Abstract
The thymus is a central lymphoid organ for T cell development. Thymic epithelial cells (TECs) constitute a major component of the thymic stroma, which provides a specialized microenvironment for survival, proliferation, and differentiation of immature T cells. In this study, subsets of TECs were examined immunohistochemically to investigate their cytokeratin (CK) expression patterns during thymus regeneration following thymic involution induced by cyclophosphamide treatment. The results demonstrated that both normal and regenerating mouse thymuses showed a similar CK expression pattern. The major medullary TECs (mTEC) subset, which is stellate in appearance, exhibited CK5 and CK14 staining, and the minor mTEC subset, which is globular in appearance, exhibited CK8 staining, whereas the vast majority of cortical TECs (cTECs) expressed CK8 during thymus regeneration. Remarkably, the levels of CK5 and CK14 expression were enhanced in mTECs, and CK8 expression was upregulated in cTECs during mouse thymus regeneration after cyclophosphamide-induced acute thymic involution. Of special interest, a relatively high number of CK5+CK8+ TEC progenitors occurred in the thymic cortex during thymus regeneration. Taken together, these findings shed more light on the role of CK5, CK8, and CK14 in the physiology of TECs during mouse thymus regeneration, and on the characterization of TEC progenitors for restoration of the epithelial network and for concomitant regeneration of the adult thymus.
Keywords: Cytokeratin, Thymic epithelial cell, Thymic epithelial progenitor, Thymus regeneration
Introduction
The thymus is a central lymphoid organ for development of T cells from bone marrow-derived immature T cells. Thymic epithelial cells (TECs) constitute a major component of the thymic stroma, which provides a specialized microenvironment for T cell development [1]. TECs in the thymic cortex are responsible for attraction of T cell precursors, commitment to the T cell lineage, expansion of immature double negative thymocytes, and positive selection of double positive thymocytes [2-4]. The thymic medulla is composed of a heterogeneous population of epithelial cells that function in negative selection of single positive thymocytes [3-5].
The morphological heterogeneity of TECs has been well documented at the light and electron microscopic level [6-8]. It is important to understand the identification of the different types of TECs, as each type has distinct properties and functions in governing T cell development. TECs are traditionally divided into two major categories; cortical TECs (cTECs) and medullary TECs (mTECs), according to their location in the thymic lobule. A subset of mTECs, particularly in humans, is often concentrically arranged and forms Hassall's corpuscles. Furthermore, human TECs can be classified into several types based on their ultrastructural characteristics, including thymic subcapsular (subcapsular/paraseptal/perivascular), cortical or pale, intermediate, dark, undifferentiated, large medullary or cystic, and spindle-shaped medullary epithelial cells, and Hassall's corpuscles [9]. Generally, TECs are broadly classified into four types: subcapsular/paraseptal/perivascular, cortical and medullary epithelial cells, and Hassall's corpuscles, based on their immunohistochemical features [6, 7, 10, 11].
A unique characteristic of TECs among the various cell populations of the thymus is the presence of cytokeratin (CK) intermediate filaments, which form tonofilaments in the cytoplasm. Thus, antibodies against various types of CKs have been very useful for identifying TECs in the thymus. In humans, CKs comprise a family of at least 30 subtypes [12, 13]. Each CK subset expressed by an epithelial cell depends mainly on the type of epithelium, the stage of development and differentiation, and the functional states and pathological conditions [14, 15]. Immunohistochemical determination of CK polypeptides with highly specific antibodies is very useful for classifying and phenotypically characterizing subsets of TEC populations. Variations in CK composition in different parts of the human thymus have been demonstrated [16, 17]. Simple epithelium CKs such as CK8, CK18, and CK19 are present in the cortex, and stellate TECs in the medulla are positive for CK19 [18]. Most mTECs express complex epithelium CKs such as CK13, CK14, and CK17, in contrast to the cortex, where only a few cells are positive for these CKs [18]. In mice, antibodies specific to CK5, CK8, and CK14 have been most commonly used in studies to identify the main TEC subtypes [19-22].
Thus, in the present study, we characterized the CK5, CK8, and CK 14 expression patterns in TECs during thymus regeneration following acute involution induced by cyclophosphamide in mice.
Materials and Methods
Experimental acute thymic involution and regeneration model
Adult male, specific pathogen-free, C57BL/6 mice were purchased from Dae Han Bio Link (Seoul, Korea). They were housed three to four per cage and maintained under a 12 hour light/dark cycle at 24℃, in a specific pathogen-free, humidity-controlled facility, and were provided with standard sterile food and water ad libitum. The mice were allowed to acclimate to their environment for 1 week, and were used at 8-10-weeks-of-age. Because cyclophosphamide, a DNA alkylating agent commonly used in chemotherapy, is a long-established method for investigating thymic regeneration [23-28], the animals were given a single intraperitoneal dose of cyclophosphamide (400 mg/kg body weight, Sigma, St. Louis, MO, USA) in normal saline and were killed in groups of four or more at 3, 7, and 14 days after injection. Mice given the same volume of normal saline were used as controls. Animal care and all experimental procedures were conducted in accordance with the "Guide for Animal Experiments" published by the Korean Academy of Medical Sciences.
Tissue preparation for immunohistochemistry
Mice were anesthetized with a single intraperitoneal injection of sodium pentobarbital (5 mg/kg body weight). For cryosections, the thymus was removed and rapidly frozen in isopentane cooled with liquid nitrogen. Frozen sections (5-µm thick) were cut on a Reichert cryostat and placed on 3-aminopropyltriethoxysilane-coated slides. After being dried, the cryosections were fixed in cold acetone for 10 minutes at -20℃.
Immunohistochemistry
Immunostaining was performed using the streptavidin-biotin complex (ABC) method, as described previously [25-27]. Briefly, the sections were incubated for 20 minutes in a solution of phosphate-buffered saline (PBS), containing 0.3% H2O2, to eliminate endogenous peroxidases. After washing in PBS, the sections were incubated with 2% bovine serum albumin (Sigma). Excess solution was shaken off, and the sections were incubated for 16-18 hours at 4℃ with a rabbit anti-CK5 monoclonal antibody (mAb) raised against a synthetic peptide mapping to the carboxy terminus of human CK8 (EP1601Y, Thermo Scientific, Rockford, IL, USA), a rabbit anti-CK8 mAb raised against a synthetic peptide mapping to the carboxy terminus of human CK8 (EP1628Y, Thermo Scientific), and a rabbit polyclonal anti-CK14 antibody raised against a synthetic peptide mapping to the carboxy terminus of human CK14 (Thermo Scientific).
Following incubation with the primary antibody, the sections were washed three times with PBS for 5 minutes and incubated for 2 hours at room temperature with an affinity-purified F(ab')2 fragment donkey anti-rabbit biotinylated antibody (1 : 200, Jackson ImmunoResearch Laboratories, West Grove, PA, USA). The sections were then rinsed in PBS and incubated for 60 minutes at room temperature with an ABC reagent (Vectastain Elite Kit, Vector Laboratories, Burlingame, CA, USA), according to the manufacturer's instructions. The sections were developed in 0.025% 3,3'-diaminobenzidine and 0.003% H2O2 medium under microscopic control at room temperature to visualize peroxidase activity. The sections were rinsed in distilled water, counterstained with or without Mayer's hematoxylin, and mounted in a xylene-based mounting medium (Entellan, Darmstadt, Germany). Controls for the staining procedure included the following: (1) omission of the primary antibody from the reaction sequence and its replacement with nonimmune rabbit serum or (2) omission of the secondary antibody or the ABC solution from the reaction sequence. Light microscopic slides were observed and photographed using an Olympus BX50 microscope (Olympus, Tokyo, Japan). All photomicrographs were taken with an Olympus C-3030 digital camera.
Immunofluorescent staining
Two-color double immunofluorescent staining was performed to identify CK5+CK8+ TECs. After the sections were rinsed in PBS and incubated with 2% bovine serum albumin (Sigma) for 60 minutes, they were incubated with the first primary antibody (anti-CK5) for 16-18 hours at 4℃. Following the primary antibody incubation, the sections were incubated with an affinity-purified F(ab')2 fragment donkey anti-rabbit rhodamine-conjugated antibody (1 : 100, Jackson ImmunoResearch Laboratories). After the sections were rinsed in PBS, they were further incubated with the second primary antibody, which was a rat anti-CK8 mAb raised against mouse cytokeratin Endo-A (Troma-1, Developmental Studies Hybridoma Bank from The University of Iowa, Ames, IA, USA). Following the second primary antibody incubation, the sections were incubated with an affinity-purified F(ab')2 fragment donkey anti-rabbit FITC-conjugated antibody (1 : 80, Jackson ImmunoResearch Laboratories). Thereafter, the labeled cells were examined with a Zeiss Axio Imager light microscope (Carl Zeiss, Oberkochen, Germany) fitted with a filter combination to visualize green and red fluorescing cells. Photomicrographs were captured digitally at a 1,300×1,030 pixel resolution with a Photometrix CoolSnap Fx CCD-camera (Roper Scientific, Trenton, NJ, USA) on a Zeiss Axio Imager microscope equipped with fluorescent epi-illumination. The digital images were processed using an image analysis program software (MetaMorph, Universal Imaging, Downingtown, PA, USA).
Results
The entire thymic epithelial network was stained with anti-CK5, -CK8, and -CK14 antibodies using an immunoperoxidase technique. Identical immunofluorescent staining of both the cortical and medullary regions was obtained with these three antibodies (data not shown). Cells immunolabeled for CK5, CK8, and CK14 were observed in the normal and regenerating rat thymus and were identifiable as TECs by their morphological features, which showed a reticular network of cells in the cortex and medulla, and by their CK expression (Figs. 1-3). In contrast to the delicate and dendritic morphology of cTECs, most mTECs were stellate in appearance and closely packed, displaying voluminous cytoplasm and short processes (Figs. 1-3).
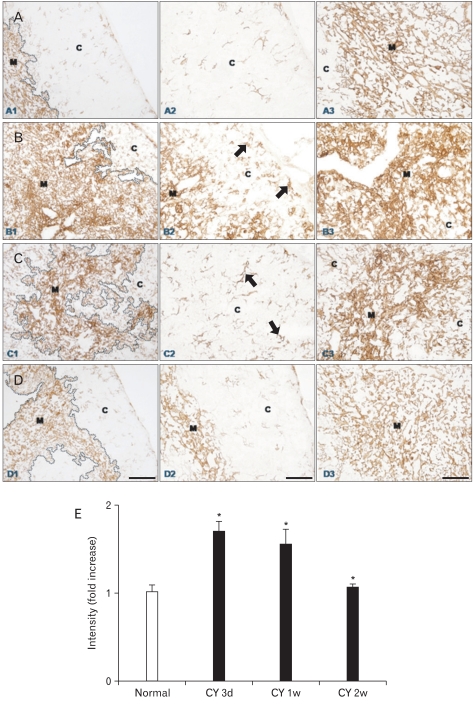
Fig. 1.
(A-D) Immunohistochemical localization of cytokeratin (CK)5 in frozen sections of mouse thymus from control animals (A1-3, n=4), and at 3 (B1-3, n=7), 7 (C1-3, n=6), and 14 (D1-3, n=4) days after cyclophosphamide treatment. C, cortex; M, medulla; arrows, CK5+ thymic epithelial cells. Scale bars=100 µm (A1, B1, C1, D1), 50 µm (A2, A3, B2, B3, C2, C3, D2, D3). (E) Data on the relative intensity of CK5 expression in the thymic medulla are also plotted in a bar graph and expressed as mean±standard deviation. CY 3d, CY 1w, and CY 2w represent 3, 7, and 14 days after cyclophosphamide treatment, respectively. *P<0.001 compared with the control, as determined by the t-test.
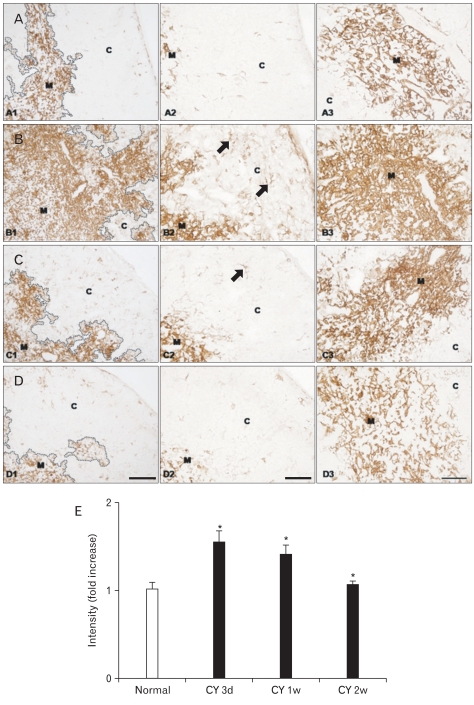
Fig. 3.
(A-D) Immunohistochemical localization of cytokeratin (CK)8 in frozen sections of mouse thymus from control animals (A1-3, n=4), and at 3 (B1-3, n=7), 7 (C1-3, n=6), and 14 (D1-3, n=4) days after cyclophosphamide treatment. C, cortex; M, medulla. Scale bars=100 µm (A1, B1, C1, D1), 50 µm (A2, A3, B2, B3, C2, C3, D2, D3). (E) Data on the relative intensity of CK8 expression in the thymic cortex are also plotted in a bar graph and expressed as mean±standard deviation. CY 3d, CY 1w, and CY 2w represent 3, 7, and 14 days after cyclophosphamide treatment, respectively. *P<0.001 compared with the control, as determined by the t-test.
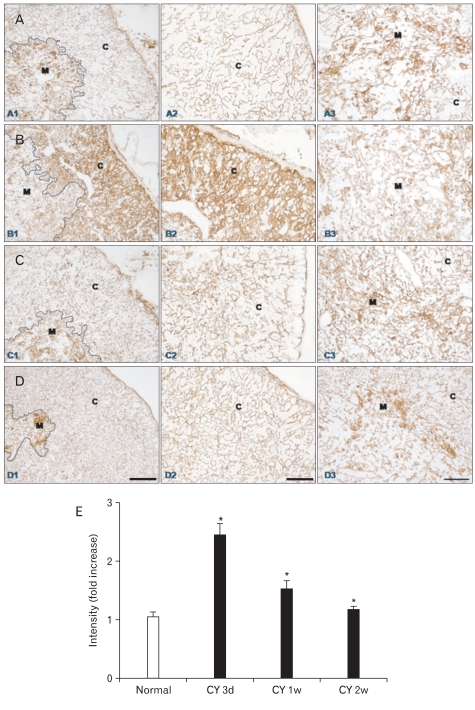
CK5 and CK14 were distributed in similar patterns in normal and regenerating thymuses with a stronger CK14 immunostaining intensity than that for CK5 (Figs. 1, 2). In normal mouse thymus, CK5 and CK14 immunoreactivity was observed in virtually all of the stellate mTECs, whereas the cTECs barely expressed CK5 and CK14 in the thymic cortex of normal mice (Figs. 1, 2, 4). We previously showed that the thymus undergoes time-dependent changes after cyclophosphamide treatment, which involve both lymphoid and stromal cells [24]. Essentially the same localization pattern of CK5 and CK14 immunoreactivity was observed in the thymuses of normal and cyclophosphamide-treated mice at all times (Figs. 1, 2). Characteristically, however, CK5 and CK14 protein expression was strongly upregulated in mTECs during thymus regeneration from acute thymic involution induced by cyclophosphamide treatment (Figs. 1, 2). Furthermore, and of particular interest, CK5- and CK14-positive TECs increased in number and in intensity in the cortex during regeneration from acute thymic involution induced by cyclophosphamide treatment (Figs. 1, 2). The CK5 and CK14 staining intensity and pattern and the number of CK5 and CK14 immunoreactive cTECs returned to levels similar to those of the normal mouse thymus 2 weeks after cyclophosphamide treatment, which coincided with the completion of thymic regeneration (Figs. 1, 2). No immunoreactivity was seen in control sections after omitting the primary antibody, secondary antibody, or the ABC reagent from the reaction sequence, or after replacing the primary antibody with a normal (nonimmune) rabbit IgG (data not shown).
Fig. 2.
(A-D) Immunohistochemical localization of cytokeratin (CK)14 in frozen sections of mouse thymus from control animals (A1-3, n=4), and at 3 (B1-3, n=7), 7 (C1-3, n=6), and 14 (D1-3, n=4) days after cyclophosphamide treatment. C, cortex; M, medulla; arrows, CK14+ thymic cortical epithelial cells. Scale bars=100 µm (A1, B1, C1, D1), 50 µm (A2, A3, B2, B3, C2, C3, D2, D3). (E) Data on the relative intensity of CK14 expression in the thymic medulla are also plotted in a bar graph and expressed as mean±standard deviation. CY 3d, CY 1w, and CY 2w represent 3, 7, and 14 days after cyclophosphamide treatment, respectively. *P<0.001 compared with the control, as determined by the t-test.
Fig. 4.
High magnification images of the stellate and globular thymic medullary epithelial cells (mTECs) by immunohistochemical localization of cytokeratin (CK)5, CK8, and CK14 in frozen sections of the mouse thymus from control animals. (A, B) CK5 and CK14 immunoreactivity was observed in virtually all of the stellate mTECs, whereas thymic cortical epithelial cells (cTECs) barely expressed CK5 and CK14 in the thymic cortex of normal mice. (C) CK8 was expressed by a minor subset of mTECs, most of which are globular in appearance, in the thymic medulla of control mice, whereas all the cTECs expressed CK8 in the thymic cortex of normal mice. C, cortex; M, medulla; arrows, CK8+ globular mTECs. Scale bars=50 µm.
CK8 immunoreactivity was observed in both cTECs and mTECs in normal mouse thymus (Fig. 3). The subcapsular/paraseptal subset of cTECs was flat and their cytoplasmic processes were very thin in control thymus (Fig. 3). These cTECs lined the capsule and the interlobular septum as a single, continuous layer, which had a smooth, even surface contour (Fig. 3). Perivascular TECs, which belong to the same subcapsular/paraseptal TEC subset, and resembled subcapsular/paraseptal TECs morphologically, surrounded blood vessels completely as a single, continuous layer (Fig. 3). Most cTECs in the thymic cortex, other than the subcapsular/paraseptal/perivascular cTEC subset, were thin and sheet like, displaying slender cytoplasmic processes and providing a large surface area for cell-cell interactions between TECs and developing thymocytes (Fig. 3). Additionally, CK8 was also expressed by the minor subset of mTECs in the thymic medulla of control mice, most of which were globular in appearance (Fig. 4).
Substantially the same localization pattern of CK8 immunoreactivity was observed in the thymuses of normal and cyclophosphamide-treated mice at all times (Fig. 3). Characteristically, however, strongly enhanced CK8 protein expression was observed in cTECs during thymus regeneration from acute thymic involution induced by cyclophosphamide treatment, particularly at 3 days after cyclophosphamide treatment (Fig. 3). However, CK8 expression by the minor globular mTEC subset decreased considerably during thymic regeneration, particularly at 3 days after cyclophosphamide treatment (Fig. 3). Morphological alterations in subcapsular/paraseptal/perivascular TECs during thymic regeneration were the same as those described in a previous study [25]. The staining intensity and expression pattern in CK8 immunoreactive TECs were similar to those of the control mouse thymus 1 week after cyclophosphamide treatment (Fig. 3).
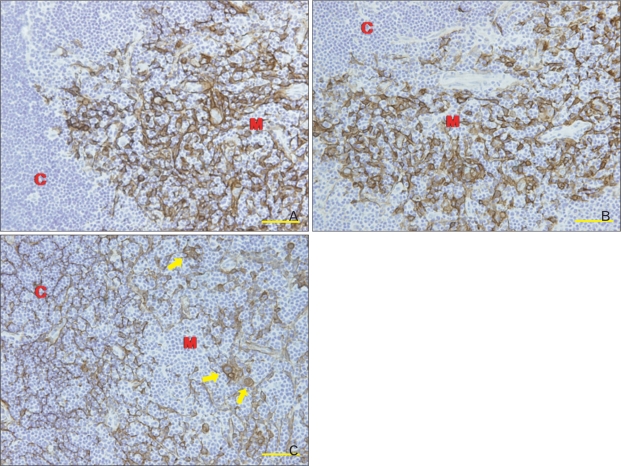
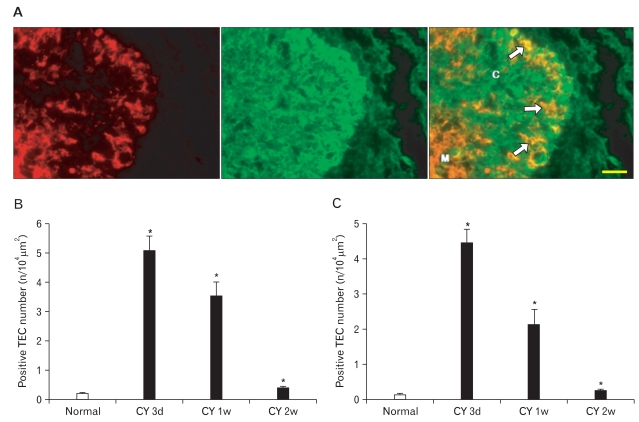
Double immunofluorescence staining of CK5 and CK8 clearly defined CK5+CK8+ TECs, which increased in number in the thymic cortex during thymus regeneration (Fig. 5).
Fig. 5.
(A) Two-color double-label immunofluorescent localization of cytokeratin (CK)8 (green) and CK5 (red) in frozen sections of mouse thymus 3 days after cyclophosphamide treatment. C, cortex; M, medulla; arrows, CK5+CK8+ thymic epithelial cells. Scale bar=50 µm. Data on the relative number of CK5+CK8+ (B) and CK14+CK8+ (C) Cotical thymic epithelial cells (cTECs) are also plotted in a bar graph and expressed as mean±standard deviation. *P<0.001 compared with the control, as determined by the t-test.
Discussion
This study was designed to examine the properties of mouse TECs from the viewpoint of CK5, CK8, and CK14 expression using immunohistochemical and immunofluorescent methods to clarify whether TEC phenotypes and expression levels change during mouse thymus regeneration following thymic involution induced by cyclophosphamide treatment. Our findings confirmed that both normal and regenerating mouse thymuses showed a substantially similar pattern of CK expression. The major mTEC subset, which was stellate in appearance, exhibited CK5 and CK14 staining, and the minor mTEC subset, which was globular in appearance, exhibited CK8 staining, whereas the vast majority of cTECs expressed CK8 during thymus regeneration. However, CK5+ and CK14+ cTECs, which were barely seen in the normal mouse thymic cortex, occurred in a relatively high number in the cortex during mouse thymus regeneration. Remarkably, the level of CK5 and CK14 expression was enhanced in mTECs, and CK8 expression was upregulated in cTECs, whereas the CK8 expression level was fairly diminished in globular mTECs during mouse thymus regeneration after cyclophosphamide-induced acute thymic involution. Generally, these changes in CK5, CK8, and CK14 expression level became similar to those of normal thymus 2 weeks after cyclophosphamide treatment at approximately the same time when thymus regeneration was almost completed. The levels of CK5, CK8, and CK14 increased in TECs during thymus regeneration. It has been demonstrated that CK5, CK8, and CK14 are overexpressed in various cancer cells including human breast carcinoma, lung squamous cell carcinoma, and gastric adenocarcinoma [29-34], suggesting that elevation of these CKs could promote cell survival and proliferation. Moreover, CK8 and CK14 suppress apoptosis induced by a variety of agents in various epithelial cells [35, 36]. Thus, it is speculated that the enhanced expression of CK5, CK8, and CK14 may be involved in various cellular activities, including survival of TECs during thymus regeneration.
Our findings strongly indicate that the anti-CK5 and anti-CK14 antibodies can be utilized as a tool for immunohistological analysis of mTECs, and that the anti-CK8 mAb used in this study can be applied for immunohistological analysis of entire cTEC populations and minor globular mTECs in both normal and regenerating mouse thymuses. Additionally and most importantly, the anti-CK5 and anti-CK14 antibodies used in this study can also be employed to identify CK5- or CK14-positive cTECs by immunohistochemical or immunofluorescence methods in both normal and regenerating mouse thymuses throughout the entire period of mouse thymus regeneration.
Our observations are in agreement with those of many other studies in which various antibodies were directed against CK5, CK8, and CK14. For example, CK5 and CK14 are expressed by the predominant mTECs using a rabbit polyclonal antibody (AF 138) specific to mouse CK5 and a rabbit polyclonal antibody (AF 64) specific to mouse CK14 obtained from Covance Research (Princeton, NJ, USA) [20-22]. These anti-CK5 and anti-CK14 antibodies were originally developed by Roop et al. [37] using synthetic peptides corresponding to the carboxyl-terminal amino acid sequences of mouse CK5 and CK14, and their immunoreactive properties against mouse thymus are well characterized [19]. Our data using a rabbit anti-CK5 mAb (clone EP1601Y, Thermo Scientific) directed against a synthetic peptide corresponding to residues on the carboxyl-terminal of human CK5, and a rabbit polyclonal antibody directed against a synthetic peptide derived from the carboxyl-terminal of human CK14 (Thermo Scientific) coincide with previous results showing that in normal mouse thymus, CK5 expression is found together with CK14 expression in stellate medullary TECs.
In agreement with previous results using a rat mAb specific for mouse CK8 (Troma-1, the Developmental Studies Hybridoma Bank), our data using a rabbit anti-CK8 mAb (EP1628Y, Thermo Scientific) directed against a synthetic peptide corresponding to residues on the carboxyl-terminal of human CK8 confirmed that in normal mouse thymus, CK8 is expressed by the vast majority of cTECs, including subcapsular TECs, which have a distinctive reticular appearance, and by the minority of mTECs, which have a distinctive globular appearance [19, 20]. This anti-CK8 mAb (Troma-1) was originally developed by Kemler et al. [38]. Thus, the medulla of the thymic lobule in mice consisted of two main populations of TECs: CK5+ or CK14+ stellate mTECs, the major mTEC subset, which did not express CK8, and CK8+ globular mTECs, the minor mTEC subset, which did not express CK5 or CK14. These two mTEC subsets were also defined by differential reactivity with the lectin UEA-1 and mAbs that recognize classical versus nonpolymorphic major histocompatibility complex molecules. The major CK5+ or CK14+ stellate mTEC subset does not express UEA-1 or Ia, but the minor CK8+ globular mTECs express UEA-1 and Ia [19, 39, 40].
One of the most salient features observed in our experimental thymus regeneration model was the occurrence of a relatively high number of CK5+CK8+ TECs in the thymic cortex during thymus regeneration. An emerging body of data supports the existence of thymic epithelial progenitor/stem cells during both the embryonic and postnatal period, which have the CK5+CK8+ phenotype [41]. Similar to our results, García-Ceca et al. [42] demonstrated that high numbers of CK5+CK8+ TEC progenitors occur in both wild-type and EphB-deficient thymic parenchyma after fetal thymus lobes were grafted under the kidney capsule of mice, and these CK5+CK8+ TEC progenitors reached higher numbers in the grafted EphB-deficient thymus lobes than in the wild-type ones. High numbers of CK5+CK8+ TEC progenitors have also been reported in other mice with defective maturation of thymic epithelium, such as Foxn1Δ/Δ mice [43], conditioned Stat3-deficient mice [44], and Krm1-/- mice [45]. Popa et al. [46] confirmed that CK5+CK8+ TEC progenitors in the thymic cortex expand significantly during thymus atrophy induced by steroid treatment or by irradiation and prior to thymic regeneration. They also showed that in involuted thymuses, cMyc and Trp63 transcription factors, known to be expressed in early epithelial cell progenitors, are expressed in a subset of cortical CK5+CK8+ TEC progenitors [46]. Thus, CK5+CK8+ cTECs, which emerged in the thymic cortex during mouse thymus regeneration, are obviously regarded as thymic epithelial progenitor cells. Although the precise role of CK5+CK8+ TEC progenitors during thymus regeneration after cyclophosphamide-induced acute thymic involution remains to be elucidated, our results suggest that these TEC progenitors may be involved in replenishing TECs during thymus regeneration.
Taken together, our data shed more light on the role of CK5, CK8, and CK14 in TECs and strongly indicate that the antibodies specific to these CKs used in this study can be utilized as a tool for immunohistological analysis of TEC subsets in the thymus during mouse thymus regeneration. In addition, our results demonstrated that critical steps in the recovery of the adult thymus include expansion of CK5+CK8+ TEC progenitors and elevated expression of CK5 and CK14 in mTECs and CK8 in cTECs during thymus regeneration, suggesting that CK5+CK8+ TEC progenitors are activated during thymus regeneration and may be involved in restoration of the epithelial network and consequent regeneration of the adult thymus.
Acknowledgements
This work was supported for 2 years by a Pusan National University Research Grant.
References
- 1.Boyd RL, Tucek CL, Godfrey DI, Izon DJ, Wilson TJ, Davidson NJ, Bean AG, Ladyman HM, Ritter MA, Hugo P. The thymic microenvironment. Immunol Today. 1993;14:445–459. doi: 10.1016/0167-5699(93)90248-J. [DOI] [PubMed] [Google Scholar]
- 2.Savage PA, Davis MM. A kinetic window constricts the T cell receptor repertoire in the thymus. Immunity. 2001;14:243–252. doi: 10.1016/s1074-7613(01)00106-6. [DOI] [PubMed] [Google Scholar]
- 3.Takahama Y. Journey through the thymus: stromal guides for T-cell development and selection. Nat Rev Immunol. 2006;6:127–135. doi: 10.1038/nri1781. [DOI] [PubMed] [Google Scholar]
- 4.Osada M, Jardine L, Misir R, Andl T, Millar SE, Pezzano M. DKK1 mediated inhibition of Wnt signaling in postnatal mice leads to loss of TEC progenitors and thymic degeneration. PLoS One. 2010;5:e9062. doi: 10.1371/journal.pone.0009062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barclay AN, Mayrhofer G. Bone marrow origin of Ia-positive cells in the medulla rat thymus. J Exp Med. 1981;153:1666–1671. doi: 10.1084/jem.153.6.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Waal EJ, Rademakers LH. Heterogeneity of epithelial cells in the rat thymus. Microsc Res Tech. 1997;38:227–236. doi: 10.1002/(SICI)1097-0029(19970801)38:3<227::AID-JEMT4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 7.Schuurman HJ, Kuper CF, Kendall MD. Thymic microenvironment at the light microscopic level. Microsc Res Tech. 1997;38:216–226. doi: 10.1002/(SICI)1097-0029(19970801)38:3<216::AID-JEMT3>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 8.Von Gaudecker B, Kendall MD, Ritter MA. Immuno-electron microscopy of the thymic epithelial microenvironment. Microsc Res Tech. 1997;38:237–249. doi: 10.1002/(SICI)1097-0029(19970801)38:3<237::AID-JEMT5>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 9.van de Wijngaert FP, Kendall MD, Schuurman HJ, Rademakers LH, Kater L. Heterogeneity of epithelial cells in the human thymus: an ultrastructural study. Cell Tissue Res. 1984;237:227–237. doi: 10.1007/BF00217140. [DOI] [PubMed] [Google Scholar]
- 10.Haynes BF. The human thymic microenvironment. Adv Immunol. 1984;36:87–142. doi: 10.1016/s0065-2776(08)60900-1. [DOI] [PubMed] [Google Scholar]
- 11.Rouse RV, Bolin LM, Bender JR, Kyewski BA. Monoclonal antibodies reactive with subsets of mouse and human thymic epithelial cells. J Histochem Cytochem. 1988;36:1511–1517. doi: 10.1177/36.12.2461413. [DOI] [PubMed] [Google Scholar]
- 12.Moll R, Franke WW, Schiller DL, Geiger B, Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982;31:11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- 13.Heid HW, Moll I, Franke WW. Patterns of expression of trichocytic and epithelial cytokeratins in mammalian tissues. II. Concomitant and mutually exclusive synthesis of trichocytic and epithelial cytokeratins in diverse human and bovine tissues (hair follicle, nail bed and matrix, lingual papilla, thymic reticulum) Differentiation. 1988;37:215–230. doi: 10.1111/j.1432-0436.1988.tb00724.x. [DOI] [PubMed] [Google Scholar]
- 14.Banks-Schlegel SP. Keratin alterations during embryonic epidermal differentiation: a presage of adult epidermal maturation. J Cell Biol. 1982;93:551–559. doi: 10.1083/jcb.93.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Linder S. Cytokeratin markers come of age. Tumour Biol. 2007;28:189–195. doi: 10.1159/000107582. [DOI] [PubMed] [Google Scholar]
- 16.Laster AJ, Itoh T, Palker TJ, Haynes BF. The human thymic microenvironment: thymic epithelium contains specific keratins associated with early and late stages of epidermal keratinocyte maturation. Differentiation. 1986;31:67–77. doi: 10.1111/j.1432-0436.1986.tb00385.x. [DOI] [PubMed] [Google Scholar]
- 17.Savino W, Dardenne M. Immunohistochemical studies on a human thymic epithelial cell subset defined by the anti-cytokeratin 18 monoclonal antibody. Cell Tissue Res. 1988;254:225–231. doi: 10.1007/BF00220038. [DOI] [PubMed] [Google Scholar]
- 18.Shezen E, Okon E, Ben-Hur H, Abramsky O. Cytokeratin expression in human thymus: immunohistochemical mapping. Cell Tissue Res. 1995;279:221–231. doi: 10.1007/BF00300707. [DOI] [PubMed] [Google Scholar]
- 19.Klug DB, Carter C, Crouch E, Roop D, Conti CJ, Richie ER. Interdependence of cortical thymic epithelial cell differentiation and T-lineage commitment. Proc Natl Acad Sci U S A. 1998;95:11822–11827. doi: 10.1073/pnas.95.20.11822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klug DB, Carter C, Gimenez-Conti IB, Richie ER. Cutting edge: thymocyte-independent and thymocyte-dependent phases of epithelial patterning in the fetal thymus. J Immunol. 2002;169:2842–2845. doi: 10.4049/jimmunol.169.6.2842. [DOI] [PubMed] [Google Scholar]
- 21.Kuraguchi M, Wang XP, Bronson RT, Rothenberg R, Ohene-Baah NY, Lund JJ, Kucherlapati M, Maas RL, Kucherlapati R. Adenomatous polyposis coli (APC) is required for normal development of skin and thymus. PLoS Genet. 2006;2:e146. doi: 10.1371/journal.pgen.0020146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liepinsh DJ, Kruglov AA, Galimov AR, Shakhov AN, Shebzukhov YV, Kuchmiy AA, Grivennikov SI, Tumanov AV, Drutskaya MS, Feigenbaum L, Kuprash DV, Nedospasov SA. Accelerated thymic atrophy as a result of elevated homeostatic expression of the genes encoded by the TNF/lymphotoxin cytokine locus. Eur J Immunol. 2009;39:2906–2915. doi: 10.1002/eji.200839191. [DOI] [PubMed] [Google Scholar]
- 23.Milićević NM, Milićević Z, Piletić O, Mujović S, Ninkov V. Patterns of thymic regeneration in rats after single or divided doses of cyclophosphamide. J Comp Pathol. 1984;94:197–202. doi: 10.1016/0021-9975(84)90040-9. [DOI] [PubMed] [Google Scholar]
- 24.Yoon S, Yoo YH, Kim BS, Kim JJ. Ultrastructural alterations of the cortical epithelial cells of the rat thymus after cyclophosphamide treatment. Histol Histopathol. 1997;12:401–413. [PubMed] [Google Scholar]
- 25.Yoon S, Lee HW, Baek SY, Kim BS, Kim JB, Lee SA. Upregulation of TrkA neurotrophin receptor expression in the thymic subcapsular, paraseptal, perivascular, and cortical epithelial cells during thymus regeneration. Histochem Cell Biol. 2003;119:55–68. doi: 10.1007/s00418-002-0486-z. [DOI] [PubMed] [Google Scholar]
- 26.Lee HW, Kim BS, Kim HJ, Lee CW, Yoo HJ, Kim JB, Yoon S. Upregulation of receptor activator of nuclear factor-kappaB ligand expression in the thymic subcapsular, paraseptal, perivascular, and medullary epithelial cells during thymus regeneration. Histochem Cell Biol. 2005;123:491–500. doi: 10.1007/s00418-005-0751-z. [DOI] [PubMed] [Google Scholar]
- 27.Lee HW, Kim SM, Shim NR, Bae SK, Jung IG, Kwak JY, Kim BS, Kim JB, Moon JO, Chung JS, Yoon S. Expression of nerve growth factor is upregulated in the rat thymic epithelial cells during thymus regeneration following acute thymic involution. Regul Pept. 2007;141:86–95. doi: 10.1016/j.regpep.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 28.Kim YM, Kim HK, Kim HJ, Lee HW, Ju SA, Choi BK, Kwon BS, Kim BS, Kim JB, Lim YT, Yoon S. Expression of 4-1BB and 4-1BBL in thymocytes during thymus regeneration. Exp Mol Med. 2009;41:896–911. doi: 10.3858/emm.2009.41.12.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lavialle C, Modjtahedi N, Lamonerie T, Frebourg T, Landin RM, Fossar N, Lhomond G, Cassingena R, Brison O. The human breast carcinoma cell line SW 613-S: an experimental system to study tumor heterogeneity in relation to c-myc amplification, growth factor production and other markers (review) Anticancer Res. 1989;9:1265–1279. [PubMed] [Google Scholar]
- 30.Tsubokawa F, Nishisaka T, Takeshima Y, Inai K. Heterogeneity of expression of cytokeratin subtypes in squamous cell carcinoma of the lung: with special reference to CK14 overexpression in cancer of high-proliferative and lymphogenous metastatic potential. Pathol Int. 2002;52:286–293. doi: 10.1046/j.1440-1827.2002.01353.x. [DOI] [PubMed] [Google Scholar]
- 31.He QY, Cheung YH, Leung SY, Yuen ST, Chu KM, Chiu JF. Diverse proteomic alterations in gastric adenocarcinoma. Proteomics. 2004;4:3276–3287. doi: 10.1002/pmic.200300916. [DOI] [PubMed] [Google Scholar]
- 32.Lau AT, Chiu JF. The possible role of cytokeratin 8 in cadmium-induced adaptation and carcinogenesis. Cancer Res. 2007;67:2107–2113. doi: 10.1158/0008-5472.CAN-06-3771. [DOI] [PubMed] [Google Scholar]
- 33.Tischkowitz M, Brunet JS, Bégin LR, Huntsman DG, Cheang MC, Akslen LA, Nielsen TO, Foulkes WD. Use of immunohistochemical markers can refine prognosis in triple negative breast cancer. BMC Cancer. 2007;7:134. doi: 10.1186/1471-2407-7-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kabos P, Haughian JM, Wang X, Dye WW, Finlayson C, Elias A, Horwitz KB, Sartorius CA. Cytokeratin 5 positive cells represent a steroid receptor negative and therapy resistant subpopulation in luminal breast cancers. Breast Cancer Res Treat. 2010 Jul 28; doi: 10.1007/s10549-010-1078-6. [Epub] DOI: 10.1007/s10549-010-1078-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Caulin C, Ware CF, Magin TM, Oshima RG. Keratin-dependent, epithelial resistance to tumor necrosis factor-induced apoptosis. J Cell Biol. 2000;149:17–22. doi: 10.1083/jcb.149.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Inada H, Izawa I, Nishizawa M, Fujita E, Kiyono T, Takahashi T, Momoi T, Inagaki M. Keratin attenuates tumor necrosis factor-induced cytotoxicity through association with TRADD. J Cell Biol. 2001;155:415–426. doi: 10.1083/jcb.200103078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roop DR, Cheng CK, Titterington L, Meyers CA, Stanley JR, Steinert PM, Yuspa SH. Synthetic peptides corresponding to keratin subunits elicit highly specific antibodies. J Biol Chem. 1984;259:8037–8040. [PubMed] [Google Scholar]
- 38.Kemler R, Brûlet P, Schnebelen MT, Gaillard J, Jacob F. Reactivity of monoclonal antibodies against intermediate filament proteins during embryonic development. J Embryol Exp Morphol. 1981;64:45–60. [PubMed] [Google Scholar]
- 39.Farr AG, Anderson SK. Epithelial heterogeneity in the murine thymus: fucose-specific lectins bind medullary epithelial cells. J Immunol. 1985;134:2971–2977. [PubMed] [Google Scholar]
- 40.Surh CD, Gao EK, Kosaka H, Lo D, Ahn C, Murphy DB, Karlsson L, Peterson P, Sprent J. Two subsets of epithelial cells in the thymic medulla. J Exp Med. 1992;176:495–505. doi: 10.1084/jem.176.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang L, Sun L, Zhao Y. Thymic epithelial progenitor cells and thymus regeneration: an update. Cell Res. 2007;17:50–55. doi: 10.1038/sj.cr.7310114. [DOI] [PubMed] [Google Scholar]
- 42.García-Ceca J, Jiménez E, Alfaro D, Cejalvo T, Muñoz JJ, Zapata AG. Cell-autonomous role of EphB2 and EphB3 receptors in the thymic epithelial cell organization. Eur J Immunol. 2009;39:2916–2924. doi: 10.1002/eji.200939437. [DOI] [PubMed] [Google Scholar]
- 43.Su DM, Navarre S, Oh WJ, Condie BG, Manley NR. A domain of Foxn1 required for crosstalk-dependent thymic epithelial cell differentiation. Nat Immunol. 2003;4:1128–1135. doi: 10.1038/ni983. [DOI] [PubMed] [Google Scholar]
- 44.Sano S, Takahama Y, Sugawara T, Kosaka H, Itami S, Yoshikawa K, Miyazaki J, van Ewijk W, Takeda J. Stat3 in thymic epithelial cells is essential for postnatal maintenance of thymic architecture and thymocyte survival. Immunity. 2001;15:261–273. doi: 10.1016/s1074-7613(01)00180-7. [DOI] [PubMed] [Google Scholar]
- 45.Osada M, Ito E, Fermin HA, Vazquez-Cintron E, Venkatesh T, Friedel RH, Pezzano M. The Wnt signaling antagonist Kremen1 is required for development of thymic architecture. Clin Dev Immunol. 2006;13:299–319. doi: 10.1080/17402520600935097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Popa I, Zubkova I, Medvedovic M, Romantseva T, Mostowski H, Boyd R, Zaitseva M. Regeneration of the adult thymus is preceded by the expansion of K5+K8+ epithelial cell progenitors and by increased expression of Trp63, cMyc and Tcf3 transcription factors in the thymic stroma. Int Immunol. 2007;19:1249–1260. doi: 10.1093/intimm/dxm092. [DOI] [PubMed] [Google Scholar]