Abstract
Rationale
The alternative activation of monocytes by IL-13 and IL-4 is a significant component of the inflammatory response. The consequences of alternative activation in inflammatory diseases remain to be determined.
Objective
In this paper we explored how integrins, receptors important for monocyte migration to inflammatory sites, regulate IL-13-mediated monocyte activation. We focused on the analysis of two proteins, which are upregulated during the alternative activation and are important for the development of atherosclerosis - an oxidative enzyme 15-lipoxygenase (15-LO) and a scavenger receptor CD36.
Methods and Results
We found that adhesion of resting monocytes through β2 integrins and inside-out activation of β2 integrins by MCP-1 did not change IL-13-stimulated 15-LO upregulation; however, preincubation of monocytes with the antibody MEM48, which generates full activation of β2 integrins, significantly inhibited 15-LO mRNA and protein expression. In contrast, activation of β1 integrins had no effect on 15-LO expression. Analysis of integrin clustering through αM, αL, αX and αD subunits demonstrated the pivotal role for integrin αMβ2 in inhibiting 15-LO expression. IL-13 treatment upregulates 15-LO-dependent CD36 expression on human monocytes, our studies showed that β2 integrin activation and αM integrin clustering significantly inhibited IL-13-dependent CD36 mRNA and protein expression as well as CD36-related foam cell formation. Moreover, IL-13 stimulation of αM-deficient peritoneal macrophages demonstrated an upregulated level of 15-LO induction, CD36 expression and lipid accumulation as compared to wild type controls.
Conclusions
The adhesion of monocytes/macrophages through activated integrin αMβ2 has a regulatory and potential athero-protective function during the alternative activation of macrophages.
Keywords: integrin αMβ2 (CD11b/CD18), 15-lipoxygenase, CD36, M2 macrophages, atherosclerosis
Introduction
Monocytes/macrophages are critical components of host defense. During the development of the inflammatory response monocytes are recruited to the site of injury and migrate to the inflamed tissue, where they differentiate into macrophages 1. A further role of macrophages in inflammation is determined by the type of activation program, which triggers specific gene expression and related cell functions. Classically activated M1 macrophages have long been known to be induced by IFN-γ, which initiates a harsh pro-inflammatory stimulus, whereas the alternatively activated M2 macrophages are induced by IL-4 and/or IL-13 and commonly believed to be associated with an anti-inflammatory response. However, recent data demonstrate both anti-inflammatory and pro-inflammatory effects for M2 macrophages on different stages of many diseases 2.
Atherosclerosis is an inflammatory disease of the vessel wall characterized by monocyte infiltration in response to proatherogenic factors then the subsequent transformation of monocytes into lipid-laden macrophage foam cells, which accumulate in the subendothelial space3. The role of alternative activation of macrophages in the development of atherosclerosis is not clear. The experimental data obtained generally for IL-4-stimulated monocytes have demonstrated that exogenous IL-4 has protective effects, while knockout models for IL-4 components show the development of milder disease, indicating a proatherogenic role for endogenous IL-4 4.
Monocyte activation and associated monocyte migration are complex and multifunctional processes, which involve simultaneous stimuli from different cell surface receptors. Integrins, adhesion receptors which guide monocyte migration, are good candidates for playing a role in regulating macrophage differentiation. During inflammation integrins are activated, which leads to the firm adhesion of monocytes to the endothelial cells, followed by monocyte transmigration through the endothelial monolayer and subsequent migration through the extracellular matrix (ECM) to the site of inflammation 5. The subfamily of β2 integrins plays a leading role in this monocyte migration and the immune-inflammatory response. The group consists of four members, αLβ2 (CD11a/CD18, LFA-1), αMβ2 (CD11b/CD18, Mac-1), αXβ2 (CD11c/CD18, p150,95) and αDβ2 (CD11d/CD18), which have a common β2 subunit and homologous α subunits 6. The role of β2 integrins in atherogenesis is controversial. In particular, opposing roles for integrin αX and αM in the development of atherosclerosis have been recently defined. While αX knockout mice on the background of ApoE-deficiency demonstrated decreased atherosclerosis 7, αM knockout on the same background showed a progression of atherosclerosis 8, indicating that αX has pro-atherogenic and αM has anti-atherogenic functions; however, the mechanisms that initiate these opposite effects by two related integrins are not clear.
In this manuscript we studied how the alternative activation of monocytes mediated by IL-13 stimulation is regulated by β2 integrins. We focused on the analysis of expression of the enzyme 15-lipoxygenase (15-LO), scavenger receptor CD36 and concomitant events related to the development of atherosclerosis. CD36 is a type II macrophage scavenger receptor, which is initially involved in the internalization of apoptotic cells, pathogens and modified low-density lipoproteins, but during atherogenesis CD36 is responsible for foam cell formation 9. The proatherogenic role of CD36 is well documented by in vitro and in vivo studies 10, 11. 15-LO catalyzes hydroperoxidation of fatty acids, a reaction of potential relevance to inflammation, membrane remodeling, and atherosclerosis 12. 15-LO is not expressed on circulating blood monocytes but is dramatically upregulated after IL-13 or IL-4 stimulation 13, 14, providing a disease-relevant marker of alternative activation of monocytes.
In this study we report that IL-13-mediated induction of 15-LO is inhibited during β2 integrin activation or clustering through αM integrin. We also found that while IL-13 stimulation promotes the upregulation and surface expression of scavenger receptor CD36, a key protein in foam cell formation, the activation of β2 integrin completely inhibited this effect. Moreover, β2 integrin activation blocked CD36 related foam cell formation on monocyte-differentiated macrophages. Based on our results we suggest a regulatory athero-protective role of integrin αMβ2 during IL-13-mediated alternative activation of macrophages.
Methods
Human peripheral blood monocytes were isolated using a Ficoll-Paque density gradient followed by adherence to bovine calf serum (BCS)-coated flasks as described earlier 15. αM-knockout mice were generated in the laboratory of Dr. Christie Ballantyne (Baylor College of Medicine) 16. The experimental protocol for isolation of peritoneal macrophages was approved by the Cleveland Clinic Institutional Animal Care and Use Committee. Statistical analyses were performed using the Student’s t-test. An expanded Methods section is available in the Online Data Supplement at http://circres.ahajournals.org and includes information regarding the reagents and antibodies used in the study and detailed protocols for isolation of human monocytes, monocyte stimulation, adhesion assays, FACS analysis, cell sorting, western blotting, real-time quantitative RT-PCR, foam cell formation assays and analysis of αM-deficient mouse peritoneal macrophages.
Results
Activation of β2 integrins, but not β1, integrins inhibits IL-13-mediated 15-Lipoxygenase expression in human monocytes
The dramatic upregulation of 15-lipoxygenase (15-LO) after monocyte stimulation with IL-4 and IL-13 is a well characterized hallmark during the alternative activation of macrophages 2, 13. In this paper we studied which conditions can modify 15-LO induction and related events. Because integrins are important surface receptors that are involved in monocyte activation and migration, we tested the effect of integrin-dependent adhesion on IL-13-mediated 15-LO expression.
For this purpose we compared the induction of 15-LO in primary human monocytes incubated in non-adhesive polypropylene tubes or on cell culture plates. Although it has been shown that cell incubation in naked cell-culture plates initiates integrin-mediated adhesion 17, we also precoated some wells with fibrinogen, a plasma protein which can specifically interact with several monocyte integrins – αMβ2, αXβ2, αDβ2 18–20. After 24 hours of incubation, cells were harvested, lysed and 15-LO expression was detected by immunoblotting. We found that all conditions evoked a similar level of 15-LO upregulation (Online Figure I), therefore adhesion through integrins on rested monocytes had no effect on IL-13-mediated 15-LO induction. However, the majority of integrins molecules on resting monocytes are expressed in the non-active conformation 21, therefore to mimic the conditions which exist during the monocyte transmigration to the inflammatory tissue we activated integrins β2 and β1, two major subfamilies of integrins expressed on monocytes. For these experiments monocytes were preincubated with anti-β2 activation antibody (clone MEM48) and placed on plates precoated with fibrinogen or preincubated with anti-β1 activation antibody (clone P4G11) and placed on plates precoated with fibronectin (a well characterized ligand for several β1 integrins). The 15-LO protein expression was analyzed by immunoblotting at 24 hours after integrin activation (Fig. 1A, left panel). We found that IL-13 mediated 15-LO induction was dramatically inhibited in the presence of activation antibody against β2 integrin, while stimulation with activation antibody against β1 integrins had no effect on 15-LO expression. Tested in parallel, the β2 blocking antibody also had no effect on 15-LO induction. Our analysis demonstrated that pretreatment with activation antibodies leads to a significant increase in monocyte adhesion, which was detected after 30 minutes (Fig. 1B), and to the marked changes in monocyte spreading, which was recorded after 24 hours (Fig. 1C). At the same time, preincubation with blocking β1 and β2 antibodies inhibited monocyte adhesion which confirmed the specificity of binding. Therefore, our data showed that activation of β2 integrins, which leads to increased monocyte adhesion and cell spreading, inhibited IL-13-mediated 15-LO induction, but activation of β1 integrins, which initiate similar events, failed to change this upregulation.
Figure 1. Induction and inhibition of 15-LO expression in primary human monocytes.
A. Activation of β2 integrins inhibits IL-13 and IL-4 mediated 15-LO induction. Monocytes were preincubated with 10μg/ml of anti-β2 function blocking antibody (IB4), anti-β2 activation antibody (MEM48) or anti-β1 activation antibody (P4G11) and then stimulated with IL-13 (left panel) or IL-4 (right panel). After 24 hours of incubation monocytes were harvested and 15-LO expression was analyzed by immunoblotting (upper panel). Equal loading was confirmed by immunoblotting with anti-β-tubulin antibody after membrane stripping (lower panel). Data are from a representative experiment of four with similar results. B. Monocyte adhesion to fibrinogen (Fg) and to fibronectin (Fn) is modified in the presence of blocking and activation antibodies. Grey Bars -Monocytes were preincubated with 10μg/ml of anti-β2 function blocking antibody (IB4) or anti-β2 activation antibody (MEM48) for 20 minutes and added to the plate with immobilized fibrinogen (10 μg/ml). White Bars - In parallel experiment monocytes were preincubated with 10μg/ml of anti-β1 function blocking antibody (TDM29) or anti-β1 activation antibody (P4G11) for 20 minutes and added to the plate with immobilized fibronectin (10 μg/ml). Adhesion assays were done as described in the Methods section. The experiment was repeated twice with a similar result. A representative experiment is shown. Data are expressed as a percentage of added cells and are the mean ± SD of triplicate wells for each experimental condition. C. Microscopic analysis of monocytes after adhesion in the presence of different antibodies. Monocytes were incubated on 6 well plates, precoated with fibrinogen or fibronectin, for 24 hours after IL-13 stimulation (original magnification x200). Data are from a representative experiment of four with similar results.
The alternative activation of monocytes can be initiated by stimulation with IL-4 and/or IL-13. To test the effect of β2 integrin activation on the IL-4-mediated 15-LO induction we performed a similar experiment where monocytes pretreated with MEM48 were stimulated with IL-4. Our experiments demonstrated that activation of β2 integrins blocked the 15-LO upregulation on IL-4 stimulated monocytes in the same manner (Fig. 1A, right panel). Therefore, activation of β2 integrin inhibited 15-LO induction mediated by either IL-13 or IL-4.
Complete integrin activation is required for the suppression of 15-LO induction
Our results led us to a more detailed analysis of integrin activation and inhibition of 15-LO expression in alternatively activated monocytes. Circulating monocytes express integrins in a non-active conformation, which prevents monocyte adhesion to the endothelial cells and other activation-dependent functions. During the activation of the inflammatory cascade integrins are activated through an inside-out mechanism by a pro-inflammatory stimulus (MCP-1, TNF-α and etc.) on the surface of endothelium 22. This activation transforms integrins into the intermediate stage of activation, while subsequent interaction with the specific ligand (outside-in integrin activation) changes integrin conformation to the fully active stage 23.
To explore how inside-out integrin signaling affects 15-LO induction we preincubated monocytes with MCP-1, a chemokine which induces inside-out activation of β2 integrins 24. To separate MCP-1 stimulation and follow integrin interaction with ligand, we performed these experiments in non-adherent polypropylene tubes. As shown above (Online Figure I), IL-13 treatment in polypropylene tubes evokes 15-LO induction. For these experiments monocytes were preincubated with 10 μg/ml of mAb MEM48 (anti-β2 activation antibody) or with 10 nmol/L MCP-1. The level of integrin activation was detected with the conformation-dependent antibody against β2 integrins mAb 24. This antibody can interact only with β2 integrins expressed in the active conformation 25, 26. Monocytes were analyzed by FACS after treatment with MEM48 and MCP-1. The right shift in fluorescence intensity indicates the more active conformation for β2 integrins (Fig. 2A). Our results confirmed that the treatment with MCP-1 only slightly increased the activation stage of β2 integrins, while incubation with mAb MEM48 significantly changed β2 integrin conformation and transforms the integrin into its fully active stage (Fig. 2B).
Figure 2. Full β2 integrin activation is required for inhibition of 15-LO induction.
The conformational stage of β2 integrins on the surface of monocytes was quantified with the conformation-dependent antibody mAb 24. Monocytes were preincubated in polypropylene tubes for 5 minutes with mAb 24 conjugated to FITC, after which 10nmol/L MCP-1 or 10μg/ml MEM48 antibody was added to the cells. After 20 minutes of incubation cells were fixed with 10% formaldehyde and analyzed by flow cytometry. A. Representative FACS is shown. Thin solid line – incubation in the presence of EDTA, dashed line – in the presence of Mg2+, dotted line – treatment with MCP-1, Thick solid line –treatment with activation antibody MEM48. B. Mean fluorescence values are plotted based on three independent experiments. (* P< 0.01 compared to Mg2+ stimulated cells) C. Human monocytes were incubated with 10 nmol/L MCP-1 or 10 μg/ml MEM48 antibody for 5 minutes and IL-13 was added for an additional 24 hours. After incubation cells were harvested and 15-LO expression was analyzed by immunoblotting (upper panel). Equal protein loading was evaluated by stripping and reprobing with β-tubulin antibody (lower panel). Data are from a representative experiment of three with similar results.
The level of 15-LO in monocytes pretreated with mAb MEM48 or MCP-1 was analyzed after 24 hours. We found that while β2 integrin activation in non-adherent conditions inhibited 15-LO induction, inside-out integrin activation with MCP-1 had no effect (Fig. 2C). Therefore, we concluded that conversion of β2 integrins to the fully active conformation is required for the inhibition of IL-13-induced expression of 15-LO.
Integrin clustering through αM integrin induces the inhibition of 15-LO induction in human monocytes
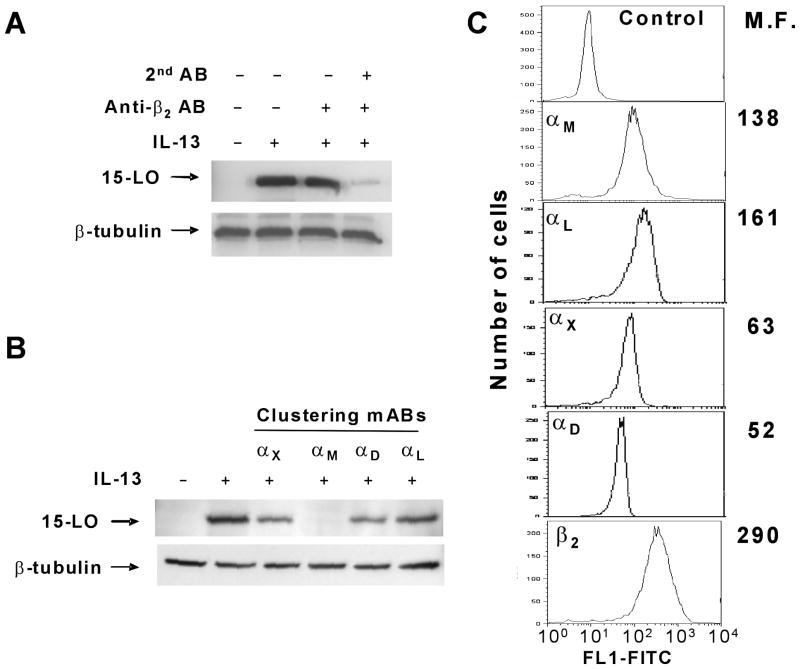
To obtain additional mechanistic insight into the role of β2 integrins in the modification of monocyte responses after IL-13 treatment we tested the changes in 15-LO induction after clustering through β2 integrin. For this purpose 5 μg/ml of β2 antibody (clone IB4) was incubated with human monocytes in non-adherent polypropylene tubes. After 20 minutes of incubation, 2 μg/ml of goat-anti-mouse antibody was added for an additional 5 minutes followed by treatment with IL-13. As a control, the cells were preincubated in the presence of only the β2 antibody for 20 minutes before IL-13 treatment. 15-LO expression was detected by immunoblotting after 24 hours of incubation (Fig. 3A). Our results demonstrated that clustering through β2 integrins inhibited 15-LO induction similar to the effect observed after activation of β2 integrin. The treatment with only the β2 antibody had no effect.
Figure 3. Integrin clustering mediated by β2 integrin AB or αM integrin AB inhibits 15-LO induction.
Human monocytes were incubated in polypropylene tubes in the presence of anti-β2 antibody (clone IB4) (A) or different anti-α integrin subunit antibodies (B) at 5 μg/ml. After 20 minutes normal mouse IgG (2 μg/ml) was added to cells to cluster the primary antibodies. After 5 minutes of incubation with normal mouse IgG IL-13 was added to cells for an additional 24 hours. As a control monocytes were incubated only in the presence of primary antibody (A, line 3). After incubation cells were harvested and 15-LO expression was analyzed by immunoblotting. Equal protein loading was evaluated by stripping and reprobing with β-tubulin antibody. C. The levels of β2 integrin expression on the surface of primary human monocytes were analyzed with anti-integrin antibodies and evaluated by FACS. Mean fluorescence (M.F.) values are shown on the graphs. Data are from a representative experiment of three with similar results.
To obtain additional information about integrin receptors involved in suppression of 15-LO induction we performed clustering through different α subunits to determine which one was responsible for inhibition (Fig. 3B). Antibody against αM, αX, αD and αL integrins were preincubated with monocytes followed by incubation with goat anti-mouse antibody as described above. Analysis of 15-LO induction at 24 hours after IL-13 stimulation demonstrated that 15-LO expression was dramatically inhibited only in the presence of antibody against αM integrin, while anti-αL antibody had no effect and anti-αX and anti-αD antibodies showed very slight inhibition. This result demonstrated that integrin αLβ2 is not involved in this process and integrin αMβ2 is a major integrin receptor responsible for the inhibition of IL-13-induced 15-LO expression. Since the reported levels of integrin expression on monocytes are vary in the literature we tested the density of all β2 integrins on primary human monocytes isolated for our studies (Fig. 3C). We found that all four integrin complexes, αMβ2, αLβ2, αXβ2, αDβ2, were expressed on the surface. While the expression of αM and αL integrins was moderate, the densities of αX and αD were lower but still readily detectable. Therefore, incubation with antibodies to all four subunits was able to induce integrin clustering, although only αM integrin ligation evoked significant inhibition of 15-LO expression.
CD16− and CD16+ monocyte subsets demonstrated a similar response to IL-13 stimulation and αMβ2 integrin activation
We performed our prior experiments on the total population of monocytes isolated from peripheral blood. To better understand the specificity of 15-LO induction after IL-13 treatment we tested two major subsets of CD14+ human monocytes - CD16− and CD16+. Corresponding to the published data the majority of freshly isolated monocytes were CD16 negative (Online Figure II, A). The amount of CD16 positive monocytes varied from 11% to 24% in different donors and additionally increased during monocyte incubation in tissue culture plates. Using double-colored FACS analysis we found that both CD16+ and CD16− subsets expressed integrin αM on the cell surface and αM densities on both subsets are similar (Online Figure II, B). To test 15-LO induction we incubated the total monocyte population in the presence of IL-13 for 24 hours. Then cells were collected from the plates and labeled with PE-conjugated anti-CD16 antibody. The monocyte population was gated and separated on CD16+ and CD16− subsets using fluorescence activated cell sorting (Online Figure II, C). Isolated subsets were lysed and analyzed by Western Blotting with 15-LO antibody. (Online Figure II, D). We found that both subsets induced 15-LO in response to IL-13 stimulation and this induction was inhibited by MEM48 on both CD16+ and CD16− monocytes. Therefore, our result demonstrated that CD16− and CD16+ monocytes have a similar mechanism of 15-LO induction and inhibition in response to IL-13 stimulation.
Activation of αM integrin inhibits 15-LO mRNA induction
To better understand the mechanism of 15-LO inhibition by β2 integrins we tested the changes in 15-LO mRNA expression after antibody treatment. Monocytes were incubated in the presence of mAb MEM48 followed by IL-13 stimulation as described previously. mRNA was isolated at different time points and analyzed by Quantitative Real Time RT-PCR. In agreement with published observations 27 the level of 15-LO mRNA was gradually upregulated after IL-13 treatment, while cells pretreated with β2-activated antibody demonstrated reduced 15-LO upregulation detectable even at 2 hours after IL-13 stimulation (Online Table I). After 24 hours, cells incubated with the β2 integrin activation antibody or clustering through αM integrin demonstrated significant inhibition of 15-LO mRNA expression (about 16 fold as compared with IL-13 stimulated cells) (Fig. 4). Therefore, this result confirmed our data obtained for 15-LO protein expression and suggested that inhibition occurs at the transcriptional level.
Figure 4. 15-LO mRNA expression is inhibited by αM integrin activation.

Human monocytes were incubated in the presence of activating antibody to β2 integrin or clustering through αM integrin (as described in the Methods section) followed by stimulation with IL-13 for 24 hours. After incubation monocyte mRNA was isolated using a Qiagen Oligotex mRNA Midi Kit and levels of 15-LO mRNA were evaluated by quantitative real time RT-PCR. After normalization with GAPDH amplification, the fold induction of 15-LO mRNA expression after β2 integrin activation or αM integrin clustering was compared with the control. Data were collected from five independent experiments and are shown as means ± SE. Statistical analysis was performed using Student’s paired t-test, (* P< 0.05 compared to IL-13 stimulated cells).
The expression of mannose receptor during alternative activation of monocytes is inhibited after β2 integrin activation
To better understand the role of β2 integrin activation in alternative activation of macrophages we analyzed the change in the expression of the mannose receptor – a well established marker of alternative activation 28. For this purpose monocytes were pretreated with the antibody MEM48 followed by activation with IL-13 as described above. After 5 days of incubation the expression of mannose receptor was tested by FACS (Online Figure III). Similar to published results we observed that the expression of mannose receptor in the presence of IL-13 was significantly increased (about 7 fold). At the same time preincubation with antibody MEM48 completely inhibited upregulation of the mannose receptor. Therefore, these data demonstrated that activation of β2 integrin is not restricted to regulation of the 15-LO pathway, but rather has a common inhibitory effect on the alternative activation of macrophages.
Activation of aM integrin inhibits IL-13 mediated CD36 expression
Recent data from our laboratory demonstrate that stimulation of human monocytes with IL-13 leads to the upregulation of scavenger receptor CD36 through a 15-LO dependent mechanism. Particularly we found that antisense oligonucleotides to 15-LO dramatically inhibited IL-13 stimulated CD36 expression (Bhattacharjee A. et al, manuscript in preparation). Based on the PPARγ dependent mechanism of CD36 expression we analyzed whether 15-LO induction is regulated by PPARγ agonist or antagonist. For this purpose human monocytes were treated with PPARγ agonist Rosiglitazone (10 μmol/L) or pretreated with PPARγ antagonist GW9662 (5μmol/L) and stimulated with IL-13. Monocytes were incubated for 24 hours and modification of 15-LO induction was analyzed by Western Blotting. We found no change in 15-LO expression after PPARγ agonist or antagonist treatment (Online Figure IV). Therefore, IL-13-mediated 15-LO induction is PPARγ independent.
Since the activation of β2 integrin inhibits 15-LO expression we hypothesized that the same mechanism would suppress CD36 upregulation. To test this hypothesis, we analyzed CD36 mRNA expression 24 hours after β2 integrin activation or αM integrin clustering followed by IL-13 treatment (Fig. 5A). We found that the level of CD36 mRNA is upregulated about fourfold after IL-13 treatment and this upregulation is completely inhibited in the presence of either activation of β2 integrin or αM integrin clustering. For further analysis we tested the change in CD36 protein expression in human monocytes. Although the change in mRNA expression was detected at 24 hours, the change in CD36 protein expression required prolonged differentiation of monocytes to macrophages. The protein expression of CD36 remained at the same low level until 4 days after IL-13 stimulation. We could detect a considerable change in CD36 protein expression only after 5 days, when IL-13 treated cells showed significant upregulation of total CD36 protein as detected by Western blotting (Fig. 5B) and CD36 protein surface expression as detected by FACS (Fig. 5C). Similar to the CD36 mRNA analysis, CD36 protein expression was completely inhibited after β2 integrin activation or clustering through αM integrin. In summary, our data demonstrate on several levels that activation of αMβ2 integrin on the surface of primary human monocytes inhibited IL-13 mediated expression of CD36.
Figure 5. CD36 expression is inhibited by αM integrin activation.
A. Human monocytes were incubated as indicated in the Fig. 4 legend. After incubation the level of CD36 mRNA was evaluated by the real time quantitative RT-PCR after normalization with the GAPDH control. Data were collected from three independent experiments and are shown as means ± SE. Statistical analysis was performed using Student’s paired t-test, (** P< 0.01 compared to IL-13 stimulated cells). B. C. In a parallel experiment cells in the presence of β2 integrin activating antibody (MEM48) or αM integrin clustering antibody were incubated 5 days with IL-13 and then harvested. CD36 protein expression was evaluated by immunoblotting (B) or by FACS analysis (C). Legend for FACS data: thin solid line – unstained control (mean fluorescence (m.f.) − 23.5); dotted line – cells incubated without IL-13 (m.f. 155); thick solid line – cells incubated in the presence of IL-13 (m.f. 536); dashed line – cells incubated in the presence of IL-13 and activation antibody MEM48 (m.f. 118); cells incubated in the presence of IL-13 after clustering through αM integrin (m.f. 92.6) demonstrated a effect similar to antibody MEM48 and result is not shown to simplify the graph. Data are from a representative experiment of three with similar results.
The foam cell formation is suppressed after αM integrin activation
The uptake of oxidized LDL by differentiated macrophages through scavenger receptors, particularly CD36, leads to foam cell formation – the hallmark of atherosclerosis 11. Based on our observation that CD36 expression is altered in our experiments we decided to analyze how foam cell formation would be affected after αM integrin activation. We pretreated monocytes with mAb MEM48 followed by IL-13 stimulation as described above and differentiated cells for 5 days in DMEM with 10% lipoprotein-deficient serum (LPDS) media. To assess the role of CD36 in foam cell formation we exposed monocyte-differentiated macrophages to a fluorophore (DiI)-tagged form of NO2 oxidized LDL (DiI-NO2 LDL) for 30 minutes at 37°C and measured DiI-NO2 LDL internalization by fluorescence microscopy (Fig. 6A). NO2-oxidazed LDL is a preferential ligand for CD36 29. Our data demonstrate that uptake of oxidized LDL was increased more than 3 fold after IL-13 stimulation and significantly diminished after integrin activation (Fig. 6B).
Figure 6. αM integrin activation reduced IL-13-mediated foam cell formation.
A. The uptake of oxLDL was assessed in human monocytes incubated in different conditions. Monocytes were plated in the presence or absence of mAb MEM48 followed by IL-13 stimulation and kept for 5 days in DMEM medium supplemented with 10% lipoprotein-deficient serum. One hour before the oxLDL treatment the media was exchanged to serum-free DMEM medium. Cells were incubated for 30 minutes in the presence of DiI-NO2 oxLDL, washed, fixed, mounted on slides in the presence of DAPI and analyzed by fluorescence microscopy. Original magnification x400. Data are from a representative experiment of three that were performed. B. Five randomly selected fields from each slide were analyzed and DiI intensity was quantified using the computer-assisted image analysis software Image-Pro Plus. Data were normalized based on the total amount of cells in a particular field and plotted as the mean ± SD. Statistical analysis was performed using Student’s t-test (* P< 0.05; ** P<0.01). The experiment was repeated three times with the similar result.
Therefore, activation of β2 integrins on alternatively activated M2 macrophages leads to functional changes in macrophage differentiation that initiate the inhibition of foam cell formation. Thus, our data demonstrate that αMβ2 integrin activation may have an athero-protective effect during the development of atherosclerosis.
αM-deficiency upregulates 15-LO induction, CD36 expression and foam cell formation on mouse peritoneal macrophages
To distinguish the role of integrin αM in the inhibition of alternative activation we analyzed 15-LO induction in αM-deficient mouse peritoneal macrophages. It has been shown previously that peritoneal macrophages express the upregulated level of 15-LO 30. Wild type and αM-deficient mice were injected with thioglycollate and after 72 hours peritoneal cells were isolated. All samples contained similar percentages of macrophages in the peritoneal exudate (around 80%) detected by FACS with F4/80 antibodies (data not shown). Macrophages were lysed and 15-LO expression was analyzed by Western Blotting. We found that endogenous stimulation led to slightly increased 15-LO induction in αM-deficient mice (1.52 fold, P<0.05) (Online Figure V). To explore the effect of exogenous IL-13 we injected mouse IL-13 into the peritoneal cavity of wild type and αM-deficient mice with pre-induced peritoneal inflammation. Mice were injected with thioglycollate and after 48 hours, when macrophages become the major subset of leukocytes in the peritoneum cavity, were injected intraperitoneally with IL-13 (100nmol/L). After an additional 24 hours, peritoneal cells were isolated, lysed and 15-LO induction was analyzed by Western Blotting. We found a significant increase in 15-LO induction in αM-deficient macrophages (2.82 fold, P<0.05). (Fig. 7A,B). Therefore, these results demonstrate that integrin αM regulates 15-LO expression and αM-deficiency promotes the additional induction of 15-LO. To analyze the changes in CD36 expression in αM-deficient macrophages in vitro, wild type and αM-deficient mouse macrophages were isolated from the peritoneal cavity at 72 hours after thioglycollate injection, plated and mouse IL-13 was added to cells for 5 days of incubation. Both wild type and αM-deficient macrophages strongly adhered to plastic and demonstrated a similar morphology and spreading. As a control we incubated macrophages in the presence of IL-13 in non-adhered polypropylene tubes. After incubation cells were harvested and analyzed by FACS. We found marked difference in the level of CD36 expression on non-adhered and adhered macrophages. The non-adhered wild type and αM-deficient cells demonstrated a similar level of CD36 expression. Whereas in adhered macrophages the CD36 level was significantly reduced in wild type cells as compared with both αM−/− adhered cells and wild type non-adhered cells (Fig. 7C). These results demonstrated that the strong adhesion via αMβ2 suppressed IL-13-induced CD36 expression, while αM-deficiency completely restored it. Moreover, because IL-13-mediated CD36 expression in the non-adhered condition is not αM-dependent, the wild type and αM-deficient cells displayed similar levels of expression. Therefore, these data demonstrated that strong adhesion through αMβ2 integrin is critical for the inhibition of IL-13-mediated CD36 expression.
Figure 7. αM -deficient mouse peritoneal macrophages demonstrated the enhanced level of 15-LO, CD36 and foam cell formation.
A. Wild type (n=4) and αM-deficient mice (n=4) were injected with 0.5 ml of 4% thioglycollate. After 48 hours IL-13 was injected in the peritoneal cavity. Peritoneal cells were collected after an additional 24 hours. Cells were lysed and analyzed by Western Blotting with anti-15-LO antibody (upper panel). The 15-LO blot was stripped and reprobed with an antibody against β-tubulin (lower panel) to assess sample loading. B. Data were normalized and 15-LO protein expression was quantified using TotalLab TL120 Software (Nonlinear Dynamics, Durham, NC). Data were plotted as the mean ± SE. Statistical analysis was performed using Student’s t-test (* P< 0.05). C. In separate experiment peritoneal macrophages from wild type and αM-deficient mice were isolated at 72 hours after thioglycollate injection. Macrophages were plated and stimulated with IL-13 for 5 days. Alternatively, macrophages were incubated in the presence of IL-13 in non-adhesive polypropylene tubes. After incubation the expression of CD36 by FACS was detected. The data represent the mean ± SE for non-adhered wild type mice (n=3); non-adhered αM−/− mice (n=3); adhered wild type mice (n=6) and adhered αM−/−mice (n=7). Statistical analysis was performed using Student’s t-test, (** P<0.01). D. Foam cells formation was evaluated on adherent peritoneal macrophages using DiI-NO2 oxLDL as described above. Data were normalized based on the total amount of cells in a particular field and plotted as the mean ± SD. Statistical analysis was performed using Student’s t-test (** P<0.01).
To obtain additional evidence of regulation of CD36 expression by αMβ2 we also tested foam cell formation in αM-deficient macrophages incubated 5 days in the presence of IL-13 as described above. After treatment, cells were incubated for 30 min in the presence of DiI-NO2 LDL and lipid uptake was analyzed. We observed a significant increase in foam cell formation by αM-deficient macrophages, indicating that the increased CD36 actively functions as a scavenger receptor (Online Figure VI and Fig. 7D). Therefore, our data demonstrated that adhesion through αMβ2 integrin is critical for the inhibition of IL-13-induced CD36 expression and foam cell formation verifying that integrin αM is a major β2 integrin responsible for the regulation of alternative M2 macrophage activation and the ability of these cells to accumulate lipids.
Discussion
IL-4 and IL-13 drive the alternative activation of macrophages to the M2 phenotype by expression of certain surface markers such as mannose receptor and also lead to the enhanced expression of 15-LO and CD36, well described participants of atherogenesis. Although IL-4 and IL-13-mediated alternative activation of macrophages has been historically attributed to anti-inflammatory stimuli, the analysis of major human diseases, including infection, inflammation, fibrosis and malignancy, demonstrate the two-edged nature of their function 2. Corresponding to these observations, the role of alternatively activated macrophages in the pathogenesis of atherosclerosis is also complex, which is confirmed by numerous reports describing pro- and anti-atherogenic effects of critical components involved in the alternative activation of macrophages.
In particular, the role of IL-4 in atherosclerosis has been investigated in several mouse models. While the injection of exogenous IL-4 decreased aortic lesion formation in mice 31, IL-4 deficiency on the background of LDLR−/− or ApoE−/− leads to significant reduction of atherosclerosis 32, 33. Taken together these data suggest that exogenous and endogenous IL-4 may have the opposite effects during the development of atherosclerosis. Based on the observations that effects of IL-4 and IL-13 are very similar because one chain (IL-4Rα) is common to both receptors 34 we can hypothesize that IL-13 may have similar functions in atherogenesis.
Opposing effects on the development of atherosclerosis were also detected for 15-lipoxygenase (15-LO). Studies of 12/15-LO deficiency (mouse analog of human 15-LO) demonstrated that 12/15-LO gene disruption lead to a significant reduction of atherosclerotic lesion progression in ApoE−/− 35 and LDLR−/− 36 mouse models of atherosclerosis. In addition, mice overexpressing human 15-LO are more susceptible to the development of atherosclerotic lesions than littermate controls 37. At odds with these observations, transgenic rabbits overexpressing human 15-LO in their macrophages developed significantly smaller atherosclerotic lesions when fed a western type diet 38. In addition, a recently discovered polymorphism in the 15-LO promoter showed a tendency to be protective against atherosclerosis in a case–controlled study for coronary artery disease involving 498 Caucasian participants 39. Therefore, 15-LO induction during IL-13 stimulation appears to have pro- or anti- atherogenic functions depending on selected models. However, in the context of upregulation of CD36 and augmentation of foam cell formation we consider that our data is consistent with studies that suggest a proatherogenic role of IL-4, IL-13 and 15-LO during alternative activation of monocytes.
Recent studies suggest a different role for two major subsets of CD14+ human monocytes CD16− and CD16+. Based on the better described mouse analog – Gr1+/Ly6Chigh and Gr1−/Ly6CLow monocytes subsets, CD16 negative monocytes are referred to as “inflammatory monocytes”, whereas CD16 positive monocytes are believed to be “patrolling” monocytes, which crawl in the luminal side of endothelium to survey surrounding tissue and probably are responsible for the scavenging of lipids and dying cells 40. Although, the expression of CD36 on CD16+ non-stimulated monocytes is slightly reduced 41, the uptake of CD36-dependent oxLDL is similar on CD16− and CD16+ monocytes isolated from the healthy donors 42. In our study we tested how these two subsets respond to IL-13-mediated 15-LO induction. We found that both CD16+ and CD16− monocytes induced 15-LO expression and this induction is inhibited by an activation antibody to αMβ2 integrin. Based on our data we conclude that 15-LO pathways are similar in both monocytes subsets, however, considering observations that CD16+ monocytes are predisposed to become migratory dendritic cells 41, we cannot exclude that while CD16− monocytes contributes to the macrophage foam cell accumulation in the atherosclerotic lesion, CD16+ monocytes capture oxLDL and emigrate from the lesion site to the lymphatics.
In this study we found that IL-13- and IL-4-mediated 15-LO induction and CD36 expression are significantly inhibited by activation of β2 integrins. Integrin activation is an important step during monocyte migration to inflammatory sites. Our data demonstrate that only complete integrin activation (outside-in integrin activation) inhibits IL-13-mediated 15-LO induction, with MCP-1 treatment alone (inside-out integrin activation) insufficient to generate that signal (Fig. 2). Monoclonal antibody MEM48 was described as an activation antibody for β2 integrins. The antibody interacts with cysteine rich repeats in the β2 subunit stalk region and activates all four integrin complexes from the β2 integrin subfamily 43. Therefore, this antibody alone (as well as other known β2 integrin activation antibodies) cannot specify which α subunit is involved in blocking IL-13 mediated functions. However, there are several lines of evidence that allow us to suggest that integrin αMβ2 is primarily responsible for this effect on monocytes. First, although the fact that mAb MEM48 activates all β2 integrins is well established, special analysis demonstrated that this antibody activates 90% of αLβ2, about 70% of αMβ2 and only 16% of αXβ2 integrins on the cell surface 44. Integrin αDβ2 was not tested in that study. Therefore, integrin αXβ2 is not activated by this antibody. Second, integrin αLβ2 is a receptor for ICAM molecules and cannot recognize fibrinogen 45, therefore integrin activation and related monocyte adhesion to fibrinogen cannot be mediated through αLβ2 integrin. Third, clustering through different integrin subunits demonstrated that only antibodies to the β2 subunit and αM subunit dramatically inhibit 15-LO induction (Fig. 3). And finally, the analysis of 15-LO and CD36 expression on αM-deficient mouse peritoneal macrophages demonstrated that strong adhesion through αMβ2 is required for the blocking of these IL-13 mediated events. β2 integrins on peritoneal macrophages are activated due to transmigration through the endothelium and the peritoneal tissue, therefore these macrophages do not require additional activation for strong adhesion and spreading. Therefore, summarizing we can propose that integrin αMβ2 is the major β2 integrin, which is responsible for the regulation of IL-13-mediated alternative activation of monocytes/macrophages.
Based on the inhibition of CD36 expression (Fig. 5) and foam cell formation (Fig. 6) our data suggest a potential athero-protective effect of integrin αMβ2 in the development of atherosclerosis. The role of β2 integrins in atherogenesis is not clear yet. It was previously suggested that β2 integrins have proatherogenic functions 46 and recent analysis of integrin αX in atherosclerosis is consistent with this hypothesis. αX-knockout mice crossbred with ApoE-deficient mice demonstrated a significant reduction of lesion formation compared to ApoE−/− control7. The examination of αM-knockout on an LDLR−/− background demonstrated no difference in the development of atherosclerosis 47. Surprisingly, the recent analysis of αM–deficiency on ApoE−/− mice showed an increase of lesion formation, suggesting that αM has an athero-protective effect on the development of atherosclerosis 8. It has been shown that CD36 expression is upregulated by oxidized LDL and IL-4 by a common signaling pathway via the transcriptional factor PPARγ. Our data demonstrated that IL-13-mediated CD36 expression is also PPARγ dependent. (Bhattacharjee A. et al, manuscript in preparation). Based on the common signaling pathways for oxLDL and IL-13 enhancement of CD36 expression we hypothesize that integrin αM may inhibit oxLDL-stimulated CD36 expression by similar mechanisms. Indeed, the analysis of lipid deposition in peritoneal macrophages isolated from αM/ApoE-deficient mice showed a marked increase in foam cell formation 8. And this result is consistent with our finding that IL-13-stimulated peritoneal macrophages demonstrated an increased level of foam cell formation in αM-deficient cells (Fig. 7D). Therefore, αM activation is required for controlling CD36 expression on macrophages, diminishing lipid deposition and potentially reducing lesion size.
In summary, although alternatively activated M2 monocyte/macrophages are often considered to be anti-inflammatory in nature, the IL-13-induced 15-LO/CD36/foam cell axis suggests that this delineation is not so simple and the role of β2 integrin activation in inhibiting 15-LO, CD36 and foam cell formation is a novel and unexpected finding, which demonstrates a potential regulatory role for the integrin αMβ2 on alternative activation of macrophages during atherogenesis.
Supplementary Material
Acknowledgments
We are grateful to Dr. Richard Morton for a generous gift of lipoprotein-deficient serum and to Drs. David Kennedy and Roy Silverstein for the oxidized LDL which they provided for our studies.
Sources of Funding: These studies were supported by NIH grants HL051068 and HL087018 and National Center for Research Resources, CTSA 1UL1RR024989 (M.K.C).
Non-standard Abbreviations and Acronyms
- 15-LO
15-lipoxygenase
- ECM
Extracellular matrix
- BCS
bovine calf serum
- DiI
1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate
- OxLDL
oxidized low density lipoprotein
Footnotes
Disclosures: None.
Reference List
- 1.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–89. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 2.Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010 May 28;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 3.Libby P. Inflammation in atherosclerosis. Nature. 2002;420(6917):868–74. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 4.Kleemann R, Zadelaar S, Kooistra T. Cytokines and atherosclerosis: a comprehensive review of studies in mice. Cardiovasc Res. 2008;79:360–76. doi: 10.1093/cvr/cvn120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krieglstein CF, Granger DN. Adhesion molecules and their role in vascular disease. Am J Hypertens. 2001;14:44S–54S. doi: 10.1016/s0895-7061(01)02069-6. [DOI] [PubMed] [Google Scholar]
- 6.Gahmberg CG. Leukocyte adhesion: CD11/CD18 integrins and intercellular adhesion molecules. Curr Opin Cell Biol. 1997;9:643–50. doi: 10.1016/s0955-0674(97)80117-2. [DOI] [PubMed] [Google Scholar]
- 7.Wu H, Gower RM, Wang H, Perrard XY, Ma R, Bullard DC, Burns AR, Paul A, Smith CW, Simon SI, Ballantyne CM. Functional role of CD11c+ monocytes in atherogenesis associated with hypercholesterolemia. Circulation. 2009;119:2708–17. doi: 10.1161/CIRCULATIONAHA.108.823740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pluskota E, Szpak D, Ballantyne CM, Smith J, Izem L, Morton R, Plow EF. CD11b Delays Development of Early Atherosclerotic Lesions in Female ApoE−/− Mice. Abstract. Arteriosclerosis, Thrombosis and Vascular Biology. 2010 Scientific Sessions, E-Poster P367. Web address: http://aha.scientificposters.com/atvb/epsWelcome.cfm.
- 9.Silverstein RL, Febbraio M. CD36, a scavenger receptor involved in immunity, metabolism, angiogenesis, and behavior. Sci Signal. 2009;2:re3. doi: 10.1126/scisignal.272re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collot-Teixeira S, Martin J, rmott-Roe C, Poston R, McGregor JL. CD36 and macrophages in atherosclerosis. Cardiovasc Res. 2007;75:468–77. doi: 10.1016/j.cardiores.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 11.Febbraio M, Hajjar DP, Silverstein RL. CD36: a class B scavenger receptor involved in angiogenesis, atherosclerosis, inflammation, and lipid metabolism. J Clin Invest. 2001;108:785–91. doi: 10.1172/JCI14006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yla-Herttuala S, Rosenfeld ME, Parthasarathy S, Sigal E, Sarkioja T, Witztum JL, Steinberg D. Gene expression in macrophage-rich human atherosclerotic lesions. 15-lipoxygenase and acetyl low density lipoprotein receptor messenger RNA colocalize with oxidation specific lipid-protein adducts. J Clin Invest. 1991;87:1146–52. doi: 10.1172/JCI115111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nassar GM, Morrow JD, Roberts LJ, Lakkis FG, Badr KF. Induction of 15-lipoxygenase by interleukin-13 in human blood monocytes. J Biol Chem. 1994;269:27631–4. [PubMed] [Google Scholar]
- 14.Conrad DJ, Kuhn H, Mulkins M, Highland E, Sigal E. Specific inflammatory cytokines regulate the expression of human monocyte 15-lipoxygenase. Proc Natl Acad Sci U S A. 1992;89:217–21. doi: 10.1073/pnas.89.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roy B, Cathcart MK. Induction of 15-lipoxygenase expression by IL-13 requires tyrosine phosphorylation of Jak2 and Tyk2 in human monocytes. J Biol Chem. 1998;273:32023–9. doi: 10.1074/jbc.273.48.32023. [DOI] [PubMed] [Google Scholar]
- 16.Lu H, Smith CW, Perrard J, Bullard D, Tang L, Entman ML, Beaudet AL, Ballantyne CM. LFA-1 is sufficient in mediating neutrophil emigration in Mac-1 deficient mice. J Clin Invest. 1997;99:1340–50. doi: 10.1172/JCI119293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yakubenko VP, Lishko VK, Lam SCT, Ugarova TP. A molecular basis for integrin αMβ2 in ligand binding promiscuity. J Biol Chem. 2002;277:48635–42. doi: 10.1074/jbc.M208877200. [DOI] [PubMed] [Google Scholar]
- 18.Yakubenko VP, Solovjov DA, Zhang L, Yee VC, Plow EF, Ugarova TP. Identification of the binding site for fibrinogen recognition peptide γ383–395 within the αM I-domain of integrin αMβ2. J Biol Chem. 2001;275:13995–4003. doi: 10.1074/jbc.M010174200. [DOI] [PubMed] [Google Scholar]
- 19.Lishko VK, Yakubenko VP, Hertzberg KM, Grieninger G, Ugarova TP. The alternatively spliced αEC domain of human fibrinogen-420 is a novel ligand for leukocyte integrins αMβ2 and αXβ2. Blood. 2001;98:2448–55. doi: 10.1182/blood.v98.8.2448. [DOI] [PubMed] [Google Scholar]
- 20.Yakubenko VP, Yadav SP, Ugarova TP. Integrin alphaDbeta2, an adhesion receptor up-regulated on macrophage foam cells, exhibits multiligand-binding properties. Blood. 2006;107:1643–50. doi: 10.1182/blood-2005-06-2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takagi J, Springer TA. Integrin activation and structural rearrangement. Immunol Rev. 2002;186:141–63. doi: 10.1034/j.1600-065x.2002.18613.x. [DOI] [PubMed] [Google Scholar]
- 22.Weber C, Alon R, Moser B, Springer TA. Sequential regulation of α4β1 and α5β1 integrin avidity by CC chemokines in monocytes: implications for transendothelial chemotaxis. J Cell Biol. 1996;134:1063–73. doi: 10.1083/jcb.134.4.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nishida N, Xie C, Shimaoka M, Cheng Y, Walz T, Springer TA. Activation of leukocyte beta2 integrins by conversion from bent to extended conformations. Immunity. 2006;25:583–94. doi: 10.1016/j.immuni.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 24.Laudanna C, Kim JY, Constantin G, Butcher E. Rapid leukocyte integrin activation by chemokines. Immunol Rev. 2002;186:37–46. doi: 10.1034/j.1600-065x.2002.18604.x. [DOI] [PubMed] [Google Scholar]
- 25.Dransfield I, Hogg N. Regulated expression of Mg2+ binding epitope on leukocyte integrin α subunit. EMBO J. 1989;8:3759–65. doi: 10.1002/j.1460-2075.1989.tb08552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu C, Shimaoka M, Zang Q, Takagi J, Springer TA. Locking in alternate conformations of the integrin αLβ2 I domain with disulfide bonds reveals functional relationships among integrin domains. Proc Natl Acad Sci USA. 2001;98:2393–8. doi: 10.1073/pnas.041618598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu B, Bhattacharjee A, Roy B, Xu HM, Anthony D, Frank DA, Feldman GM, Cathcart MK. Interleukin-13 induction of 15-lipoxygenase gene expression requires p38 mitogen-activated protein kinase-mediated serine 727 phosphorylation of Stat1 and Stat3. Mol Cell Biol. 2003;23:3918–28. doi: 10.1128/MCB.23.11.3918-3928.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009;27:451–83. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- 29.Podrez EA, Febbraio M, Sheibani N, Schmitt D, Silverstein RL, Hajjar DP, Cohen PA, Frazier WA, Hoff HF, Hazen SL. Macrophage scavenger receptor CD36 is the major receptor for LDL modified by monocyte-generated reactive nitrogen species. J Clin Invest. 2000;105:1095–108. doi: 10.1172/JCI8574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun D, Funk CD. Disruption of 12/15-lipoxygenase expression in peritoneal macrophages. Enhanced utilization of the 5-lipoxygenase pathway and diminished oxidation of low density lipoprotein. J Biol Chem. 1996;271:24055–62. [PubMed] [Google Scholar]
- 31.Huber SA, Sakkinen P, David C, Newell MK, Tracy RP. T helper-cell phenotype regulates atherosclerosis in mice under conditions of mild hypercholesterolemia. Circulation. 2001;103:2610–6. doi: 10.1161/01.cir.103.21.2610. [DOI] [PubMed] [Google Scholar]
- 32.Davenport P, Tipping PG. The role of interleukin-4 and interleukin-12 in the progression of atherosclerosis in apolipoprotein E-deficient mice. Am J Pathol. 2003;163:1117–25. doi: 10.1016/S0002-9440(10)63471-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.King VL, Szilvassy SJ, Daugherty A. Interleukin-4 deficiency decreases atherosclerotic lesion formation in a site-specific manner in female LDL receptor−/− mice. Arterioscler Thromb Vasc Biol. 2002;22:456–61. doi: 10.1161/hq0302.104905. [DOI] [PubMed] [Google Scholar]
- 34.Zurawski SM, Chomarat P, Djossou O, Bidaud C, McKenzie AN, Miossec P, Banchereau J, Zurawski G. The primary binding subunit of the human interleukin-4 receptor is also a component of the interleukin-13 receptor. J Biol Chem. 1995;270:13869–78. doi: 10.1074/jbc.270.23.13869. [DOI] [PubMed] [Google Scholar]
- 35.Cyrus T, Witztum JL, Rader DJ, Tangirala R, Fazio S, Linton MF, Funk CD. Disruption of the 12/15-lipoxygenase gene diminishes atherosclerosis in apo E-deficient mice. J Clin Invest. 1999;103:1597–604. doi: 10.1172/JCI5897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.George J, Afek A, Shaish A, Levkovitz H, Bloom N, Cyrus T, Zhao L, Funk CD, Sigal E, Harats D. 12/15-Lipoxygenase gene disruption attenuates atherogenesis in LDL receptor-deficient mice. Circulation. 2001;104:1646–50. doi: 10.1161/hc3901.095772. [DOI] [PubMed] [Google Scholar]
- 37.Harats D, Shaish A, George J, Mulkins M, Kurihara H, Levkovitz H, Sigal E. Overexpression of 15-lipoxygenase in vascular endothelium accelerates early atherosclerosis in LDL receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2000;20:2100–5. doi: 10.1161/01.atv.20.9.2100. [DOI] [PubMed] [Google Scholar]
- 38.Shen J, Herderick E, Cornhill JF, Zsigmond E, Kim HS, Kuhn H, Guevara NV, Chan L. Macrophage-mediated 15-lipoxygenase expression protects against atherosclerosis development. J Clin Invest. 1996;98:2201–8. doi: 10.1172/JCI119029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wittwer J, Bayer M, Mosandl A, Muntwyler J, Hersberger M. The c.-292C>T promoter polymorphism increases reticulocyte-type 15-lipoxygenase-1 activity and could be atheroprotective. Clin Chem Lab Med. 2007;45:487–92. doi: 10.1515/CCLM.2007.103. [DOI] [PubMed] [Google Scholar]
- 40.Woollard KJ, Geissmann F. Monocytes in atherosclerosis: subsets and functions. Nat Rev Cardiol. 2010;7:77–86. doi: 10.1038/nrcardio.2009.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Randolph GJ, Sanchez-Schmitz G, Liebman RM, Schakel K. The CD16(+) (FcgammaRIII(+)) subset of human monocytes preferentially becomes migratory dendritic cells in a model tissue setting. J Exp Med. 2002;196:517–27. doi: 10.1084/jem.20011608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mosig S, Rennert K, Krause S, Kzhyshkowska J, Neunubel K, Heller R, Funke H. Different functions of monocyte subsets in familial hypercholesterolemia: potential function of CD14+ CD16+ monocytes in detoxification of oxidized LDL. FASEB J. 2009;23:866–74. doi: 10.1096/fj.08-118240. [DOI] [PubMed] [Google Scholar]
- 43.Bazil V, Stefanova I, Hilgert I, Kristofova H, Vanek S, Horejsi V. Monoclonal antibodies against human leucocyte antigens. IV. Antibodies against subunits of the LFA-1 (CD11a/CD18) leucocyte-adhesion glycoprotein. Folia Biol (Praha) 1990;36:41–50. [PubMed] [Google Scholar]
- 44.Lu C, Ferzly M, Takagi J, Springer TA. Epitope mapping of antibodies to the C-terminal region of the integrin beta 2 subunit reveals regions that become exposed upon receptor activation. J Immunol. 2001;166:5629–37. doi: 10.4049/jimmunol.166.9.5629. [DOI] [PubMed] [Google Scholar]
- 45.Shimizu Y, Van Seventer GA, Horgan KJ, Shaw S. Roles of adhesion molecules in T-cell recognition: Fundamental similarrities between four integrins on resting human T cells (LFA-1, VLA-4, VLA-5, VLA-6) in expression, binding, and costimulation. Immunol Rev. 1990;114:109–43. doi: 10.1111/j.1600-065x.1990.tb00563.x. [DOI] [PubMed] [Google Scholar]
- 46.Nageh MF, Sandberg ET, Marotti KR, Lin AH, Melchior EP, Bullard DC, Beaudet AL. Deficiency of inflammatory cell adhesion molecules protects against atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 1997;17:1517–20. doi: 10.1161/01.atv.17.8.1517. [DOI] [PubMed] [Google Scholar]
- 47.Kubo N, Boisvert WA, Ballantyne CM, Curtiss LK. Leukocyte CD11b expression is not essential for the development of atherosclerosis in mice. J Lipid Res. 2000;41(7):1060–6. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.