Summary
Heme oxygenase (HO)-1 is a cytoprotective enzyme that plays a critical role in defending the body against oxidant-induced injury during inflammatory processes. HO catalyzes the degradation of heme to carbon monoxide (CO), biliverdin, and ferrous iron. Biliverdin is converted to bilirubin, a potent endogenous antioxidant. CO has a number of biological functions, including anti-inflammatory properties. In various models of disease, HO-1 is known to play a critical role by ameliorating the pathological consequences of injury. In many of these models, the beneficial effects of HO-1 and its products of heme catabolism are by suppressing an inflammatory response. However, when investigating diseases due to microbial infections, inhibition of the inflammatory response could disrupt the ability of the immune system to eradicate an invading pathogen. Thus, questions remain regarding the role of HO-1 in microbial host defense. This microreview will address our present understanding of HO-1 and its functional significance in a variety of microbial infections.
Introduction
Infectious diseases constitute a high percentage of deaths in the United States and throughout the world each year (Mathers and Loncar, 2006; Mokdad et al., 2004). Microbial agents were the fourth leading cause of death in the United States in the year 2000, with influenza and pneumonia, and sepsis, contributing most to this mortality (Mokdad et al., 2004). In the year 2002, infectious etiologies including lower respiratory infections, HIV/AIDS, tuberculosis, and malaria ranked in the top fifteen causes of death worldwide (Mathers and Loncar, 2006). Other than HIV/AIDS, the projection is for mortality due to microbial agents to fall by the year 2030 (Mathers and Loncar, 2006), however, infections of various etiologies will still have a great impact on morbidity and mortality throughout the world.
Our laboratory has an ongoing interest in sepsis, and its pathophysiology. Sepsis is a complex process of cellular events induced by a severe underlying infection. In the past, sepsis has been defined as a clinical syndrome defined by both the presence of infection and a systemic inflammatory response (Levy et al., 2003). The invading microorganisms activate immune cells, resulting in the production of pro-inflammatory mediators that trigger cellular defense mechanisms to fight the infection (Pinsky, 2001; Bone et al., 1997). If this inflammatory response is too exaggerated, tissue injury will ensue and potentially result in death. However, if the immune response is suppressed or not adequate to eradicate the pathogen, the infectious process will progress leading to organ dysfunction followed by refractory hypotension, circulatory failure, and ultimately death. Thus, a critical balance exists between pro- and anti-inflammatory mediators to allow the resolution of microbial infections, and eventual resolution of the infectious process.
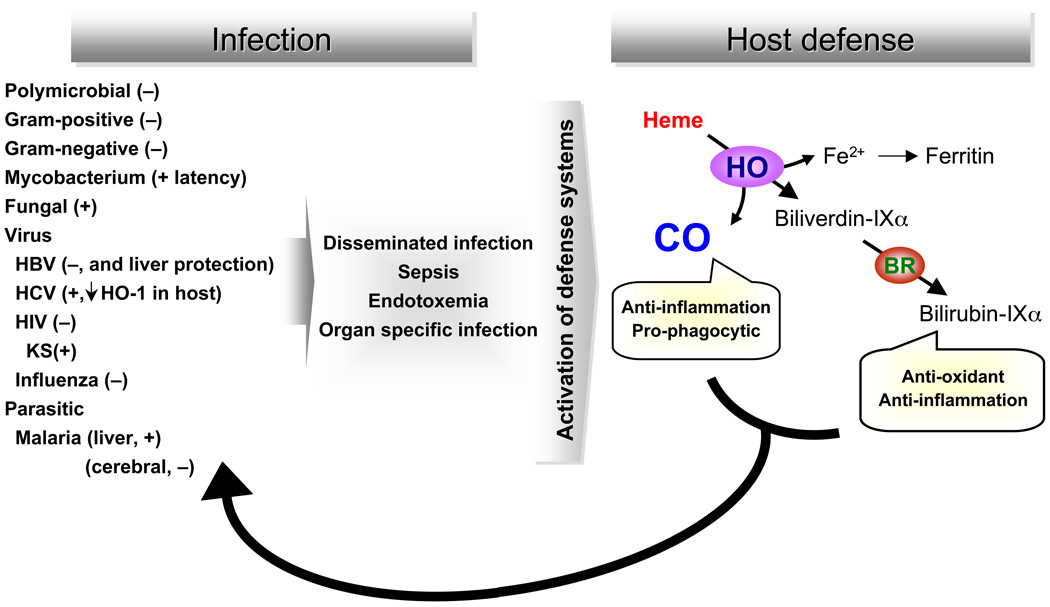
In an effort to further understand the balance between pro- and anti-inflammatory mediators during an infectious insult, we have been interested in the cytoprotective enzyme heme oxygenase (HO)-1. HO-1 is an enzyme that plays a critical role in defending the body against oxidant-induced injury during inflammatory processes (Wiesel et al., 2000; Yet et al., 1999; Poss and Tonegawa, 1997). HO catalyzes the first and rate-limiting step in the degradation of heme to carbon monoxide (CO), biliverdin, and ferrous iron (Ryter et al., 2006; Abraham and Kappas, 2005; Maines and Gibbs, 2005) (Figure 1, depicted in right panel). Biliverdin is converted to bilirubin, a potent endogenous antioxidant (Stocker et al., 1987), with recently recognized anti-inflammatory properties (Sarady-Andrews et al., 2005). CO has numerous biological functions, including anti-inflammatory properties (Ryter et al., 2007; Abraham and Kappas, 2005; Maines and Gibbs, 2005; Otterbein et al., 2000). In purely inflammatory models of disease, such as endotoxin exposure from gram-negative bacteria, HO-1 deficient mice are susceptible to oxidant-induced tissue injury and death (Wiesel et al., 2000; Yet et al., 1999; Poss and Tonegawa, 1997). In contrast, administration of CO or biliverdin/bilirubin to animals exposed to endotoxin decreases inflammation and attenuates end-organ injury (Ryter et al., 2006; Foresti et al., 2005; Mazzola et al., 2005; Morse et al., 2003; Otterbein et al., 2000). These studies support the beneficial effects of HO-1, and its products, during purely inflammatory processes. However, there is less certainty to the role of HO-1 during a microbial-based model of disease. For instance, there is a possibility that inhibition of the inflammatory response to infection, by HO-1 and it products, could disrupt the ability of the immune system to eliminate an invading pathogen. Thus, this review will focus on our present understanding of HO-1, and its products of heme metabolism, on the pathobiology of microbial agents. Figure 1 provides an overview of the microbes to be discussed, and the potential role of HO-1 in suppressing the microbial response, or promoting microbial survival, during disease.
Fig. 1. The HO-1 pathway and its effect on microbial infections.
Microbial infections that may involve the HO-1 pathway include those due to bacteria (polymicrobial, gram-positive, and gram negative); mycobacteria; fungi; viruses including hepatitis B virus (HBV), hepatitis C virus (HCV), HIV (and its complication Kaposi’s sarcoma – KS), and influenza; and the parasitic species leading to malaria (both the exoerythrocytic liver stage and the erythrocytic blood stage). These microbes may lead to either disseminated disease (which may result in sepsis) or organ specific infections. Microbial infections typically lead to an induction of HO-1, which degrades heme generating carbon monoxide (CO), biliverdin-IXα (subsequently reduced to biliverdin-IXα, and iron. These metabolites have a number of properties including the anti-inflammatory and pro-phagocytic effects of CO, and the anti-oxidant and anti-inflammatory properties of biliverdin/bilirubin. The properties of HO-1, and the products of heme catabolism, then have an ability to suppress (−) the microbial response or promoter (+) microbial survival.
Bacterial infections
Bacterial infections are a common etiology of sepsis in hospitalized patients (Angus et al., 2001). Sepsis affects approximately 750,000 patients per year, with an overall mortality of 29%, resulting in 215,000 deaths per year (Angus et al., 2001). Severe sepsis is the most common cause of death in intensive care units (Parrillo, 1993; Bone, 1991), and the medical expenses related to the treatment of patients with sepsis approach $16.7 billion annually (Angus et al., 2001). Infections of the abdomen, chest, genitourinary tract, and primary bloodstream account for more than 80% of sepsis cases, and the incidence of sepsis continues to rise. Until the late 1980s, sepsis was considered by many to be consistent with a Gram-negative infection and associated endotoxemia. However, in the early 1990s, it became more and more obvious that the concept of sepsis can arise from microbial infections in the absence of endotoxin. Interestingly, Gram-negative sepsis has diminished over the years to an incidence of 25–30% in 2000, while Gram-positive and polymicrobial infections account for 30–50% and 25% respectively (Annane et al., 2005).
Recently, our laboratory has demonstrated that during microbial sepsis, HO-1– derived CO plays an important role in the antimicrobial process without inhibiting the inflammatory response. Mice with a deficiency in HO-1 demonstrated an increased rate of death, compared with wild-type mice, when exposed to a model of abdominal sepsis (Chung et al., 2008) caused by cecal ligation and puncture (CLP) (Buras et al., 2005). These studies demonstrated the importance of endogenous HO-1 expression in protecting against the lethal effects of polymicrobial sepsis. Interestingly, Jao and colleagues assessed the expression of HO-1 message during the early stages of sepsis, and found that early induction of HO-1 was associated with more elevated levels of plasma lipid peroxidation, a marker of oxidative stress, and organ dysfunction (liver and renal) during the later stages of sepsis in rats (Jao et al., 2005). Thus, early HO-1 expression in leukocytes correlated negatively with oxidative stress and predicted organ dysfunction. These data suggest that HO-1 is an important cytoprotective molecule induced during sepsis, however endogenous upregulation of HO-1 is often not adequate to resolve the pathobiology of sepsis. To understand this concept further, additional work in microbial sepsis has been done in transgenic mice overexpressing HO-1 or in mice treated with the products of heme catabolism by HO-1.
Since the route of infectious dissemination in sepsis is the bloodstream, we decided to overexpress HO-1 in the vasculature of mice using the promoter of aortic carboxypeptidase-like protein (ACLP) to target expression. This promoter has been shown previously to be active in smooth muscle cells, not only in large- and medium-sized vessels, but also small vessels down to the level of the arteriole (Layne et al., 2002). We also determined that HO-1 is expressed in the myofibroblasts of the villi and crypts of the ileum and colon, respectively, in these transgenic mice (Chung et al., 2008). Overexpression of HO-1, directed by the ACLP promoter, ameliorated death due to polymicrobial sepsis in the CLP model. Investigation of individual organisms leading to sepsis showed that transgenic overexpression of HO-1 decreased sepsis-induced death associated with gram-positive Enterococcus faecalis (E. faecalis), but not gram-negative Escherichia coli (E. coli), infection (Chung et al., 2008). Interestingly, while HO-1 has anti-inflammatory effects in pure inflammatory models of disease (such as endotoxemia), in this microbial model of sepsis the increased expression of HO-1 did not suppress circulating inflammatory cells or their accumulation at the site of injury. However, HO-1 did enhance bacterial clearance, in part, by increasing phagocytic activity against E. faecalis (Chung et al., 2008). We confirmed these effects were via CO, as a CO-releasing molecule (CO-RM) was able to enhance phagocytosis in wild-type mice. Finally, we demonstrated that CO-RM was able to rescue HO-1 deficient mice from the lethality of CLP-induced sepsis, and CO-RM administered six hours after the onset of polymicrobial sepsis was able to improve survival in wild-type mice (Chung et al., 2008). These data advocate HO-1-derived CO as an important mediator of the host defense response to sepsis with therapeutic implications.
Otterbein and colleagues previously reported the effects of CO on the ability of a macrophage cell line, RAW 264.7 cells, to phagocytize E. coli in culture conditions. In these experiments, exposure to CO augmented E. coli phagocytosis but had no effect on inert particulate internalization into cells in vitro (Otterbein et al., 2005). The ability of CO to increase uptake of the bacteria was in part mediated by the redistribution and increased expression of Toll-like receptor 4 (TLR4) on the cell surface (Otterbein et al., 2005). In our experiments, when assessing the neutrophilic response in vivo, transgenic overexpression of HO-1 in mice increased the phagocytic response to E. faecalis, but not E. coli (Chung et al., 2008). In addition, the preferential phagocytosis of E. faecalis involved nucleotide-binding oligomerization domain (NOD) proteins, specifically NOD2, and not TLR4. The differences in these studies may involve the use of primary neutrophils versus a macrophage cell line, in vivo versus in vitro, respectively. Nevertheless, both studies suggest HO-1–derived CO plays a beneficial role in the enhanced phagocytosis of bacteria.
Overhaus and colleagues also investigated the effects of biliverdin against sepsis-induced inflammation and intestinal dysmotility (Overhaus et al., 2006). CLP-induced sepsis was performed in rats, with and without the administration of biliverdin. Exogenous administration of biliverdin before CLP resulted in decreased neutrophil infiltration into the bowel (jejunum), and ameliorated sepsis-induced delay in the upper gastrointestinal transit time (Overhaus et al., 2006). Biliverdin also significantly reduced expression of pro-inflammatory mediators (interleukin-6 and monocyte chemoattractant protein-1), and increased expression of an anti-inflammatory mediator (IL-10), in the small bowel. These findings suggest biliverdin is able to attenuate sepsis-induced inflammation and its subsequent sequelae on intestinal dysfunction during a model of abdominal sepsis. However, with this decrease in inflammatory response, it will be important to know the effects of biliverdin on CLP-induced bacteremia and lethality.
Mycobacterial infection
Another bacterial infectious process that has a great impact on global health is Mycobacterium tuberculosis (MTB) (Pieters, 2008). MTB infects approximately one third of the human population and results in death in an estimated two million people per year worldwide (Pieters, 2008)). While antimycobacterial therapy exists, MTB infection is difficult to treat due to impermeability of the organism’s cell wall, the ability of MTB to develop drug resistance, and a number of other survival strategies (Pieters, 2008). Another challenging issue, which is different from the organisms typically leading to bacterial sepsis, is that MTB organisms have the capacity to remain viable within an infected host for years as a latent infection. It is felt that MTB expresses a set of genes, known as the dormancy regulon, which allows the organism to persist during a latent infection (Shiloh et al., 2008). Mediators of the dormancy regulon include nitric oxide, generated within macrophages by the nitric oxide synthase 2 enzyme, and hypoxia.
Recently it has been determined that HO-1, and HO-1–derived CO, may also play a role in the dormancy regulon (Kumar et al., 2008; Shiloh et al., 2008). HO-1 is induced in macrophages infected with MTB both ex vivo and in vivo, and in fact both moderate and high concentrations of CO are well tolerated by MTB (Shiloh et al., 2008). Moreover, carbon monoxide is able to induce the MTB dormancy regulon. To confirm that this affect on the dormancy regulon was related to HO-1, gene deficient macrophages were infected with MTB. In the absence of HO-1, expression of the dormancy regulon was reduced (Shiloh et al., 2008). The results of this study led to the hypothesis that as MTB establishes its latency infection, the organism induces a host immune response that results in the induction of HO-1, NOS2, and the formation of a granuloma. Consequently, MTB is exposed to HO-1–derived CO, NOS2–derived NO, and lower oxygen levels in granulomas to activate the dormancy regulon and allow survival of the organisms. Thus, very different than the bacterial organisms associated with abdominal sepsis in which HO-1–derived CO enhances the phagocytic response and leads to resolution of the infection, HO-1–derived CO may in fact contribute to the survival of MTB during a latent infection.
Fungal infections
Fungal organisms, some of which are normal components of the gastrointestinal flora, have the potential to be pathogenic. For instance, Candida albicans, is a commensal organism in the gastrointestinal tract of healthy people, but in the setting of an impaired immune response this organism may become an invasive pathogen that colonizes host organs and may produce a bloodstream infection (candidemia) (Pendrak et al., 2004). The consequences of Candida species as an invasive pathogen leading to disseminated disease are very detrimental, with high morbidity and mortality (Wisplinghoff et al., 2004). It has been reported that the incidence of candidemia has increased significantly in recent years, and Candida species account for 9% of nosocomial bloodstream infections, making Candida the fourth most common cause of nosocomial bloodstream infections in the United States (Wisplinghoff et al., 2004).
Pendrak and colleagues have been investigating the adhesion of C. albicans to host cells, in an effort to understand the pathogenesis of disseminated disease (Pendrak et al., 2004). These investigators determined that exposure of C. albicans to hemoglobin rapidly increased its ability to express the fibronectin receptor, thus hemoglobin may play a role in regulating its adhesion to specific cells or tissues. These data led to the hypothesis that hemoglobin may be an important host factor for triggering disseminated infections (Pendrak et al., 2004). These investigators also determined that CaHMX1, encoding a putative HO, was a very inducible gene by hemoglobin in C. albicans. In addition, HO enzyme activity was confirmed in C. albicans because exposure to hemoglobin produced biliverdin in wild-type, but not homozygous null yeast. The authors hypothesized that HO in C. albicans was able to provide a nutritional advantage for growth in a mammalian host (Pendrak et al., 2004), and that hemoglobin may provide spatial cues to yeast at sites of vascularization and tissue injury. The authors also speculated that since biliverdin may protect against oxidative killing by host phagocytes, and CO has anti-inflammatory properties, that HO might provide other advantages for yeast as an opportunistic pathogen in a host. However, additional studies will need to be performed to confirm these hypotheses in vivo, and to understand the role of HO-1 in the host response to a fungal infection.
Viral infections
Hepatitis B
Hepatitis represents one of the most common causes of liver disease. The etiology of hepatitis remains quite varied but viral infection is the leading cause of most cases and is characterized by the infiltration of inflammatory cells and destruction of parenchymal cells, and persistence of the disease can lead to cirrhosis, liver failure and hepatocellular carcinoma (Rehermann and Nascimbeni, 2005). It is estimated that 2 billion people worldwide are infected with the virus and more than 350 million people are currently living with chronic liver disease, where the majority of cases are found in Africa and Asia (Shepard et al., 2006).
Currently, no effective treatments are available for acute hepatitis B virus (HBV) infection. However, for persistent chronic infection, several antiviral drugs are in use, and vaccination has proved effective in preventing infection (Shepard et al., 2006; Rehermann and Nascimbeni, 2005). Despite this, there is a growing need for the development of novel interventional and cytoprotective strategies to protect the liver from the destructive consequences of chronic hepatitis. HBV targets hepatocytes and culminates in the interference of host liver function. However, it is the secondary wave of recruitment and chronic activation of inflammatory cells in the liver that is thought to be responsible for the pathogenesis of liver disease in hepatitis B, as the host coordinates a humoral and cell-mediated immune response to rid itself of the virus (Rehermann and Nascimbeni, 2005). To date, a direct viral oncogenic action of HBV has not been demonstrated. Because of its main role in the resolution phase of inflammation, HO-1 may protect the liver from the deleterious consequences of the host immune response to HBV. In a mouse model of acute human HBV infection, Protzer and colleagues demonstrated that induction of HO-1 protects the liver from HBV-mediated liver damage (using adenoviral expression) due to a direct antiviral activity (Protzer et al., 2007). To confirm this, the authors employed the HBV transgenic mouse model, representing the chronic carrier state of the HBV infection devoid of inflammation or liver damage. This model offers the advantage to study the effects of HO-1 induction in the setting of established and ongoing HBV replication. Induction of HO-1 using cobalt protoporphyrin IX (CoPP) or adenoviral overexpression of HO-1 resulted in potent and direct antiviral activity on HBV replication, secondary to its well-known anti-inflammatory effects (Protzer et al., 2007). The direct antiviral activity of HO-1 was confirmed in stable HBV-transfected hepatoma cells. Mechanistically, ferritin, whose synthesis is increased by a transient increase in chelatable iron caused by HO-mediated heme degradation, was ruled out as the cytoprotective agent, as the concentration of CoPP used in this study did not induce ferritin production. A role for CO as well as biliverdin was speculated (Protzer et al., 2007); however, it remains to be determined whether these products of heme catabolism by HO-1 have direct antiviral activity providing protection against HBV infection and consequent liver damage.
Hepatitis C
An estimated 30% of the world’s population is infected with the hepatitis C virus (HCV). Chronic hepatitis C is the major cause of morbidity and mortality, as it is responsible for over 50% of all cases of liver cancer. In the US alone, some 4 million people are infected, where it is a major reason for liver transplantation (Shepard et al., 2005). Despite the widespread prevalence of this disease, there is a paucity of effective treatments and no approved vaccine. Because of this, there is an urgent need of novel therapies to treat persistent disease. The etiology behind the pathogenesis of HCV remains incompletely understood. However, HCV core proteins, as well as nonstructural proteins, are believed to lead to generation of reactive oxygen species (ROS) and oxidative stress and consequent severe inflammation leading to liver damage (Abdalla et al., 2005). Because of this, HO-1 may exert a protective benefit in infected individuals. Liver samples obtained from patients with HCV infection demonstrated downregulation of HO-1 expression restricted to hepatocytes at all stages of fibrosis, as reported by Abdalla and colleagues (Abdalla et al., 2004). These same authors reported an enhanced HO-1 expression in HBV-infected livers; data that supports the findings in HBV replicating mice with concomitant liver injury (Protzer et al., 2007). Interestingly, in the absence of inflammation, HBV may target HO-1 directly leading to downregulation (Protzer et al., 2007). In this respect, one may hypothesize that HCV core proteins also may regulate the expression of HO-1 directly, irrespective of a generalized inflammatory response. Moreover, the efficacy of known pharmacological inducers of HO-1 was reduced in hepatoma cell lines expressing HCV core proteins (Abdalla et al., 2004). These data suggest that part of the host’s innate immune response may be compromised following HCV infection due to a downregulation of HO-1 resulting in increased susceptibility to ROS-induced injury (Foy et al., 2003).
There is emerging evidence to suggest that microRNAs (miRNAs), small endogenous non-coding RNAs that mediate post-transcriptional regulation of gene expression, contribute functionally to several biological processes in health and disease (Ambros Nature 2004). miR-122 is abundantly expressed in the human liver (Esau et al., 2006) and facilitates the replication of HCV (Jopling et al., 2005). Efficient silencing of miR-122 has been demonstrated both in vitro (Elmén et al., 2008a; Shan et al., 2007) and in vivo in mouse and non-human primates (Elmén et al., 2008b). Moreover, delivery of antagomirs of miR-122 resulted in significant downregulation of HCV replication in replicon cells in vitro (Elmén et al., 2008a; Shan et al., 2007). miR-122 antagonism resulted in a concomitant upregulation in HO-1 levels (Shan et al., 2007). The mechanism whereby miR-122 leads to the suppression of HO-1 may be mediated via Bach1, a basic region leucine zipper (bZip) transcriptional repressor of HO-1 expression (Hou et al., 2008; Sun et al., 2004; Sun et al., 2002). The interplay between miR-122 and HO-1 may represent a novel means to induce protection against the damaging effects following HCV infection, both at the level of viral replication and the ensuing inflammatory response. These data are encouraging; however, further studies are needed to determine the longterm effects of silencing mirRNA on organ function.
HIV infection
Human immunodeficiency virus (HIV) is a RNA containing lentivirus, and the known cause of acquired immunodeficiency disorder referred to as AIDS. To date, HIV-1, the more virulent strain and the causative agent of the majority of the HIV infectious complications, shows tropism for host immune cells necessary for a coordinated immune response, ultimately leading to a loss of cell-mediated immunity. Devadas and Dhawan investigated the biological consequences of pharmacological induction of HO-1 using hemin as a putative host defense mechanism against HIV infection (Devadas and Dhawan, 2006). Hemin-induction of HO-1 in infected human monocytes and T cells resulted in substantially lower infectivity, as well as suppression of HIV-1 replication in previously inoculated cells. Moreover, in a humanized mouse model to study HIV pathogenesis, HO-1 induction suppressed infection at a clinically relevant dose (Devadas and Dhawan, 2006). Thus, HO-1 may represent a novel strategy to combat the detrimental consequences of HIV-1 infection on host cell-mediated immunity. In support of this, the protease inhibitor rotinavir, an anti-retroviral therapy targeting HIV, has been suggested to function in part by inducing HO-1 (Mühl et al., 2004).
In contrast to the putative anti-viral properties against HIV-1, induction of HO-1 may play a contributory role in Kaposi’s sarcoma (KS), a multifocal angioproliferative disorder following infection with human herpesvirus-8 (HH8, also known as Kaposi’s sarcoma-associated herpes virus, KSHV) (McAllister et al., 2004). KS represents one of the most common AIDS-associated malignancies. An upregulation of both HO-1 mRNA and protein were found in AIDS-KS tissue, as well as in KSHV-infected dermal microvascular endothelial cells (McAllister et al., 2004; Cornelissen et al., 2003). The fact that HO-1 is upregulated is not an unexpected occurrence, as KS is characterized by angiogenesis, a physiological process that critically depends on HO-1 (Dulak et al., 2008; Dulak et al., 2004). The oncogenic G protein-coupled receptor (vGPCR), one of the main KSHV genes involved in KS development, leads to the activation of HO-1 (Marinissen et al., 2006), which may be linked to the ability of HO-1 to control VEGF expression at the transcriptional level through HIF-1α. In addition, targeted knockdown of HO-1 via shRNA or chemical inhibition with tin protoporphyrin IX (SnPP) interferes with the transforming activity of vGPCR.
Influenza infection
Influenza is a respiratory infection caused by a member of the orthomyxovirus family. Currently, three types of RNA containing influenza viruses have been identified: A, B and C, where type A is the most virulent strain and responsible for the majority of morbidity and mortality following influenza outbreaks (Rambaut et al., 2008). According to the Centers for Disease Control, there are over 200,000 hospitalizations per year and some 36,000 deaths in the US alone following influenza (Thompson et al., 2004). Small children, the elderly and immunocompromised individuals or people with on-going health problems are at high-risk for complications following infection. The etiology of the pathogenesis following influenza most likely results from replication of the pathogen and dysregulated immune response, most notably active T-cells, ultimately leading to destruction of respiratory epithelium (Humphreys et al., 2003; Yap et al., 1978). Because of this, a coordinated immune response to dampen viral-induced lung injury and subsequent clearance of the virus from the lung represents an obligatory step required for survival. Besides vaccination or antiviral drugs, HO-1 has been shown to modulate immune function in response to viral infection and may serve to protect the lung in a similar setting, as discussed in the above sections (Willis et al., 1996). It has been demonstrated previously that infection with influenza virus leads to induction of HO-1 expression and activity, independently of inflammation (Choi et al., 1996). In a murine model of acute lung injury caused by type A influenza (H1N1), Hashiba and colleagues demonstrate that overexpression of HO-1, using the adenoviral system prior to influenza exposure, prevented the death of the animals via protection of respiratory epithelium from cascpase-8-mediated cell death (Hashiba et al., 2001). However, it will be important to determine whether overexpression of HO-1 after the onset of influenza infection could have a role in protecting the host from the ensuing pulmonary complications, and if this occurs via decreasing viral load. Moreover, the use of HO-1 as adjunct therapy to vaccination in high-risk patients also will need to be investigated.
There appears to be evidence, though preliminary, that HO-1 exhibits direct anti-viral activity, irrespective of its well-described anti-inflammatory effects.
Parasitic infections
Malaria is a vector-borne infectious disease found mostly in tropical and subtropical regions, including parts of the Americas, Asia, and sub-Saharan Africa. Malaria claims the lives of over one million persons per year, mainly affecting children under the age of five and pregnant women (Snow et al., 2005).
Of the 4 major protozoan parasite species, Plasmodium falciparum is responsible for the majority of the morbidity and mortality associated with malaria infection in humans (Miller et al., 2002). Malaria consists of two distinct stages: an exoerythrocytic (liver) and erythrocytic (blood) stage. In the exoerythrocytic stage, Plasmodium infects liver hepatocytes where they multiply and then escape into the circulation infecting red blood cells (RBCs), causing them to adhere to the lining of small blood vessels, which is a key feature to protect the parasite from being cleared in the spleen. It is during the second stage of RBC infection where parasites induce hemolysis leading to the significant accumulation of free heme, which is highly cytotoxic to the infected host and thought to be responsible for the symptoms of malaria. The coordination of a combined coagulation-inflammatory cascade plays a critical role in the pathogenesis of severe malaria due to the integrated response of both the innate and adaptive immune systems trying to rid itself of the infectious agent (van der Heyde et al., 2006). Because HO-1 plays a critical role in the control of the immune system, as well as vascular function (Dulak et al., 2008), its protective benefit is an emerging theme.
A major cause of death following P. falciparum infection is cerebral malaria. Schluesener and colleagues reported an upregulation of HO-1 in lesions of human cerebral malaria (Schluesener et al., 2001), and HO-1 expression was found in activated monocytes and microglia in close vicinity to sites of hemorrhage. However, it was difficult to determine whether HO-1 induction was a cause or consequence of cerebral injury. In studying the role of HO-1 in a mouse model of cerebral malaria, Pamplona and colleagues demonstrated that HO-1 and its breakdown product CO mitigate the blood brain barrier breakdown and consequent microvascular congestion, hemorrhage, and neuroinflammation seen in the erythrocytic or blood stage (Pamplona et al., 2007). More importantly, the suppression of the onset of cerebral malaria in response to HO-1 induction or exogenous administration of CO was accomplished despite a lack in the modulation in parasitic burden (Pamplona et al., 2007). The protective effect of HO-1 induction or inhaled CO was attributed to the binding to cell-free hemoglobin ultimately suppressing free heme accumulation in the circulation, which is deleterious to the infected host (Jeney et al., 2002). Interestingly, biliverdin/bilirubin were not shown to afford protection in this model, despite evidence to support their putative cytoprotective role in other disease states. In a follow-up study, Epiphanio and colleagues demonstrated that Plasmodium infection in the liver induced host HO-1 expression, and HO-1 served to protect the infected hepatocytes thereby promoting the exoerythrocytic or liver stage of infection (Epiphanio et al., 2008). Thus, in contrast to its beneficial role for the host during cerebral malaria, induction of host HO-1 serves as an obligatory step in the establishment of liver disease by controlling host innate inflammatory response and protecting infected hepatocytes (Epiphanio et al., 2008). Thus, there is a significant difference in the role of HO-1 depending on the stage of the disease.
Conclusion
HO-1 is a cytoprotective enzyme with anti-inflammatory and anti-oxidant properties, which plays a fundamental role in the control of systemic inflammation. The biological properties of HO-1 are controlled, in part, by its products of heme catabolism—CO and biliverdin/bilirubin. HO-1 and its products have been shown to be beneficial during purely inflammatory disease processes, however much less is known during infection with live organisms. As is shown in this microreview, HO-1 can have both beneficial and detrimental consequences toward the host, depending upon the infecting microbe. Moreover, while CO and biliverdin/bilirubin demonstrate overlapping favorable properties against inflammatory insults, they may not always function in a comparable manner during microbial infections. Thus, future therapeutic considerations regarding HO-1, or its products of heme catabolism, will need to consider the disease process, the infecting organism(s), the stage of disease, and the host response to the microbe.
Acknowledgements
We apologize to the authors’ whose articles could not be sited due to space limitations. This work was supported by grants HL60788, AI061246, and GM53249 from the National Institutes of Health to M.A.P. Also, grants from Ulsan University (2008-0014) and the Korea Research Foundation (KRF-2008-331-C00216) to S.W.C.
References
- Abdalla MY, I/M/ A, D/R/ S, W/N/ S, B/E B. Hepatitis C virus-core and non structural proteins lead to different effects on cellular antioxidant defenses. J. Med. Virol. 2005;76:489–497. doi: 10.1002/jmv.20388. [DOI] [PubMed] [Google Scholar]
- Abdalla MY, Britigan BE, Wen F, Icardi M, McCormick ML, LaBrecque DR, et al. Down-regulation of heme oxygenase-1 by hepatitis C virus infection in vivo and by the in vitro expression of hepatitis C core protein. J. Infect. Dis. 2004;190:1109–1118. doi: 10.1086/423488. [DOI] [PubMed] [Google Scholar]
- Abraham NG, Kappas A. Heme oxygenase and the cardiovascular-renal system. Free Radic. Biol. Med. 2005;39:1–25. doi: 10.1016/j.freeradbiomed.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- Annane D, Bellissant E, Cavaillon JM. Septic shock. Lancet. 2005;35:63–78. doi: 10.1016/S0140-6736(04)17667-8. [DOI] [PubMed] [Google Scholar]
- Bone RC. The pathogenesis of sepsis. Ann. Intern. Med. 1991;115:457–469. doi: 10.7326/0003-4819-115-6-457. [DOI] [PubMed] [Google Scholar]
- Bone RC, Grodzin CJ, Balk RA. Sepsis: A new hypothesis for pathogenesis of the disease process. Chest. 1997;112:235–243. doi: 10.1378/chest.112.1.235. [DOI] [PubMed] [Google Scholar]
- Buras JA, Holzmann B, Sitkovsky M. Animal models of sepsis: setting the stage. Nat. Rev. Drug Discov. 2005;4:854–865. doi: 10.1038/nrd1854. [DOI] [PubMed] [Google Scholar]
- Choi AM, Knobil K, Otterbein SL, Eastman DA, Jacoby DB. Oxidant stress responses in influenza virus pneumonia: gene expression and transcription factor activation. Am. J. Physiol. 1996;271:L383–L391. doi: 10.1152/ajplung.1996.271.3.L383. [DOI] [PubMed] [Google Scholar]
- Chung SW, Liu X, Macias AA, Baron RM, Perrella MA. Heme oxygenase-1-derived carbon monoxide enhances the host defense response to microbial sepsis in mice. J. Clin. Invest. 2008;118:239–247. doi: 10.1172/JCI32730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelissen M, van der Kuyl AC, van den Burg R, Zorgdrager F, van Noesel CJ, Goudsmit J. Gene expression profile of AIDS-related Kaposi's sarcoma. BMC Cancer. 2003;3:7. doi: 10.1186/1471-2407-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devadas K, Dhawan S. Hemin activation ameliorates HIB-1 infection via heme oxygenase-1 induction. J. Immunol. 2006;176:4252–4257. doi: 10.4049/jimmunol.176.7.4252. [DOI] [PubMed] [Google Scholar]
- Dulak J, Loboda A, Zagórska A, Józkowicz A. Complex role of heme oxygenase-1 in angiogenesis. Antioxid. Redox. Signal. 2004;6:858–866. doi: 10.1089/ars.2004.6.858. [DOI] [PubMed] [Google Scholar]
- Dulak J, Deshane JS, Jozkowicz A, Agarwal A. Heme oxygenase-1 and carbon monoxide in vascular pathobiology: focus on angiogenesis. Circulation. 2008;117:231–241. doi: 10.1161/CIRCULATIONAHA.107.698316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmén J, Lindow M, Silahtaroglu A, Bak M, Christensen M, Lind-Thomsen A, et al. Antagonism of microRNA-122 in mice by systemically administered LNA-antimiR leads to up-regulation of a large set of predicted target mRNAs in the liver. Nucleic Acids Res. 2008a;36:1153–1156. doi: 10.1093/nar/gkm1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmén J, Lindow M, Schütz S, Lawrence M, Petri A, Obad S, et al. LNA-mediated microRNA silencing in non-human primates. Nature. 2008b;452:896–899. doi: 10.1038/nature06783. [DOI] [PubMed] [Google Scholar]
- Epiphanio S, Mikolajczak SA, Gonçalves LA, Pamplona A, Portugal S, Albuquerque S, et al. Heme oxygenase-1 is an anti-inflammatory host factor that promotes murine plasmodium liver infection. Cell Host Microbe. 2008;3:331–338. doi: 10.1016/j.chom.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Esau C, Davis S, Murray SF, Yu XX, Pandey SK, Pear M, et al. miR-122 regulation of lipid metabolism revealed by in vivo antisense targeting. Cell Metab. 2006;3:87–98. doi: 10.1016/j.cmet.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Foresti R, Shurey C, Ansari T, Sibbons P, Mann BE, Johnson TR, et al. Reviewing the use of carbon monoxide-releasing molecules (CO-RMs) in biology: implications in endotoxin-mediated vascular dysfunction. Cell. Mol. Biol. 2005;51:409–423. [PubMed] [Google Scholar]
- Foy E, Li K, Wang C, Sumpter RJ, Ikeda M, Lemon SM, Gale MJ. Regulation of interferon regulatory factor-3 by the hepatitis C virus serine protease. Science. 2003;300:1145–1148. doi: 10.1126/science.1082604. [DOI] [PubMed] [Google Scholar]
- Hashiba T, Suzuki M, Nagashima Y, Suzuki S, Inoue S, Tsuburai T, et al. Adenovirus-mediated transfer of heme oxygenase-1 cDNA attenuates severe lung injury induced by the influenza virus in mice. Gene Ther. 2001;8:499–507. doi: 10.1038/sj.gt.3301540. [DOI] [PubMed] [Google Scholar]
- Hou W, Shan Y, Zheng J, Lambrecht RW, Donohue SE, Bonkovsky HL. Zinc mesoporphyrin induces rapid and marked degradation of the transcription factor Bach1 and up-regulates HO-1. Biochim. Biophys. Acta. 2008;1779:195–203. doi: 10.1016/j.bbagrm.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys IR, Walzl G, Edwards L, Rae A, Hill S, Hussell T. A critical role for OX40 in T cell-mediated immunopathology during lung viral infection. J. Exp. Med. 2003;198:1237–1242. doi: 10.1084/jem.20030351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jao HC, Lin YT, Tsai LY, Wang CC, Liu HW, Hsu C. Early expression of heme oxygenase-1 in leukocytes correlates negatively with oxidative stress and predicts hepatic and renal dysfunction at late stage of sepsis. Shock. 2005;23:464–469. doi: 10.1097/01.shk.0000158117.15446.5a. [DOI] [PubMed] [Google Scholar]
- Jeney V, Balla J, Yachie A, Varga Z, Vercellotti GM, Eaton JW, Balla G. Pro-oxidant and cytotoxic effects of circulating heme. Blood. 2002;100:879–887. doi: 10.1182/blood.v100.3.879. [DOI] [PubMed] [Google Scholar]
- Jopling CL, Yi M, Lancaster AM, Lemon SM, P S. Modulation of hepatitis C virus RNA abundance by a liver-specific MicroRNA. Science. 2005;309:1577–1581. doi: 10.1126/science.1113329. [DOI] [PubMed] [Google Scholar]
- Kumar A, Deshane JS, Crossman DK, Bolisetty S, Yan B-S, Kramnik I, et al. Heme oxygenase-1-derived carbon monoxide induces the Mycobacterium tuberculosis dormancy regulon. J. Biol. Chem. 2008;283:18032–18039. doi: 10.1074/jbc.M802274200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layne MD, Yet S-F, Maemura K, Hsieh C-M, Liu X, Ith B, et al. Characterization of the mouse aortic carboxypeptidase-like protein promoter reveals activity in differentiated and dedifferentiated vascular smooth muscle cells. Circ. Res. 2002;90:728–736. doi: 10.1161/01.res.0000013289.97650.c8. [DOI] [PubMed] [Google Scholar]
- Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit. Care Med. 2003;31:1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- Maines MD, Gibbs PEM. 30 years of heme oxygenase: From a "molecular wrecking ball" to a "mesmerizing" trigger of cellular events. Biochem. Biophys. Res. Commun. 2005;338:568–577. doi: 10.1016/j.bbrc.2005.08.121. [DOI] [PubMed] [Google Scholar]
- Marinissen MJ, Tanos T, Bolós M, de Sagarra MR, Coso OA, Cuadrado A. Inhibition of heme oxygenase-1 interferes with the transforming activity of the Kaposi sarcoma herpesvirus-encoded G protein-coupled receptor. J. Biol. Chem. 2006;281:11332–11346. doi: 10.1074/jbc.M512199200. [DOI] [PubMed] [Google Scholar]
- Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:2011–2029. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzola S, Forni M, Albertini M, Bacci ML, Zannoni A, Gentilini F, et al. Carbon monoxide pretreatment prevents respiratory derangement and ameliorates hyperacute endotoxic shock in pigs. FASEB J. 2005 doi: 10.1096/fj.05-3782fje. 10.1096/fj.05-3782fje. [DOI] [PubMed] [Google Scholar]
- McAllister SC, Früh K, Moses AV. Functional genomics and the development of pathogenesis-targeted therapies therapies for Kaposi's sarcoma. Blood. 2004;103:3465–3473. doi: 10.1517/14622416.6.3.235. [DOI] [PubMed] [Google Scholar]
- Miller LH, Baruch DI, Marsh K, Doumbo OK. The pathogenic basis of malaria. Nature. 2002;415:673–679. doi: 10.1038/415673a. [DOI] [PubMed] [Google Scholar]
- Mokdad AH, Marks JS, Strop DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA. 2004;291:1238–1245. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- Morse D, Pischke SE, Zhou Z, Davis RJ, Flavell RA, Loop T, et al. Suppression of inflammatory cytokine production by carbon monoxide involves the JNK pathway and AP-1. J. Biol. Chem. 2003;278:36993–36998. doi: 10.1074/jbc.M302942200. [DOI] [PubMed] [Google Scholar]
- Mühl H, Paulukat J, Höfler S, Hellmuth M, Franzen R, Pfeilschifter J. The HIV protease inhibitor ritonavir synergizes with butyrate for induction of apoptotic cell death and mediates expression of heme oxygenase-1 in DLD-1 colon carcinoma cells. Br. J. Pharmacol. 2004;143:890–898. doi: 10.1038/sj.bjp.0706023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otterbein LE, May A, Chin BY. Carbon monoxide increases macrophage bacterial clearance through Toll-like receptor (TLR)4 expression. Cell. Mol. Biol. 2005;51:433–440. [PubMed] [Google Scholar]
- Otterbein LE, Bach FH, Alam J, Soares M, Tao Lu H, Wysk M, et al. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat. Med. 2000;6:422–428. doi: 10.1038/74680. [DOI] [PubMed] [Google Scholar]
- Overhaus M, Moore BA, Barbato JE, Behrendt FF, Doering JG, Bauer AJ. Biliverdin protects against polymicrobial sepsis by modulating inflammatory mediators. Am. J. Physiol. (Gastrointest. Liver Physiol.) 2006;290:G295–G703. doi: 10.1152/ajpgi.00152.2005. [DOI] [PubMed] [Google Scholar]
- Pamplona A, Ferreira A, Balla J, Jeney V, Balla G, Epiphanio S, et al. Heme oxygenase-1 and carbon monoxide suppress the pathogenesis of experimental cerebral malaria. Nat. Med. 2007;13:703–710. doi: 10.1038/nm1586. [DOI] [PubMed] [Google Scholar]
- Parrillo JE. Pathogenetic mechanisms of septic shock. N. Engl. J. Med. 1993;328:1471–1477. doi: 10.1056/NEJM199305203282008. [DOI] [PubMed] [Google Scholar]
- Pendrak ML, Yan SS, Roberts DD. Sensing the host environment: recognition of hemoglobin by the pathogenic yeast Candida albicans. Arch. Biochem. Biophys. 2004;426:148–156. doi: 10.1016/j.abb.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Pieters J. Mycobacterium tuberculosis and the macrophage: Maintaining a balance. Cell Host Microbe. 2008;3:399–407. doi: 10.1016/j.chom.2008.05.006. [DOI] [PubMed] [Google Scholar]
- Pinsky MR. Sepsis: a pro- and anti-inflammatory disequilibrium syndrome. Contrib. Nephrol. 2001;132:354–366. doi: 10.1159/000060100. [DOI] [PubMed] [Google Scholar]
- Poss KD, Tonegawa S. Reduced stress defense in heme oxygenase 1-deficient cells. Proc. Natl. Acad. Sci. U.S.A. 1997;94:10925–10930. doi: 10.1073/pnas.94.20.10925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protzer U, Seyfried S, Quasdorff M, Sass G, Svorcova M, Webb D, et al. Antiviral activity and hepatoprotection by heme oxygenase-1 in hepatitis B virus infection. Gastroenterology. 2007;133:1156–1165. doi: 10.1053/j.gastro.2007.07.021. [DOI] [PubMed] [Google Scholar]
- Rambaut A, Pybus OG, Nelson MI, Viboud C, Taubenberger JK, Holmes EC. The genomic and epidemiological dynamics of human influenza A virus. Nature. 2008;453:615–619. doi: 10.1038/nature06945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehermann B, Nascimbeni M. Immunology of hepatitis B virus and hepatitis C virus infection. Nat. Rev. Immunol. 2005;5:215–229. doi: 10.1038/nri1573. [DOI] [PubMed] [Google Scholar]
- Ryter SW, Alam J, Choi AMK. Heme oxygenase-1/carbon monoxide: From basic science to therapeutic implications. Physiol. Rev. 2006;86:583–650. doi: 10.1152/physrev.00011.2005. [DOI] [PubMed] [Google Scholar]
- Ryter SW, Morse D, Choi AMK. Carbon monoxide and bilirubin: Potential therapies for pulmonary/vascular injury and disease. Am. J. Respir. Cell Mol. Biol. 2007;36:175–182. doi: 10.1165/rcmb.2006-0333TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarady-Andrews JK, Liu F, Gallo D, Nakao A, Overhaus M, Ollinger R, et al. Biliverdin administration protects against endotoxin-induced acute lung injury in rats. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005;289:L1131–L1137. doi: 10.1152/ajplung.00458.2004. [DOI] [PubMed] [Google Scholar]
- Schluesener HJ, Kremsner PG, Meyermann R. Heme oxygenase-1 in lesions of human cerebral malaria. Acta Neuropathol. 2001;101:65–68. doi: 10.1007/s004010000250. [DOI] [PubMed] [Google Scholar]
- Shan Y, Zheng J, Lambrecht RW, Bonkovsky HL. Reciprocal effects of micro-RNA-122 on expression of heme oxygenase-1 and hepatitis C virus genes in human hepatocytes. Gastroenterology. 2007;133:1166–1174. doi: 10.1053/j.gastro.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect. Dis. 2005;5:558–567. doi: 10.1016/S1473-3099(05)70216-4. [DOI] [PubMed] [Google Scholar]
- Shepard CW, Simard EP, Finelli L, Fiore AE, Bell BP. Hepatitis B virus infection: epidemiology and vaccination. Epidemiol Rev. 2006;2006:112–125. doi: 10.1093/epirev/mxj009. [DOI] [PubMed] [Google Scholar]
- Shiloh MU, Manzanillo P, Cox JS. Mycobacterium tuberculosis senses host-derived carbon monoxide during macrophage infection. Cell Host Microbe. 2008;15:323–330. doi: 10.1016/j.chom.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow RW, Guerra CA, Noor AM, Myint HY, Hay SI. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature. 2005;434:214–217. doi: 10.1038/nature03342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN. Bilirubin is an antioxidant of possible physiological importance. Science. 1987;235:1042–1046. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- Sun J, Brand M, Zenke Y, Tashiro S, Groudine M, Igarashi K. Heme regulates the dynamic exchange of Bach1 and NF-E2-related factors in the Maf transcription factor network. Proc. Natl. Acad. Sci. USA. 2004;101:1461–1466. doi: 10.1073/pnas.0308083100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Hoshino H, Takaku K, Nakajima O, Muto A, Suzuki H, et al. Hemoprotein Bach1 regulates enhancer availability of heme oxygenase-1 gene. EMBO J. 2002;21:5216–5224. doi: 10.1093/emboj/cdf516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson WW, Shay DK, Weintraub E, Brammer L, Bridges CB, Cox NJ, Fukuda K. Influenza-associated hospitalizations in the United States. JAMA. 2004;292:1333–1340. doi: 10.1001/jama.292.11.1333. [DOI] [PubMed] [Google Scholar]
- van der Heyde HC, Nolan J, Combes V, Gramaglia I, G/E G. A unified hypothesis for the genesis of cerebral malaria: sequestration, inflammation and hemostasis leading to microcirculatory dysfunction. Trends Parasitol. 2006;22:503–508. doi: 10.1016/j.pt.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Wiesel P, Patel AP, DiFonzo N, Marria PB, Sim CU, Pellacani A, et al. Endotoxin-induced mortality is related to increased oxidative stress and end-organ dysfunction, not refractory hypotension, in heme oxygenase-1 deficient mice. Circulation. 2000;102:3015–3022. doi: 10.1161/01.cir.102.24.3015. [DOI] [PubMed] [Google Scholar]
- Willis D, Moore AR, Frederick R, Willoughby DA. Heme oxygenase: a novel target for the modulation of the inflammatory response. Nat. Med. 1996;2:87–90. doi: 10.1038/nm0196-87. [DOI] [PubMed] [Google Scholar]
- Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin. Infect. Dis. 2004;39:309–317. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- Yap KL, Ada GL, McKenzie IF. Transfer of specific cytotoxic T lymphocytes protects mice inoculated with influenza virus. Nature. 1978;273:238–239. doi: 10.1038/273238a0. [DOI] [PubMed] [Google Scholar]
- Yet S-F, Perrella MA, Layne MD, Hsieh C-M, Maemura K, Kobzik L, et al. Hypoxia induces severe right ventricular dilatation and infarction in heme oxygenase-1 null mice. J. Clin. Invest. 1999;103:R23–R29. doi: 10.1172/JCI6163. [DOI] [PMC free article] [PubMed] [Google Scholar]