Abstract
Non-alcoholic fatty liver disease (NAFLD) represents a burgeoning public health concernin westernized nations. The obesity-related disorder is associated with an increased risk of cardiovascular disease, type 2 diabetes and liver failure. Although the underlying pathogenesis of NAFLD is unclear, increasing evidence suggests that excess saturated fatty acids presented to or stored within the liver may play a role in both the development and progression of the disorder. Aputative mechanism linking saturated fatty acids to NAFLD may been doplasmic reticulum (ER) stress. Specifically, excess saturated fatty acids may induce an ER stress response that, if left unabated, can activate stress signaling pathways, cause hepatocyte cell death, and eventually lead to liver dysfunction. In the current review we discuss the involvement of saturated fatty acids in the pathogenesis of NAFLD with particular emphasis on the role of ER stress.
Keywords: saturated fatty acids, ER stress, liver injury, NAFLD
The Burden of NAFLD
Nonalcoholic fatty liver disease (NAFLD) is a chronic metabolic disorder characterized by hepatic fat accumulation (steatosis) in the absence of excessive alcohol consumption (1–2). The prevalence of NAFLD has nearly doubled since 1980, and current US estimates indicate that NAFLD may affect up to 25% of the general population and 80% of obese and diabetic individuals (3–4). Clinically, NAFLD encompasses a broad spectrum of hepatic derangements ranging from steatosis to nonalcoholic steatohepatitis (NASH), the latter characterized by hepatic fat accumulation coincident with inflammation, reduced liver function, and fibrosis (5–6). Progression to NASH occurs in approximately 10% of NAFLD patients, and 20% of these individuals, in turn, progress to cirrhosis within 10 years (7–9). Individuals with NAFLD are also at an increased risk of cardiovascular disease, type 2 diabetes and overall-and obesity-related mortality (10–12). Therefore, given the increasing prevalence and clinical consequences of NAFLD, a thorough understanding of its underlying pathology is crucial for the development of effective therapeutic strategies.
Lipids and NAFLD
The initial stage of NAFLD involves accumulation of triglycerides in the liver. Hepatic lipids are derived from three potential sources, 1) the diet, 2) de novo lipogenesis, and 3) circulating fatty acids released from adipose tissue (13). Donnelly et al., recently found that the latter source accounts for approximately 60% of the hepatic triglyceride content in NAFLD patients (14), highlighting the importance of circulating fatty acids in the development of NAFLD.
Accumulating data suggest that, in addition to their role in the development of NAFLD, fatty acids play an important role in the progression from NAFLD to NASH. Free fatty acids are elevated in NASH patients and are positively correlated with disease severity (15–16). Experimental suppression of circulating fatty acids improves hepatic insulin sensitivity and reduces liver enzymes in healthy individuals (17). These data have led to the emerging concept that elevated fatty acids and products of fatty acid metabolism, rather than triglycerides per se, promote hepatotoxicity. Indeed, hepatic triglycerides are higher in patients with benign steatosis compared to those with NASH (18), and esterification of fatty acids into triglycerides prevents fatty acid toxicity in hepatocytes and reduces liver damage in experimental animals (19–21).
Numerous studies have indicated that saturated fatty acids are more deleterious to hepatocytes and liver function than unsaturated fatty acids (22–23, 21). Wang et al., reported that a high saturated fat diet fed to experimental animals resulted in significant liver injury, greater susceptibility to endotoxin, and a reduced liver proliferative capacity. In contrast, a high unsaturated fat diet did not induce liver damage despite similar levels of total hepatic triglyceride accumulation (24). The toxic effects of saturated fatty acids maybe due to their inability, relative to unsaturated fatty acids, to be esterified and incorporated into triglyceride. As such, genetic and pharmacologic manipulations that channel saturated fatty acids towards triglyceride accumulation attenuate liver cell dysfunction and death (19, 25, 21). These data are consistent with the notion that the composition of fatty acids delivered to and stored within the liver is an important determinant of liver cell integrity, and potentially an independent risk factor for the progression to NASH.
The Endoplasmic Reticulum
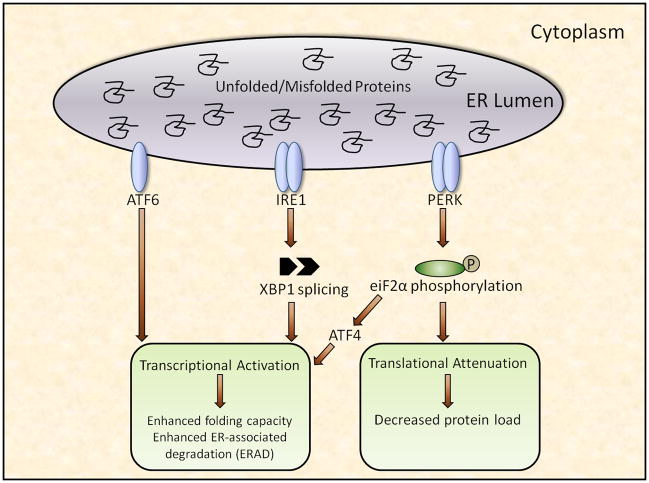
The mechanisms by which saturated fatty acids contribute to liver injury are not completely understood, although accumulating data implicate disruption of endoplasmic reticulum (ER) homeostasis, or ER stress, as a proximal event. ER stress, in turn, may lead to activation of various intracellular stress pathways that can initiate or exacerbate inflammation and, in some cases, culminate in hepatocyte cell death and liver damage. The ER, one of the largest cellular organelles, is responsible for the proper assembly and posttranslational modification of proteins destined for intracellular organelles and the cell surface(26). When the demand for protein modification exceeds the capacity of the ER to fold or degrade proteins, unfolded proteins accumulate in the ER lumen, causing disruption of ER homeostasis, or ER stress. Because unfolded proteins can impair cell function, the ER has evolved a highly specialized quality control system, the unfolded protein response (UPR), that monitors the status of ER protein assembly and serves to restore ER homeostasis (27). Specifically, the UPR monitors ER protein status via three transmembrane proteins, RNA-dependent protein kinase-like ER eukaryotic initiation factor-2α kinase (PERK), activating transcription factor 6 (ATF6) and inositol-requiring ER-to-nucleus signaling protein 1 (IRE1). These proteins act as proximal sensors of ER homeostasis(28–30), and when activated, initiate three distinct but inter-related signaling cascades that reduce unfolded proteins by attenuating protein translation, enhancing ER folding capacity, and facilitating protein degradation (figure 1) (31–34).
Figure 1.
The diversity of ER stress-mediated UPR signaling likely yields outcomes that are specific to the imposed stress and the needs of the involved cell; however, signaling can be broadly categorized into three successive stages: adaptation, alarm and cell death(28, 35). The initial stage, adaptation, involves transcriptional and translational modifications aimed at clearing unfolded proteins from the ER lumen. These adaptive responses likely occur under normal physiological conditions, especially in cells such as hepatocytes where high secretory rates demand elevated rates of protein folding. The second stage, alarm, involves induction of cellular stress pathways that inform the cell and surrounding tissues that homeostasis is compromised and cellular function is jeopardized. These signaling cascades, which are commonly associated with innate immunity and host defense, represent a compensatory response aimed at protecting the cell, but can themselves compromise cellular integrity if chronically activated (36). The final stage, cell death, occurs when the initial insult is sufficiently excessive or chronic that the adaptation and alarm responses are incapable of mitigating ER stress and restoring ER homeostasis (37). A detailed discussion of these categories and the proposed mechanisms that mediate the transition from adaptation to cell death is beyond the scope of this review. The reader is referred to several excellent reviews pertaining to these issues (35, 38, 36).
Recent evidence suggests that, in addition to promoting the progression of NAFLD, saturated fatty acids can cause ER stress and activate the UPR in numerous cell types. This activation, and in particular the signaling pathways initiated during the alarm and cell death stages, may in turn mediate the toxic effects of saturated fatty acids. In the remainder of this review, we will describe the association between excess saturated fatty acids and ER stress, particularly as it pertains to NAFLD, and discuss the potential mechanisms by which ER stress mediates the development and progression of saturated fatty acid-induced liver damage.
Saturated Fatty Acids and ER Stress
In two seminal publications, Ozcan et al., reported that dietary and genetic models of obesity display markers of ER stress in adipose tissue and liver, and administration of chemical chaperones that improve ER folding capacity abrogate systemic insulin resistance, hepatic lipid accumulation, and markers of liver injury (39–40). Subsequent experiments incorporating genetic manipulations of the UPR have supported the concept that ER stress contributes to the pathogenesis of NAFLD (41–44). Further, clinical studies have indicated that patients suffering from metabolic disorders, including NAFLD, display markers of ER stress in the liver and other tissues (45–48). Collectively, these data indicate that certain feature(s) of an obese/over nutrition environment are sufficient to disrupt ER homeostasis, and increasing evidence suggests that the accumulation of saturated fatty acids may be one feature of the obese environment that causes this disruption. The ensuing ER stress, if left unabated, may in turn mediate saturated fatty acid-induced dysfunction in metabolic tissues such as the liver. In support of this concept, high saturated fat diets, but not high unsaturated fat diets, induce hepatic ER stress and liver damage in male Wistar rats; importantly, evidence of ER stress precedes the development of liver dysfunction in these animals(49). Numerous in vitro studies have found that saturated fatty acid-induced liver cell dysfunction and death are accompanied by increased ER stress, and can be mitigated by co-incubation with chemical chaperones that enhance ER folding capacity (23, 50, 25, 51–54).
These data beget the clinically relevant question of how saturated fatty acids induce ER stress. Several lines of evidence suggest that this induction may occur via selective, structural effects to the ER. As mentioned above, compared to unsaturated fatty acids, saturated fatty acids are less readily converted into triglycerides, and are thus left free to travel to the ER where they may disrupt ER morphology and function. In vitro data suggest that palmitoyl CoA can inhibit ER assembly and propagate ER membrane fission (55). Moffitt et al., reported that palmitate-mediated cell dysfunction and death in INS-1 cells was caused by conversion of palmitate to tripalmitin, an insoluble triglyceride formed and retained in the ER that ultimately disrupts ER architecture (56). Through a series of experiments, Schaffer and colleagues demonstrated that palmitate-induced ER stress in CHO cells and H9c2 cardiomyocytes was associated with the rapid incorporation of palmitate into lipid components of the ER followed by disruption of ER structure and function(19, 57). Incorporation of saturated fatty acids into the ER may also disrupt ER folding capacity and chaperone function by altering ER calcium homeostasis (58–60, 52). Thus, selective trafficking of saturated fatty acids to the ER membrane may be an important determinant of ER homeostasis and may ultimately mediate the toxic effects of these lipids.
Saturated Fatty Acids and the UPR-Mediated Alarm Response
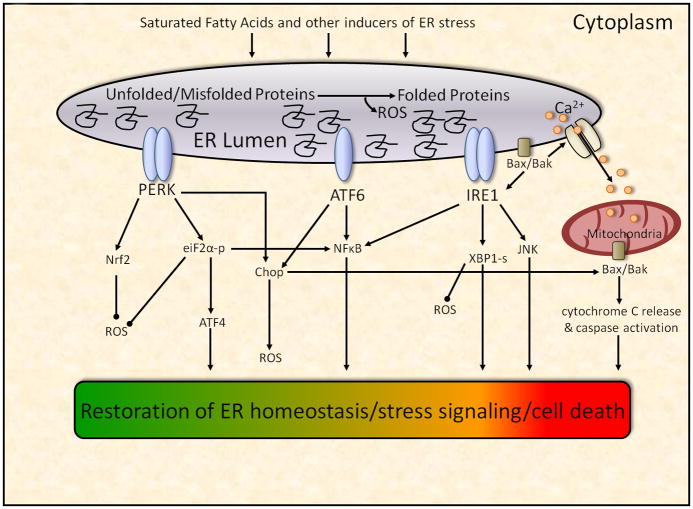
As described above, the initial stage of the UPR, adaptation, is likely a common physiological response to increased folding demands in the ER that does not necessarily result in cell damage. If the steps taken during the adaptation stage do not restore ER homeostasis, the UPR has the capacity to activate stress signaling pathways (figure 2). C-Jun NH2 terminal kinase (JNK), a member of the mitogen-activated protein kinase (MAPK) family of proteins, has emerged as a critical mediator of cellular stress responses and has been implicated in the pathogenesis of various metabolic disorders, including NAFLD (61–62, 39). JNK activation is present in human NAFLD and correlates with disease severity, whereas JNK inhibition protects experimental animals from NASH (63, 46–47, 64–65). The mechanisms by which JNK may potentiate liver damage include regulation of inflammatory genes, disruption of hepatic insulin signaling and induction of hepatocyte apoptosis (66, 61, 22).
Figure 2.
A link between ER stress and JNK was first provided by Srivastava et al., who demonstrated that JNK was activated by thapsigargin, a chemical that disrupts ER homeostasis via inhibition of the endoplasmic reticulum-associated calcium ATPase (67). Urano et al., found that ER stress-mediated JNK activation is specific to the proximal UPR sensor IRE1α, which, upon its phosphorylation following ER stress, activates JNK by complexing with the adaptor protein TRAF2 (68). Collectively, these data support a sequence of events whereby elevated fatty acids, in particular saturated fatty acids, induce a protracted ER stress response in hepatocytes that leads to JNK activation and subsequent inflammation, cell injury and death. Indeed, saturated fatty acids, but not unsaturated fatty acids, activate JNK in hepatocytes, and inhibition of JNK signaling prevents saturated fatty acid-induced hepatocyte injury (22, 50, 69–70, 53).
NFκB is a critical transcriptional regulator of inflammation and may also play a role in the development of NASH. Under normal physiological conditions, NFκB remains inactive through binding to IκB, and signal-induced phosphorylation and degradation of IκB allow for the activation and nuclear translocation of NFκB. Hepatic levels of NFκB are increased in NASH patients and correlate with disease severity (71–72). These data are consistent with most (73–74), but not all studies (75) that have reported deleterious effects of NFκB on liver function. Saturated fatty acids activate NFκB in various cell types, including liver cells, and this activation may play a role in saturated fatty acid-induced liver cell inflammation/toxicity (76–77). Disruption of ER homeostasis also activates NFκB (78–80), and all three proximal sensors of the UPR-ATF6, IRE1a, and PERK-appear capable of mediating this activation (figure 2) (81–82). The mechanisms of ATF6 activation of NFκB are unclear, whereas IRE1α-mediated activation occurs, much like JNK, by an IRE1α-TRAF2 complex. PERK-mediated activation occurs via an eiF2α-mediated reduction in general translation, which leads to an increase in the NFκB-IκB ratio and therefore frees NFκB to translocate to the nucleus (83–84). In support of a role for NFκB in ER stress-induced cell dysfunction, inhibition of NFκB prevents thapsigargin-mediated apoptosis in INS1E cells (85).
NFκB-mediated liver damage occurs largely by transcriptional up-regulation of proinflammatory cytokines, such as IL-6 and TNFα, which have been implicated in hepatocyte apoptosis and liver fibrosis (73, 86–88). TNFα, in particular, may play an important role in the development and progression of NAFLD. Patients suffering from NAFLD display increased circulating and hepatic levels of TNFα (89–92) and certain TNFα polymorphisms are associated with disease prevalence and severity (93–94). Inhibition of TNFα protects animals from dietary-and genetically-induced NASH (95–96), and early clinical studies utilizing TNFα inhibitors have yielded promising results in patients with NAFLD (97–98). Although TNFα is increased by fatty acids in hepatocytes and has been linked to ER stress (99), future studies are needed to determine whether TNFα mediates saturated fatty acid-induced ER stress and liver injury.
CREBh is a recently identified, liver-specific, endoplasmic reticulum (ER)-localized transcription factor (100). Genetically altered mice lacking CREBh have scarcely detectable levels of the acute phase reactant, C-reactive protein, in the basal state and following stimulation with the ER stress inducer tunicamycin, suggesting that CREBh is required for initiation of the acute phase response and systemic inflammation subsequent to ER stress (101–102). More recent data have demonstrated that fatty acids up-regulate CREBh expression in vitro and in vivo, but interestingly, both saturated and unsaturated fatty acids appear capable of mediating this up-regulation (103–104). Thus, CREBh may represent a link between excess fatty acids and inflammation, although more studies are needed to address how different fatty acids regulate CREBh, as well as the effects of CREBh activation on liver function.
Oxidative stress has been linked to the development and progression of NAFLD and to saturated fat-induced cell damage (105–109). Recent evidence suggests that the ER may be a potent source of reactive oxygen species (ROS) production. Each disulfide bond formed during oxidative protein folding in the ER produces a single ROS, and it has been estimated that this process accounts for ~25% of all ROS generated in a cell (110–113). Malhotra et al., using cells that were engineered for transcriptional induction of wild-type human FVIII, demonstrated that FVIII misfolds in the ER lumen, activates the UPR, causes oxidative stress and induces apoptosis. All of these responses to FVIII misfolding were reduced by antioxidant treatment (114).
UPR-mediated ROS generation is at least partly due to activation of CCAAT/enhancer-binding protein homologous protein (Chop), also known as growth arrest-and DNA damage-inducible gene 153 (GADD153) (115). Chop expression is regulated by the ATF6 and PERK arms of the UPR, the latter via activation of the transcription factor ATF4 (116–117). As described in the following section, Chop is best known as an important mediator of ER stress-induced cell death, but Song et al., recently found that Chop deletion reduces oxidative damage in mouse models of diabetes, suggesting that Chop activation may enhance oxidative stress.
To manage the endogenous creation of ROS, the UPR is linked to the regulation of antioxidant defense systems via numerous pathways (figure 2). Most notably, the antioxidant transcription factor Nrf2 is a substrate of the proximal UPR sensor PERK, and PERK-mediated activation of Nrf2 maintains redox homeostasis and prevents cell death following ER stress (118–119). Interestingly, Nrf2 is highly expressed in the liver, and its deletion results in rapid onset and progression of steatohepatitis in mice provided a methionine-choline deficient diet (120). The PERK pathway may also provide protection from ROS via the downstream effector, eiF2α. Mice lacking the ability to phosphorylate eiF2α are characterized by a severe diabetic phenotype that can be attenuated by a high antioxidant diet, suggesting a role for eiF2α phosphorylation in preventing oxidative stress (121). The UPR protein XBP1 may also protect cells from oxidative damage. Liu et al., reported that mouse embryonic fibroblasts deficient in XBP1 were more prone to cell death and less able to activate antioxidant defenses following exposure to hydrogen peroxide (122). Future studies are necessary to determine if ROS play a role in ER stress and liver injury secondary to saturated fatty acids, although substantial evidence indicates that direct links exist among ER stress, ROS, and NAFLD.
Several points regarding our overview of the alarm stage of the UPR warrant consideration. First, the signaling molecules/pathways discussed above do not represent an exhaustive list of those that may mediate liver injury secondary to saturated fatty acids and/or ER stress. Second, the signaling molecules/pathways discussed above are not mutually exclusive, but may interact with one another to facilitate and exacerbate liver injury, and it is probable that some of these deleterious effects are independent of ER stress. Third, the alarm and cell death stages of the UPR are not entirely dichotomous, and it is likely that several of the mediators of the alarm stage also play an important role in hepatocyte cell death secondary to saturated fatty acids and ER stress. An important issue for future studies is to identify the conditions under which the UPR transitions from a survival response to a cell death response. Finally, the relation between ER stress and many of the molecules/pathways described above appears to bebi-directional, which raises the intriguing possibility that saturated fatty acids initiate a self-perpetuating cycle whereby ER stress activates intracellular stress pathways, which further exacerbate ER stress, with NASH developing as collateral damage.
Saturated Fatty Acids and the UPR-Mediated Cell Death Response
If ER stress is particularly severe or protracted, and the UPR cannot re-establish homeostasis, downstream signaling molecules can initiate apoptotic cell death. ER stress-related apoptosis can occurvia the intrinsic pathway, whereby mitrochondrial outer membrane permeabilization (MOMP)leads to cytochrome c release from the mitochondria and subsequent activation of initiator and effector caspases (123–124). The importance of this pathway in the pathogenesis of NAFLD is supported by data demonstrating that elevated caspase activity is a prominent feature of NAFLD and correlates with disease severity (125–126). Furthermore, inhibition of caspase activity via the general caspase inhibitor, FMK (Z-Val-Ala-Asp-fluoromethylketone), reduces apoptosis and fibrosis in an animal model of NASH and prevents saturated fatty acid-induced apoptosis in hepatocytes (22–23, 127). Thus, hepatocyte apoptosis secondary to prolonged ER stress may be an important mechanism by which excess saturated fatty acids play a role in liver injury and the development of NASH.
As mentioned in the previous section, the transition of the UPR from a cell survival pathway to anapoptotic pathway is, at least in part, mediated by the same effectors that are activated in the alarm stage. For example, JNK has been identified as an important mediator of ER stress-related apoptosis, and pharmacologic or genetic inhibition of JNK attenuates saturated fatty acid-induced ER stress and cell death in liver cells (22, 50). Chop, an ER resident transcription factor that lies downstream of the transmembrane proteinsATF6 and PERK, is perhaps the most well characterized mediator of ER stress-induced cell death. Silencing Chop reduces hepatocyte apoptosis in alcohol-induced liver disease, and attenuates indices of liver injury in some, but not all models of liver disease (128–130, 54). Furthermore, Chop appears to mediate liver cell death induced by higher (i.e. 400–800 μM), but not lower (i.e. 100–300 μM) saturated fatty acid concentrations (131–132, 54), suggesting that Chopactivation reflects the presence of ER stress and UPR activation, and may play a role in, but is not required for, saturated fatty acid-induced death in liver cells.
The ER lumen is a major site of calcium storage, and calcium homeostasis is critical in maintaining both ER folding capacity and cell viability (133–135). As such, disruption of ER calcium homeostasis, for example via inhibition of the sarco/endoplasmic reticulum ATPase (SERCA) uptake pump, reduces the folding capacity of the ER, induces ER stress, and is an important mediator of ER-associated apoptosis (136–137). Recent data also indicate that disruption in ER calcium stores may be an important mechanism by which saturated fatty acids induce cell death in H4IIE liver cells and primary rat hepatocytes (52), although future studies are needed to determine whether disruption of intracellular calcium homeostasis secondary to excess saturated fatty acids plays a direct role in the development and progression of NAFLD.
The aforementioned pathways appear to all converge on the Bcl-2 family of proteins, which in turn propagates the apoptotic signal and acts at the mitochondria to induce MOMP and cytochrome c release (138–140, 141). The Bcl-2 protein family consists of approximately 20 members that can be broadly divided into three categories: multi-domain anti-apoptotic proteins (e.g. Bcl-2, Bcl-XL); multi-domain pro-apoptotic proteins (e.g. Bax and Bak); BH-3 domain pro-apoptotic proteins (e.g. Bim, Bid, Bad, Puma). Collectively, these proteins monitor incoming stress signals and help determine cell fate by altering the balance between pro-and anti-apoptotic family members. Several lines of evidence suggest tht Bcl-2 proteins may play a direct role in saturated fatty acid-induced hepatocyte cell death and the progression of NAFLD. First, the expression of Bcl-2 proteins is altered towards a pro-apoptotic profile in patients with NAFLD and NASH (142, 69). Second, saturated fatty acid-mediated hepatocyte cell death is accompanied by increases in pro-apoptotic Bcl-2 family members (e.g. Bax) and decreases in anti-apoptotic members, such as Bcl-2 and BclxL (22, 143, 69, 53). Third, alterations in Bcl-2 proteins occur downstream of Chop and JNK, and inhibition of the BH-3 domain proteins Bim or Puma prevents saturated fatty acid-mediated hepatocyte cell death (22, 69, 53).
Although best known for their actions on mitochondria, recent data suggest that Bcl-2 proteins may localize at the ER and directly modulate ER function and the UPR (144–148). For example, Hetz et al., reported that Bax and Bak function at the ER membrane to regulate IRE1α activation, and Bax and Bak double knockout mice demonstrate deficient IREα signaling (146). ER localization of Bcl-2 proteins may also affect ER function and promote apoptosis by modulating ER calcium stores (149–151, 140). Collectively, these data strongly suggest that Bcl-2 proteins may mediate saturated fatty acid-induced apoptosis by acting at both mitochondria and the ER.
Conclusion
NAFLD is a progressive disorder that can lead to impaired liver function and ultimately liver failure. An accumulation of saturated fatty acids in the liver may play an important role in the pathogenesis of NAFLD, and a growing body of literature suggests that ER stress may mediate the toxic effects of saturated fatty acids. Chronic ER stress induces numerous intracellular pathways that, if left unabated, can lead to systemic inflammation, hepatic fibrosis and hepatocyte cell death. Future studies are necessary to determine the precise mechanisms by which saturated fatty acids cause ER stress, and the proximal pathways that mediate ER stress-induced liver damage and hepatocyte cell death. Such studies could identify novel therapeutic strategies for the prevention and treatment of NAFLD.
References
- 1.Festi D, Colecchia A, Sacco T, Bondi M, Roda E, Marchesini G. Hepatic steatosis in obese patients: clinical aspects and prognostic significance. Obes Rev. 2004;5:27–42. doi: 10.1111/j.1467-789x.2004.00126.x. [DOI] [PubMed] [Google Scholar]
- 2.Lewis JR, Mohanty SR. Nonalcoholic fatty liver disease: a review and update. Dig Dis Sci. 2010;55:560–578. doi: 10.1007/s10620-009-1081-0. [DOI] [PubMed] [Google Scholar]
- 3.Szczepaniak LS, Victor RG, Orci L, Unger RH. Forgotten but not gone: the rediscovery of fatty heart, the most common unrecognized disease in America. Circ Res. 2007;101:759–767. doi: 10.1161/CIRCRESAHA.107.160457. [DOI] [PubMed] [Google Scholar]
- 4.Wieckowska A, McCullough AJ, Feldstein AE. Noninvasive diagnosis and monitoring of nonalcoholic steatohepatitis: present and future. Hepatology. 2007;46:582–589. doi: 10.1002/hep.21768. [DOI] [PubMed] [Google Scholar]
- 5.Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD Single Topic Conference. Hepatology. 2003;37:1202–1219. doi: 10.1053/jhep.2003.50193. [DOI] [PubMed] [Google Scholar]
- 6.Tiniakos DG, Vos MB, Brunt EM. Nonalcoholic fatty liver disease: pathology and pathogenesis. Annu Rev Pathol. 2010;5:145–171. doi: 10.1146/annurev-pathol-121808-102132. [DOI] [PubMed] [Google Scholar]
- 7.Powell EE, Cooksley WG, Hanson R, Searle J, Halliday JW, Powell LW. The natural history of nonalcoholic steatohepatitis: a follow-up study of forty-two patients for up to 21 years. Hepatology. 1990;11:74–80. doi: 10.1002/hep.1840110114. [DOI] [PubMed] [Google Scholar]
- 8.Bacon BR, Farahvash MJ, Janney CG, Neuschwander-Tetri BA. Nonalcoholic steatohepatitis: an expanded clinical entity. Gastroenterology. 1994;107:1103–1109. doi: 10.1016/0016-5085(94)90235-6. [DOI] [PubMed] [Google Scholar]
- 9.Clark JM, Diehl AM. Nonalcoholic fatty liver disease: an underrecognized cause of cryptogenic cirrhosis. Jama. 2003;289:3000–3004. doi: 10.1001/jama.289.22.3000. [DOI] [PubMed] [Google Scholar]
- 10.Targher G, Bertolini L, Poli F, Rodella S, Scala L, Tessari R, Zenari L, Falezza G. Nonalcoholic fatty liver disease and risk of future cardiovascular events among type 2 diabetic patients. Diabetes. 2005;54:3541–3546. doi: 10.2337/diabetes.54.12.3541. [DOI] [PubMed] [Google Scholar]
- 11.Soderberg C, Stal P, Askling J, Glaumann H, Lindberg G, Marmur J, Hultcrantz R. Decreased survival of subjects with elevated liver function tests during a 28-year follow-up. Hepatology. 2010;51:595–602. doi: 10.1002/hep.23314. [DOI] [PubMed] [Google Scholar]
- 12.Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med. 2010;363:1341–1350. doi: 10.1056/NEJMra0912063. [DOI] [PubMed] [Google Scholar]
- 13.Postic C, Girard J. The role of the lipogenic pathway in the development of hepatic steatosis. Diabetes Metab. 2008;34:643–648. doi: 10.1016/S1262-3636(08)74599-3. [DOI] [PubMed] [Google Scholar]
- 14.Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005;115:1343–1351. doi: 10.1172/JCI23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nehra V, Angulo P, Buchman AL, Lindor KD. Nutritional and metabolic considerations in the etiology of nonalcoholic steatohepatitis. Dig Dis Sci. 2001;46:2347–2352. doi: 10.1023/a:1012338828418. [DOI] [PubMed] [Google Scholar]
- 16.Diraison F, Moulin P, Beylot M. Contribution of hepatic de novo lipogenesis and reesterification of plasma non esterified fatty acids to plasma triglyceride synthesis during non-alcoholic fatty liver disease. Diabetes Metab. 2003;29:478–485. doi: 10.1016/s1262-3636(07)70061-7. [DOI] [PubMed] [Google Scholar]
- 17.Rigazio S, Lehto HR, Tuunanen H, Nagren K, Kankaanpaa M, Simi C, Borra R, Naum AG, Parkkola R, Knuuti J, Nuutila P, Iozzo P. The lowering of hepatic fatty acid uptake improves liver function and insulin sensitivity without affecting hepatic fat content in humans. Am J Physiol Endocrinol Metab. 2008;295:E413–419. doi: 10.1152/ajpendo.00744.2007. [DOI] [PubMed] [Google Scholar]
- 18.Puri P, Baillie RA, Wiest MM, Mirshahi F, Choudhury J, Cheung O, Sargeant C, Contos MJ, Sanyal AJ. A lipidomic analysis of nonalcoholic fatty liver disease. Hepatology. 2007;46:1081–1090. doi: 10.1002/hep.21763. [DOI] [PubMed] [Google Scholar]
- 19.Listenberger LL, Han X, Lewis SE, Cases S, Farese RV, Jr, Ory DS, Schaffer JE. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc Natl Acad Sci U S A. 2003;100:3077–3082. doi: 10.1073/pnas.0630588100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamaguchi K, Yang L, McCall S, Huang J, Yu XX, Pandey SK, Bhanot S, Monia BP, Li YX, Diehl AM. Inhibiting triglyceride synthesis improves hepatic steatosis but exacerbates liver damage and fibrosis in obese mice with nonalcoholic steatohepatitis. Hepatology. 2007;45:1366–1374. doi: 10.1002/hep.21655. [DOI] [PubMed] [Google Scholar]
- 21.Li ZZ, Berk M, McIntyre TM, Feldstein AE. Hepatic lipid partitioning and liver damage in nonalcoholic fatty liver disease: role of stearoyl-CoA desaturase. J Biol Chem. 2009;284:5637–5644. doi: 10.1074/jbc.M807616200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malhi H, Bronk SF, Werneburg NW, Gores GJ. Free fatty acids induce JNK-dependent hepatocyte lipoapoptosis. J Biol Chem. 2006;281:12093–12101. doi: 10.1074/jbc.M510660200. [DOI] [PubMed] [Google Scholar]
- 23.Wei Y, Wang D, Topczewski F, Pagliassotti MJ. Saturated fatty acids induce endoplasmic reticulum stress and apoptosis independently of ceramide in liver cells. Am J Physiol Endocrinol Metab. 2006;291:E275–281. doi: 10.1152/ajpendo.00644.2005. [DOI] [PubMed] [Google Scholar]
- 24.Wang D, Wei Y, Schmoll D, Maclean KN, Pagliassotti MJ. Endoplasmic reticulum stress increases glucose-6-phosphatase and glucose cycling in liver cells. Endocrinology. 2006;147:350–358. doi: 10.1210/en.2005-1014. [DOI] [PubMed] [Google Scholar]
- 25.Wei Y, Wang D, Pagliassotti MJ. Saturated fatty acid-mediated endoplasmic reticulum stress and apoptosis are augmented by trans-10, cis-12-conjugated linoleic acid in liver cells. Mol Cell Biochem. 2007;303:105–113. doi: 10.1007/s11010-007-9461-2. [DOI] [PubMed] [Google Scholar]
- 26.Zhang K, Kaufman RJ. Protein folding in the endoplasmic reticulum and the unfolded protein response. Handb Exp Pharmacol. 2006:69–91. doi: 10.1007/3-540-29717-0_3. [DOI] [PubMed] [Google Scholar]
- 27.Malhotra JD, Kaufman RJ. The endoplasmic reticulum and the unfolded protein response. Semin Cell Dev Biol. 2007;18:716–731. doi: 10.1016/j.semcdb.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu C, Bailly-Maitre B, Reed JC. Endoplasmic reticulum stress: cell life and death decisions. J Clin Invest. 2005;115:2656–2664. doi: 10.1172/JCI26373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu J, Kaufman RJ. From acute ER stress to physiological roles of the Unfolded Protein Response. Cell Death Differ. 2006;13:374–384. doi: 10.1038/sj.cdd.4401840. [DOI] [PubMed] [Google Scholar]
- 30.Zhang K, Kaufman RJ. The unfolded protein response: a stress signaling pathway critical for health and disease. Neurology. 2006;66:S102–109. doi: 10.1212/01.wnl.0000192306.98198.ec. [DOI] [PubMed] [Google Scholar]
- 31.Hong M, Luo S, Baumeister P, Huang JM, Gogia RK, Li M, Lee AS. Underglycosylation of ATF6 as a novel sensing mechanism for activation of the unfolded protein response. J Biol Chem. 2004;279:11354–11363. doi: 10.1074/jbc.M309804200. [DOI] [PubMed] [Google Scholar]
- 32.Thuerauf DJ, Morrison L, Glembotski CC. Opposing roles for ATF6alpha and ATF6beta in endoplasmic reticulum stress response gene induction. J Biol Chem. 2004;279:21078–21084. doi: 10.1074/jbc.M400713200. [DOI] [PubMed] [Google Scholar]
- 33.Back SH, Schroder M, Lee K, Zhang K, Kaufman RJ. ER stress signaling by regulated splicing: IRE1/HAC1/XBP1. Methods. 2005;35:395–416. doi: 10.1016/j.ymeth.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 34.Shen J, Prywes R. ER stress signaling by regulated proteolysis of ATF6. Methods. 2005;35:382–389. doi: 10.1016/j.ymeth.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 35.Kim I, Xu W, Reed JC. Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat Rev Drug Discov. 2008;7:1013–1030. doi: 10.1038/nrd2755. [DOI] [PubMed] [Google Scholar]
- 36.Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140:900–917. doi: 10.1016/j.cell.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rasheva VI, Domingos PM. Cellular responses to endoplasmic reticulum stress and apoptosis. Apoptosis. 2009;14:996–1007. doi: 10.1007/s10495-009-0341-y. [DOI] [PubMed] [Google Scholar]
- 38.Zhang K, Kaufman RJ. From endoplasmic-reticulum stress to the inflammatory response. Nature. 2008;454:455–462. doi: 10.1038/nature07203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Gorgun C, Glimcher LH, Hotamisligil GS. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 40.Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Gorgun CZ, Hotamisligil GS. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–1140. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee AH, Scapa EF, Cohen DE, Glimcher LH. Regulation of hepatic lipogenesis by the transcription factor XBP1. Science. 2008;320:1492–1496. doi: 10.1126/science.1158042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oyadomari S, Harding HP, Zhang Y, Oyadomari M, Ron D. Dephosphorylation of translation initiation factor 2alpha enhances glucose tolerance and attenuates hepatosteatosis in mice. Cell Metab. 2008;7:520–532. doi: 10.1016/j.cmet.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rutkowski DT, Wu J, Back SH, Callaghan MU, Ferris SP, Iqbal J, Clark R, Miao H, Hassler JR, Fornek J, Katze MG, Hussain MM, Song B, Swathirajan J, Wang J, Yau GD, Kaufman RJ. UPR pathways combine to prevent hepatic steatosis caused by ER stress-mediated suppression of transcriptional master regulators. Dev Cell. 2008;15:829–840. doi: 10.1016/j.devcel.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kammoun HL, Chabanon H, Hainault I, Luquet S, Magnan C, Koike T, Ferre P, Foufelle F. GRP78 expression inhibits insulin and ER stress-induced SREBP-1c activation and reduces hepatic steatosis in mice. J Clin Invest. 2009;119:1201–1215. doi: 10.1172/JCI37007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marchetti P, Bugliani M, Lupi R, Marselli L, Masini M, Boggi U, Filipponi F, Weir GC, Eizirik DL, Cnop M. The endoplasmic reticulum in pancreatic beta cells of type 2 diabetes patients. Diabetologia. 2007;50:2486–2494. doi: 10.1007/s00125-007-0816-8. [DOI] [PubMed] [Google Scholar]
- 46.Boden G, Duan X, Homko C, Molina EJ, Song W, Perez O, Cheung P, Merali S. Increase in endoplasmic reticulum stress-related proteins and genes in adipose tissue of obese, insulin-resistant individuals. Diabetes. 2008;57:2438–2444. doi: 10.2337/db08-0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Puri P, Mirshahi F, Cheung O, Natarajan R, Maher JW, Kellum JM, Sanyal AJ. Activation and dysregulation of the unfolded protein response in nonalcoholic fatty liver disease. Gastroenterology. 2008;134:568–576. doi: 10.1053/j.gastro.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 48.Sharma NK, Das SK, Mondal AK, Hackney OG, Chu WS, Kern PA, Rasouli N, Spencer HJ, Yao-Borengasser A, Elbein SC. Endoplasmic reticulum stress markers are associated with obesity in nondiabetic subjects. J Clin Endocrinol Metab. 2008;93:4532–4541. doi: 10.1210/jc.2008-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang D, Wei Y, Pagliassotti MJ. Saturated fatty acids promote endoplasmic reticulum stress and liver injury in rats with hepatic steatosis. Endocrinology. 2006;147:943–951. doi: 10.1210/en.2005-0570. [DOI] [PubMed] [Google Scholar]
- 50.Pagliassotti MJ, Wei Y, Wang D. Insulin protects liver cells from saturated fatty acid-induced apoptosis via inhibition of c-Jun NH2 terminal kinase activity. Endocrinology. 2007;148:3338–3345. doi: 10.1210/en.2006-1710. [DOI] [PubMed] [Google Scholar]
- 51.Rahman SM, Qadri I, Janssen RC, Friedman JE. Fenofibrate and PBA prevent fatty acid-induced loss of adiponectin receptor and pAMPK in human hepatoma cells and in hepatitis C virus-induced steatosis. J Lipid Res. 2009;50:2193–2202. doi: 10.1194/jlr.M800633-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wei Y, Wang D, Gentile CL, Pagliassotti MJ. Reduced endoplasmic reticulum luminalcalcium links saturated fatty acid-mediated endoplasmic reticulum stress and cell death in liver cells. Mol Cell Biochem. 2009;331:31–40. doi: 10.1007/s11010-009-0142-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Akazawa Y, Cazanave S, Mott JL, Elmi N, Bronk SF, Kohno S, Charlton MR, Gores GJ. Palmitoleate attenuates palmitate-induced Bim and PUMA up-regulation and hepatocyte lipoapoptosis. J Hepatol. 2010;52:586–593. doi: 10.1016/j.jhep.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pfaffenbach KT, Gentile CL, Nivala AM, Wang D, Wei Y, Pagliassotti MJ. Linking endoplasmic reticulum stress to cell death in hepatocytes: roles of C/EBP homologous protein and chemical chaperones in palmitate-mediated cell death. Am J Physiol Endocrinol Metab. 2010;298:E1027–1035. doi: 10.1152/ajpendo.00642.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Turner MD. Fatty acyl CoA-mediated inhibition of endoplasmic reticulum assembly. Biochim Biophys Acta. 2004;1693:1–4. doi: 10.1016/j.bbamcr.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 56.Moffitt JH, Fielding BA, Evershed R, Berstan R, Currie JM, Clark A. Adverse physicochemical properties of tripalmitin in beta cells lead to morphological changes and lipotoxicity in vitro. Diabetologia. 2005;48:1819–1829. doi: 10.1007/s00125-005-1861-9. [DOI] [PubMed] [Google Scholar]
- 57.Borradaile NM, Han X, Harp JD, Gale SE, Ory DS, Schaffer JE. Disruption of endoplasmic reticulum structure and integrity in lipotoxic cell death. J Lipid Res. 2006;47:2726–2737. doi: 10.1194/jlr.M600299-JLR200. [DOI] [PubMed] [Google Scholar]
- 58.Burdakov D, Petersen OH, Verkhratsky A. Intraluminal calcium as a primary regulator of endoplasmic reticulum function. Cell Calcium. 2005;38:303–310. doi: 10.1016/j.ceca.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 59.Gorlach A, Klappa P, Kietzmann T. The endoplasmic reticulum: folding, calcium homeostasis, signaling, and redox control. Antioxid Redox Signal. 2006;8:1391–1418. doi: 10.1089/ars.2006.8.1391. [DOI] [PubMed] [Google Scholar]
- 60.Gwiazda KS, Yang TL, Lin Y, Johnson JD. Effects of palmitate on ER and cytosolic Ca2+ homeostasis in beta-cells. Am J Physiol Endocrinol Metab. 2009;296:E690–701. doi: 10.1152/ajpendo.90525.2008. [DOI] [PubMed] [Google Scholar]
- 61.Hirosumi J, Tuncman G, Chang L, Gorgun CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 62.Bogoyevitch MA, Boehm I, Oakley A, Ketterman AJ, Barr RK. Targeting the JNK MAPK cascade for inhibition: basic science and therapeutic potential. Biochim Biophys Acta. 2004;1697:89–101. doi: 10.1016/j.bbapap.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 63.Schattenberg JM, Singh R, Wang Y, Lefkowitch JH, Rigoli RM, Scherer PE, Czaja MJ. JNK1 but not JNK2 promotes the development of steatohepatitis in mice. Hepatology. 2006;43:163–172. doi: 10.1002/hep.20999. [DOI] [PubMed] [Google Scholar]
- 64.Kodama Y, Kisseleva T, Iwaisako K, Miura K, Taura K, De Minicis S, Osterreicher CH, Schnabl B, Seki E, Brenner DA. c-Jun N-terminal kinase-1 from hematopoietic cells mediates progression from hepatic steatosis to steatohepatitis and fibrosis in mice. Gastroenterology. 2009;137:1467–1477. e1465. doi: 10.1053/j.gastro.2009.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Singh R, Wang Y, Xiang Y, Tanaka KE, Gaarde WA, Czaja MJ. Differential effects of JNK1 and JNK2 inhibition on murine steatohepatitis and insulin resistance. Hepatology. 2009;49:87–96. doi: 10.1002/hep.22578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 67.Srivastava RK, Sollott SJ, Khan L, Hansford R, Lakatta EG, Longo DL. Bcl-2 and Bcl-X(L) block thapsigargin-induced nitric oxide generation, c-Jun NH(2)-terminal kinase activity, and apoptosis. Mol Cell Biol. 1999;19:5659–5674. doi: 10.1128/mcb.19.8.5659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, Ron D. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287:664–666. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
- 69.Cazanave SC, Mott JL, Elmi NA, Bronk SF, Werneburg NW, Akazawa Y, Kahraman A, Garrison SP, Zambetti GP, Charlton MR, Gores GJ. JNK1-dependent PUMA expression contributes to hepatocyte lipoapoptosis. J Biol Chem. 2009;284:26591–26602. doi: 10.1074/jbc.M109.022491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nakamura S, Takamura T, Matsuzawa-Nagata N, Takayama H, Misu H, Noda H, Nabemoto S, Kurita S, Ota T, Ando H, Miyamoto K, Kaneko S. Palmitate induces insulin resistance in H4IIEC3 hepatocytes through reactive oxygen species produced by mitochondria. J Biol Chem. 2009;284:14809–14818. doi: 10.1074/jbc.M901488200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ribeiro PS, Cortez-Pinto H, Sola S, Castro RE, Ramalho RM, Baptista A, Moura MC, Camilo ME, Rodrigues CM. Hepatocyte apoptosis, expression of death receptors, and activation of NF-kappaB in the liver of nonalcoholic and alcoholic steatohepatitis patients. Am J Gastroenterol. 2004;99:1708–1717. doi: 10.1111/j.1572-0241.2004.40009.x. [DOI] [PubMed] [Google Scholar]
- 72.Videla LA, Tapia G, Rodrigo R, Pettinelli P, Haim D, Santibanez C, Araya AV, Smok G, Csendes A, Gutierrez L, Rojas J, Castillo J, Korn O, Maluenda F, Diaz JC, Rencoret G, Poniachik J. Liver NF-kappaB and AP-1 DNA binding in obese patients. Obesity (Silver Spring) 2009;17:973–979. doi: 10.1038/oby.2008.601. [DOI] [PubMed] [Google Scholar]
- 73.Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, Shoelson SE. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med. 2005;11:183–190. doi: 10.1038/nm1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dela Pena A, Leclercq I, Field J, George J, Jones B, Farrell G. NF-kappaB activation, rather than TNF, mediates hepatic inflammation in a murine dietary model of steatohepatitis. Gastroenterology. 2005;129:1663–1674. doi: 10.1053/j.gastro.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 75.Iimuro Y, Nishiura T, Hellerbrand C, Behrns KE, Schoonhoven R, Grisham JW, Brenner DA. NFkappaB prevents apoptosis and liver dysfunction during liver regeneration. J Clin Invest. 1998;101:802–811. doi: 10.1172/JCI483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Novak TE, Babcock TA, Jho DH, Helton WS, Espat NJ. NF-kappa B inhibition by omega -3 fatty acids modulates LPS-stimulated macrophage TNF-alpha transcription. Am J Physiol Lung Cell Mol Physiol. 2003;284:L84–89. doi: 10.1152/ajplung.00077.2002. [DOI] [PubMed] [Google Scholar]
- 77.Senn JJ. Toll-like receptor-2 is essential for the development of palmitate-induced insulin resistance in myotubes. J Biol Chem. 2006;281:26865–26875. doi: 10.1074/jbc.M513304200. [DOI] [PubMed] [Google Scholar]
- 78.Pahl HL, Baeuerle PA. A novel signal transduction pathway from the endoplasmic reticulum to the nucleus is mediated by transcription factor NF-kappa B. EMBO J. 1995;14:2580–2588. doi: 10.1002/j.1460-2075.1995.tb07256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pahl HL, Baeuerle PA. Activation of NF-kappa B by ER stress requires both Ca2+ and reactive oxygen intermediates as messengers. FEBS Lett. 1996;392:129–136. doi: 10.1016/0014-5793(96)00800-9. [DOI] [PubMed] [Google Scholar]
- 80.Hung JH, Su IJ, Lei HY, Wang HC, Lin WC, Chang WT, Huang W, Chang WC, Chang YS, Chen CC, Lai MD. Endoplasmic reticulum stress stimulates the expression of cyclooxygenase-2 through activation of NF-kappaB and pp38 mitogen-activated protein kinase. J Biol Chem. 2004;279:46384–46392. doi: 10.1074/jbc.M403568200. [DOI] [PubMed] [Google Scholar]
- 81.Deng J, Lu PD, Zhang Y, Scheuner D, Kaufman RJ, Sonenberg N, Harding HP, Ron D. Translational repression mediates activation of nuclear factor kappa B by phosphorylated translation initiation factor 2. Mol Cell Biol. 2004;24:10161–10168. doi: 10.1128/MCB.24.23.10161-10168.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yamazaki H, Hiramatsu N, Hayakawa K, Tagawa Y, Okamura M, Ogata R, Huang T, Nakajima S, Yao J, Paton AW, Paton JC, Kitamura M. Activation of the Akt-NF-kappaB pathway by subtilase cytotoxin through the ATF6 branch of the unfolded protein response. J Immunol. 2009;183:1480–1487. doi: 10.4049/jimmunol.0900017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jiang HY, Wek SA, McGrath BC, Scheuner D, Kaufman RJ, Cavener DR, Wek RC. Phosphorylation of the alpha subunit of eukaryotic initiation factor 2 is required for activation of NF-kappaB in response to diverse cellular stresses. Mol Cell Biol. 2003;23:5651–5663. doi: 10.1128/MCB.23.16.5651-5663.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wu S, Tan M, Hu Y, Wang JL, Scheuner D, Kaufman RJ. Ultraviolet light activates NFkappaB through translational inhibition of IkappaBalpha synthesis. J Biol Chem. 2004;279:34898–34902. doi: 10.1074/jbc.M405616200. [DOI] [PubMed] [Google Scholar]
- 85.Tonnesen MF, Grunnet LG, Friberg J, Cardozo AK, Billestrup N, Eizirik DL, Storling J, Mandrup-Poulsen T. Inhibition of nuclear factor-kappaB or Bax prevents endoplasmic reticulum stress-but not nitric oxide-mediated apoptosis in INS-1E cells. Endocrinology. 2009;150:4094–4103. doi: 10.1210/en.2009-0029. [DOI] [PubMed] [Google Scholar]
- 86.Guicciardi ME, Bronk SF, Werneburg NW, Yin XM, Gores GJ. Bid is upstream of lysosome-mediated caspase 2 activation in tumor necrosis factor alpha-induced hepatocyte apoptosis. Gastroenterology. 2005;129:269–284. doi: 10.1053/j.gastro.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 87.Carter-Kent C, Zein NN, Feldstein AE. Cytokines in the Pathogenesis of Fatty Liver and Disease Progression to Steatohepatitis: Implications for Treatment. Am J Gastroenterol. 2008 doi: 10.1111/j.1572-0241.2007.01709.x. [DOI] [PubMed] [Google Scholar]
- 88.Tilg H, Moschen AR. Insulin resistance, inflammation, and non-alcoholic fatty liver disease. Trends Endocrinol Metab. 2008;19:371–379. doi: 10.1016/j.tem.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 89.Crespo J, Cayon A, Fernandez-Gil P, Hernandez-Guerra M, Mayorga M, Dominguez-Diez A, Fernandez-Escalante JC, Pons-Romero F. Gene expression of tumor necrosis factor alpha and TNF-receptors, p55 and p75, in nonalcoholic steatohepatitis patients. Hepatology. 2001;34:1158–1163. doi: 10.1053/jhep.2001.29628. [DOI] [PubMed] [Google Scholar]
- 90.Wigg AJ, Roberts-Thomson IC, Dymock RB, McCarthy PJ, Grose RH, Cummins AG. The role of small intestinal bacterial overgrowth, intestinal permeability, endotoxaemia, and tumour necrosis factor alpha in the pathogenesis of non-alcoholic steatohepatitis. Gut. 2001;48:206–211. doi: 10.1136/gut.48.2.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Abiru S, Migita K, Maeda Y, Daikoku M, Ito M, Ohata K, Nagaoka S, Matsumoto T, Takii Y, Kusumoto K, Nakamura M, Komori A, Yano K, Yatsuhashi H, Eguchi K, Ishibashi H. Serum cytokine and soluble cytokine receptor levels in patients with non-alcoholic steatohepatitis. Liver Int. 2006;26:39–45. doi: 10.1111/j.1478-3231.2005.01191.x. [DOI] [PubMed] [Google Scholar]
- 92.Haukeland JW, Damas JK, Konopski Z, Loberg EM, Haaland T, Goverud I, Torjesen PA, Birkeland K, Bjoro K, Aukrust P. Systemic inflammation in nonalcoholic fatty liver disease is characterized by elevated levels of CCL2. J Hepatol. 2006;44:1167–1174. doi: 10.1016/j.jhep.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 93.Valenti L, Fracanzani AL, Dongiovanni P, Santorelli G, Branchi A, Taioli E, Fiorelli G, Fargion S. Tumor necrosis factor alpha promoter polymorphisms and insulin resistance in nonalcoholic fatty liver disease. Gastroenterology. 2002;122:274–280. doi: 10.1053/gast.2002.31065. [DOI] [PubMed] [Google Scholar]
- 94.Tokushige K, Takakura M, Tsuchiya-Matsushita N, Taniai M, Hashimoto E, Shiratori K. Influence of TNF gene polymorphisms in Japanese patients with NASH and simple steatosis. J Hepatol. 2007;46:1104–1110. doi: 10.1016/j.jhep.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 95.Li Z, Yang S, Lin H, Huang J, Watkins PA, Moser AB, Desimone C, Song XY, Diehl AM. Probiotics and antibodies to TNF inhibit inflammatory activity and improve nonalcoholic fatty liver disease. Hepatology. 2003;37:343–350. doi: 10.1053/jhep.2003.50048. [DOI] [PubMed] [Google Scholar]
- 96.Tomita K, Tamiya G, Ando S, Ohsumi K, Chiyo T, Mizutani A, Kitamura N, Toda K, Kaneko T, Horie Y, Han JY, Kato S, Shimoda M, Oike Y, Tomizawa M, Makino S, Ohkura T, Saito H, Kumagai N, Nagata H, Ishii H, Hibi T. Tumour necrosis factor alpha signalling through activation of Kupffer cells plays an essential role in liver fibrosis of non-alcoholic steatohepatitis in mice. Gut. 2006;55:415–424. doi: 10.1136/gut.2005.071118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Adams LA, Zein CO, Angulo P, Lindor KD. A pilot trial of pentoxifylline in nonalcoholic steatohepatitis. Am J Gastroenterol. 2004;99:2365–2368. doi: 10.1111/j.1572-0241.2004.40064.x. [DOI] [PubMed] [Google Scholar]
- 98.Satapathy SK, Sakhuja P, Malhotra V, Sharma BC, Sarin SK. Beneficial effects of pentoxifylline on hepatic steatosis, fibrosis and necroinflammation in patients with non-alcoholic steatohepatitis. J Gastroenterol Hepatol. 2007;22:634–638. doi: 10.1111/j.1440-1746.2006.04756.x. [DOI] [PubMed] [Google Scholar]
- 99.Feldstein AE, Werneburg NW, Canbay A, Guicciardi ME, Bronk SF, Rydzewski R, Burgart LJ, Gores GJ. Free fatty acids promote hepatic lipotoxicity by stimulating TNF-alpha expression via a lysosomal pathway. Hepatology. 2004;40:185–194. doi: 10.1002/hep.20283. [DOI] [PubMed] [Google Scholar]
- 100.Omori Y, Imai J, Watanabe M, Komatsu T, Suzuki Y, Kataoka K, Watanabe S, Tanigami A, Sugano S. CREB-H: a novel mammalian transcription factor belonging to the CREB/ATF family and functioning via the box-B element with a liver-specific expression. Nucleic Acids Res. 2001;29:2154–2162. doi: 10.1093/nar/29.10.2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang K, Shen X, Wu J, Sakaki K, Saunders T, Rutkowski DT, Back SH, Kaufman RJ. Endoplasmic reticulum stress activates cleavage of CREBH to induce a systemic inflammatory response. Cell. 2006;124:587–599. doi: 10.1016/j.cell.2005.11.040. [DOI] [PubMed] [Google Scholar]
- 102.Luebke-Wheeler J, Zhang K, Battle M, Si-Tayeb K, Garrison W, Chhinder S, Li J, Kaufman RJ, Duncan SA. Hepatocyte nuclear factor 4alpha is implicated in endoplasmic reticulum stress-induced acute phase response by regulating expression of cyclic adenosine monophosphate responsive element binding protein H. Hepatology. 2008;48:1242–1250. doi: 10.1002/hep.22439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Danno H, Ishii KA, Nakagawa Y, Mikami M, Yamamoto T, Yabe S, Furusawa M, Kumadaki S, Watanabe K, Shimizu H, Matsuzaka T, Kobayashi K, Takahashi A, Yatoh S, Suzuki H, Yamada N, Shimano H. The liver-enriched transcription factor CREBH is nutritionally regulated and activated by fatty acids and PPARalpha. Biochem Biophys Res Commun. 2009 doi: 10.1016/j.bbrc.2009.12.046. [DOI] [PubMed] [Google Scholar]
- 104.Gentile CL, Wang D, Pfaffenbach KT, Cox R, Wei Y, Pagliassotti MJ. Fatty acids regulate CREBh via transcriptional mechanisms that are dependent on proteasome activity and insulin. Mol Cell Biochem. 2010 doi: 10.1007/s11010-010-0533-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Listenberger LL, Ory DS, Schaffer JE. Palmitate-induced apoptosis can occur through a ceramide-independent pathway. J Biol Chem. 2001;276:14890–14895. doi: 10.1074/jbc.M010286200. [DOI] [PubMed] [Google Scholar]
- 106.Chalasani N, Deeg MA, Crabb DW. Systemic levels of lipid peroxidation and its metabolic and dietary correlates in patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2004;99:1497–1502. doi: 10.1111/j.1572-0241.2004.30159.x. [DOI] [PubMed] [Google Scholar]
- 107.Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Videla LA, Rodrigo R, Orellana M, Fernandez V, Tapia G, Quinones L, Varela N, Contreras J, Lazarte R, Csendes A, Rojas J, Maluenda F, Burdiles P, Diaz JC, Smok G, Thielemann L, Poniachik J. Oxidative stress-related parameters in the liver of non-alcoholic fatty liver disease patients. Clin Sci (Lond) 2004;106:261–268. doi: 10.1042/CS20030285. [DOI] [PubMed] [Google Scholar]
- 109.Narasimhan S, Gokulakrishnan K, Sampathkumar R, Farooq S, Ravikumar R, Mohan V, Balasubramanyam M. Oxidative stress is independently associated with non-alcoholic fatty liver disease (NAFLD) in subjects with and without type 2 diabetes. Clin Biochem. 2010;43:815–821. doi: 10.1016/j.clinbiochem.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 110.Tu BP, Weissman JS. Oxidative protein folding in eukaryotes: mechanisms and consequences. J Cell Biol. 2004;164:341–346. doi: 10.1083/jcb.200311055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chakravarthi S, Jessop CE, Bulleid NJ. The role of glutathione in disulphide bond formation and endoplasmic-reticulum-generated oxidative stress. EMBO Rep. 2006;7:271–275. doi: 10.1038/sj.embor.7400645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Malhotra JD, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid Redox Signal. 2007;9:2277–2293. doi: 10.1089/ars.2007.1782. [DOI] [PubMed] [Google Scholar]
- 113.Shimizu Y, Hendershot LM. Oxidative folding: cellular strategies for dealing with the resultant equimolar production of reactive oxygen species. Antioxid Redox Signal. 2009;11:2317–2331. doi: 10.1089/ars.2009.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Malhotra JD, Miao H, Zhang K, Wolfson A, Pennathur S, Pipe SW, Kaufman RJ. Antioxidants reduce endoplasmic reticulum stress and improve protein secretion. Proc Natl Acad Sci U S A. 2008;105:18525–18530. doi: 10.1073/pnas.0809677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004;11:381–389. doi: 10.1038/sj.cdd.4401373. [DOI] [PubMed] [Google Scholar]
- 116.Ma Y, Brewer JW, Diehl JA, Hendershot LM. Two distinct stress signaling pathways converge upon the CHOP promoter during the mammalian unfolded protein response. J Mol Biol. 2002;318:1351–1365. doi: 10.1016/s0022-2836(02)00234-6. [DOI] [PubMed] [Google Scholar]
- 117.Wu J, Rutkowski DT, Dubois M, Swathirajan J, Saunders T, Wang J, Song B, Yau GD, Kaufman RJ. ATF6alpha optimizes long-term endoplasmic reticulum function to protect cells from chronic stress. Dev Cell. 2007;13:351–364. doi: 10.1016/j.devcel.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 118.Cullinan SB, Zhang D, Hannink M, Arvisais E, Kaufman RJ, Diehl JA. Nrf2 is a direct PERK substrate and effector of PERK-dependent cell survival. Mol Cell Biol. 2003;23:7198–7209. doi: 10.1128/MCB.23.20.7198-7209.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nguyen T, Nioi P, Pickett CB. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem. 2009;284:13291–13295. doi: 10.1074/jbc.R900010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sugimoto H, Okada K, Shoda J, Warabi E, Ishige K, Ueda T, Taguchi K, Yanagawa T, Nakahara A, Hyodo I, Ishii T, Yamamoto M. Deletion of nuclear factor-E2-related factor-2 leads to rapid onset and progression of nutritional steatohepatitis in mice. Am J Physiol Gastrointest Liver Physiol. 2010;298:G283–294. doi: 10.1152/ajpgi.00296.2009. [DOI] [PubMed] [Google Scholar]
- 121.Back SH, Scheuner D, Han J, Song B, Ribick M, Wang J, Gildersleeve RD, Pennathur S, Kaufman RJ. Translation attenuation through eIF2alpha phosphorylation prevents oxidative stress and maintains the differentiated state in beta cells. Cell Metab. 2009;10:13–26. doi: 10.1016/j.cmet.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Liu Y, Adachi M, Zhao S, Hareyama M, Koong AC, Luo D, Rando TA, Imai K, Shinomura Y. Preventing oxidative stress: a new role for XBP1. Cell Death Differ. 2009;16:847–857. doi: 10.1038/cdd.2009.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB, Korsmeyer SJ. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kuwana T, Mackey MR, Perkins G, Ellisman MH, Latterich M, Schneiter R, Green DR, Newmeyer DD. Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell. 2002;111:331–342. doi: 10.1016/s0092-8674(02)01036-x. [DOI] [PubMed] [Google Scholar]
- 125.Feldstein AE, Canbay A, Angulo P, Taniai M, Burgart LJ, Lindor KD, Gores GJ. Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology. 2003;125:437–443. doi: 10.1016/s0016-5085(03)00907-7. [DOI] [PubMed] [Google Scholar]
- 126.Papatheodoridis GV, Hadziyannis E, Tsochatzis E, Georgiou A, Kafiri G, Tiniakos DG, Margariti K, Manolakopoulos S, Manesis EK, Archimandritis AJ. Serum apoptotic caspase activity in chronic hepatitis C and nonalcoholic Fatty liver disease. J Clin Gastroenterol. 2010;44:e87–95. doi: 10.1097/MCG.0b013e3181c0945a. [DOI] [PubMed] [Google Scholar]
- 127.Witek RP, Stone WC, Karaca FG, Syn WK, Pereira TA, Agboola KM, Omenetti A, Jung Y, Teaberry V, Choi SS, Guy CD, Pollard J, Charlton P, Diehl AM. Pan-caspase inhibitor VX-166 reduces fibrosis in an animal model of nonalcoholic steatohepatitis. Hepatology. 2009;50:1421–1430. doi: 10.1002/hep.23167. [DOI] [PubMed] [Google Scholar]
- 128.Zinszner H, Kuroda M, Wang X, Batchvarova N, Lightfoot RT, Remotti H, Stevens JL, Ron D. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev. 1998;12:982–995. doi: 10.1101/gad.12.7.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ji C, Mehrian-Shai R, Chan C, Hsu YH, Kaplowitz N. Role of CHOP in hepatic apoptosis in the murine model of intragastric ethanol feeding. Alcohol Clin Exp Res. 2005;29:1496–1503. doi: 10.1097/01.alc.0000174691.03751.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tamaki N, Hatano E, Taura K, Tada M, Kodama Y, Nitta T, Iwaisako K, Seo S, Nakajima A, Ikai I, Uemoto S. CHOP deficiency attenuates cholestasis-induced liver fibrosis by reduction of hepatocyte injury. Am J Physiol Gastrointest Liver Physiol. 2008;294:G498–505. doi: 10.1152/ajpgi.00482.2007. [DOI] [PubMed] [Google Scholar]
- 131.Cunha DA, Hekerman P, Ladriere L, Bazarra-Castro A, Ortis F, Wakeham MC, Moore F, Rasschaert J, Cardozo AK, Bellomo E, Overbergh L, Mathieu C, Lupi R, Hai T, Herchuelz A, Marchetti P, Rutter GA, Eizirik DL, Cnop M. Initiation and execution of lipotoxic ER stress in pancreatic beta-cells. J Cell Sci. 2008;121:2308–2318. doi: 10.1242/jcs.026062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cazanave SC, Elmi NA, Akazawa Y, Bronk SF, Mott JL, Gores GJ. CHOP and AP-1 cooperatively mediate PUMA expression during lipoapoptosis. Am J Physiol Gastrointest Liver Physiol. 2010;299:G236–243. doi: 10.1152/ajpgi.00091.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sambrook JF. The involvement of calcium in transport of secretory proteins from the endoplasmic reticulum. Cell. 1990;61:197–199. doi: 10.1016/0092-8674(90)90798-j. [DOI] [PubMed] [Google Scholar]
- 134.Rapoport TA. Transport of proteins across the endoplasmic reticulum membrane. Science. 1992;258:931–936. doi: 10.1126/science.1332192. [DOI] [PubMed] [Google Scholar]
- 135.Pinton P, Giorgi C, Siviero R, Zecchini E, Rizzuto R. Calcium and apoptosis: ER-mitochondria Ca2+ transfer in the control of apoptosis. Oncogene. 2008;27:6407–6418. doi: 10.1038/onc.2008.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhou YP, Teng D, Dralyuk F, Ostrega D, Roe MW, Philipson L, Polonsky KS. Apoptosis in insulin-secreting cells. Evidence for the role of intracellular Ca2+ stores and arachidonic acid metabolism. J Clin Invest. 1998;101:1623–1632. doi: 10.1172/JCI1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Luciani DS, Gwiazda KS, Yang TL, Kalynyak TB, Bychkivska Y, Frey MH, Jeffrey KD, Sampaio AV, Underhill TM, Johnson JD. Roles of IP3R and RyR Ca2+ channels in endoplasmic reticulum stress and beta-cell death. Diabetes. 2009;58:422–432. doi: 10.2337/db07-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.McCullough KD, Martindale JL, Klotz LO, Aw TY, Holbrook NJ. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol Cell Biol. 2001;21:1249–1259. doi: 10.1128/MCB.21.4.1249-1259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Puthalakath H, O’Reilly LA, Gunn P, Lee L, Kelly PN, Huntington ND, Hughes PD, Michalak EM, McKimm-Breschkin J, Motoyama N, Gotoh T, Akira S, Bouillet P, Strasser A. ER stress triggers apoptosis by activating BH3-only protein Bim. Cell. 2007;129:1337–1349. doi: 10.1016/j.cell.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 140.Szegezdi E, Macdonald DC, Ni Chonghaile T, Gupta S, Samali A. Bcl-2 family on guard at the ER. Am J Physiol Cell Physiol. 2009;296:C941–953. doi: 10.1152/ajpcell.00612.2008. [DOI] [PubMed] [Google Scholar]
- 141.Chipuk JE, Moldoveanu T, Llambi F, Parsons MJ, Green DR. The BCL-2 family reunion. Mol Cell. 2010;37:299–310. doi: 10.1016/j.molcel.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Panasiuk A, Dzieciol J, Panasiuk B, Prokopowicz D. Expression of p53, Bax and Bcl-2 proteins in hepatocytes in non-alcoholic fatty liver disease. World J Gastroenterol. 2006;12:6198–6202. doi: 10.3748/wjg.v12.i38.6198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Barreyro FJ, Kobayashi S, Bronk SF, Werneburg NW, Malhi H, Gores GJ. Transcriptional regulation of Bim by FoxO3A mediates hepatocyte lipoapoptosis. J Biol Chem. 2007;282:27141–27154. doi: 10.1074/jbc.M704391200. [DOI] [PubMed] [Google Scholar]
- 144.Klee M, Pimentel-Muinos FX. Bcl-X(L) specifically activates Bak to induce swelling and restructuring of the endoplasmic reticulum. J Cell Biol. 2005;168:723–734. doi: 10.1083/jcb.200408169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bailly-Maitre B, Fondevila C, Kaldas F, Droin N, Luciano F, Ricci JE, Croxton R, Krajewska M, Zapata JM, Kupiec-Weglinski JW, Farmer D, Reed JC. Cytoprotective gene bi-1 is required for intrinsic protection from endoplasmic reticulum stress and ischemia-reperfusion injury. Proc Natl Acad Sci U S A. 2006;103:2809–2814. doi: 10.1073/pnas.0506854103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hetz C, Bernasconi P, Fisher J, Lee AH, Bassik MC, Antonsson B, Brandt GS, Iwakoshi NN, Schinzel A, Glimcher LH, Korsmeyer SJ. Proapoptotic BAX and BAK modulate the unfolded protein response by a direct interaction with IRE1alpha. Science. 2006;312:572–576. [Google Scholar]
- 147.Lisbona F, Rojas-Rivera D, Thielen P, Zamorano S, Todd D, Martinon F, Glavic A, Kress C, Lin JH, Walter P, Reed JC, Glimcher LH, Hetz C. BAX inhibitor-1 is a negative regulator of the ER stress sensor IRE1alpha. Mol Cell. 2009;33:679–691. doi: 10.1016/j.molcel.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bailly-Maitre B, Belgardt BF, Jordan SD, Coornaert B, von Freyend MJ, Kleinridders A, Mauer J, Cuddy M, Kress CL, Willmes D, Essig M, Hampel B, Protzer U, Reed JC, Bruning JC. Hepatic Bax inhibitor-1 inhibits IRE1alpha and protects from obesity-associated insulin resistance and glucose intolerance. J Biol Chem. 2010;285:6198–6207. doi: 10.1074/jbc.M109.056648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Scorrano L, Oakes SA, Opferman JT, Cheng EH, Sorcinelli MD, Pozzan T, Korsmeyer SJ. BAX and BAK regulation of endoplasmic reticulum Ca2+: a control point for apoptosis. Science. 2003;300:135–139. doi: 10.1126/science.1081208. [DOI] [PubMed] [Google Scholar]
- 150.Palmer AE, Jin C, Reed JC, Tsien RY. Bcl-2-mediated alterations in endoplasmic reticulum Ca2+ analyzed with an improved genetically encoded fluorescent sensor. Proc Natl Acad Sci U S A. 2004;101:17404–17409. doi: 10.1073/pnas.0408030101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Oakes SA, Scorrano L, Opferman JT, Bassik MC, Nishino M, Pozzan T, Korsmeyer SJ. Proapoptotic BAX and BAK regulate the type 1 inositol trisphosphate receptor and calcium leak from the endoplasmic reticulum. Proc Natl Acad Sci U S A. 2005;102:105–110. doi: 10.1073/pnas.0408352102. [DOI] [PMC free article] [PubMed] [Google Scholar]