Abstract
Previous studies have indicated that thyroid cancer risk after a first childhood malignancy is curvilinear with radiation dose, increasing at low to moderate doses and decreasing at high doses. Understanding factors that modify the radiation dose response over the entire therapeutic dose range is challenging and requires large numbers of subjects. We quantified the long-term risk of thyroid cancer associated with radiation treatment among 12,547 5-year survivors of a childhood cancer (leukemia, Hodgkin lymphoma and non-Hodgkin lymphoma, central nervous system cancer, soft tissue sarcoma, kidney cancer, bone cancer, neuroblastoma) diagnosed between 1970 and 1986 in the Childhood Cancer Survivor Study using the most current cohort follow-up to 2005. There were 119 subsequent pathologically confirmed thyroid cancer cases, and individual radiation doses to the thyroid gland were estimated for the entire cohort. This cohort study builds on the previous case-control study in this population (69 thyroid cancer cases with follow-up to 2000) by allowing the evaluation of both relative and absolute risks. Poisson regression analyses were used to calculate standardized incidence ratios (SIR), excess relative risks (ERR) and excess absolute risks (EAR) of thyroid cancer associated with radiation dose. Other factors such as sex, type of first cancer, attained age, age at exposure to radiation, time since exposure to radiation, and chemotherapy (yes/no) were assessed for their effect on the linear and exponential quadratic terms describing the dose–response relationship. Similar to the previous analysis, thyroid cancer risk increased linearly with radiation dose up to approximately 20 Gy, where the relative risk peaked at 14.6-fold (95% CI, 6.8–31.5). At thyroid radiation doses >20 Gy, a downturn in the dose–response relationship was observed. The ERR model that best fit the data was linear-exponential quadratic. We found that age at exposure modified the ERR linear dose term (higher radiation risk with younger age) (P < 0.001) and that sex (higher radiation risk among females) (P = 0.008) and time since exposure (higher radiation risk with longer time) (P < 0.001) modified the EAR linear dose term. None of these factors modified the exponential quadratic (high dose) term. Sex, age at exposure and time since exposure were found to be significant modifiers of the radiation-related risk of thyroid cancer and as such are important factors to account for in clinical follow-up and thyroid cancer risk estimation among childhood cancer survivors.
INTRODUCTION
Previous studies have indicated that long-term survivors of childhood malignancy have an increased incidence of second primary thyroid cancer after radiotherapy that is curvilinear with dose, such that risk steadily increases up to approximately 20 Gy, above which there is a downturn in the dose response (1, 2). The complex relationship of radiation dose and risk for thyroid cancer complicates risk estimation and assessment of factors that may modify the dose–response relationship.
Prior studies have evaluated fewer than 70 thyroid cancer cases among childhood cancer survivors (1–5), and there is conflicting evidence as to modifiers of the radiation dose response (1). We have conducted one of the largest cohort studies to date of treatment-related secondary thyroid cancers based on the Childhood Cancer Survivor Study (CCSS) population. A previous case-control study in the CCSS cohort evaluated 69 thyroid cancer cases with follow-up to the year 2000 (1, 2). With 5 years of additional follow-up, 119 thyroid cancer diagnoses and the availability of thyroid gland radiation dose estimates for all survivors, we have estimated absolute risks, and due to increased precision, we have evaluated radiation effect modifiers in greater detail than previously possible. To our knowledge, the present study is among the first to describe the effect of age at exposure, type of first cancer, sex, attained age and time since exposure on the ascending and descending portions of the dose–response curve for excess relative and excess absolute risk of thyroid cancer after a childhood malignancy.
MATERIALS AND METHODS
Study Population
The design of the CCSS and characteristics of the study population have been described in detail previously (6, 7). Briefly, subjects eligible for study were diagnosed before age 21 years with leukemia, central nervous system (CNS) cancer, Hodgkin or non-Hodgkin lymphoma, kidney tumor, neuroblastoma, soft tissue sarcoma or bone cancer during 1970–1986 at one of 26 institutions in the U.S. or Canada and had survived for at least 5 years. In addition, for the present analysis, participants had to agree to the release of their medical records so radiation treatment and chemotherapy status could be determined. Of the 12,756 potentially eligible participants in the CCSS cohort, three were excluded due to missing information about follow-up, 204 due to missing information about whether radiation or chemotherapy treatments were received, and two who developed thyroid cancer within 5 years of treatment for their initial cancer, leaving 12,547 childhood cancer survivors for analysis.
Data Collection and Case Ascertainment
The CCSS research protocol and procedures were approved by the human subjects committees at each participating institution. A baseline self-administered questionnaire sent in 1994 collected data on demographic characteristics, education, income, employment history, marital status, height, weight, personal health habits, family cancer history, medication use, reproductive history, and new malignancies and other health outcomes (see http://ccss.stjude.org for detailed study methods and instruments). Subsequent surveys were mailed to cohort members in 2000, 2003 and 2005. Less extensive information was collected in these follow-up surveys, but each inquired about the occurrence of new malignancies.
Cases were defined as patients who developed a subsequent thyroid cancer at least 5 years after a first childhood malignancy. Thyroid cancers were ascertained through self-report (including surrogate respondents) on questionnaires. Pathology reports were obtained, and thyroid cancers were verified by a CCSS pathologist (S.H.). From 1970 to 2005, 119 confirmed thyroid cancer cases were identified (by histology: n = 96 papillary, n = 14 follicular, n = 3 other, n = 6 unknown). One hundred and eleven of these cases were second primary cancers and eight cases were third primary cancers. For these eight cases, the intervening cancers included breast cancer, lung cancer, non-Hodgkin lymphoma (n = 2), osteosarcoma, soft tissue sarcoma (n = 2) or melanoma skin cancer.
Radiation Dosimetry
Copies of radiation therapy records pertaining to the original cancer diagnosis and treatment, plus any additional treatment that occurred during the ensuing 10 years, were obtained from the treating institution and forwarded to the CCSS Radiation Physics Center at the University of Texas M. D. Anderson Cancer Center for thyroid dosimetry assessment. Absorbed organ doses in Gy to the left and right lobes of the thyroid gland were calculated separately. If the organ was outside the nearest treatment field, doses were based on out-of-beam measurements in a water phantom (8, 9). Doses to organs in the beam were derived using standard radiotherapy techniques (10), which took into account typical blocking procedures. Due to the large number of patients who had radiotherapy (n = 8,538), it was not possible to evaluate individual patient records to take into account each instance when the thyroid gland was or was not under blocking. If important therapy details were missing from medical records (e.g., date of treatment, treatment dose or body site treated), total doses were set to unknown (n = 449). For each patient, doses from all radiation treatments given within 10 years after the first cancer were included; 1805 patients received subsequent radiation therapy 1 to 10 years after their initial treatment. For analysis, radiation dose (calculated as the mean of the total doses received by the left and right lobes of the thyroid gland) was treated as a time-dependent variable with a 5-year lag to account for a minimum 5-year interval between radiation exposure and development of thyroid cancer (11, 12).
Statistical Analysis
To conduct Poisson regression analysis, the DATAB module of Epicure (Hirosoft International Corporation, Seattle, WA) was used to arrange the data in a multidimensional table with each cell providing case counts and person-years of follow-up for separate combinations of various categorized demographic, diagnosis and treatment-related variables. These included sex, year of birth, race, type of childhood cancer, age at childhood cancer diagnosis, time since first cancer, attained age, calendar year of childhood cancer diagnosis, attained calendar year, type of treatment, thyroid radiation dose, radiation treatment status and chemotherapy status (see Table 1 for categories). Each cell in the table also provided the mean values of year of birth, attained age, attained calendar year, age at childhood cancer diagnosis, time since childhood cancer diagnosis, year of childhood cancer diagnosis, and radiation dose to the thyroid.
TABLE 1.
Standardized Thyroid Cancer Incidence Ratios by Demographic and Treatment-Related Factors in the Childhood Cancer Survivor Study Cohort
| Characteristic | Person-years/10,000 | Thyroid cancer cases | SIR (95% CI)a | P valueb |
|---|---|---|---|---|
| Overall | 20.3 | 119 | 14.0 (11.7, 16.8) | |
| Sex | ||||
| Male | 10.5 | 40 | 25.0 (18.5, 34.4) | <0.001 |
| Female | 9.8 | 79 | 11.5 (9.2, 14.3) | |
| Year of birth | ||||
| <1960 | 2.0 | 8 | 5.2 (2.6, 10.5) | 0.004 |
| 1960–1969 | 7.1 | 62 | 15.6 (12.2, 20.0) | |
| 1970–1979 | 8.5 | 43 | 16.2 (12.0, 21.8) | |
| ≥1980 | 2.7 | 6 | 18.9 (8.5, 42.1) | |
| Race | ||||
| White, Non-Hispanic | 17.3 | 100 | 13.6 (11.2, 16.6) | >0.5 |
| Black | 0.7 | 4 | 15.4 (5.8, 40.9) | |
| Hispanic/Latino | 0.8 | 7 | 21.2 (10.1, 44.5) | |
| Other/Unspecified | 1.4 | 8 | 14.7 (7.3, 29.3) | |
| Type of first cancer | ||||
| Leukemia | 6.6 | 27 | 11.5 (7.9, 16.7) | 0.02 |
| Hodgkin lymphoma | 2.8 | 39 | 21.6 (15.8, 29.6) | |
| Central nervous system cancer | 2.5 | 14 | 14.3 (8.5, 24.2) | |
| Soft tissue sarcoma | 1.9 | 8 | 9.2 (4.6, 18.5) | |
| Kidney cancer (Wilms) | 1.8 | 3 | 5.7 (1.8, 17.7) | |
| Bone cancer | 1.7 | 12 | 11.5 (6.5, 20.3) | |
| Non-Hodgkin lymphoma | 1.5 | 7 | 12.1 (5.8, 25.4) | |
| Neuroblastoma | 1.4 | 9 | 27.4 (14.3, 52.7) | |
| Age at first cancer, years | ||||
| <5 | 8.2 | 32 | 17.2 (12.2, 24.3.) | 0.004 |
| 5–9 | 4.4 | 26 | 15.7 (10.7, 23.0) | |
| 10–14 | 4.1 | 42 | 17.7 (13.1, 24.0) | |
| ≥15 | 3.6 | 19 | 7.4 (4.7, 11.5) | |
| Time since first cancer, years | ||||
| 5–14 | 11.7 | 33 | 12.6 (8.9, 17.7) | >0.5 |
| 15–19 | 4.6 | 41 | 16.6 (12.3, 22.6) | |
| 20–24 | 2.6 | 27 | 13.0 (8.9,19.0) | |
| ≥25 | 1.2 | 18 | 13.8 (8.7, 21.9) | |
| Attained age, years | ||||
| <20 | 8.4 | 19 | 24.6 (15.7, 38.5) | 0.02 |
| 20–24 | 4.3 | 23 | 13.4 (8.9, 20.2) | |
| 25–29 | 3.5 | 21 | 9.6 (6.3, 14.8) | |
| 30–34 | 2.3 | 33 | 18.1 (12.9, 25.5) | |
| ≥35 | 1.8 | 23 | 11.6 (7.7, 17.5) | |
| Calendar year of childhood cancer diagnosis | ||||
| <1974 | 4.9 | 38 | 15.4 (11.2, 21.1) | >0.5 |
| 1975–1979 | 6.5 | 41 | 13.7 (10.1, 18.7) | |
| ≥1980 | 8.9 | 40 | 13.3 (9.7, 18.1) | |
| Attained calendar year | ||||
| <1980 | 2.3 | 6 | 15.6 (7.0, 34.6) | >0.5 |
| 1980–1984 | 3.6 | 12 | 14.6 (8.3, 25.8) | |
| 1985–1989 | 5.5 | 22 | 13.5 (8.9, 20.4) | |
| 1990–1994 | 5.1 | 35 | 13.3 (9.6, 18.6) | |
| ≥1995 | 3.2 | 40 | 14.7 (10.8, 20.0) | |
| Type of treatment | ||||
| Radiation and chemotherapy | 11.3 | 89 | 19.3 (15.6, 23.7) | <0.001 |
| Chemotherapy only | 4.8 | 11 | 6.2 (3.4, 11.2) | |
| Radiation only | 2.7 | 18 | 12.8 (8.1, 20.3) | |
| No radiation and no chemotherapy | 1.6 | 1 | 1.5 (0.2, 10.4) | |
| Radiation | ||||
| No | 6.3 | 12 | 4.9 (2.8, 8.6) | <0.001 |
| Yes | 13.3 | 107 | 17.9 (14.8, 21.8) | |
| Thyroid radiation dose, Gy | ||||
| 0 | 6.5 | 12 | 4.8 (2.7, 8.5) | <0.001c |
| >0<5 | 8.2 | 17 | 5.4 (3.4, 8.7) | |
| 5<10 | 0.4 | 6 | 40.2 (18.0, 89.4) | |
| 10<15 | 0.6 | 10 | 47.3 (25.5, 88.0) | |
| 15–<20 | 0.5 | 14 | 61.9 (36.7, 104.6) | |
| 20–<25 | 0.6 | 18 | 69.2 (43.6, 109.8) | |
| 25–<30 | 0.5 | 10 | 43.8 (23.6, 81.4) | |
| 30–<35 | 0.4 | 10 | 42.7 (23.0, 79.4) | |
| 35–<40 | 0.6 | 7 | 17.3 (8.2, 36.3) | |
| ≥40 | 1.2 | 11 | 12.9 (7.1, 23.3) | |
| Unknown | 0.7 | 4 | – | |
| Chemotherapy | ||||
| No | 4.2 | 19 | 9.1 (5.8, 14.3) | 0.02 |
| Yes | 16.0 | 100 | 15.6 (12.8, 19.0) | |
Standardized incidence ratio (SIR) and 95% confidence interval (CI) using the Surveillance, Epidemiology and End Results database to estimate expected numbers by applying the age-, gender- and calendar year-specific incidence rates to the person-year distribution in the CCSS cohort.
Likelihood ratio test for heterogeneity of SIRs.
Unknown category excluded from test of heterogeneity.
Poisson regression analyses were used to calculate standardized incidence ratios (SIR) and estimate excess relative risks (ERR) and excess absolute risks (EAR) associated with radiation dose using the AMFIT module of Epicure. Corresponding P values and 95% CI were also calculated, and all statistical tests were two-sided with statistical significance assessed at P < 0.05.
The SIR was defined as the ratio of observed to expected numbers of thyroid cancer cases. The expected number was calculated by multiplying sex-, age- and calendar year-specific U.S. incidence thyroid cancer rates by the number of person-years at risk in the cohort. The general population incidence rates were based on data collected through the Surveillance, Epidemiology, and End Results (SEER) program (13). For various demographic and treatment variables, the heterogeneity of SIRs among categories of those variables was evaluated using two-sided likelihood ratio tests (LRT).
The ERR and EAR for thyroid cancer were assessed in Poisson regression models that were based on purely internal analyses because the CCSS cohort included unexposed cases and non-cases, allowing for the estimation of the baseline risk. The models were based on radiobiological principles, with an ascending term allowing risk to increase with increasing dose in a linear, quadratic or linear-quadratic fashion and a negative log-linear or log-quadratic term to allow for the effects of cell killing at high doses (1, 2). The ERR and EAR models have the general form
| (1) |
| (2) |
where the first exponential term is the baseline risk as a function of a group of continuous or categorical variables (xi) that are potential confounders (e.g., sex, attained age etc.) and the second exponential term allows for the modification of ERR(d) and EAR(d) by particular covariates (αi and γi are the parameters to be estimated for the covariates). ERR(d) and EAR(d) are the excess relative risk and excess absolute risk, respectively, expressed as functions of dose (d); 10 of these functions, which had one to three dose parameters, were evaluated. For example, the simplest function assessed was the linear dose response, β1d, while one of the more complex functions evaluated was the linear-quadratic exponential quadratic dose response, (β1d + β2d2) × exp(β3d2).
Likelihood ratio tests (LRT) were used to evaluate various nested ERR and EAR models to determine the most parsimonious models that best fit the data. Then LRT were used to sequentially assess possible effect modification of the dose parameters in these models. For example, with a linear-exponential model of the following form,
| (3) |
Effect modification by a pertinent variable was first assessed for the linear ascending risk term (β1d), comparing a model with the effect modification term (Eq. 4) to a model without this term (Eq. 3):
| (4) |
To assess effect modification of the exponential downturn risk term [exp(β2d)], a model with both an exponential effect modification term and a linear effect modification term (Eq. 5) was compared to the model that contained only the linear effect modification term (Eq. 4):
| (5) |
While stratified dose–response parameters for categories of potential effect modifiers are presented, continuous versions of the covariates (where appropriate) were used to assess the presence of effect modification as a test for trend. Sex, year of birth, race, type of first cancer, attained age, attained calendar year and treatment with chemotherapy (yes/no) were evaluated as potential effect modifiers. Because radiation treatment was delivered near the time of cancer diagnosis, we used age at cancer diagnosis, time since cancer diagnosis and year of cancer diagnosis as proxies for age at exposure, time since exposure and year of exposure, respectively, and analyzed these variables as potential effect modifiers. When we evaluated each potential effect modifier, it was also included in the baseline risk term, except for age at, time since and year of radiation exposure since individuals included in the baseline are unexposed to radiation and thus are not assigned values for these variables.
The natural logarithm of attained age, sex and type of childhood cancer (Hodgkin lymphoma, leukemia or other) were included in the baseline risk term in all models that were evaluated. No major differences were observed in the results when including categories of attained age or more differentiated categories of type of childhood cancer. Since inclusion of other variables in the baseline term as potential confounders, such as race, year of birth or chemotherapy (yes/no) did not have a significant influence on the dose–response parameters in the ERR and EAR models, these variables were not included in the baseline term.
RESULTS
Table 1 provides distributions of person-years and cases, together with SIRs, by various demographic and treatment related-factors. The 449 patients with unknown radiation doses were excluded from all further analyses, leaving 115 cases and a total of 19.6 × 104 person-years of follow-up. Table 2 provides relative risks for select variables adjusted for categories of radiation exposure, attained age, sex and type of first cancer, where appropriate. When examining categories of thyroid dose received from radiation therapy, the SIR and RR increased steadily up to the 20 to <25 Gy category and then declined at higher doses. While the SIR among males was significantly elevated compared to females, the adjusted RR indicates that females had a twofold increased risk of developing thyroid cancer compared to males. Significant heterogeneity by type of first cancer was seen for the SIR but not the RR, though patients with childhood bone cancer and neuroblastoma still demonstrated significant twofold increased RRs of developing thyroid cancer compared to patients with childhood leukemia. There was also evidence of heterogeneity of the SIR and RR by age at first cancer, with a trend of decreasing adjusted RRs with increasing age. Among patients receiving chemotherapy, the SIR and RR were both elevated, but the latter was of borderline statistical significance.
TABLE 2.
Thyroid Cancer Relative Risks for Selected Demographic and Treatment-Related Factors in the Childhood Cancer Survivor Study Cohort
| Characteristic | Relative risk (95% confidence interval)a | P valueb |
|---|---|---|
| Thyroid radiation dose, Gy (mean) | ||
| 0 | 1.0 | <0.001 |
| >0–<5 (0.8) | 1.2 (0.6, 2.5) | |
| 5–<10 (7.4) | 8.5 (3.2, 22.6) | |
| 10–<15 (12.3) | 10.6 (4.5, 24.9) | |
| 15–<20 (17.4) | 13.8 (6.3, 30.3) | |
| 20–<25 (22.0) | 14.6 (6.8, 31.5) | |
| 25–<30 (27.3) | 9.3 (3.9, 21.9) | |
| 30–<35 (32.4) | 8.9 (3.6, 21.7) | |
| 35–<40 (37.5) | 3.6 (1.3, 10.2) | |
| ≥40 (45.6) | 2.8 (1.1, 7.1) | |
| Sex | ||
| Male | 1.0 | <0.001 |
| Female | 2.3 (1.6, 3.4) | |
| Type of first cancer | ||
| Leukemia | 1.0 | 0.2 |
| Hodgkin lymphoma | 1.1 (0.5, 2.1) | |
| Central nervous system cancer | 1.0 (0.5, 2.0) | |
| Soft tissue sarcoma | 1.2 (0.6, 2.8) | |
| Kidney cancer (Wilms) | 0.9 (0.3, 3.1) | |
| Bone cancer | 2.1 (1.0, 4.4) | |
| Non-Hodgkin lymphoma | 0.6 (0.2, 1.5) | |
| Neuroblastoma | 2.2 (1.0, 4.9) | |
| Age at first cancer, years | ||
| <5 | 1.0 | <0.001 |
| 5–9 | 0.7 (0.4, 1.2) | |
| 10–14 | 0.6 (0.4, 1.2) | |
| ≥15 | 0.2 (0.1, 0.4) | |
| Type of treatment | <0.001 | |
| Radiation and chemotherapy | 1.0 | |
| Chemotherapy only | 0.3 (0.2, 0.6) | |
| Radiation only | 0.6 (0.3, 1.0) | |
| No radiation and no chemotherapy | 0.08 (0.01, 0.6) | |
| Chemotherapy | ||
| No | 1.0 | 0.06 |
| Yes | 1.6 (1.0, 2.7) | |
Note. Adjusted relative risk for radiation yes versus no = 3.4 (95% CI: 1.8, 6.2).
Based on Poisson regression analysis with adjustment for categories of thyroid radiation dose, natural logarithm of attained age, sex and type of first cancer (Hodgkin lymphoma, leukemia or other) where appropriate; e.g., when estimating effects of type of first cancer, type of first cancer was not adjusted for in the underlying model.
Likelihood ratio test for heterogeneity of relative risks.
Table 3 presents descriptive statistics for radiation dose and mean age at radiation exposure by type of first cancer. Patients diagnosed with Hodgkin lymphoma received the highest doses of radiation to the thyroid gland (mean = 35.0 Gy), while the lowest doses were received by patients diagnosed with kidney cancer (mean = 1.5 Gy). The youngest ages at radiation exposure were for patients diagnosed with neuroblastoma (2 years), and the oldest ages were for patients diagnosed with Hodgkin lymphoma (mean = 15).
TABLE 3.
Radiation Treatment Characteristics by Type of First Cancer in the Childhood Cancer Survivor Study Cohort
| Type of first cancer | N | N (%) patients treated with radiation | Mean (range) thyroid radiation dose (Gy)a | Mean (range) age at first radiation exposurea,b |
|---|---|---|---|---|
| Leukemia | 4109 | 2805 (68) | 3.9 (0–39.3) | 7 (<1–20) |
| Hodgkin lymphoma | 1600 | 1502 (94) | 35.0 (0.01–63.1) | 15 (3–20) |
| Central nervous system cancer | 1562 | 1098 (70) | 11.1 (0.05–53.6) | 8 (<1–20) |
| Soft tissue sarcoma | 1050 | 652 (62) | 6.5 (0–59.4) | 9 (<1–20) |
| Kidney cancer (Wilms) | 1051 | 669 (64) | 1.5 (0.03–31.5) | 4 (<1–20) |
| Bone cancer | 1017 | 362 (36) | 3.9 (0–49.5) | 13 (2–20) |
| Non-Hodgkin lymphoma | 893 | 606 (68) | 11.4 (0–67.3) | 11 (<1–20) |
| Neuroblastoma | 816 | 395 (48) | 5.2 (0.01–59.4) | 2 (<1–20) |
| Overall | 12098 | 8089 (67) | 11.3 (0–67.3) | 9 (<1–20) |
Among treated.
Assumed to be the same as age at first cancer diagnosis.
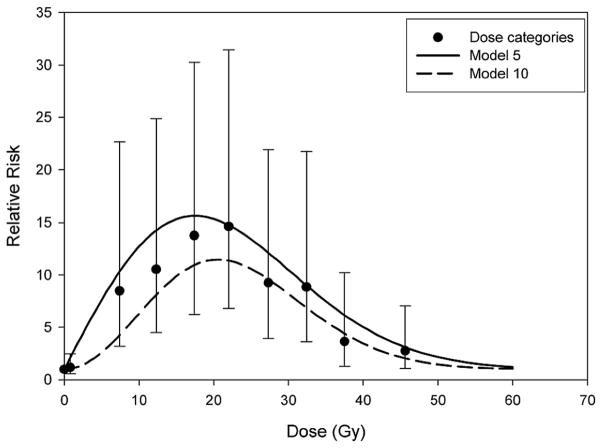
Based on the difference in deviance of nested models, statistical significance and parsimony, model 5, with two dose parameters [linear parameter (ERR/Gy) and exponential quadratic parameter for the high-dose downturn], appeared to best describe the data (Table 4). Model 10, also a two-dose parameter model, could be argued as the best model; however, the change in deviance compared to model 5 was not large, and model 5 is more congruent with radiobiological theory (14) (i.e., linear dose response at lower doses). Figure 1 shows the RR of thyroid cancer as a function of mean thyroid radiation dose for models 5 and 10 and also includes RRs and corresponding 95% CI for dose categories.
TABLE 4.
Models for Excess Relative Risk (ERR) as a Function of Radiation Dose to the Thyroid Gland in the Childhood Cancer Survivor Study Cohort
| Model number | Description | Equation | β1 | β2 | β3 | Deviance | Referent model | P valuea |
|---|---|---|---|---|---|---|---|---|
| 1 | Baselineb | αc | _ | _ | _ | 1532.5 | ||
| 2 | Linear | α[1 + β1d] | 0.404 | _ | _ | 1469.9 | 1 | <0.001 |
| 3 | Quadratic | α[1 + β1d2] | 0.00798 | _ | _ | 1515.0 | 1 | <0.001 |
| 4 | Linear exponential | α[1 + β1d × exp(β2d)] | 2.420 | −0.0665 | _ | 1430.5 | 2 | <0.001 |
| 5 | Linear exponential quadratic model-1 | α[1 + β1d × exp(β2d2)] | 1.381 | −0.00164 | _ | 1419.2 | 2 | <0.001 |
| 6 | Linear exponential quadratic model-2 | α[1 + β1d × exp(β2d + β3d2)] | 0.670 | 0.0615 | −0.00292 | 1417.1 | 5 | 0.1 |
| 7 | Linear quadratic | α[1 + β1d + β2d2] | 0.758 | −0.0115 | _ | 1441.8 | 2 | <0.001 |
| 8 | Linear quadratic exponential | α[1 + (β1d + β2d2) × exp(β3d)] | 2.215 | −0.0362 | −0.0402 | 1426.7 | 7 | <0.001 |
| 9 | Linear quadratic exponential quadratic | α[1 + (β1d + β2d2) × exp(β3d2)] | 0.433 | 0.0709 | −0.00242 | 1416.6 | 5 | 0.1 |
| 10 | Quadratic exponential quadratic | α[1 + β1d2 × exp(β2d2)] | 0.0674 | −0.00237 | _ | 1417.5 | 3 | <0.001 |
Note. Italics represent the most parsimonious models with the lowest deviance and compatibility with radiobiological theory.
Likelihood ratio test comparing to referent (nested) model (e.g. model 2 compared to model 1, model 5 compared to model 2).
Baseline function includes natural logarithm of attained age, sex and type of childhood cancer (Hodgkin lymphoma, leukemia or other).
α represents the baseline function as described in footnote b.
FIG. 1.
Observed relative risk of thyroid cancer as a function of mean radiation dose to the thyroid gland for categories of dose and fitted values based on model 5, α[1 + 1.4d × exp(−0.002d2)], and model 10, α[1 + 0.07d2 × exp(−0.002d2)], adjusted for attained age, sex and type of first cancer.
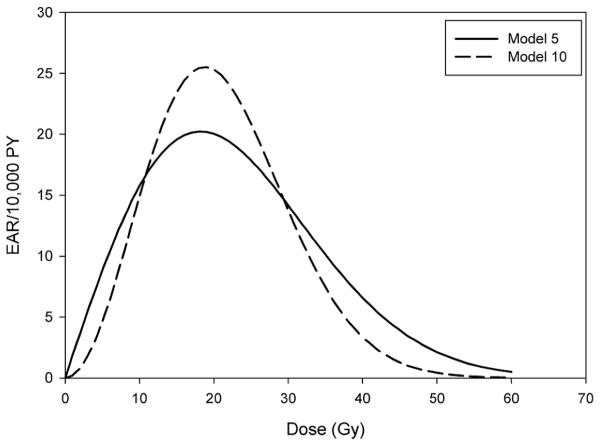
When examining EAR dose–response models, there were significant improvements in fit when comparing models with three dose parameters to model 5, which again is the most consistent with radiobiological theory (Table 5). However, based on deviance values, it appeared that model 10, with only two dose parameters, fit the data just as well as the three-parameter models. Figure 2 shows the EAR/10,000 person-years for thyroid cancer as a function of mean thyroid radiation dose for models 5 and 10. In Table 6 predicted baseline (specified for model covariates as follows: males diagnosed with childhood leukemia with an attained age >20 years) and excess cases of thyroid cancer are provided based on EAR models 5 and 10 of the dose response applied to the mean dose and number of person-years in various dose categories. The total number of excess cases is the sum of all stratum-specific excess cases for each combination of the variables sex, type of childhood cancer and attained age.
TABLE 5.
Models for Excess Absolute Risk per 10,000 Person-Years (EAR) as a Function of Radiation Dose to the Thyroid Gland in the Childhood Cancer Survivor Study Cohort
| Model number | Description | Equation | β1 | β2 | β3 | Deviance | Referent model | P valuea |
|---|---|---|---|---|---|---|---|---|
| 1 | Baselineb | αc | _ | _ | _ | 1532.5 | ||
| 2 | Linear | α + β1d | 0.458 | _ | _ | 1478.1 | 1 | <0.001 |
| 3 | Quadratic | α + β1d 2 | 0.00805 | _ | _ | 1516.1 | 1 | <0.001 |
| 4 | Linear exponential | α + β1d × exp(β2d) | 2.547 | −0.0582 | _ | 1439.8 | 2 | <0.001 |
| 5 | Linear exponential quadratic model-1 | α + β1d × exp(β2d2) | 1.830 | −0.00151 | _ | 1429.4 | 2 | <0.001 |
| 6 | Linear exponential quadratic model-2 | α + β1d × exp(β2d + β3d2) | 0.759 | 0.100 | −0.00381 | 1425.0 | 5 | 0.04 |
| 7 | Linear quadratic | α + β1d + β2d2 | 1.030 | −0.0156 | _ | 1448.9 | 2 | <0.001 |
| 8 | Linear quadratic exponential | α + (β1d + β2d2) × exp(β3d) | 2.351 | −0.0370 | −0.0327 | 1436.5 | 7 | <0.001 |
| 9 | Linear quadratic exponential quadratic | α + (β1d + β2d2) × exp(β3d2) | 0.284 | 0.169 | −0.00270 | 1424.1 | 5 | 0.02 |
| 10 | Quadratic exponential quadratic | α + β1d2 × exp(β2d2) | 0.197 | −0.00284 | 1424.3 | 3 | <0.001 |
Note. Italics represent the most parsimonious models with the lowest deviance and compatibility with radiobiological theory.
Likelihood ratio test comparing to referent model.
Baseline function includes natural logarithm of attained age, sex and type of childhood cancer (Hodgkin lymphoma, leukemia or other).
α represents the baseline function as described in footnote b.
FIG. 2.
Fitted excess absolute risk (per 10,000 person-years) of thyroid cancer as a function of mean radiation therapy dose to the thyroid gland for model 5, α + 1.8d × exp(−0.002d2), and model 10, α + 0.2d2 × exp(−0.003d2), adjusted for attained age, sex and type of first cancer.
TABLE 6.
Predicted Number of Baseline and Excess Cases of Thyroid Cancer at Various Doses of Radiation to the Thyroid Gland in the Childhood Cancer Survivor Study Cohort
| Dose category (Gy) | Mean dose (Gy) | PY/10,000 | Estimated number of baseline cases |
Estimated number of excess cases |
||
|---|---|---|---|---|---|---|
| Model 5a | Model 10b | Model 5c | Model 10d | |||
| 0 | 0 | 6.540 | 1.3 | 2.4 | 0 | 0 |
| >0–<5 | 0.79 | 8.186 | 1.7 | 3.0 | 11.8 | 1.0 |
| 5–<10 | 7.39 | 0.367 | 0.1 | 0.1 | 4.6 | 3.4 |
| 10–<15 | 12.31 | 0.556 | 0.1 | 0.2 | 10.0 | 10.8 |
| 15–<20 | 17.40 | 0.537 | 0.1 | 0.2 | 10.8 | 13.6 |
| 20–<25 | 21.99 | 0.607 | 0.1 | 0.2 | 11.8 | 14.6 |
| 25–<30 | 27.26 | 0.506 | 0.1 | 0.2 | 8.2 | 9.0 |
| 30–<35 | 32.41 | 0.433 | 0.1 | 0.2 | 5.3 | 4.5 |
| 35–<40 | 37.52 | 0.643 | 0.1 | 0.2 | 5.3 | 3.3 |
| ≥40 | 45.62 | 1.213 | 0.2 | 0.4 | 4.4 | 1.3 |
For each dose category: baseline cases = (PY/10,000) × 0.2019; specified for males diagnosed with childhood leukemia with an attained age <20 years.
For each dose category: baseline cases = (PY/10,000) × 0.3690; specified for males diagnosed with childhood leukemia with an attained age <20 years.
For each dose category: Excess cases = (PY/10,000) × 1.830 × (mean dose) × exp(−0.00151 × (mean dose)2).
For each dose category: Excess cases = (PY/10,000) × 0.197 × (mean dose)2 × exp(−0.00284 × (mean dose)2).
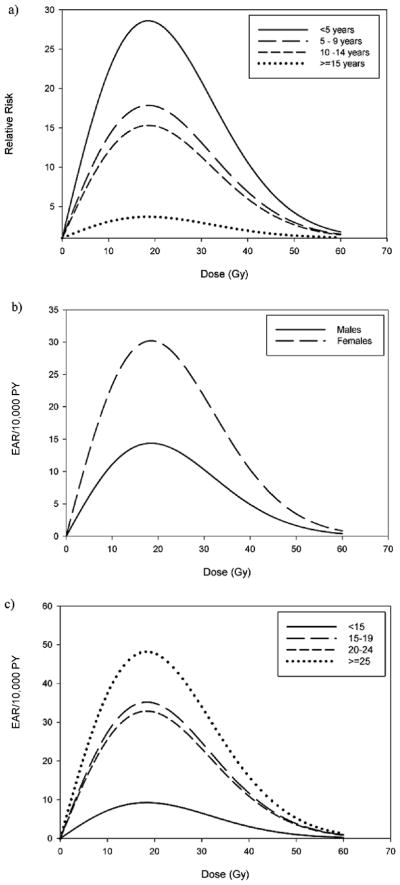
Type of first cancer, attained age, age at radiation exposure and time since radiation exposure all significantly modified the linear dose term in ERR model 5 (Table 7). However, when we assessed two effect modifiers at a time in the same models, only age at radiation exposure remained statistically significant, with a trend of smaller linear dose terms with later ages at exposure. For example, when we added an effect modification term for type of first cancer to a model that already had an effect modification term for attained age, the LRT for effect modification by type of first cancer was not statistically significant. However, when adding an effect modification term for attained age to a model that already had an effect modification term for type of first cancer, the LRT for effect modification by attained age was statistically significant. Though not statistically significant, the ERR/Gy among those who did not receive chemotherapy was approximately fivefold higher than that among those who did receive chemotherapy. Table 7 provides P values for effect modification for the covariates in which the nested models were adjusted for effect modification by age at radiation exposure. None of the variables in Table 7 significantly modified the exponential quadratic term (results not shown). See Fig. 3a for the relative risk dose–response curves stratified by age at exposure, which shows a marked reduction in risk over the dose range among those diagnosed after age 15 compared to those diagnosed before age 5.
TABLE 7.
Effect Modification of the Linear Ascending Terms of The Excess Relative Risk and Excess Absolute Risk of Thyroid Cancer by Demographic and Treatment-Related Variables in the Childhood Cancer Survivor Study Cohort
| Potential effect modifier | ERR model 5a |
EAR model 5a |
||||
|---|---|---|---|---|---|---|
| Linear term (ERR/Gy) (95% CI)b | P valuec | P valued | Linear term (EAR/10,000 PY/Gy) (95% CI)b | P valuec | P valuee | |
| Sex | ||||||
| Male | 1.8 (0.7, 6.3) | >0.5 | >0.5 | 1.3 (0.8, 2.0) | 0.007 | 0.008 |
| Female | 1.2 (0.6, 2.7) | 2.7 (1.6, 3.7) | ||||
| Year of birth | ||||||
| <1960 | 0.2 (−0.1, 1.0) | 0.07f | >0.5f | 0.4 (−0.4, 1.7) | >0.5f | 0.4f |
| 1960–1969 | 1.1 (0.6, 2.4) | 1.9 (1.1, 3.1) | ||||
| 1970–1979 | 1.6 (0.8, 3.4) | 1.7 (1.0, 2.7) | ||||
| ≥1980 | 1.6 (0.2, 6.3) | 0.7 (−0.5, 2.5) | ||||
| Race | ||||||
| White, Non-Hispanic | 1.4 (0.7, 2.8) | >0.5 | >0.5 | 1.8 (1.2, 2.6) | 0.4 | >0.5 |
| Black | 0.9 (−2.1, 3.9)g | 0.6 (−1.3, 2.4)g | ||||
| Hispanic/Latino | 1.8 (−0.3, 37.7) | 3.6 (1.0, 8.4) | ||||
| Other/Unspecified | 0.8 (−0.4, 80.6) | 1.1 (−0.6, 3.4) | ||||
| Type of first cancer | ||||||
| Leukemia | 5.6 (−0.1, 98.9) | 0.04 | 0.4 | 2.0 (1.2, 3.1) | >0.5 | >0.5 |
| Hodgkin lymphoma | 0.4 (0.06, 1,5) | 1.6 (0.5, 3.3) | ||||
| Other | 1.3 (0.6, 3.2) | 1.6 (0.9, 2.7) | ||||
| Attained age, years | ||||||
| <20 | 1.5 (0.5, 4.4) | 0.01f | 0.3f | 1.1 (0.5, 1.9) | 0.007f | 0.4f |
| 20–24 | 1.4 (0.6, 3.0) | 1.8 (0.9, 3.2) | ||||
| 25–29 | 1.1 (0.5, 2.4) | 2.1 (1.1, 3.8) | ||||
| 30–34 | 1.9 (0.9, 4.2) | 4.8 (2.7, 8.0) | ||||
| ≥35 | 0.9 (0.2, 2.7) | 3.3 (1.2, 6.9) | ||||
| Attained calendar year | ||||||
| <1980 | 1.2 (−0.4, 26.5) | >0.5f | >0.5f | 0.9 (0.2, 2.1) | <0.001f | 0.1f |
| 1980–1984 | 10.5 (−40.0, 60.9)g | 1.1 (0.3, 2.5) | ||||
| 1985–1989 | 2.7 (0.7, 17.6) | 1.6 (0.8, 2.8) | ||||
| 1990–1994 | 0.8 (0.3, 2.3) | 1.6 (0.8, 2.9) | ||||
| ≥1995 | 1.5 (0.5, 5.3) | 5.2 | ||||
| Age at radiation exposure, years | ||||||
| <5 | 2.5 (1.2, 5.2) | <0.001f | _ | 1.7 (1.0, 2.7) | 0.2f | 0.4f |
| 5–9 | 1.5 (0.7, 3.3) | 1.8 (1.0, 3.0) | ||||
| 10–14 | 1.3 (0.6, 2.7) | 2.2 (1.2, 3.7) | ||||
| ≥15 | 0.2 (0.03, 0.8) | 0.5 (0.07, 1.4) | ||||
| Time since radiation exposure, years | ||||||
| 5–14 | 0.7 (0.3, 1.6) | 0.02f | 0.3f | 0.8 (0.4, 1.4) | <0.001f | _ |
| 15–19 | 2.1 (1.1, 4.3) | 3.2 (1.9, 4.9) | ||||
| 20–24 | 1.8 (0.8, 3.9) | 3.0 (1.5, 5.1) | ||||
| ≥25 | 2.0 (0.7, 5.2) | 4.3 (1.9, 8.5) | ||||
| Calendar year of exposure | ||||||
| <1974 | 1.5 (0.7, 3.1) | >0.5f | >0.5f | 2.3 (1.3, 3.7) | >0.5f | >0.5f |
| 1975–1979 | 1.2 (0.6, 2.6) | 1.7 (0.9, 2.9) | ||||
| ≥1980 | 1.4 (0.7, 2.9) | 1.6 (0.9, 2.6) | ||||
| Chemotherapy | ||||||
| No | 5.0 (−0.6, 95.6) | 0.1 | 0.07 | 2.0 (1.0, 3.8) | >0.5 | >0.5 |
| Yes | 1.1 (0.6, 2.1) | 1.7 (1.1, 2.4) | ||||
ERR model 5: α[1 + β1d × exp(β2d2)]; EAR model 5: α + β1d × exp(β2d2); Baseline function includes natural logarithm of attained age, sex, type of childhood cancer (Hodgkin lymphoma, leukemia or other) and effect modification variable of interest (e.g. when evaluating race as an effect modifier, it is also included in the baseline term).
95% confidence interval based on the likelihood ratio profile of the parameter estimates (more appropriate than Wald estimates for linear dose–response terms).
Likelihood ratio test comparing models with and without effect modification terms.
Likelihood ratio test comparing models with and without effect modification terms with adjustment for effect modification by age at radiation exposure.
Likelihood ratio test comparing models with and without effect modification terms with adjustment for effect modification by time since radiation exposure.
Likelihood ratio test using the underlying continuous variable.
Likelihood-based confidence interval incalculable, thus Wald-based 95% confidence interval presented.
FIG. 3.
Effect modification of fitted relative risk and excess absolute risk dose responses. Panel a: Relative risk by age at radiation exposure; panel b: excess absolute risk by sex; panel c: excess absolute risk by time since radiation exposure.
Sex, attained age, attained calendar year and time since radiation exposure all significantly modified the linear dose term in EAR model 5 (Table 7). However, when we assessed two effect modifiers at a time in the same models (results not shown), only sex and time since radiation exposure remained significant modifiers of the EAR/Gy term in model 5. Females had a twofold larger EAR/Gy than males (P = 0.008) (Table 7), and there was a general trend of larger linear dose–response coefficients with increasing time since exposure. The similarity of the EAR/Gy estimates, but differences in ERR/Gy estimates by chemotherapy status suggest an additive rather than multiplicative effect of radiation and chemotherapy on the risk of second primary thyroid cancer. Table 7 provides P values for effect modification for the covariates in which the nested models were adjusted for effect modification by time since radiation exposure. None of the variables in Table 7 significantly modified the exponential quadratic term (results not shown). The excess absolute risk dose–response curves stratified by sex and time since exposure in Fig. 3b and c, respectively, show the increased EAR over the dose range for females and for those whose time since exposure exceeded 25 years.
Sex and time since radiation exposure were also significant modifiers of the ascending quadratic risk term in EAR model 10 (results not shown), demonstrating very similar patterns to those seen with the linear term in EAR model 5. This quadratic term was larger in females compared to males (0.3 compared to 0.1, P = 0.02), and there were trends of larger quadratic dose–response terms with increasing time since exposure age (P = <0.001). None of the variables significantly modified the exponential quadratic term.
DISCUSSION
In the largest study to date of radiation exposure for the treatment of childhood cancers and subsequent thyroid cancer risk, we confirmed the downturn in risk of developing thyroid cancer after radiation doses exceeding approximately 20–25 Gy that was demonstrated in the previous case-control study conducted in the CCSS cohort (1, 2) and is thought to be attributable to cell killing (15). We also found that age at radiation exposure significantly modified the ascending linear portion of the ERR dose response, for which the previous case-control study found only suggestive evidence. Unlike the previous study, we were able to evaluate the EAR, which demonstrated a similar dose–response pattern to the ERR; however, significant modifiers of the EAR linear term were sex and time since radiation exposure. Results were similar when restricting analyses to the 111 second primary thyroid cancers (results not shown) and when evaluating different lag periods (0, 10 and 20 years) for the radiation doses to the thyroid gland (results not shown). It is also important to note that the downturn in risk remained significant in the ERR and EAR models when accounting for effect modification of the linear portion of the dose response by age at exposure and time since radiation exposure, respectively (results not shown).
As with the previous case-control study, we found the linear exponential quadratic model (model 5) to best describe the ERR for thyroid cancer in relation to radiation dose, and the values of the parameters in our model (β1 = 1.4, β2 = −0.002) were nearly identical to the parameters estimated in the case-control study (β1 = 1.3, β2 = −0.002). While this model is the most consistent with radiobiological theory (14), the quadratic exponential quadratic model (model 10) was also consistent with our data, particularly when examining the EAR. Both models 5 and 10, however, demonstrated similar patterns, and sex and time since radiation exposure were identified as modifiers of the EAR dose–response relationships in each of the models (results not shown). It is important to note that at low doses (<1 Gy) model 5 predicts an excess number of thyroid cancers that is nearly 12 times larger than those predicted by model 10 (Table 6). At larger doses, however, there were no substantial differences in the predicted number of excess cases between the models.
Also similar to the previous CCSS case-control study, we found neuroblastoma to be a risk factor for thyroid cancer, independent of radiation exposure, which has been observed in another cohort of childhood cancer survivors (3). However, our finding was based on only nine cases, four of which were diagnosed with neuroblastoma before 1 year of age; only two patients were diagnosed with cancer before age 1 among the other 110 thyroid cancer cases. Given the strong interrelationship between type of first cancer, age at exposure and radiation dose (Table 3), our observation for neuroblastoma may be attributable to an age-at-exposure effect for which statistical adjustment was not completely possible. It is also worth mentioning that when patients diagnosed with Hodgkin lymphoma were excluded from the analysis, the thyroid cancer dose–response relationship remained virtually unchanged (results not shown), meaning that our findings are not entirely attributable to Hodgkin lymphoma patients.
We also observed suggestive evidence of an increased risk of thyroid cancer in association with chemotherapy that was independent of radiation exposure, which was not observed in the previous case-control study (1, 2). However, after adjustment for radiation treatment the association with chemotherapy was relatively weak, of the order of 1.6-fold increased risk, demonstrating that the risk of second primary thyroid cancer is typically dominated by the radiation effect, particularly at the highest radiation doses where cell killing would presumably remove cells from chemotherapy-related cancer risk. Chemotherapy was not found to be a confounder of the association between radiation and thyroid cancer risk. The similarity of the EAR/Gy estimates by chemotherapy status but suggestive heterogeneity of the ERR/Gy estimates by chemotherapy status points to a potentially additive rather than multiplicative interaction between chemotherapy and radiation for the risk of developing a second primary thyroid cancer. Because of the variety of drug regimens and combinations, doses and schedules of treatments, however, it was beyond the scope of the present analysis to assess in detail the effects of chemotherapy as a modifier of radiation-associated risk.
Small numbers of thyroid cancer cases have precluded previous studies of childhood cancer survivors from conclusively demonstrating a downturn in risk at high doses and from examining effect modifiers of the dose response (3, 5). There are larger studies in other settings with childhood radiation exposure, but doses to the thyroid gland were considerably lower (16–23). These lower-dose studies have been restricted to the linear portion of the dose–response relationship, though results from a few studies have suggested a leveling or downward curvature of thyroid cancer risk at the upper dose ranges (4 to 10 Gy) (16, 20, 21).
Results for effect modification by sex have varied among previous studies, including no difference in ERR/Gy by sex (16, 22), a higher ERR/Gy in men compared to women (19, 20) and a higher ERR/Gy in female subjects compared to male subjects (21, 23). The EAR/Gy, however, has consistently been shown to be elevated in women compared to men (two- to fourfold) (20–22), as in our study, reflecting the higher background rate of thyroid cancer among women.
The radiation-related relative risk of thyroid cancer has also consistently been shown to decrease with increasing age at exposure (19–23). When considering time since exposure, a pooled analysis of seven studies of thyroid cancer, primarily among those exposed to radiation as children, found significant heterogeneity in the ERR/Gy, with an increase up to about 30 years, after which the risk began to decline (21). This pattern was also apparent in an updated analysis of a study of thyroid cancer risk after childhood treatment with radiation for tinea capitis that originally contributed to the pooled analysis (22). However, a recent case-control study of children in Belarus exposed to radiation from the Chernobyl accident found no significant heterogeneity in radiation-related risk by time since the accident (16).
A downturn in risk at high doses has generally not been observed for solid tumors other than thyroid cancer. Risks of breast cancer, central nervous system tumors, osteosarcoma and lung cancer have demonstrated no evidence for departure from linearity for organ doses in excess of 30 to 60 Gy (24–28) among childhood cancer survivors and adults treated for Hodgkin lymphoma. Small study size, limited case numbers and narrow ranges of radiation exposure could explain why a downturn in risk was not observed in these studies. To our knowledge, the only other cancer type to demonstrate a downturn in risk at high doses is leukemia. Among patients treated for cervical cancer, an increased risk of leukemia was observed up to bone marrow doses of 4 Gy, after which the risk began to decrease (29).
Our study had several strengths and limitations. Strengths included its large size, detailed treatment data and individual level dose estimates to the thyroid gland allowing for complex dose–response modeling. The dosimetry was not as detailed as for the previous case-control study because of the impracticality of evaluating each instance when the thyroid gland was under blocking, given the large number of patients who had radiotherapy. However, our findings are nearly identical to those of the case-control study, suggesting that the less detailed dosimetry was not a major limitation. We relied on self-reported thyroid cancers that were subsequently confirmed by study pathologists, and it may be possible that some cancer survivors neglected to report their second cancers or may not have completed the follow-up questionnaire. Unless such omissions are related to dose, it is unlikely to have introduced bias but would have added some imprecision to the risk estimates. Contrary to findings from the case-control study (2), we did not observe a significant difference in mean tumor size by type of first cancer, such as might be expected if surveillance was greater after some types of cancer (e.g., Hodgkin lymphoma) than for others. While there appeared to be an independent effect of neuroblastoma, we were unable to discount confounding by age at exposure, given that neuroblastoma patients tended to be younger at time of diagnosis.
In the largest study to date of second primary thyroid cancers among childhood cancer survivors, we have confirmed and strengthened the results of previous studies of populations exposed to radiation during childhood. Sex, age at exposure and time since exposure were found to be significant modifiers of the radiation-related risk of thyroid cancer and as such are important factors to take into account for clinical follow-up and thyroid cancer risk estimation among childhood cancer survivors.
Acknowledgments
This study was funded by National Cancer Institute grant U24 CA55727, the Children’s Cancer Research Fund, the Lance Arm-strong Foundation grant 147149 and the Intramural Research Program of the NIH, National Cancer Institute, Division of Cancer Epidemiology and Genetics. We thank Drs. Ethel Gilbert, Dale Preston and Jay Lubin for their assistance with the statistical analysis and for helpful comments on the manuscript.
References
- 1.Ronckers CM, Sigurdson AJ, Stovall M, Smith SA, Mertens AC, Liu Y, Hammond S, Land CE, Neglia JP, Inskip PD. Thyroid cancer in childhood cancer survivors: a detailed evaluation of radiation dose response and its modifiers. Radiat Res. 2006;166:618–628. doi: 10.1667/RR3605.1. [DOI] [PubMed] [Google Scholar]
- 2.Sigurdson AJ, Ronckers CM, Mertens AC, Stovall M, Smith SA, Liu Y, Berkow RL, Hammond S, Neglia JP, Inskip PD. Primary thyroid cancer after a first tumour in childhood (the Childhood Cancer Survivor Study): a nested case-control study. Lancet. 2005;365:2014–2023. doi: 10.1016/S0140-6736(05)66695-0. [DOI] [PubMed] [Google Scholar]
- 3.de Vathaire F, Hardiman C, Shamsaldin A, Campbell S, Grimaud E, Hawkins M, Raquin M, Oberlin O, Diallo I, Bonaïti C. Thyroid carcinomas after irradiation for a first cancer during childhood. Arch Intern Med. 1999;159:2713–2719. doi: 10.1001/archinte.159.22.2713. [DOI] [PubMed] [Google Scholar]
- 4.Taylor AJ, Croft AP, Palace AM, Winter DL, Reulen RC, Stiller CA, Stevens MC, Hawkins MM. Risk of thyroid cancer in survivors of childhood cancer: results from the British Childhood Cancer Survivor Study. Int J Cancer. 2009;125:2400–2405. doi: 10.1002/ijc.24581. [DOI] [PubMed] [Google Scholar]
- 5.Tucker MA, Jones PH, Boice JD, Jr, Robison LL, Stone BJ, Stovall M, Jenkin RD, Lubin JH, Baum ES the Late Effects Study Group. Therapeutic radiation at a young age is linked to secondary thyroid cancer. Cancer Res. 1991;51:2885–2888. [PubMed] [Google Scholar]
- 6.Robison LL, Armstrong GT, Boice JD, Chow EJ, Davies SM, Donaldson SS, Green DM, Hammond S, Meadows AT, Zeltzer LK. The Childhood Cancer Survivor Study: a National Cancer Institute-supported resource for outcome and intervention research. J Clin Oncol. 2009;27:2308–2318. doi: 10.1200/JCO.2009.22.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robison LL, Mertens AC, Boice JD, Breslow NE, Donaldson SS, Green DM, Li FP, Meadows AT, Mulvihill JJ, Zeltzer LK. Study design and cohort characteristics of the Childhood Cancer Survivor Study: a multi-institutional collaborative project. Med Pediatr Oncol. 2002;38:229–239. doi: 10.1002/mpo.1316. [DOI] [PubMed] [Google Scholar]
- 8.Stovall M, Blackwell CR, Cundiff J, Novack DH, Palta JR, Wagner LK, Webster EW, Shalek RJ. Fetal dose from radiotherapy with photon beams: report of AAPM Radiation Therapy Committee Task Group No. 36. Med Phys. 1995;22:63–82. doi: 10.1118/1.597525. [DOI] [PubMed] [Google Scholar]
- 9.Stovall M, Smith SA, Rosenstein M. Tissue doses from radiotherapy of cancer of the uterine cervix. Med Phys. 1989;16:726–733. doi: 10.1118/1.596331. [DOI] [PubMed] [Google Scholar]
- 10.Central axis depth dose data for use in radiotherapy. A survey of depth doses and related data measured in water or equivalent media. Br J Radiol Suppl. 1983;17:1–147. [PubMed] [Google Scholar]
- 11.Inskip PD. Thyroid cancer after radiotherapy for childhood cancer. Med Pediatr Oncol. 2001;36:568–573. doi: 10.1002/mpo.1132. [DOI] [PubMed] [Google Scholar]
- 12.Shore RE. Issues and epidemiological evidence regarding radiation-induced thyroid cancer. Radiat Res. 1992;131:98–111. [PubMed] [Google Scholar]
- 13.Surveillance, Epidemiology, and End Results (SEER) Program Populations (1969–2006) National Cancer Institute; Bethesda, MD: 2009. [Available online at www.seer.cancer.gov/popdata] [Google Scholar]
- 14.Brenner DJ, Doll R, Goodhead DT, Hall EJ, Land CE, Little JB, Lubin JH, Preston DL, Preston RJ, Zaider M. Cancer risks attributable to low doses of ionizing radiation: assessing what we really know. Proc Natl Acad Sci USA. 2003;100:13761–13766. doi: 10.1073/pnas.2235592100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gray LH. Cellular Radiation Biology: a collection of works presented at the 18th Annual Symposium on Experimental Cancer Research 1964. Williams and Wilkins; Baltimore: 1965. Radiation biology and cancer; pp. 7–25. [Google Scholar]
- 16.Cardis E, Kesminiene A, Ivanov V, Malakhova I, Shibata Y, Khrouch V, Drozdovitch V, Maceika E, Zvonova I, Williams D. Risk of thyroid cancer after exposure to 131I in childhood. J Natl Cancer Inst. 2005;97:724–732. doi: 10.1093/jnci/dji129. [DOI] [PubMed] [Google Scholar]
- 17.Davis S, Stepanenko V, Rivkind N, Kopecky KJ, Voilleque P, Shakhtarin V, Parshkov E, Kulikov S, Lushnikov E, Tsyb A. Risk of thyroid cancer in the Bryansk Oblast of the Russian Federation after the Chernobyl Power Station accident. Radiat Res. 2004;162:241–248. doi: 10.1667/rr3233. [DOI] [PubMed] [Google Scholar]
- 18.Kerber RA, Till JE, Simon SL, Lyon JL, Thomas DC, Preston-Martin S, Rallison ML, Lloyd RD, Stevens W. A cohort study of thyroid disease in relation to fallout from nuclear weapons testing. J Am Med Assoc. 1993;270:2076–2082. [PubMed] [Google Scholar]
- 19.Likhtarov I, Kovgan L, Vavilov S, Chepurny M, Ron E, Lubin J, Bouville A, Tronko N, Bogdanova T, Howe G. Post-Chernobyl thyroid cancers in Ukraine. Report 2: risk analysis. Radiat Res. 2006;166:375–386. doi: 10.1667/RR3593.1. [DOI] [PubMed] [Google Scholar]
- 20.Preston DL, Ron E, Tokuoka S, Funamoto S, Nishi N, Soda M, Mabuchi K, Kodama K. Solid cancer incidence in atomic bomb survivors: 1958–1998. Radiat Res. 2007;168:1–64. doi: 10.1667/RR0763.1. [DOI] [PubMed] [Google Scholar]
- 21.Ron E, Lubin JH, Shore RE, Mabuchi K, Modan B, Pottern LM, Schneider AB, Tucker MA, Boice JD., Jr Thyroid cancer after exposure to external radiation: a pooled analysis of seven studies. Radiat Res. 1995;141:259–277. [PubMed] [Google Scholar]
- 22.Sadetzki S, Chetrit A, Lubina A, Stovall M, Novikov I. Risk of thyroid cancer after childhood exposure to ionizing radiation for tinea capitis. J Clin Endocrinol Metab. 2006;91:4798–4804. doi: 10.1210/jc.2006-0743. [DOI] [PubMed] [Google Scholar]
- 23.Tronko MD, Howe GR, Bogdanova TI, Bouville AC, Epstein OV, Brill AB, Likhtarev IA, Fink DJ, Markov VV, Beebe GW. A cohort study of thyroid cancer and other thyroid diseases after the Chornobyl accident: thyroid cancer in Ukraine detected during first screening. J Natl Cancer Inst. 2006;98:897–903. doi: 10.1093/jnci/djj244. [DOI] [PubMed] [Google Scholar]
- 24.Gilbert ES, Stovall M, Gospodarowicz M, Van Leeuwen FE, Andersson M, Glimelius B, Joensuu T, Lynch CF, Curtis RE, Travis LB. Lung cancer after treatment for Hodgkin’s disease: focus on radiation effects. Radiat Res. 2003;159:161–173. doi: 10.1667/0033-7587(2003)159[0161:lcatfh]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 25.Inskip PD, Robison LL, Stovall M, Smith SA, Hammond S, Mertens AC, Whitton JA, Diller L, Kenney L, Neglia JP. Radiation dose and breast cancer risk in the childhood cancer survivor study. J Clin Oncol. 2009;27:3901–3907. doi: 10.1200/JCO.2008.20.7738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Le Vu B, de Vathaire F, Shamsaldin A, Hawkins MM, Grimaud E, Hardiman C, Diallo I, Vassal G, Bessa E, Lemerle J. Radiation dose, chemotherapy and risk of osteosarcoma after solid tumours during childhood. Int J Cancer. 1998;77:370–377. doi: 10.1002/(sici)1097-0215(19980729)77:3<370::aid-ijc11>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 27.Travis LB, Hill DA, Dores GM, Gospodarowicz M, van Leeuwen FE, Holowaty E, Glimelius B, Andersson M, Wiklund T, Gilbert E. Breast cancer following radiotherapy and chemotherapy among young women with Hodgkin disease. J Am Med Assoc. 2003;290:465–475. doi: 10.1001/jama.290.4.465. [DOI] [PubMed] [Google Scholar]
- 28.Tucker MA, D’Angio GJ, Boice JD, Jr, Strong LC, Li FP, Stovall M, Stone BJ, Green DM, Lombardi F the Late Effects Study Group. Bone sarcomas linked to radiotherapy and chemotherapy in children. N Engl J Med. 1987;317:588–593. doi: 10.1056/NEJM198709033171002. [DOI] [PubMed] [Google Scholar]
- 29.Boice JD, Jr, Blettner M, Kleinerman RA, Stovall M, Moloney WC, Engholm G, Austin DF, Bosch A, Cookfair DL, Hutchison GB. Radiation dose and leukemia risk in patients treated for cancer of the cervix. J Natl Cancer Inst. 1987;79:1295–1311. [PubMed] [Google Scholar]