic reference for the numbness caused by nerve injury, for trigeminal neuralgia, and to provide an anatomical guide for various surgical interventions (Fig. 2).
Fig. 2.
For observing the exact distribution of the buccal nerve and its anatomical relationship with mental nerve and infraorbital nerve which is innervating the oral mucosa applied a Sihler's staining (Sihler's-staining study of buccal nerve distribution and its clinical implications, focusing on its relationship with adjacent sensory nerves in the angular area of the mouth. Reproduced from J Craniofac Surg Submitted, with permission).
Nerve distribution pattern of the masticatory muscles
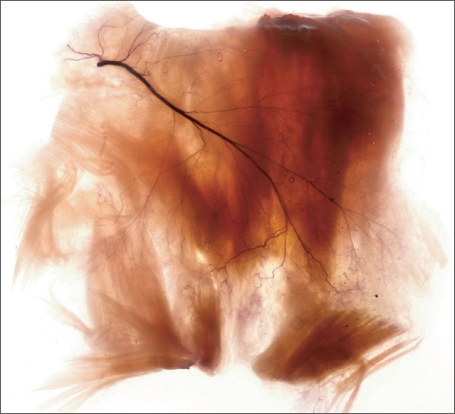
The masseter muscle: The masseter muscle is of clinical importance with regard to masseteric hypertrophy, bruxism, and disorders of the temporomandibular joint. Conventional treatment includes mandibular angle resection and reduction of the masseter muscle through surgical intervention and injection of botulinum toxin A (BTX-A).
Gurney [16] suggested an extraoral approach for the resection of a hypertrophied masseter muscle, but he encountered postoperative complications such as bleeding, trismus, and swelling [16-18]. BTX-A injection can be used as an alternative and noninvasive treatment for avoiding the complications associated with surgical intervention. For an effective BTX-A injection, the needle should be inserted as near as possible to the region where most of the neuromuscular synapses are located [19]. Therefore, we performed Sihler's staining to reveal the nerve distribution pattern within the masseter muscle; thus, providing critical information for determining the most efficient and safe sites for BTX-A injection (Fig. 3) [13].
Fig. 3.
For observing the intramuscular distribution of masseteric nerve innervating the masseter muscle applied a Sihler's staining (intramuscular nerve distribution of the masseter muscle for botulinum toxin injection. Reproduced form J Craniofac Surg 2010;21:588-91, with permission) [13].
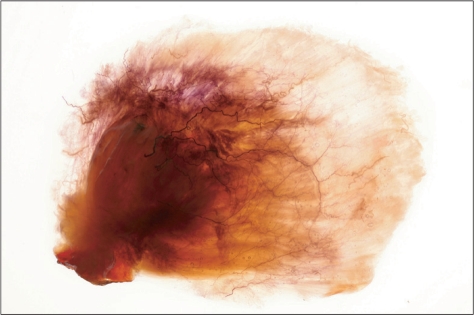
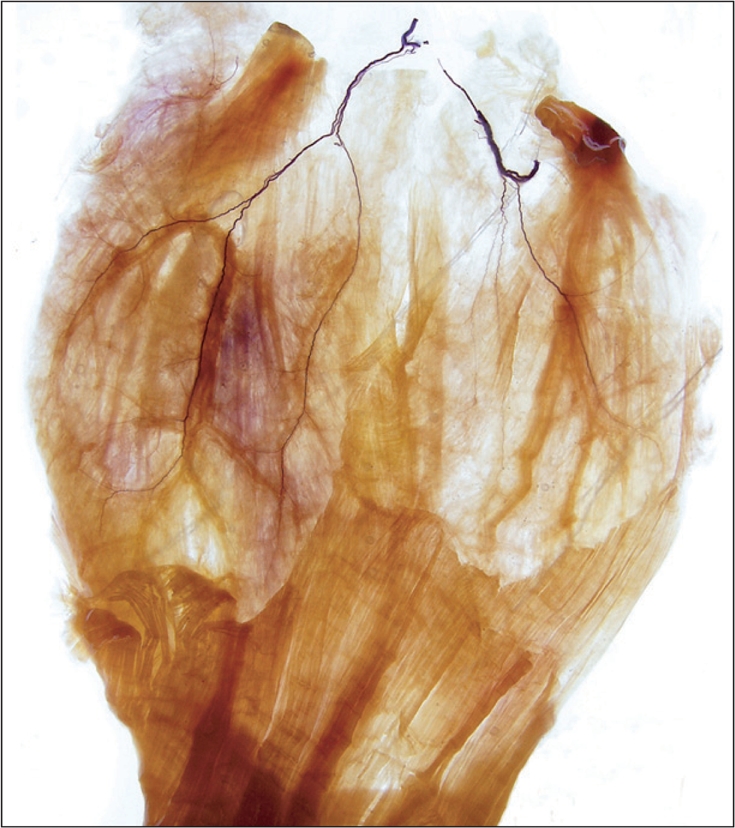
The temporalis muscle: We performed Sihler's staining simply to observe the intramuscular nerve pattern of the temporalis muscle, which is divided into three topographical portions: anterior, middle, and posterior portions. The temporalis muscle is one of the masticatory muscles and plays an essential part in upward and backward elevation of the mandible [20]. Upward elevation occurs by contracting the anterior fibers of the temporalis muscle, and backward elevation is caused by contraction of the posterior fibers. The anterior fibers of the temporalis muscle were innervated by the anterior deep temporal nerve, the middle fibers by the middle deep temporal nerve, and the posterior fibers by the posterior deep temporal nerve, which are derived from the third branch of the trigeminal nerve (Fig. 4).
Fig. 4.
For observing the intramuscular distribution of deep temporal nerve innervating the temporalis muscle which is divided into three portions as regards topographical shape applied a Sihler's staining.
Nerve distribution pattern of the anterior compartment of the forearm muscle
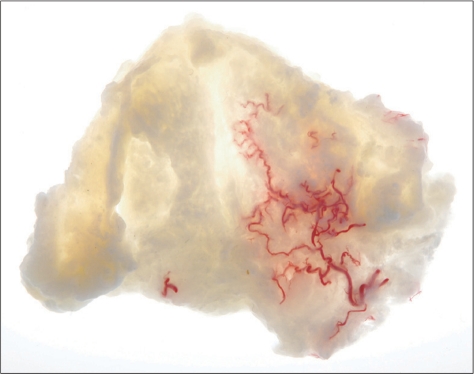
In patients with a damaged central nervous system, spasticity of the forearm muscles can lead to excessive pronation of the forearm as well as inflexible flexion and extension of the elbow, wrist, and fingers. It can induce pain, joint contracture, skin breakdown, and functional impairments [21]. Spasticity can be reduced by things like antispastic medication, nerve block, or kinesiology. BTX-A injection is the most effective and safe method for reducing forearm spasticity, but it is necessary to place the needle as near as possible to the neuromuscular synapses due to unwanted side effects and weakness of the neighboring muscles [22]. Therefore, we used a detailed dissection and Sihler's stain to reveal the distribution pattern of the extra- and intramuscular nerves innervating the forearm muscles to determine the accurate injection point for BTX-A (Fig. 5) [21].
Fig. 5.
For finding the region as near as possible to the neuromuscular synapses in a forearm muscles applied a Sihler's staining (intramuscular nerve distribution of the masseter muscle for botulinum toxin injection. Reproduced from Am J Phys Med Rehabil 2010;89:644-52, with permission) [21].
Nerve distribution pattern of the thigh muscles (adductor longus and gracilis)
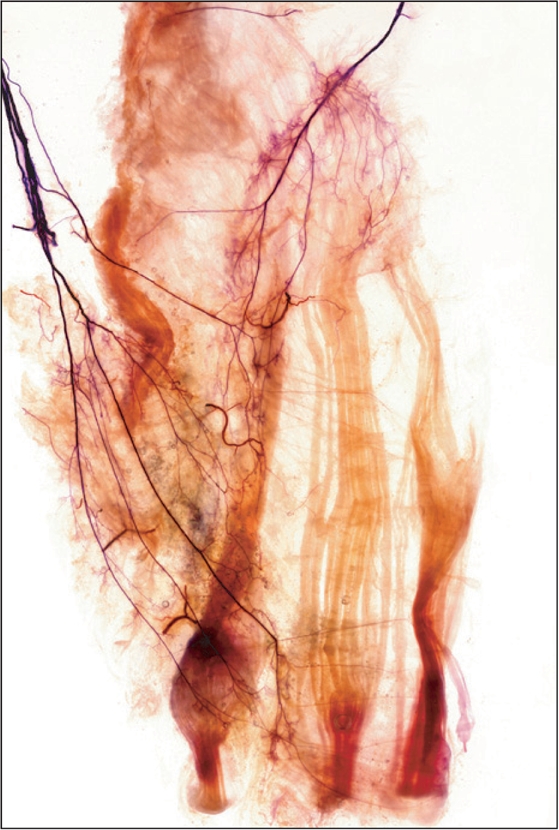
Hip adductors act as synergists of gait activity and controllers of posture in the supine position and adduction of the flexed thigh when standing [23]. Patients with hip adductor spasticity exhibit gait-pertaining signs such as a narrow base of support, poor balance, and an unstable gait [24]. Common treatment modalities for hip adductor spasticity have been applied to operations such as an obturator neurectomy, obturator neurolysis, and motor point block [25]. BTX-A is a particularly effective treatment, not only because of its effectiveness, but also because of easy accessibility. The injection point is often assumed to be situated halfway along the length of a muscle belly [26, 27], or at the motor point, which is where the muscular branch enters the muscle belly, rather than at the neuromuscular junctions [28]. However, previous studies have noted that the neuromuscular junction is usually more distal than the motor point [29, 30]. Therefore, we performed Sihler's staining to clarify the intramuscular branching pattern and arborizing area of the hip adductor muscle with reference to the surface landmarks of the thigh (Fig. 6).
Fig. 6.
For observing the intramuscular branching pattern and arborizing area of hip adductor muscle with reference to surface landmarks of the thigh applied a Sihler's staining (This research is in the middle of writing an article. Reproduced with permission).
Nerve distribution pattern of the leg muscles
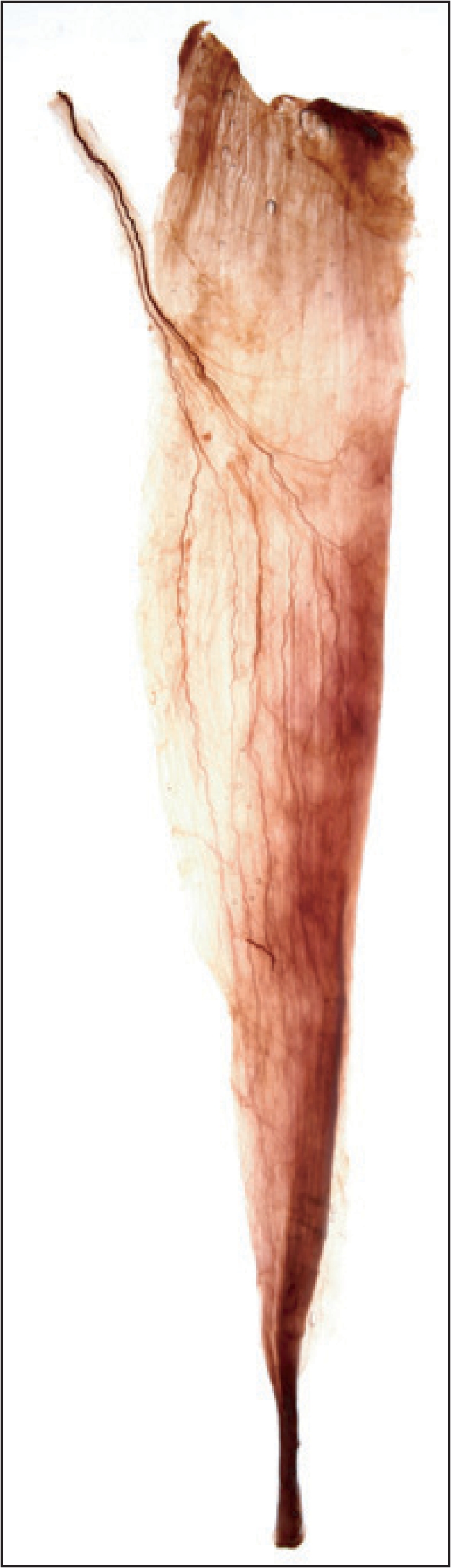
Anatomical information regarding the triceps surae muscles is clinically significant due to the increasing number of medical interventions, such as neurectomy [31, 32], a gastrocnemius resection [33], and BTX-A injection [34], that are used to reduce spasticity and improve the esthetic appearance of bulky calves. Improvements in terms of efficacy and safety have been investigated in many studies by examining the nerve innervation supplying the triceps surae [35]. However, the distribution pattern in the triceps surae muscles has been reported only rarely, because it is difficult to visualize and to analyze the intramuscular branching patterns therein. Liu et al. [36] mentioned that tracing becomes more difficult as the end of the nerve is approached. However, we can be stained successfully using modified Sihler's technique, thus enabling analysis of the intra- and extramuscular branching patterns, and hence anatomical substantiation (Fig. 7) [37].
Fig. 7.
For observing the intramuscular nerve distribution innervating the triceps surae muscles applied a Sihler's staining (extra- and intramuscular nerves distributions of the triceps surae muscle as a basis for muscle resection and botulinum toxin injections. Reproduced form Surg Radiol Anat 2009;31:615-21, with permission) [37].
Arterial distribution pattern of the palatal mucosa
The palatal mucosal and submucosal tissues are used for transplants when performing periodontal procedures involving dental implant fixation [38]. Thus, the topographical location of the greater palatine artery (GPA) is important, not only to avoid bleeding but also to maintain the vitality of donor tissues for transplant [39]. In particular, esthetic subepithelial connective tissue grafting is often used in periodontal interventions for the incisive region relative to implant fixation [40]. In this case, the two-dimensional course of the GPA and its depth from the epithelial tissue are important considerations. We have used Sihler's staining to provide the the branching patterns of GPA twigs from its main trunk (Fig. 8).
Fig. 8.
For observing the accurate courses of main trunk of greater palatine artery with reference to cevix of individual teeth was used only in step 2 of Sihler's staining (This research is in the middle of writing an article. Reproduced with permission).
References
- 1.Sihler C. Ueber muskelspindeln und intramuskuläre nervenendigungen bei schlangen und röschen. Arch Mikrosk Anat. 1895;46:709–723. [Google Scholar]
- 2.Sihler C. Die Muskelspindeln Kerne und Lage der motorischen Nervenendigungen Zugleich em Nachtrag zu der Arbeit: Ueber Muskelspindeln etc Dieses Archiv Bd. XXXXVI, pg. 709. Archiv Mikrosk Anat. 1900;56:334–354. [Google Scholar]
- 3.Wharton LR. A technique for studying the innervation of organs. Anat Rec. 1937;67:469–475. [Google Scholar]
- 4.Williams TW., Jr A technique for the gross differential staining of peripheral nerves in cleared vertebrate tissue. Anat Rec. 1943;86:189–195. [Google Scholar]
- 5.Freihofer WC. The Sihler technique of staining nerves for systematic study especially of fishes. Copeia. 1966;3:470–475. [Google Scholar]
- 6.Liem RS, Douwe van Willigen J. In toto staining and preservation of peripheral nervous tissue. Stain Technol. 1988;63:113–120. doi: 10.3109/10520298809107170. [DOI] [PubMed] [Google Scholar]
- 7.Wu BL, Sanders I. A technique for demonstrating the nerve supply of whole larynges. Arch Otolaryngol Head Neck Surg. 1992;118:822–827. doi: 10.1001/archotol.1992.01880080044011. [DOI] [PubMed] [Google Scholar]
- 8.Sanders I, Wu BL, Mu L, Li Y, Biller HF. The innervation of the human larynx. Arch Otolaryngol Head Neck Surg. 1993;119:934–939. doi: 10.1001/archotol.1993.01880210022003. [DOI] [PubMed] [Google Scholar]
- 9.Sanders I, Wu BL, Mu L, Biller HF. The innervation of the human posterior cricoarytenoid muscle: evidence for at least two neuromuscular compartments. Laryngoscope. 1994;104:880–884. doi: 10.1288/00005537-199407000-00019. [DOI] [PubMed] [Google Scholar]
- 10.Mu L, Sanders I. The innervation of the human upper esophageal sphincter. Dysphagia. 1996;11:234–238. doi: 10.1007/BF00265207. [DOI] [PubMed] [Google Scholar]
- 11.Mu L, Sanders I. Neuromuscular organization of the human upper esophageal sphincter. Ann Otol Rhinol Laryngol. 1998;107(5 Pt 1):370–377. doi: 10.1177/000348949810700502. [DOI] [PubMed] [Google Scholar]
- 12.Mu L, Sanders I. Neuromuscular organization of the canine tongue. Anat Rec. 1999;256:412–424. doi: 10.1002/(SICI)1097-0185(19991201)256:4<412::AID-AR8>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 13.Kim DH, Hong HS, Won SY, Kim HJ, Hu KS, Choi JH, Kim HJ. Intramuscular nerve distribution of the masseter muscle as a basis for botulinum toxin injection. J Craniofac Surg. 2010;21:588–591. doi: 10.1097/SCS.0b013e3181d08bb3. [DOI] [PubMed] [Google Scholar]
- 14.Maeda S, Miyawaki T, Kuboki T, Shimada M. A trigeminal neuralgia-like paroxysmal pain condition presumably due to buccal nerve compression in the temporalis muscle. Cranio. 2001;19:56–60. doi: 10.1080/08869634.2001.11746152. [DOI] [PubMed] [Google Scholar]
- 15.Touré G, Bicchieray L, Selva J, Vacher C. The intra-lingual course of the nerves of the tongue. Surg Radiol Anat. 2005;27:297–302. doi: 10.1007/s00276-005-0335-6. [DOI] [PubMed] [Google Scholar]
- 16.Gurney CE. Chronic bilateral benign hypertrophy of the masseter muscles. Am J Surg. 1947;73:137–139. doi: 10.1016/0002-9610(47)90304-8. [DOI] [PubMed] [Google Scholar]
- 17.Park MY, Ahn KY, Jung DS. Botulinum toxin type A treatment for contouring of the lower face. Dermatol Surg. 2003;29:477–483. doi: 10.1046/j.1524-4725.2003.29116.x. [DOI] [PubMed] [Google Scholar]
- 18.Kim NH, Chung JH, Park RH, Park JB. The use of botulinum toxin type A in aesthetic mandibular contouring. Plast Reconstr Surg. 2005;115:919–930. doi: 10.1097/01.prs.0000153236.79775.a0. [DOI] [PubMed] [Google Scholar]
- 19.Lepage D, Parratte B, Tatu L, Vuiller F, Monnier G. Extra- and intramuscular nerve supply of the muscles of the anterior antebrachial compartment: applications for selective neurotomy and for botulinum toxin injection. Surg Radiol Anat. 2005;27:420–430. doi: 10.1007/s00276-005-0012-9. [DOI] [PubMed] [Google Scholar]
- 20.Standring S. Gray's anatomy: the anatomical basis of clinical practice. 39th ed. New York: Churchill Livingstone; 2005. pp. 520–521. [Google Scholar]
- 21.Won SY, Hur MS, Rha DW, Park HD, Hu KS, Fontaine C, Kim HJ. Extra- and intramuscular nerve distribution patterns of the muscles of the ventral compartment of the forearm. Am J Phys Med Rehabil. 2010;89:644–652. doi: 10.1097/PHM.0b013e3181d8a116. [DOI] [PubMed] [Google Scholar]
- 22.Park ES, Rha DW. Botulinum toxin type A injection for management of upper limb spasticity in children with cerebral palsy: a literature review. Yonsei Med J. 2006;47:589–603. doi: 10.3349/ymj.2006.47.5.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Standring S. Gray's anatomy: the anatomical basis of clinical practice. 39th ed. New York: Churchill Livingstone; 2005. pp. 1467–1468. [Google Scholar]
- 24.Ofluoglu D, Esquenazi A, Hirai B. Temporospatial parameters of gait after obturator neurolysis in patients with spasticity. Am J Phys Med Rehabil. 2003;82:832–836. doi: 10.1097/01.PHM.0000091986.32078.CD. [DOI] [PubMed] [Google Scholar]
- 25.Kwon JY, Kim JS, Lee WI. Anatomic localization of motor points of hip adductors. Am J Phys Med Rehabil. 2009;88:336–341. doi: 10.1097/PHM.0b013e3181619435. [DOI] [PubMed] [Google Scholar]
- 26.Childers MK. Targeting the neuromuscular junction in skeletal muscles. Am J Phys Med Rehabil. 2004;83(10 Suppl):S38–S44. doi: 10.1097/01.phm.0000141129.23219.42. [DOI] [PubMed] [Google Scholar]
- 27.Christensen E. Topography of terminal motor innervation in striated muscles from stillborn infants. Am J Phys Med. 1959;38:65–78. [PubMed] [Google Scholar]
- 28.Crystal R, Malone AA, Eastwood DM. Motor points for neuromuscular blockade of the adductor muscle group. Clin Orthop Relat Res. 2005;(437):196–200. doi: 10.1097/01.blo.0000165856.38166.9a. [DOI] [PubMed] [Google Scholar]
- 29.Van Campenhout A, Molenaers G. Localization of the motor endplate zone in human skeletal muscles of the lower limb: anatomical guidelines for injection with botulinum toxin. Dev Med Child Neurol. 2011;53:108–119. doi: 10.1111/j.1469-8749.2010.03816.x. [DOI] [PubMed] [Google Scholar]
- 30.Van Campenhout A, Hubens G, Fagard K, Molenaers G. Localization of motor nerve branches of the human psoas muscle. Muscle Nerve. 2010;42:202–207. doi: 10.1002/mus.21660. [DOI] [PubMed] [Google Scholar]
- 31.Kim SC, Kang MH, Ock JJ. Calf-contouring surgery of gastrocnemius hypertrophy: selective neurectomy of the sural nerve. Aesthetic Plast Surg. 2008;32:889–893. doi: 10.1007/s00266-007-9107-5. [DOI] [PubMed] [Google Scholar]
- 32.Tsai FC, Mardini S, Fong TH, Kang JH, Chou CM. Selective neurectomy of the gastrocnemius and soleus muscles for calf hypertrophy: an anatomical study and 700 clinical cases. Plast Reconstr Surg. 2008;122:178–187. doi: 10.1097/PRS.0b013e3181774199. [DOI] [PubMed] [Google Scholar]
- 33.Lemperle G, Exner K. The resection of gastrocnemius muscles in aesthetically disturbing calf hypertrophy. Plast Reconstr Surg. 1998;102:2230–2236. doi: 10.1097/00006534-199811000-00064. [DOI] [PubMed] [Google Scholar]
- 34.Han KH, Joo YH, Moon SE, Kim KH. Botulinum toxin A treatment for contouring of the lower leg. J Dermatolog Treat. 2006;17:250–254. doi: 10.1080/09546630600899070. [DOI] [PubMed] [Google Scholar]
- 35.Yoo WK, Chung IH, Park CI. Anatomic motor point localization for the treatment of gastrocnemius muscle spasticity. Yonsei Med J. 2002;43:627–630. doi: 10.3349/ymj.2002.43.5.627. [DOI] [PubMed] [Google Scholar]
- 36.Liu J, Kumar VP, Shen Y, Lau HK, Pereira BP, Pho RW. Modified Sihler's technique for studying the distribution of intramuscular nerve branches in mammalian skeletal muscle. Anat Rec. 1997;247:137–144. doi: 10.1002/(SICI)1097-0185(199701)247:1<137::AID-AR16>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 37.Sheverdin VA, Hur MS, Won SY, Song WC, Hu KS, Koh KS, Kim HJ. Extra- and intramuscular nerves distributions of the triceps surae muscle as a basis for muscle resection and botulinum toxin injections. Surg Radiol Anat. 2009;31:615–621. doi: 10.1007/s00276-009-0490-2. [DOI] [PubMed] [Google Scholar]
- 38.Dominiak M, Konopka T. Gingival esthetics in periodontology. E-Dentico. 2005;(2):6–15. [Google Scholar]
- 39.Reiser GM, Bruno JF, Mahan PE, Larkin LH. The subepithelial connective tissue graft palatal donor site: anatomic considerations for surgeons. Int J Periodontics Restorative Dent. 1996;16:130–137. [PubMed] [Google Scholar]
- 40.Cordioli G, Mortarino C, Chierico A, Grusovin MG, Majzoub Z. Comparison of 2 techniques of subepithelial connective tissue graft in the treatment of gingival recessions. J Periodontol. 2001;72:1470–1476. doi: 10.1902/jop.2001.72.11.1470. [DOI] [PubMed] [Google Scholar]