Abstract
Objectives
To study how apatite crystal alignment of an enamel-like substrate affects DPSC cellular adhesion and growth as a precursor to produce an in vitro enamel/dentin superstructure for future studies.
Methods
The cells were subcultured in 10% FBS DMEM up to 7 weeks on the two surfaces. Specimens were observed under SEM, counted, and analyzed using the human pathway-focused matrix and adhesion PCR array.
Results
After 3 days, the cell number on ordered FA surface was significantly higher than on the disordered surface. Of the 84 focused pathway genes, a total of 20 genes were either up or down regulated in the cells on ordered FA surface compared to the disordered surface. More interestingly, of the cell-matrix adhesion molecules, integrin alpha 7 and 8 (ITGA 7 and 8), integrin beta 3 and 4 (ITGB3 and 4), and the vitronectin receptor-integrin alpha V (ITGAV) and the key adhesion protein-fibronectin1 (FN1) were up-regulated. In SEM, both surfaces showed good biocompatibility and supported long term growth of DPSC cells but with functional cell-matrix interaction on the ordered FA surfaces.
Significance
The enhanced cellular response of DPSC cell to the ordered FA crystal surface involves a set of delicately regulated matrix and adhesion molecules which could be manipulated by treating the cells with a dentin extract, to produce a dentin/enamel superstructure.
Keywords: Fluorapatite, stem cell, PCR array, Matrix, Adhesion
Introduction
In primary dentinogenesis during tooth development, epithelial signals are essential to induce the dental papilla ectomesenchymal cells to terminally differentiate into odontoblasts. After tooth eruption, the dental pulp still retains its defensive and regenerative potential. Under certain physical and pathological stimuli, bound bioactive molecules, including growth factors and some noncollagenous proteins, may be released from the dentin matrix and recapitulate developmental events allowing reparative dentin to form 1–3. Recently, a distinct population of human postnatal dental pulp stem cells (DPSCs) have been described 4, 5 and similar cells have been isolated from shed deciduous teeth 6. These postnatal stem/progenitor cells have offered us promising possibilities either for the regeneration from resident populations in the pulp or for the engineering tissue constructs. Most recently, an enamel-dentin-like complex has been generated in vivo by transplantation of the odontogenic epithelial cells and dental mesenchymal cells co-culture construct 7 and the odontogenic epithelial cells and dental pulp cells co-culture construct 8. For many years, the methodology to “grow a tooth” for transplantation into humans has been the subject of much conjecture and a large number of studies. The complex nature of the tooth may preclude this being done in an in vitro situation. The ability to synthesize a fluorapatite superstructure in vitro which has the prismatic appearance of normal enamel will make the biological situation less complex and perhaps, a tooth grown in culture a reality.
We have described the in vitro induction of these DPSCs into odontoblast-like cells after treatment with a mineralization supplement (MS) containing AA and β-GP and an EDTA-soluble dentin extract (DE), which includes a cocktail of growth factors and bio-active molecules sequestered in the dentin 9. In a more recent study the gene profile changes during the DPSC cell mineralization process induced in the same experimental conditions as above were also analyzed 10 which lead us to speculate whether the DPSC adhere to and grow long term on an enamel-like substrate in vitro.
Promisingly, an ordered enamel-like fluorapatite (FAP) film via a hydrothermal method has been established in our laboratory. The crystals are very well aligned over a large area after being deposited on metal surface and the rods spontaneously formed bundles (prism-like structures) 11. It should be possible, therefore, that under the control of the appropriate signaling molecules and the DPSC culture conditions to induce dentin formation on the synthetic FA layer in vitro without using the epithelial cells, thus, producing a model enamel/dentin superstructure formation.
In this study we used fluorapatite (FA) substrates, with ordered (enamel-like) or disordered surface organization, to study their effect on the biocompatibility, initial cellular response and long term growth of the DPSC cells. The underlying mechanisms during these processes were also investigated.
Materials and Methods
Synthesis of the FA apatite surfaces
For a typical synthesis of FA crystals, 9.36 g ethylenedeiaminetetraacetic acid calcium disodium salt (EDTA-Ca-Na2) and 2.07 g NaH2PO4.H2O were mixed with about 90 mL distilled water. The suspensions were stirred continuously until the powder dissolved. The pH was adjusted to 6.0 using NaOH. Prior to mixing 0.21 g NaF in 90 mL of the EDTA-Ca-Na2 and NaH2PO4 solution, it was dissolved in 10 mL water (pH 7.0) and stirred continuously. The FA crystal growth on the substrates (15 mm 316 stainless steel discs) was achieved by adding the plates to 100 mL of newly prepared EDT-Ca-Na2/NaH2PO4 / NaF mixture and then autoclaving at 121 °C at pressure of 2.4× 105 Pa for 10 hours. Ordered and disordered films were produced individually on the undersurfaces and upper surfaces of the stainless steel discs respectively.
Cell culture and cell attachment assay
DPSC cells (kindly donated by Dr. S. Shi, USC) were subcultured on the FA films in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% FBS, 100 units/ml of penicillin and 100 μg/ml of streptomycin, at a seeding density of 0.5× 105, in 12-well plates. The cultures were maintained at 37°C in a humidified atmosphere containing 5% CO2 and 95% air. Before cell seeding, the ordered and disordered FA substrates were equilibrated with 10% FBS culture media for 2 hours. After being counted, harvested cells were then seeded onto 12-well cell culture plates containing different FA samples under the above serum adsorption conditions. Cells were seeded on the two sterilized coating surfaces at a density of 1× 105cells/mL and cultured for 3 days. At the end of culture, the samples were taken out and removed to new 12-well plates. After being washed twice with phosphate buffered saline (PBS) solution, cells were detached with trypsin/EDTA, stained with trypan blue and then counted using a haemocytometer.
RNA isolation and Reverse Transcription
Total cellular RNA was isolated from DPSC cells grown for 3 days on the two surfaces using the RNeasy Mini kit (Qiagen, CA, USA) according to the manufacturer’s instructions. The RNA was treated with the RNase-free DNase Set (Qiagen, CA, USA) during RNA isolation. The cDNA samples were prepared from the isolated RNA using the RT first strand kit (Cat. No. C-03, Qiagen, CA, USA) according to the manufacturer’s protocols. An average of 6–8 replicates of each FA crystal substrate on which the cells were grown has been used for the total cellular RNA isolation and cDNA sample preparation.
RT2 profiler PCR array analysis
Specimens were analyzed using the human pathway-focused matrix and adhesion PCR array (Qiagen, CA, USA), which combines the PCR sensitivity and the multi-gene profiling capability of a microarray. Briefly, the above prepared cDNA samples were added to the RT2 qPCR master mix containing SYBR Green and reference dye. The above mixture was then aliquoted across the PCR array templates which contain 84 pathway-specific genes plus controls. The real-time PCR analysis was carried out using an ABI 7700 sequence detector (Applied Biosystems, Foster, USA). Relative gene expression values were analyzed using the Superarray web-based software package performing all ΔΔCt based fold-change calculations. Duplicate 96-well plates were used in this PCR array analyses.
Scanning Electron Microscopy (SEM)
After 1, 3, 5, and 7 weeks of culture, the DPSC cells grown on the two FA surfaces were rinsed and fixed in 2.5% glutaraldehyde in distilled water, serially dehydrated and critical point dried. SEM analysis was conducted on a Phillips XL30FEG Scanning Electron Microscope (SEM) FEI company, Hillsboro, OR, USA) operated at 10kV (Resolution: 2.0nm at 30kV, 5.0nm at 1kV). The SEM specimen was coated with Au/Pd film to prevent specimen charging.
Statistical analysis
The cell counting results were analyzed statistically using GraphPad Prism 5 for unpaired t test of an average of 3–5 replicates and significance was considered at p < 0.05. Data are expressed as means ± standard deviation.
Results
Hydrothermal Synthesis of ordered and disordered FA crystal surfaces
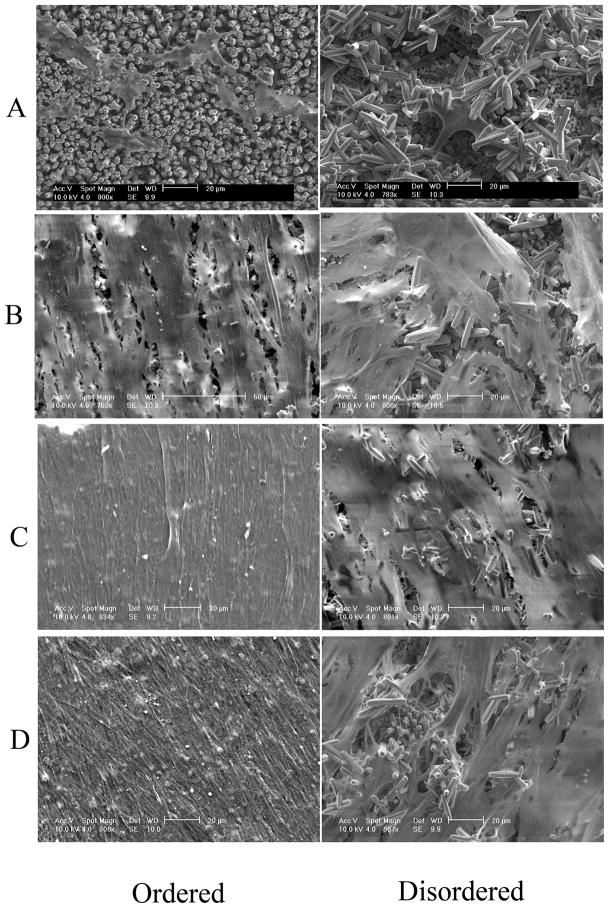
The ordered rod-like FA crystals were very well aligned along the c-axis after being grown on the undersurfaces of the 316 stainless steel discs. The densely-packed growth mode along the c-axis forced the rod-like crystals to align parallel to each other (Figure 1A). On the upper surfaces of the discs the FA crystals are randomly arranged without forming the well aligned structure (Figure 1B).
Figure 1.
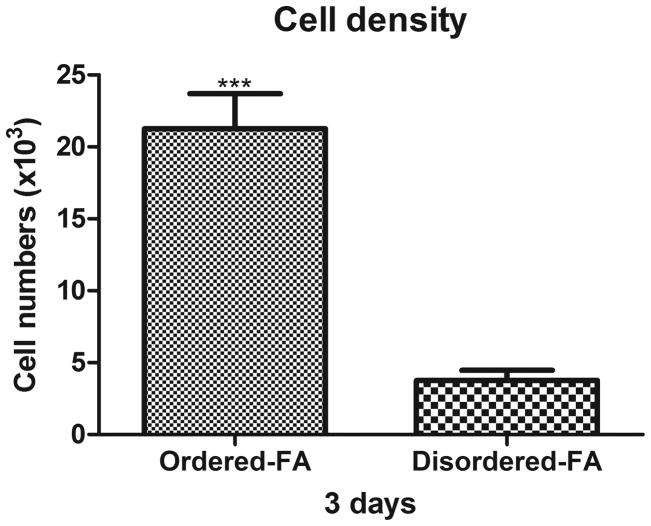
Cell density on ordered and disordered FA surfaces
The initial attached cell numbers on the ordered FA surfaces were significantly higher than that on the disordered FA surfaces (unpaired t test, P<0.001, n=3) (Figure 2).
Figure 2.
Human matrix and adhesion pathway-focused PCR array analysis
Of the 84 focused pathway genes, a total of 20 genes were either up or down regulated in the cells on ordered FA surface compared to the disordered surface. Of the cell-matrix adhesion molecules, secreted phosphoprotein 1 (SPP1), thrombospondin 3 (THBS3), integrin alpha 7 and 8 (ITGA 7 and 8), integrin beta 3 and 4 (ITGB3 and 4), and more interestingly, the vitronectin receptor-integrin alpha V (ITGAV) were up-regulated. Other adhesion molecules for example collagen type VII alpha 1 (COL7A1), COL8A1, COL12A1, laminin alpha1 (LAMA1) and the key adhesion protein-fibronectin1 (FN1) were also up-regulated. Among the ECM proteases, the up-regulation of the matrix metalloproteinase 13 (MMP13) was coordinated with the down-regulation of 3 other proteases (Figure 3, Table 1). The expression change of at least 1.5 fold was set as the “cutoff” value for the transcripts being differentially expressed on the two crystal surfaces. In our preliminary studies, this “cutoff” value for the detection of the differentially regulated individual genes was further confirmed by the single real-time PCR amplifications. A similar methodology was adopted in our earlier publication 12.
Figure 3.
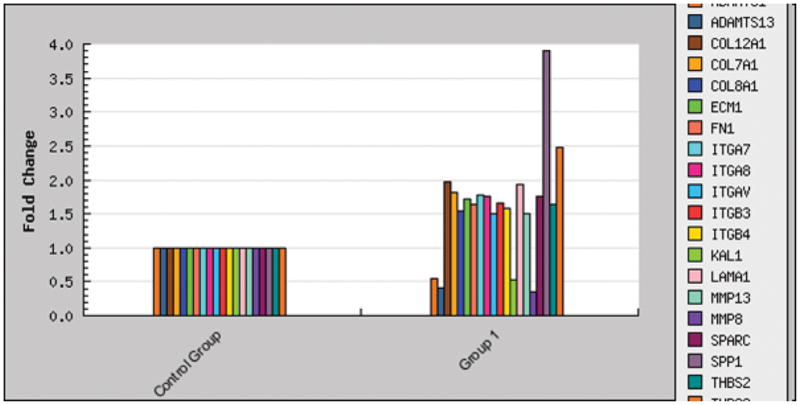
Table 1.
Fold change of the human extracellular matrix and adhesion molecules expressed by the DPSC cells grown on the ordered FA surface relative to the disordered surface.
| Symbol | Unigene | Refseq | Gname | Fold Change |
|---|---|---|---|---|
| SPP1 | Hs.313 | NM_000582 | BNSP/BSPI | 3.91 |
| THBS3 | Hs.169875 | NM_007112 | TSP3 | 2.47 |
| COL12A1 | Hs.101302 | NM_004370 | COL12A1L | 1.97 |
| LAMA1 | Hs.270364 | NM_005559 | LAMA | 1.94 |
| COL7A1 | Hs.476218 | NM_000094 | EBD1/EBDCT | 1.82 |
| ITGA7 | Hs.524484 | NM_002206 | FLJ25220 | 1.77 |
| ITGA8 | Hs.171311 | NM_003638 | Integrin a8 | 1.76 |
| SPARC | Hs.111779 | NM_003118 | ON | 1.75 |
| ECM1 | Hs.81071 | NM_004425 | ECM1 | 1.71 |
| ITGB3 | Hs.218040 | NM_000212 | CD61/GP3A | 1.65 |
| FN1 | Hs.203717 | NM_002026 | CIG/DKFZp686F10164 | 1.64 |
| THBS2 | Hs.371147 | NM_003247 | TSP2 | 1.63 |
| ITGB4 | Hs.632226 | NM_000213 | CD104 | 1.58 |
| COL8A1 | Hs.134830 | NM_001850 | MGC9568 | 1.54 |
| ITGAV | Hs.436873 | NM_002210 | CD51/MSK8 | 1.51 |
| MMP13 | Hs.2936 | NM_002427 | CLG3 | 1.50 |
| MMP8 | Hs.161839 | NM_002424 | CLG1/HNC | −2.88 |
| ADAMTS13 | Hs.131433 | NM_139028 | C9orf8/DKFZp434C2322 | −2.45 |
| KAL1 | Hs.521869 | NM_000216 | ADMLX/HHA | −1.92 |
| ADAMTS1 | Hs.643357 | NM_006988 | C3-C5/METH1 | −1.83 |
After the real-time PCR amplification, relative gene expression values were analyzed using the Superarray web-based software package performing all ΔΔCt based fold-change calculations.
SEM observation
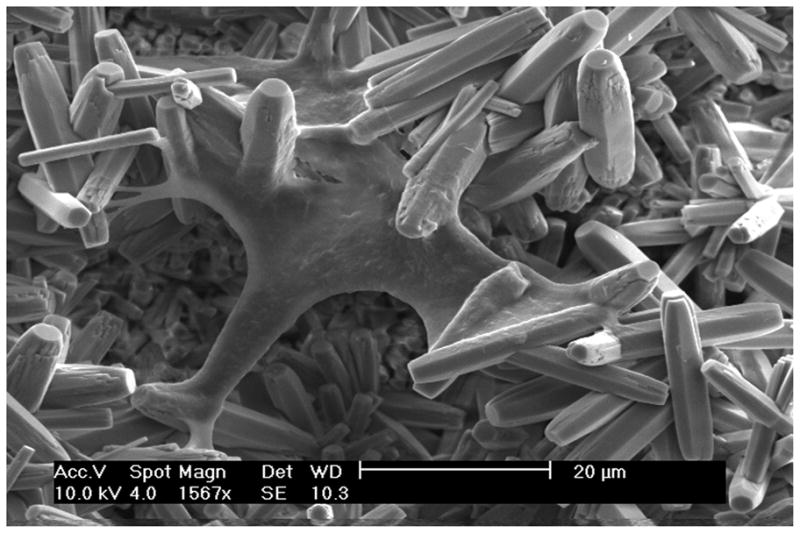
Scanning Electron Microscopy (SEM) of the DPSC cells grown on both the ordered and disordered FA crystal surfaces showed good biocompatibility. The cells spread and flattened on both surfaces; however, a greater number of cells were identified on the ordered FA crystal surface compared to the disordered surface after 1 week of cell growth (Figure 4A). After culturing for 3 weeks, dense cell nodule formation was observed on the ordered FA surface with more cellular extensions and extracellular matrix; whereas isolated or fused cell nodules were seen on the disordered FA surface (Figure 4B). At 5 weeks, the cells were fused together to completely cover the FA surfaces with denser matrix formation on the ordered FA surfaces than on the disordered (Figure 4C). After 7 weeks, multi-layers of cells with cell bodies extensively elongated and polarized were seen on the ordered FA surfaces. Further a much denser matrix was seen on the ordered surface as opposed to the disordered surfaces (Figure 4D). At greater magnification of the DPSC cells grown on ordered FA crystal surfaces, cytoplasmic extensions of the cells adhering to the individual FA crystals can be seen (Figure 5).
Figure 4.
Figure 5.
Discussion
In this study, we focused on the initial cellular response of DPSC cells to ordered enamel-like and disordered FA surfaces and explored the mechanisms underlying this process, which will provide valuable information in understanding of the biocompatibility of these newly developed biomedical materials and for the future application of these materials in creating a functional enamel-dentin superstructure.
Correlations among surface properties, protein adsorption, and cell response will dictate the value of the synthetic FA material to be used in a model for the creation in vitro of the enamel/dentin superstructure. Proteins will be adsorbed onto FA substrates in different quantities, densities, conformations, and orientations depending on the chemical and physical characteristics of the surface and involves van der Waals, hydrophobic and electrostatic interactions, and hydrogen bonding. Adsorbed proteins may act as mediators for cell adhesion if they have the correct geometry (i.e. exposed RGD sequence in fibronectin) to mediate cell attachment 13. In this study, the initial cellular attachment of the DPSC cells was significantly higher on the ordered than the disordered FA surfaces, though both surfaces showed excellent biocompatibility and support the long term growth of the cells. Thus, the composition and conformation of the adsorbed protein layer might be responsible for the enhanced DPSC cellular response on the ordered FA surfaces. Surface characteristics such as chemistry affect cell-surface interactions, whereas neither the structural or topographical effects on the cell adhesion have been fully elucidated 13.
Following protein adsorption, the cell-matrix interactions control further cell adhesion, cell survival and the cellular proliferation and differentiation process. Adsorption of adhesion proteins, such as fibronectin (FN) and vitronectin has been reported to mediate cell adhesion 14. During tooth development, FN has been found in the dental pulp and predentin at different stages of dentinogenesis; FN has also been observed in the basement membrane which is between the inner enamel epithelium and the underlying dental mesenchyme, and in the mantle predentin 15. In bone, it is involved in the early stages of osteogenesis and the structural organization of its fibrillogenesis has been suggested to be essential in mineral nucleation 16.
It has been suggested that studies of the gene regulation responsible for cell adhesion selectivity in cell-surface interactions is critical at an early stage (3 days). However, if applied too early before 3 days or too late after 3 days, the cells will either not sense the related environment or have already remodeled their micro-environment to give inaccurate adhesion information 17. Therefore, in this study, after the growth of DPSC cells on the two surfaces for 3 days, the expression of human pathway-focused matrix and adhesion molecules of the cells were analyzed using PCR array. Of the cell-matrix adhesion molecules, secreted phosphoprotein 1 (SPP1), thrombospondin 3 (THBS3), integrin alpha 7 and 8 (ITGA 7 and 8), integrin beta 3 and 4 (ITGB3 and 4), and more interestingly, the vitronectin receptor-integrin alpha V (ITGAV) were up-regulated on the ordered enamel-like FA surfaces. Other adhesion molecules for example collagen type VII alpha 1 (COL7A1), COL8A1, COL12A1, laminin alpha1 (LAMA1) and importantly, the key adhesion protein-fibronectin1 (FN1) were also up-regulated on the ordered FA surfaces. As key adhesion molecules, FN and vitronectin along with fibrinogen, collagens, laminin, osteopontin, and some other trace proteins, mediates the specific interaction between cells and surfaces via cell integrin receptors 18, 19. Thus, the differential expression of adhesion and matrix related molecules especially the up regulation of vitronectin receptor-integrin alpha V and FN itself may play important role in the enhanced initial cellular response of DPSC cells to the ordered enamel-like FA surfaces. Among the ECM proteases, the up-regulation of the matrix metalloproteinase 13 (MMP13) was coordinated with the down-regulation of 3 other proteases which also appears to be important in establishing the favorable micro-environment (i.e. favorable focal adhesions) for the DPSC cells grown on the ordered FA surfaces.
The long term differential growth pattern of the DPSC cells on the two FA surfaces may be closely related to the differential initial cellular adhesion characteristics of these surfaces. It has been suggested that the first phase of cell-material interaction involving attachment, adhesion and spreading of the cell may probably influence the second phase of the interaction involving proliferation and differentiation of the DPSCs into odontoblast-like cells. As we had shown earlier the induction of DPSCs into odontoblast-like cells after treatment with a mineralizing supplement and a dentin extract, we did not attempt to recapitulate this process 9, 10.
Similarly, it has been shown that surface characteristics not only affect the focal adhesion and extracellular matrix formation of the human bone marrow mesenchymal stem cell but also the differentiation of these cells 20. In a most recent study, it has been reported that surface characteristics such as mechanical strength or rigidity of biomaterials not only affected the cell focal adhesions, cytoskeletal contractility but also stem cell differentiation21. Therefore it would not be surprising that the different topographical characteristics of the crystal surface might affect the DPSC differentiation and mineralization process. Thus, experiments marrying an enamel-like synthetic fluorapatite (FA) coated with basement membrane component (FN, collagen type IV etc) and the induction of DPSC cells to create a dentin-like structure together with a functional enamel/dentin junction will be our future line of investigation. In the future application, it should be possible to synthesize these FA superstructures on the inner surface of metal crowns in order to obtain the appropriate anatomical crown shapes. Then we would generate the “dentin” on the inner surface of these synthetic enamel-like crown shaped superstructures.
Conclusion
From this study, the newly created ordered FA crystal surface provides a favorable environment for the long term growth and the functional cell-matrix interaction of the DPSC cells. The enhanced cellular response of DPSC cell to the ordered FA crystal surface involves a set of delicately regulated matrix and adhesion molecules. As a consequence of this study a biomimetic model for the formation of the enamel/dentin junction will be developed.
Acknowledgments
This work was supported by grant DE015599 from the National Institutes of Health.
References
- 1.Smith AJ, Tobias RS, Plant CG, Browne RM, Lesot H, Ruch JV. In vivo morphogenetic activity of dentine matrix proteins. Journal de biologie buccale. 1990;18:123–9. [PubMed] [Google Scholar]
- 2.Smith AJ, Tobias RS, Cassidy N, Plant CG, Browne RM, Begue-Kirn C, Ruch JV, Lesot H. Odontoblast stimulation in ferrets by dentine matrix components. Archives of oral biology. 1994;39:13–22. doi: 10.1016/0003-9969(94)90029-9. [DOI] [PubMed] [Google Scholar]
- 3.Smith AJ, Lesot H. Induction and regulation of crown dentinogenesis: embryonic events as a template for dental tissue repair? Crit Rev Oral Biol Med. 2001;12:425–37. doi: 10.1177/10454411010120050501. [DOI] [PubMed] [Google Scholar]
- 4.Gronthos S, Brahim J, Li W, Fisher LW, Cherman N, Boyde A, DenBesten P, Robey PG, Shi S. Stem cell properties of human dental pulp stem cells. Journal of dental research. 2002;81:531–5. doi: 10.1177/154405910208100806. [DOI] [PubMed] [Google Scholar]
- 5.Shi S, Bartold PM, Miura M, Seo BM, Robey PG, Gronthos S. The efficacy of mesenchymal stem cells to regenerate and repair dental structures. Orthodontics & craniofacial research. 2005;8:191–9. doi: 10.1111/j.1601-6343.2005.00331.x. [DOI] [PubMed] [Google Scholar]
- 6.Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, Shi S. SHED: stem cells from human exfoliated deciduous teeth. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:5807–12. doi: 10.1073/pnas.0937635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Honda MJ, Shinohara Y, Hata KI, Ueda M. Subcultured odontogenic epithelial cells in combination with dental mesenchymal cells produce enamel-dentin-like complex structures. Cell transplantation. 2007;16:833–47. doi: 10.3727/000000007783465208. [DOI] [PubMed] [Google Scholar]
- 8.Honda MJ, Shinmura Y, Shinohara Y. Enamel Tissue Engineering Using Subcultured Enamel Organ Epithelial Cells in Combination with Dental Pulp Cells. Cells, tissues, organs. 2008 doi: 10.1159/000151743. [DOI] [PubMed] [Google Scholar]
- 9.Liu J, Jin T, Ritchie HH, Smith AJ, Clarkson BH. In vitro differentiation and mineralization of human dental pulp cells induced by dentin extract. In vitro cellular & developmental biology. 2005;41:232–8. doi: 10.1290/0502014.1. [DOI] [PubMed] [Google Scholar]
- 10.Liu J, Jin T, Chang S, Ritchie HH, Smith AJ, Clarkson BH. Matrix and TGF-beta-related gene expression during human dental pulp stem cell (DPSC) mineralization. In vitro cellular & developmental biology. 2007;43:120–8. doi: 10.1007/s11626-007-9022-8. [DOI] [PubMed] [Google Scholar]
- 11.Chen HFTZ, Liu J, Sun K, Chang SR, Peters MC, Mansfield JF, Czajka-Jakubowska A, Clarkson BH. Acellular synthesis of a human enamel-like microstructure. Advanced Materials. 2006;18:1846–51. [Google Scholar]
- 12.Liu J, Jin T, Chang S, Czajka-Jakubowska A, Zhang Z, Nör JE, Clarkson BH. The effect of novel fluorapatite surfaces on osteoblast-like cell adhesion, growth, and mineralization. Tissue Eng Part A. 2010;16:2977–86. doi: 10.1089/ten.tea.2009.0632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roach P, Farrar D, Perry CC. Interpretation of protein adsorption: surface-induced conformational changes. J Am Chem Soc. 2005;127:8168–73. doi: 10.1021/ja042898o. [DOI] [PubMed] [Google Scholar]
- 14.Steele JG, Dalton BA, Johnson G, Underwood PA. Adsorption of fibronectin and vitronectin onto Primaria and tissue culture polystyrene and relationship to the mechanism of initial attachment of human vein endothelial cells and BHK-21 fibroblasts. Biomaterials. 1995;16:1057–67. doi: 10.1016/0142-9612(95)98901-p. [DOI] [PubMed] [Google Scholar]
- 15.Linde A, Johansson S, Jonsson R, Jontell M. Localization of fibronectin during dentinogenesis in rat incisor. Archives of oral biology. 1982;27:1069–73. doi: 10.1016/0003-9969(82)90013-9. [DOI] [PubMed] [Google Scholar]
- 16.Pellenc D, Berry H, Gallet O. Adsorption-induced fibronectin aggregation and fibrillogenesis. Journal of colloid and interface science. 2006;298:132–44. doi: 10.1016/j.jcis.2005.11.059. [DOI] [PubMed] [Google Scholar]
- 17.Liu X, Lim JY, Donahue HJ, Dhurjati R, Mastro AM, Vogler EA. Influence of substratum surface chemistry/energy and topography on the human fetal osteoblastic cell line hFOB 1.19: Phenotypic and genotypic responses observed in vitro. Biomaterials. 2007;28:4535–50. doi: 10.1016/j.biomaterials.2007.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D'Souza SE, Ginsberg MH, Burke TA, Lam SC, Plow EF. Localization of an Arg-Gly-Asp recognition site within an integrin adhesion receptor. Science. 1988;242:91–3. doi: 10.1126/science.3262922. [DOI] [PubMed] [Google Scholar]
- 19.Reinholt FP, Hultenby K, Oldberg A, Heinegård D. Osteopontin--a possible anchor of osteoclasts to bone. Proc Natl Acad Sci. 1990;87:4473–5. doi: 10.1073/pnas.87.12.4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Curran JM, Chen R, Hunt JA. The guidance of human mesenchymal stem cell differentiation in vitro by controlled modifications to the cell substrate. Biomaterials. 2006;27:4783–93. doi: 10.1016/j.biomaterials.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 21.Fu J, Wang YK, Yang MT, Desai RA, Yu X, Liu Z, Chen CS. Mechanical regulation of cell function with geometrically modulated elastomeric substrates. Nat Methods. 2010;7:733–6. doi: 10.1038/nmeth.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]