Abstract
It is known that angiotensin converting enzyme inhibitors (ACEIs) and angiotensin II type-1 receptor blockers (ARBs) can be used to mitigate radiation-induced renal injury. However, for a variety of reasons, these previous results are not directly applicable to the development of agents for the mitigation of injuries caused by terrorism-related radiation exposure. As part of an effort to develop an animal model that would fit the requirements of the U.S. Food and Drug Administration (FDA) “Animal Efficacy Rule”, we designed new studies which used an FDA-approved ACEI (captopril) or an FDA-approved ARB (losartan, Cozaar®) started 10 days after a single total-body irradiation (TBI) at drug doses that are equivalent (on a g/m2/day basis) to the doses prescribed to humans. Captopril and losartan were equally effective as mitigators, with DMFs of 1.23 and 1.21, respectively, for delaying renal failure. These studies show that radiation nephropathy in a realistic rodent model can be mitigated with relevant doses of FDA-approved agents. This lays the necessary groundwork for pivotal rodent studies under the FDA Animal Efficacy Rule and provides an outline of how the FDA-required large-animal studies could be designed.
INTRODUCTION
A major effort is under way in the U.S. to develop agents that could be used to mitigate and/or treat radiation injuries in the event of a radiological terrorism incident (1). In this context, mitigation refers to therapies begun after irradiation but before there is clinical evidence of injury, whereas treatment refers to therapies begun after there is such evidence (2). We have previously shown that angiotensin converting enzyme inhibitors (ACEIs) and an angiotensin II type-1 receptor blocker (ARB) could be used to mitigate radiation-induced renal injury (3, 4). However, these previous results are not directly applicable to the development of agents for the mitigation of injuries caused by terrorism-related radiation exposure because the ARB we had been using, L-158,809 (5), was not approved for human use, we had used the ACEIs at doses above those commonly used in humans, and we had shown efficacy only after fractionated irradiation. As part of an effort to develop an animal model and an experimental protocol that would fit the requirements of the U.S. Food and Drug Administration (FDA) “Animal Efficacy Rule” (6, 7), we designed and conducted new studies that used an FDA-approved ACEI (captopril) or an FDA-approved ARB (losartan, Cozaar®) given after a single total-body irradiation (TBI) at drug doses that are equivalent (on a g/m2/day basis) to the doses prescribed to humans. We then examined the merits of a wide range of methods for quantifying the relative efficacy of the agents.
MATERIALS AND METHODS
Rat Syngeneic Bone Marrow Transplant (BMT) Model
Single-dose total-body radiation regimens were used to cause radiation nephropathy (8, 9). This syndrome is characterized by proteinuria, azotemia and progressive hypertension that leads to renal failure as early as 15 weeks after TBI (8, 9). The studies were performed in syngeneic WAG/RijCmcr rats that were bred and housed in a moderate-security barrier. The animals were free of Mycoplasma pulmonis, Pseudomonas and common rat viruses. No antibiotics or immunosuppressive drugs were used. The rats were maintained in the Biomedical Resource Center of the Medical College of Wisconsin, which is fully accredited by the American Association of Accreditation of Laboratory Animal Care. The animal protocols were approved by the Institutional Animal Care and Use Committee (IACUC).
Seven- to 8-week-old male rats underwent TBI given as a single dose at a dose rate of 1.95 Gy/min. Irradiation was done with a 300 kVp orthovoltage source with a half-value layer of 1.4 mm copper; the radiation dosimetry is described in detail in Cohen et al. (10). For irradiation, unanesthetized rats were immobilized in a specially constructed jig (8). Within 24 h after TBI, the rats received a BMT from a syngeneic donor (8).
Experimental Design
Radiation doses of 7.8, 8.9, 10, 11.1, 12.2 or 13.3 Gy were used. The lower dose limit was set at 7.8 Gy because doses appreciably below this do not produce significant renal injury within 26 weeks (8). The upper dose was set at 13.3 Gy because higher doses cause acute gastrointestinal mortality (8). After irradiation, animals were randomized to receive no further treatment, captopril in the drinking water at 150 mg/liter, or losartan in the drinking water at 100 mg/liter; the 7.8-Gy dose was used only for animals receiving no further treatment, and the 13.3-Gy dose was used only for animals receiving captopril or losartan. There were six animals in all groups except the 10-Gy captopril-treated group, where only five animals were available. Drug therapy was begun at 10 days after irradiation and was continued for 26 weeks. The start of therapy was delayed because in a mass-casualty situation it is unlikely that therapies could be started immediately. In addition, prior experimental studies have shown that such a delay has no impact on the efficacy of mitigation (11) and, in fact, that the use of captopril only in the days immediately before or after irradiation is ineffective (12). Drug therapy was stopped at 6 months for two reasons. First, if a human subject did not develop renal injury after irradiation, drug therapy would not be continued indefinitely; second, we know from prior animal studies that if a mitigation agent does not work after 6 months of therapy, it is not going to work for longer treatments (12). The resulting schedule closely resembles the way in which captopril was used to mitigate radiation injuries in humans receiving TBI in preparation for BMT (13).
Captopril was obtained in USP grade from Sigma-Aldrich (St. Louis, MO); pharmaceutical-grade losartan (Cozaar®) was a gift from Merck & Co (Rahway, NJ). The drug doses were chosen to match, on a mg/m2/day basis, those commonly used in humans for treatment of hypertension, kidney disease and heart disease. For captopril, a typical adult human dose is 75–150 mg/day or 45–90 mg/m2/day; captopril in the drinking water at 150 mg/liter delivers 70–90 mg/m2/day to a rat, or about 17 mg/kg/day. For losartan, a typical adult human dose is 50–100 mg/day; a rat dose of 100 mg/liter in drinking water will deliver an equivalent dose (11 mg/kg/day) on a mg/m2/day basis.
Monitoring the Development of Radiation Nephropathy
Animals were monitored daily. Development of azotemia (as BUN) was assessed for up to 47 weeks after TBI, and animals who were moribund or whose blood urea nitrogen (BUN) exceeded 120 mg/dl were euthanized. The BUN >120 mg/dl criterion for euthanasia was based on negotiations with the IACUC, which had decided that our previous criteria for euthanasia were no longer satisfactory (see the Results for further discussion of the euthanasia criteria). The study was terminated after 47 weeks because the number of survivors was too low to justify further follow-up. BUN, urine protein and urine creatinine were determined for all animals at 17, 26 and 47 weeks using commercial kits (14); additional BUN determinations were done if animals were moribund and at intermediate times for animals that had high BUN at a prior check. Urine protein excretion is expressed as the ratio of urine protein to urine creatinine (UP/UC) in the same urine sample; this is done to account for the known urine-concentrating defect that occurs in renal radiation injury and to normalize for animal size differences. Systolic blood pressure (BP) was measured at 17, 26 and 47 weeks with a tail cuff (IITC Life Science Inc., Woodland Hills, CA). Animals were conditioned to the apparatus and the reported BP was the average of readings on three consecutive days.
In the 13.3-Gy groups, nine animals were lost to acute GI toxicity or pneumonitis by 10 weeks, and these groups were excluded from further analysis. Three other animals died without high BUN and were also excluded from further analysis; they were all in TBI-alone groups, one 7.8-Gy animal at 2 weeks, one 8.9-Gy animal at 7 weeks, and one 12.2-Gy animal at 1 day.
Statistical Methods
Depending on the type of data analyzed, three different methods were used to calculate dose-modifying factors (DMFs). Where the end point was a real number and there were no missing data (e.g., time to renal failure at doses where all animals developed renal failure), a linear regression technique was used (15) that is mathematically equivalent to that used to derive hypoxic fractions from paired survival curve data (16). Parallel dose–response curves (log dose as the independent variable and end point as the dependent variable) were fitted to the raw data by linear regression and tested for goodness of fit by assessing whether there were deviations from linearity and parallelism. The horizontal difference between the parallel curves, log (DMF), and the 95% confidence interval on this difference were then derived by linear regression analysis.
Where the end point was a real number but data were missing because some animals had already been euthanized for high BUN (e.g., BUN after 47 weeks of follow-up), the nonparametric method of Kellerer and Brenot (17) was used. In this procedure all animals that had been euthanized for high BUN were assigned an outcome more severe than that for any survivor.
Where the end point was quantal (i.e., incidence of renal failure), probit analysis (18) was used to calculate DMFs using a technique that is mathematically equivalent to that used to derive hypoxic fractions from paired tumor control data (16). Curves (log dose as the independent variable and incidence as the dependent variable) were fitted to the raw data by probit analysis and tested for goodness of fit by assessing whether there were deviations from linearity and parallelism. The horizontal difference between the parallel curves, log (DMF), and the 95% confidence interval on this difference were then derived by probit analysis.
Correlations were analyzed with the Kendall rank correlation test.
RESULTS
Development of a Clinically Relevant End Point for Radiation Nephropathy
The FDA Animal Efficacy Rule (6) requires that the end points in an animal model be “clearly related to the desired benefit in humans, generally the enhancement of survival or prevention of major morbidity.” In this case, the objective is to prevent, or at least delay, radiation-induced renal failure. In previous studies with the rat radiation nephropathy model we defined renal failure as “uremia” (19) or as “lethargy, dehydration and severe azotemia” (20). These definitions have a subjective component, and they require a degree of morbidity that our current IACUC requires us to avoid. We reevaluated animals from several previous studies (14, 19, 20) and found that a BUN level greater than 120 mg/dl was always followed by “renal failure” (Fig. 1). More precisely, 41 of 44 animals with BUN ≥ 120 mg/dl had developed renal failure within 8.5 weeks (median of 5.3 weeks) of the BUN measurement, and all had developed renal failure within 13 weeks. This relationship was valid regardless of whether the animals had received TBI alone or were treated with an effective mitigator (Fig. 1). Based on this analysis we now define renal failure as development of BUN ≥ 120 mg/dl. Time to renal failure is based on logarithmic interpolation between the last BUN < 120 mg/dl and the first BUN ≥ 120 mg/dl.
FIG. 1.

Relationship of measured blood urea nitrogen (BUN) after TBI and time to development of uremia, where uremia is defined as a degree of azotemia incompatible with survival. Data are from animals described previously by Moulder et al. (19, 20) and Cohen et al. (14). Open circles show medians and ranges (minimum–maximum) for groups of animals (n = 20) that received TBI alone or TBI plus ineffective mitigators (i.e., low-salt diets, verapamil); closed circles are for individual animals that were given effective mitigators (i.e., captopril, enalapril, high-salt diet). Up-arrows indicate that some animals in the group had not developed azotemia sufficient to require euthanasia after more than 23 weeks of further follow-up. BUN was measured between 17 and 47 weeks after irradiation.
Effect of Captopril and Losartan on Development of TBI-Induced Renal Failure
Total-body irradiation at doses of 8.9 Gy and above caused renal failure in animals by 41 weeks after irradiation if no mitigation therapy was used (Fig. 2). Both captopril (17 mg/kg/day) and losartan (11 mg/kg/day), when used in a mitigation regimen (drug started 10 days after TBI and continued for 26 weeks), delayed renal failure by 10–30 weeks (Fig. 2). DMFs were calculated using both linear regression analysis and by probit analysis, because each technique has theoretical advantages and disadvantages.
FIG. 2.

Time to reach BUN ≥ 120 mg/dl as a function of total-body radiation dose. Data are shown as medians and ranges (minimum–maximum, n = 5–6 per group) for animals receiving TBI alone (●), TBI plus captopril at 17 mg/kg/day (○), or TBI plus losartan at 11 mg/kg/day (□). Drug therapy started 10 days after TBI and continued for 26 weeks. Up-arrows indicate that some animals in the group had still not reached 120 mg/dl after 47 weeks. At 8.9 Gy, four of six animals in the captopril group and three of six animals in the losartan group had still not reached 120 mg/dl after 47 weeks.
DMFs were calculated by applying linear regression analysis to all groups in which all animals developed renal failure; this meant excluding the 7.8-Gy TBI-alone group, the captopril group at 8.9 Gy, and the losartan groups at 8.9 and 10 Gy. The DMFs (with 95% confidence intervals) were 1.23 for captopril and 1.21 for losartan (Table 1). When the captopril and losartan groups are compared to each other, there is no statistically significant difference between the efficacy of these two agents (Table 1).
TABLE 1.
Dose-Modifying Factors for the Mitigating Action of Captopril and Losartan
| End point | Follow-up time (weeks) | Analysis | Captopril DMFa | Losartan DMFa | Captopril vs losartanb |
|---|---|---|---|---|---|
| Time to renal failure | all | Regression | 1.23 (1.18–1.29) | 1.21 (1.15–1.29) | 1.01 |
| Incidence of renal failure | 23 | Probit | 1.29 (1.14–1.61) | 1.25 (1.12–1.54) | 1.03 |
| Incidence of renal failure | 26 | Probit | 1.18 (1.06–1.34) | 1.19 (1.07–1.36) | 1.00 |
| Incidence of renal failure | 31 | Probit | 1.22 (1.06–1.45) | 1.16 (1.02–1.35) | 1.05 |
| Incidence of renal failure | 36 | Probit | 1.19 (1.06–1.37) | 1.19 (1.05–1.36) | 1.00 |
| Incidence of renal failure | 41 | Probit | 1.14 (1.01–1.32) | 1.18 (1.01–1.35) | 0.97 |
| Azotemia (BUN) | 17 | Regression | 1.25 (1.19–1.35) | 1.15 (1.10–1.24) | 1.07c |
| Azotemia (BUN) | 17 | Nonparametric | 1.32 (1.00–1.42) | 1.18 (1.00–1.42) | 1.00 |
| Azotemia (BUN) | 26 | Nonparametric | 1.32 (1.12–1.42) | 1.27 (1.00–1.42) | 1.01 |
| Azotemia (BUN) | 37 | Nonparametric | 1.19 (1.12–1.49) | 1.19 (1.00–1.42) | 1.01 |
| Blood pressure | 17 | Regression | 1.35 (1.25–1.62) | 1.29 (1.20–1.51) | 1.06 |
| Proteinuria | 17 | Regression | 1.42 (1.25–3.24) | 1.14 (1.03–1.36) | 1.19zI |
Dose-modifying factor with 95% CI.
Number greater than one indicates that captopril is a more effective mitigator than losartan.
Significantly different from 1.0 (P < 0.05).
The linear regression technique gives tight confidence intervals (Table 1) but requires exclusion of some data because time to renal failure in some animals was indeterminate (i.e., greater than 47 weeks). To avoid such exclusion, DMFs were also calculated on the basis of whether renal failure had occurred prior to a specific follow-up time. This was done by probit analysis, which does not exclude any groups as long as a follow-up time is picked prior to any data censoring (i.e., prior to 47 weeks in these studies). This calculation can be done at any follow-up time at which there are at least two dose groups in each schedule with failure rates that are neither 0 nor 100%; for these studies, this means 23–41 weeks. For follow-up of less than 23 weeks, there were not enough failures in the captopril and losartan groups to allow a DMF calculation, whereas after 41 weeks, there were not enough survivors in the TBI-alone group. DMF calculations were done for five different follow-up times from 23 to 41 weeks (Fig. 3, Table 1). The DMF for captopril ranged from 1.29 at 23 weeks to 1.14 at 41 weeks, while for losartan it ranged from 1.25 at 23 weeks to 1.16 at 31 weeks. There were no significant trends in DMF with follow-up time; however, the confidence intervals were narrower for follow-up times with overall failure rates close to 50% (e.g., 26 weeks, Fig. 3). All DMFs were statistically significant, and in no case was there a significant difference between captopril and losartan (Table 1). The DMFs derived by probit analysis of failure rates are similar to those derived by regression analysis of time to renal failure, but the confidence intervals are wider (Table 1).
FIG. 3.
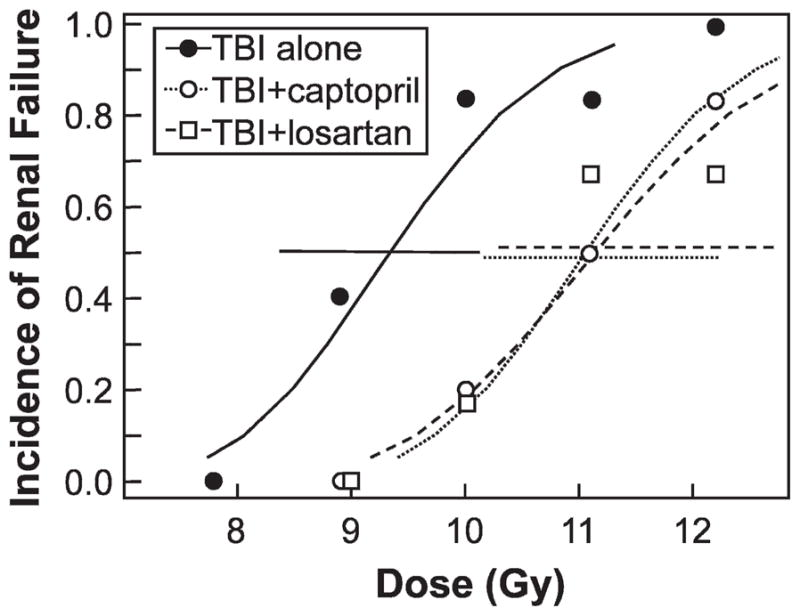
Incidence of renal failure (defined as BUN ≥ 120 mg/dl) after 26 weeks of follow-up as a function of total-body radiation dose. Data are shown for groups (n = 5–6 per group) of animals receiving TBI alone (●), TBI plus captopril at 17 mg/kg/day (○), or TBI plus losartan at 11 mg/kg/day (□). Drug therapy started 10 days after TBI and continued for 26 weeks. Curves are fitted by probit analysis, and horizontal lines at an incidence of 0.5 show the 95% confidence interval for that incidence.
Effect of Captopril and Losartan on Development of TBI-Induced Azotemia
We also evaluated whether there were surrogate measures of radiation nephropathy that could be used to evaluate mitigation efficacy after shorter follow-up times. The primary surrogate end point evaluated was azotemia (BUN), because azotemia after irradiation is tightly correlated with the probability of eventual renal failure (Fig. 1), and it is closely correlated with the degree of histopathological renal injury (21). BUN was measured in all surviving animals at 17, 26 and 37 weeks after irradiation. At 17 weeks, BUN values (Fig. 4) were available for all animals at doses below 12.2 Gy, and the DMFs calculated by regression analysis were 1.25 for captopril and 1.15 for losartan (Table 1). The captopril DMF derived from BUN analysis after 17 weeks of follow-up was slightly higher than that derived from analysis of the renal failure data, and the losartan DMF was slightly lower; in addition, by BUN analysis after 17 weeks of follow-up, captopril had a significantly higher DMF than losartan (Table 1). By 26 weeks, the amount of missing data (because animals had developed renal failure) was large enough that regression analysis could not be used. However, the nonparametric method of Keller and Brenot (17) could be applied to the 26- and 37-week BUN data. The resulting DMFs were compatible with those derived from the 17-week data, but the confidence intervals were much wider (Table 1). The relative lack of power of the nonparametric procedure was obvious when it was applied to the BUN analysis after 17 weeks of follow-up (Table 1); while the DMF values for the two analytical methods were similar, the nonparametric method gave wider confidence intervals and did not detect a significant difference between the two agents (Table 1).
FIG. 4.

Azotemia (as BUN) after 17 weeks of follow-up as a function of total-body radiation dose. Data are shown as medians and ranges (minimum–maximum, n = 5–6 per group) for animals receiving TBI alone (●), TBI plus captopril at 17 mg/kg/day (○), or TBI plus losartan at 11 mg/kg/day (□). Drug therapy started 10 days after TBI and continued for 26 weeks. Two animals each in the captopril and losartan groups at 12.2 Gy were euthanized prior to 17 weeks because of high BUN levels. At 12.2 Gy, where animals were euthanized prior to 17 weeks because of high BUN levels, the medians assume infinite BUN values for those animals. Reproduced with permission from Cohen et al. (33).
Effect of Captopril and Losartan on Development of TBI-Induced Hypertension and Proteinuria
We also evaluated hypertension and proteinuria as surrogate measures of radiation nephropathy. Blood pressure (BP) and proteinuria (as UP/UC) were measured in all surviving animals at 17 weeks after irradiation. BP and UP/UC values were available for all animals at doses below 12.2 Gy. The hypertension DMFs calculated by regression analysis were 1.35 for captopril and 1.29 for losartan; the DMFs for captopril and losartan were not significantly different (Table 1). For proteinuria, the DMFs calculated by regression analysis were 1.42 for captopril and 1.14 for losartan, and the DMF for captopril was significantly higher than that for losartan (Table 1). Nonparametric analysis of BP and UP/UC at later times gave very wide confidence intervals (data not shown).
Summary of DMF Studies
The most robust and relevant method of measuring the DMF was time to renal failure, with DMFs of 1.23 for captopril and 1.21 for losartan (Table 1). DMFs based on the incidence of renal failure were of similar magnitude to those based on time to renal failure (1.14–1.29) but had wider confidence intervals (Table 1). The surrogate end points (azotemia, proteinuria, hypertension) gave an even wider range of DMFs (Table 1), but they were broadly compatible with the DMFs based on renal failure. For surrogate measures, the narrowest confidence intervals were for azotemia but only if analysis was done prior to appreciable loss of animals from renal failure. Regardless of the end point or method of analysis, all DMFs were statistically significant at P < 0.05; with two exceptions (Table 1), none showed a difference between captopril and losartan.
DISCUSSION
The studies described here take us part of the way toward a “pivotal study”(6) of the efficacy of an ACEI (captopril) or an ARB (losartan, Cozaar®) for the mitigation of the radiation nephropathy that could be caused by a radiation accident or a radiological terrorism incident. The two agents are FDA-approved for clinical use and were tested in these studies at doses equivalent (on a g/m2/day basis) to the doses prescribed to humans. The radiation was delivered in a single dose and efficacy was demonstrated for end points (e.g., azotemia and renal failure) of direct relevance to the prevention of radiation nephropathy in humans.
For our primary end point, time to renal failure, DMFs of 1.21–1.23 were found. To illustrate the impact that such a DMF would have on a population with a range of radiation doses, we have plotted renal failure incidence curves for all animals treated with doses between 8.9 and 12.2 Gy (Fig. 5). For these animals captopril mitigation increased median survival from 23.5 weeks to 31 weeks and losartan mitigation increased it to 33 weeks. Perhaps more importantly, there were 12 long-term survivors in the groups that received mitigators compared to none in the animals not receiving therapy, and half of these long-term survivors still had BUN less than 75 mg/dl when the study was terminated after 47 weeks. Based on previous studies with fractionated irradiation (22), higher doses of ACEIs and ARBs would give substantially higher DMFs and even more long-term survivors.
FIG. 5.

Cumulative incidence of renal failure in animals after 8.9–12.2 Gy TBI (10.5 Gy median dose). Data are shown for animals receiving TBI alone (solid line, n = 22), animal receiving TBI plus captopril at 17 mg/kg/day (dotted line, n = 23), and animals receiving TBI plus losartan at 11 mg/kg/day (dashed line, n = 24). Number in parentheses is the number at risk at 38 weeks. Renal failure is significantly delayed by either captopril or losartan (P < 0.001), but the results for the two mitigators did not differ significantly from each other (P > 0.20).
An ACEI was chosen for this study because of prior data showing that these drugs can mitigate a wide range of experimental radiation injuries (4) and because these agents are very well tolerated and safe in clinical use (23, 24), including use in patients who are not hypertensive (25, 26). Captopril was specifically chosen because it has been tested prospectively as a mitigator in radiation oncology patients. A Radiation Therapy Oncology (RTOG) trial of captopril mitigation after irradiation for lung cancer (27) did not accrue enough patients to assess efficacy, but it did accrue enough to show the absence of enhanced toxicity (28). A trial of captopril to mitigate toxicity in patients who received high-dose total-body radiation (14 Gy in nine fractions) in preparation for bone marrow transplantation showed both safety and efficacy (13).
An ARB was chosen for this study to help establish that the efficacy of captopril was due to its suppression of the renin-angiotensin system (29). In humans, the ARBs have few side effects and are generally well tolerated (30, 31). Losartan was specifically chosen because it was the most common ARB in use in the clinic and the laboratory at the time these studies were initiated.
One issue posed by the “Animal Efficacy Rule” (6) is that for ACEIs and ARBs to be effective for mitigation in the rat they must be given for at least 3–6 weeks (12). The ACEIs and ARBs are given to the rats in drinking water, but humans generally take these agents by mouth. If the FDA does not consider agents in drinking water to be equivalent to agents taken in tablet form, then animals would have to be given pills or gavaged daily for perhaps 6 weeks; this is impractical. The counterargument is that we know that delivering ACEIs or ARBs to rats in drinking water suppresses the renin-angiotensin system [e.g., they lower the BP (14), they increase plasma renin activity (32), and they increase urinary AcSDKP2 (33)].
The model (single-dose, high-dose-rate, total-body exposure of unanesthetized animals) was designed to mimic exposure to a nuclear device or a high-dose-rate source; it may not be as relevant to low-dose-rate exposures (e.g., from environmental or food contamination). The relevance of the model to radiological terrorism scenarios could also be questioned because of the BMT and the relatively high radiation doses (≥7.8 Gy). We know that BMT in this model can be replaced with leg shielding without affecting the progression of renal injury, so that aspect of the model can be changed. Lower radiation doses can also be used, but much longer follow-up times (>12 months) would be required to get life-threatening renal dysfunction. In any case, the dose–response curves (Figs. 3 and 4) show no indication that the efficacy of captopril or losartan is decreased at the lower radiation doses.
The FDA “Animal Efficacy Rule” (6) also requires that testing be done in a model “expected to react with a response predictive for humans.” This model of radiation nephropathy after TBI is pathophysiologically analogous to the radiation nephropathy that occurs in humans after TBI (34, 35), and the 10-Gy single-fraction dose in these studies is identical to the dose reported to cause TBI-related radiation nephropathy in children (36) and adults (37). This rat model has also predicted the efficacy of ACEIs and ARBs for treatment of renal injury in patients receiving TBI (4), and the success of mitigation with captopril in the model was our major justification for the successful test of captopril to mitigate chronic renal failure after BMT in humans (26).
Finally, the FDA “Animal Efficacy Rule” (6) requires “a reasonably well-understood pathophysiological mechanism” for the efficacy of the agent. It is now clear that suppression of the renin-angiotensin system can account for the efficacy of both ACEIs and ARBs for mitigation of radiation nephropathy (4, 33). All effective mitigators of radiation nephropathy [ARBs (4,33), ACEIs (4, 33) and a high-salt diet (20)] suppress the renin-angiotensin system, whereas infusion of excess AII makes radiation nephropathy worse (38). Antihypertensive agents that work via mechanisms other than suppression of the renin-angiotensin system are not effective mitigators (39). Other actions of ACEIs [i.e., on the aldosterone, AcSDKP and angiotensin (1–7) pathways] have largely been ruled out by other experimental studies (33), and involvement of the AcSDKP and bradykinin pathways has largely been ruled out by the demonstration that ACEIs and ARBs are equally effective as mitigators.
These studies demonstrate that experimental radiation nephropathy in a realistic rodent model can be mitigated with relevant doses of FDA-approved ACEIs or ARBs and create a gold standard to which other proposed mitigators of late effects can be compared. The studies also lay the necessary groundwork for pivotal rodent studies under the FDA Animal Efficacy Rule (6) and provide an outline of how the FDA-required large-animal studies could be designed.
Acknowledgments
These studies were supported by an NCI grant (CA-24652) and an NIH cooperative agreement (AI-067734). The Irradiation Core of the Medical College of Wisconsin Center for Medical Countermeasures against Radiological Terrorism assisted with the irradiations and with the radiation dosimetry. Yvonne A. Morauski assisted with preparation of the manuscript, and Marylou Mäder provided expert technical assistance. Losartan (Cozaar®) was a gift from Merck & Co (Rahway, NJ).
Footnotes
AcSDKP is a tetrapeptide (N-acetyl-Ser-Asp-Lys-Pro) hematopoietic cytokine (40) that is degraded by ACE, so that serum AcSDKP levels can be used to monitor compliance with ACEI therapy (41). AcSDKP also has anti-fibrotic (42) and anti-proliferative (43, 44) activity in the kidney, and Azizi et al. (44) have speculated that the anti-proliferative action of AcSDKP could explain some of the efficacy of captopril in irradiated kidneys.
References
- 1.Coleman CN, Stone HB, Moulder JE, Pellmar TC. Modulation of radiation injury. Science. 2004;304:693–694. doi: 10.1126/science.1095956. [DOI] [PubMed] [Google Scholar]
- 2.Stone HB, Moulder JE, Coleman CN, Ang KK, Anscher MS, Barcellos-Hoff MH, Dynan WS, Fike JR, Grdina DJ, Zaharevitz D. Models for evaluating agents intended for the prophylaxis, mitigation and treatment of radiation injuries. Radiat Res; Report of an NCI workshop; December 3–4, 2003; 2004. pp. 711–728. [DOI] [PubMed] [Google Scholar]
- 3.Moulder JE, Fish BL, Cohen EP. ACE inhibitors and AII receptor antagonists in the treatment and prevention of bone marrow transplant nephropathy. Curr Pharm Design. 2003;9:737–749. doi: 10.2174/1381612033455422. [DOI] [PubMed] [Google Scholar]
- 4.Cohen EP, Joines MM, Moulder JE. Prevention and treatment of radiation injuries – The role of the renin-angiotensin system. In: Rubin P, Constine LS, Mark LB, Okunieff P, editors. Late Effects of Cancer Treatment on Normal Tissues. Springer-Verlag; Heidelberg: 2008. pp. 69–76. [Google Scholar]
- 5.Siegl PKS, Chang RSL, Mantlo NB, Chakavarty PK, Ondeyka DL, Greenlee WJ, Patchett AA, Sweet CS, Lotti VJ. In vivo pharmacology of L-158,809, a highly potent and selective non-peptide angiotensin II receptor blocker. J Pharmacol Exp Ther. 1992;262:139–144. [PubMed] [Google Scholar]
- 6.Food and Drug Administration. New drug and biological drug products: evidence needed to demonstrate effectiveness of new drugs when human efficacy studies are not ethical or feasible. Fed Regist. 2002;67:37988–37998. [PubMed] [Google Scholar]
- 7.Williams JP, Brown SL, Georges GE, Huser AK, Hill RP, Hauer-Jensen M, Kirsch DG, MacVittie TJ, Mason KA, McBride WH. Animal models for medical countermeasures. Radiat Res. 2010;173:557–578. doi: 10.1667/RR1880.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moulder JE, Fish BL. Late toxicity of total body irradiation with bone marrow transplantation in a rat model. Int J Radiat Oncol Biol Phys. 1989;16:1501–1509. doi: 10.1016/0360-3016(89)90955-3. [DOI] [PubMed] [Google Scholar]
- 9.Cohen EP, Fish BL, Moulder JE. The renin-angiotensin system in experimental radiation nephropathy. J Lab Clin Med. 2002;139:251–257. doi: 10.1067/mlc.2002.122279. [DOI] [PubMed] [Google Scholar]
- 10.Cohen EP, Fish BL, Sharma M, Li XA, Moulder JE. Role of the angiotensin II type-2 receptor in radiation nephropathy. Transl Res. 2007;150:106–115. doi: 10.1016/j.trsl.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen EP, Fish BL, Moulder JE. Successful brief captopril treatment in radiation nephropathy. J Lab Clin Med. 1997;129:536–547. doi: 10.1016/s0022-2143(97)90008-1. [DOI] [PubMed] [Google Scholar]
- 12.Moulder JE, Fish BL, Cohen EP. Brief pharmacologic intervention in experimental radiation nephropathy. Radiat Res. 1998;150:535–541. [PubMed] [Google Scholar]
- 13.Cohen EP, Irving AA, Drobyski WR, Klein JP, Passweg J, Talano J, Juckett M, Moulder JE. Captopril to mitigate chronic renal failure after hematopoietic stem cell transplantation: a randomized controlled trial. Int J Radiat Oncol Biol Phys. 2008;70:1546–1551. doi: 10.1016/j.ijrobp.2007.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen EP, Moulder JE, Fish BL, Hill P. Prophylaxis of experimental bone marrow transplant nephropathy. J Lab Clin Med. 1994;124:371–380. [PubMed] [Google Scholar]
- 15.Brownlee KA. Statistical Theory and Methodology in Science and Engineering. 2. Wiley; New York: 1965. Simple linear regression; pp. 605–611. [Google Scholar]
- 16.Moulder JE, Rockwell S. Hypoxic fractions of solid tumors: Experimental techniques, methods of analysis, and a survey of existing data. Int J Radiat Oncol Biol Phys. 1984;10:695–712. doi: 10.1016/0360-3016(84)90301-8. [DOI] [PubMed] [Google Scholar]
- 17.Kellerer AM, Brenot J. Nonparametric determination of modifying factors in radiation action. Radiat Res. 1973;56:28–39. [PubMed] [Google Scholar]
- 18.Finney DJ. Statistical Methods in Biological Assay. Macmillan; New York: 1978. [Google Scholar]
- 19.Moulder JE, Fish BL, Regner KR, Cohen EP. Angiotensin II blockade reduces radiation-induced proliferation in experimental radiation nephropathy. Radiat Res. 2002;157:393–401. doi: 10.1667/0033-7587(2002)157[0393:aibrri]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 20.Moulder JE, Fish BL, Cohen EP. Dietary sodium modification and experimental radiation nephropathy. Int J Radiat Biol. 2002;79:903–911. doi: 10.1080/09553000210155897. [DOI] [PubMed] [Google Scholar]
- 21.Cohen EP, Molteni A, Hill P, Fish BL, Ward WF, Moulder JE, Carone FA. Captopril preserves function and ultrastructure in experimental radiation nephropathy. Lab Invest. 1996;75:349–360. [PubMed] [Google Scholar]
- 22.Moulder JE, Fish BL, Cohen EP. Treatment of radiation nephropathy with ACE inhibitors and AII type-1 and type-2 receptor antagonists. Curr Pharm Design. 2007;13:1317–1325. doi: 10.2174/138161207780618821. [DOI] [PubMed] [Google Scholar]
- 23.McFate Smith W, Davies RO, Gabriel MA, Kramsch DM, Moncloa F, Rush JE, Walker JF. Tolerance and safety of enalapril. Br J Clin Pharmacol. 1984;18(Suppl 2):249S–253S. doi: 10.1111/j.1365-2125.1984.tb02604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. N Engl J Med. 1993;329:1456–1462. doi: 10.1056/NEJM199311113292004. [DOI] [PubMed] [Google Scholar]
- 25.Rengo F, Acanfora D, Furgi G, Papa A, Nicolino A, Picone C, Vitale DF, Rengo C. Comparison of the safety and efficacy of delapril with enalapril in patients with congestive heart failure. Am J Cardiol. 1995;75:25F–28F. doi: 10.1016/s0002-9149(99)80511-6. [DOI] [PubMed] [Google Scholar]
- 26.Mason J, Young P, Freemantle N, Hobbs R. Safety and costs of initiating angiotensin converting enzyme inhibitors for heart failure in primary care: analysis of individual patient data from studies of left ventricular dysfunction. BMJ. 2000;321:1113–1116. doi: 10.1136/bmj.321.7269.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.RTOG-0123: A Phase II randomized trial with captopril in patients who have received radiation therapy±chemotherapy for stage II-IIIB non-small cell lung cancer, stage I central non-small cell lung cancer, or limited-stage small cell lung cancer. Radiation Therapy Oncology Group; Philadelphia: 2004. [Google Scholar]
- 28.A Phase II Randomized Trial with Captopril in Patients Who Have Received Radiation Therapy +/− Chemotherapy for Stage II-IIIB Non-Small Cell Lung Cancer, Stage I Central Non-Small Cell Lung Cancer, or Limited-Stage Small-Cell Lung Cancer (Report June 2009) Radiation Therapy Oncology Group; Philadelphia: 2009. [Google Scholar]
- 29.Moulder JE, Fish BL, Cohen EP, Bonsib SM. Angiotensin II receptor antagonists in the prevention of radiation nephropathy. Radiat Res. 1996;146:106–110. [PubMed] [Google Scholar]
- 30.Bönner G, Smolka W, Jung C, Bestehorn K. Efficacy and safety of losartan 100 mg or losartan 100 mg plus hydrochlorothiazide 25 mg in the treatment of patients with essential arterial hypertension and CV risk factors: observational, prospective study in primary care. Curr Med Res Opin. 2009;25:981–990. doi: 10.1185/03007990902809876. [DOI] [PubMed] [Google Scholar]
- 31.McInnes GT. Clinical advantage of valsartan. Cardiology. 1999;91(Suppl 1):14–18. doi: 10.2196/47283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Molteni A, Moulder JE, Cohen EP, Fish BL, Taylor JM, Veno PA, Wolfe LF, Ward WF. Prevention of radiation-induced nephropathy and fibrosis in a model of bone marrow transplant by an angiotensin II receptor blocker. Exp Biol Med. 2001;226:1016–1023. doi: 10.1177/153537020122601108. [DOI] [PubMed] [Google Scholar]
- 33.Cohen EP, Fish BL, Moulder JE. Mitigation of radiation injuries via suppression of the renin-angiotensin system: Emphasis on radiation nephropathy. Curr Drug Targets. 2010;11:1423–1429. doi: 10.2174/1389450111009011423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cohen EP, Lawton CA, Moulder JE. Bone marrow transplant nephropathy: Radiation nephritis revisited. Nephron. 1995;70:217–222. doi: 10.1159/000188587. [DOI] [PubMed] [Google Scholar]
- 35.Cohen EP, Robbins MEC. Radiation nephropathy. Semin Nephrol. 2003;23:486–499. doi: 10.1016/s0270-9295(03)00093-7. [DOI] [PubMed] [Google Scholar]
- 36.Antignac C, Gubler MC, Leverger G, Broyer M, Habib R. Delayed renal failure with extensive mesangiolysis following bone marrow transplantation. Kidney Int. 1989;35:1336–1344. doi: 10.1038/ki.1989.132. [DOI] [PubMed] [Google Scholar]
- 37.Kal HB, van Kempen-Harteveld ML. Renal dysfunction after total body irradiation: Dose–effect relationship: In regard to Kal and Van Kempen-Harteveld (Int J Radiat Oncol Biol Phys 2006; 65: 1228–1232) – In reply to Drs. Moulder and Cohen. Int J Radiat Oncol Biol Phys. 2007;67:319–320. doi: 10.1016/j.ijrobp.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cohen EP, Fish BL, Moulder JE. Angiotensin II infusion exacerbates radiation nephropathy. J Lab Clin Med. 1999;134:283–291. doi: 10.1016/s0022-2143(99)90209-3. [DOI] [PubMed] [Google Scholar]
- 39.Moulder JE, Robbins MEC, Cohen EP, Hopewell JW, Ward WF. Pharmacologic modification of radiation-induced late normal tissue injury. Cancer Treat Res. 1998;93:129–151. doi: 10.1007/978-1-4615-5769-2_6. [DOI] [PubMed] [Google Scholar]
- 40.Lenfant M, Itoh K, Sakoda H, Sotty D, Sasaki NA, Wdzieczak-Bakala J, Mori KJ. Enhancement of the adherence of hematopoietic stem cells to mouse bone marrow-derived stromal cell line MS-1-T by a tetrapeptide acetyl-N-Ser-Asp-Lys-Pro. Exp Hematol. 1989;17:898–902. [PubMed] [Google Scholar]
- 41.Azizi M, Ezan E, Nicolet L, Grognet JM, Menard J. High plasma level of N-acetyl-seryl-aspartyl-lysyl-proline: a new marker of chronic angiotensin-converting enzyme inhibition. Hypertension. 1997;30:1015–1019. doi: 10.1161/01.hyp.30.5.1015. [DOI] [PubMed] [Google Scholar]
- 42.Peng H, Carretero OA, Brigstock DR, Oja-Tebbe N, Rhaleb NE. Ac-SDKP reverses cardiac fibrosis in rats with renovascular hypertension. Hypertension. 2003;42:1164–1170. doi: 10.1161/01.HYP.0000100423.24330.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iwamoto N, Xano HJ, Yoshioka T, Shiraga H, Nitta K, Muraki T, Ito K. Acetyl-seryl-aspartyl-lysyl-proline is a novel natural cell cycle regulator of renal cells. Life Sci. 2000;66:221–226. doi: 10.1016/s0024-3205(00)00460-4. [DOI] [PubMed] [Google Scholar]
- 44.Azizi M, Rousseau A, Ezan E, Guyene T, Michelet TS, Grognet JM, Lenfant M, Corvol P, Menard J. Acute angiotensin-converting enzyme inhibition increases the plasma level of the natural stem cell regulator N-acetyl-seryl-aspartyl-lysyl-proline. J Clin Invest. 1996;97:839–844. doi: 10.1172/JCI118484. [DOI] [PMC free article] [PubMed] [Google Scholar]


