Abstract
Background
Recent cross-sectional studies have reported an association between retinal vessel caliber and chronic kidney disease (CKD), but the direction of the association between these two processes is not clear. In a prospective study with multiple measurements of retinal vessel diameters and serum creatinine, we examined if baseline retinal vessel diameters are associated with future risk of CKD, or vice versa.
Study design
Population-based cohort study
Setting and participants
3,199 Wisconsin adults aged 43-84 years who were followed prospectively for 15 years.
Predictor
Baseline retinal arteriolar and venular diameters for analysis 1, and baseline estimated glomerular filtration rate (eGFR) categories for analysis 2.
Outcomes and measurements
For analysis 1, incident CKD defined as eGFR <60 mL/min/1.73m2 accompanied by a 25% decrease in eGFR during follow up. For analysis 2, incident retinal arteriolar narrowing defined as a central retinal arteriolar equivalent measurement of <144.0μm and incident retinal venular dilation defined as a central retinal venular equivalent measurement of >243.8μm.
Results
Baseline retinal arteriolar and venular diameters were not found to be associated with the 15-year risk of incident CKD. After adjustment for age, sex, diabetes, hypertension and other confounders, the multivariable hazard ratio (HR) (95% confidence interval (CI) of incident CKD comparing the narrowest with the widest quartile was 1.15 (0.74-1.80) for retinal arteriolar and 1.05 (0.67-1.67) for retinal venular diameter. Similarly, there was no significant association between eGFR and 15-year risk of incident retinal arteriolar narrowing or retinal venular widening. Compared to eGFR >90 mL/min/1.73m2 (referent), the multivariable HR (95% CI) among those with eGFR <45 mL/min/1.73m2 was 1.66 (0.93-2.96) for incident retinal arteriolar narrowing and 0.60 (0.17-1.85) for retinal venular widening.
Limitations
Lack of data on albuminuria and loss to follow-up.
Conclusions
Retinal vessel diameters and CKD may run together through shared mechanisms but are not causally related.
Keywords: Retinal arteriolar diameter, retinal venular diameter, retinal vessel diameter, chronic kidney disease, eGFR, CKD, glomerular filtration rate
Chronic kidney disease (CKD) is a major public health problem worldwide associated with premature morbidity and mortality.1 Animal studies have shown that microvascular damage contributes to the development and progression of renal disease.2 Conversely, deterioration in renal function can also lead to end organ microvascular damage.2 Retinal microvasculature is accessible for non-invasive visualization, and reflects changes in the systemic microcirculation.3 Population-based studies have shown that retinal microvascular abnormalities such as retinal arteriolar narrowing and venular widening are associated with diabetes4;5 and hypertension,6;7 the two major risk factors of CKD. However, few previous studies have examined the independent association between retinal vessel diameters and CKD.
Two recent cross-sectional studies have documented an association between retinal arteriolar narrowing and CKD.8;9 Few prospective studies have examined the role of retinal vessel diameters in the development of renal dysfunction.10-12 In a prospective study of subjects with type 1 diabetes, retinal venular widening was found to be associated with incident CKD.11 However, subjects with younger onset type 1 diabetes may have a different baseline risk of kidney disease than the general population due to the presence of hyperglycemia, a risk factor for kidney disease, from a young age.13 Two other prospective studies conducted in general population samples reported mutually inconsistent findings.10;12 In the Atherosclerosis Risk in Communities Study (ARIC), lower arteriolar-to-venule ratio (AVR) a summary measure for arteriolar narrowing and venular widening, was associated with a longitudinal increase in serum creatinine.12 In contrast, in the Cardiovascular Health Study (CHS), lower AVR was not associated with longitudinal decrease in estimated glomerular filtration rate (GFR).10 However, despite the advantage of being longitudinal follow-up studies, these analyses were based on one single measurement and included subjects with both normal GFR as well as CKD subjects at baseline. Therefore beyond the information that retinal microvascular processes and reduced kidney function are mutually correlated, these studies may not be able to provide a clear answer as to whether retinal microvascular abnormalities leads to an increased risk of CKD, or whether the presence of CKD leads to a greater incidence of retinal microvascular abnormalities. In this context, we examined the temporal association between retinal vessel diameters and CKD in a large population-based study with 15 years of follow-up data, including up to four repeat retinal vessel and serum creatinine measurements on subjects.
METHODS
Study Population
The Beaver Dam Chronic Kidney Disease (BDCKD) Study is a longitudinal study designed as an ancillary study within the population-based Beaver Dam cohort to study the risk factors for CKD. The exposure, outcome, and covariate information for BDCKD were obtained by combining 1) newly measured biomarker data, including serum creatinine and cystatin C, measured from stored blood in two related prospective cohort studies that followed the same set of study subjects of the Beaver Dam cohort, the Beaver Dam Eye study,14;15 and the Epidemiology of Hearing Loss Study,16;17 and (2) questionnaire, physical examination, and laboratory measurement data already collected in these studies. The methods used to identify and describe the Beaver Dam population have been described elsewhere.14-17
In brief, a private census of the population of the town of Beaver Dam, Wisconsin, was performed from September 1987 to May 1988 to identify all residents who were 43-84 years of age. Of the 5,924 eligible individuals (98% Caucasians), 4,926 (83.1%) participated in the baseline examination of the eye study between March 1, 1988 and September 14, 1990.
Therefore, the BDCKD Study involved a baseline examination from 1988 to 1990 followed by a 5-year follow-up examination from 1993 to 1995, a 10-year follow-up examination from 1998-2000, and a 15-year follow-up examination from 2003-2005. The study was approved by the Human Subjects Committee of the University of Wisconsin School of Medicine and Public Health, Madison, WI.
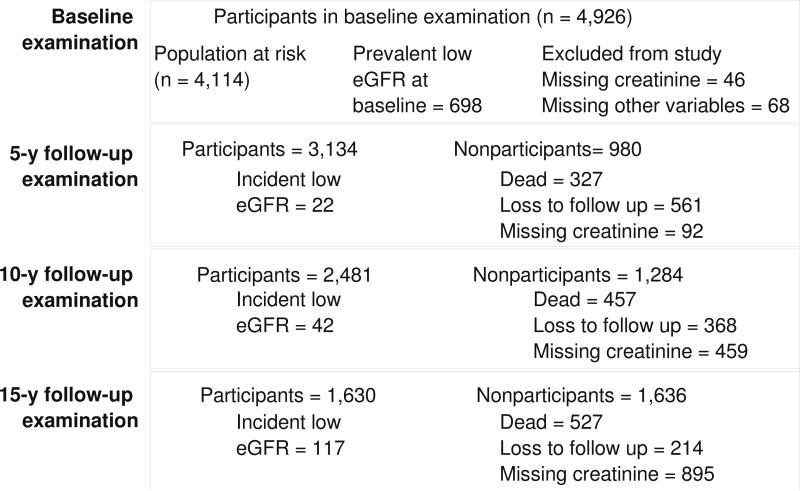
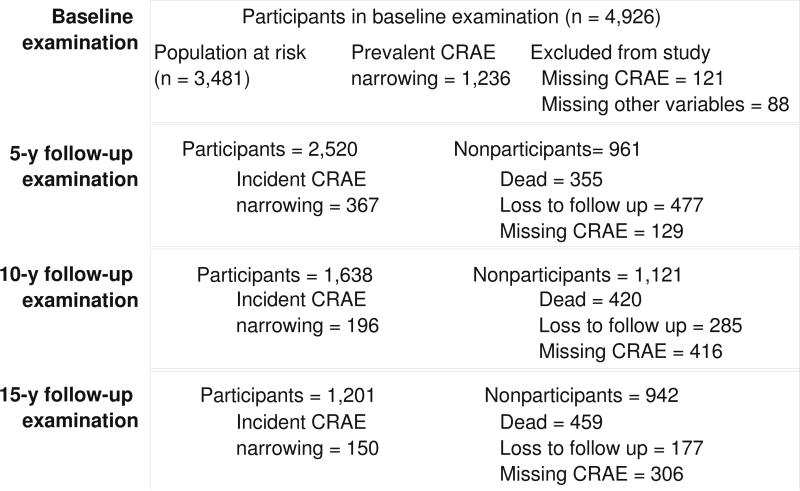
We performed two sets of analyses, first, examining the incidence of low eGFR, and second, examining incident retinal microvascular changes (arteriolar narrowing and venular widening). Figure 1 shows the participation at each follow-up for the incident low eGFR outcome. Out of the 4,926 baseline participants, 3,770 individuals participated in at least one follow-up examination. Out of the 3,770 individuals, we first excluded those with ungradable retinal photographs (n=43), and those with missing information on covariates used in the multivariable model (n=27). We further excluded those with eGFR below 60 mL/min per 1.73m2 at baseline (n=398), leaving 3,302 for the final analysis. Figure 2 shows the participation at each follow-up for the incident retinal arteriolar narrowing outcome. Out of the 4,926 baseline participants, 3,691 individuals participated in at least one follow-up examination. After excluding those with missing information on covariates (n=53) and those with prevalent retinal arteriolar narrowing (n=952), 2686 participants were available for the retinal arteriolar narrowing analysis; of the 3,691 participants, after excluding those with missing information on other relevant covariates (n=59), and those with prevalent retinal venular widening (n=909), 2723 participants were available for the retinal venular analysis.
Figure 1.
Participation at each follow-up examination for analysis exploring the incidence of low eGFR
Figure 2.
Participation at each follow-up examination for the incident retinal arteriolar (CRAE) narrowing analysis
Retinal vessel measurement
All participants had 30° color retinal photographs taken of both eyes. Details of retinal measurement from these photographs have been published before.18 After converting the field 1 photographs to digitized images, retinal measurements were carried out by four trained graders masked to participant characteristics using a computer-assisted software. All arterioles and venules coursing through a specified zone of 0.5-1 disc diameter surrounding the optic disc margin were measured and summarized as central retinal arteriolar equivalent (CRAE) or central retinal venular equivalent (CRVE) using a modification of the Parr-Hubbard formula19 as described by Knudtson et al.20 These equivalents are the projected diameters of the central retinal vessels, measured away from the optic disc. Reproducibility of these retinal measurements has been previously reported with intragrader and intergrader intraclass correlation coefficients ranging from 0.78-0.99.21 For the current analysis, average of right and left eye measurements were then taken as the CRAE and CRVE for that participant.
Kidney function measurement
Serum creatinine was measured from all study examinations by reflectance spectrophotometry on the Vitros analyzer (Johnson & Johnson Clinical Diagnostics, Inc., Rochester, NY) consistent with the current National Kidney Disease Education Program (NKEDP) recommendations.22 The laboratory coefficient of variability (CV) was 2.2%. The glomerular filtration rate was estimated from serum creatinine using the recently developed Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation as follows: 141 × min(Scr/k, 1)α× max(Scr/k, 1)- 1.209×0.993Age ×1.018 (for women), where Scr is serum creatinine, k is 0.7 for females and 0.9 for males, α is -0.329 for females and -0.411 for males, min indicates the minimum of Scr/k or 1, and max indicates the maximum of Scr/k or 1.23 The CKD-EPI equation is more accurate for estimating GFR in the general population than the Modification of Diet in Renal Disease (MDRD) Study equation and overcomes the limitation of MDRD study equation at higher GFR values.23 Serum cystatin C was determined from serum at the baseline and 5-year follow-up examinations nephelometically using the Dade Behring BN100 nephelometer (Deerfield, IL). The inter-assay precision was determined at two control levels: 1.72 mg/L (CV 6.4%) and 0.78 mg/L (CV 5.2%).
Outcomes of interest
We had two outcomes of interest: 1) 15-year incidence of low eGFR based on serum creatinine measurements, and 2) 15-year incidence of retinal microvascular changes (arteriolar narrowing or venular widening) based on retinal vessel diameter measurements.
Incident low eGFR was defined as eGFR <60 mL/min/1.73 m2 accompanied by a decrease in eGFR of at least 25% over the follow-up period as defined by Bash et al24 among subjects free of CKD at baseline. For sensitivity analyses, we also considered three alternate definitions of CKD 1) serum cystatin C ≥1.00 mg/dL,25 2) eGFR <45 mL/min/1.73 m2 corresponding to stage 3B CKD26 and 3) composite end point including incident low eGFR or death due to CKD.
Retinal arteriolar narrowing was defined as a CRAE of <144.0 μm, corresponding to the 25th percentile CRAE in the Beaver Dam study population; and retinal venular widening was defined as a CRVE of >243.8 μm, corresponding to the 75th CRVE percentile quartile. We defined incident retinal arteriolar narrowing as the new development of retinal arteriolar narrowing at the 5-, 10- or 15-year follow-up examination among subjects free of retinal arteriolar narrowing at baseline. Similarly we defined incident retinal venular widening as the new development of retinal venular widening at the 5-, 10- or 15-year follow-up examination among subjects free of retinal venular widening at baseline. For sensitivity analyses, we included only those with all 4 retinal vessel measurements (n=1382).
Measurement of other variables
Participants underwent a standardized interview and examination at each visit. Information on demographic characteristics, smoking, alcohol intake, self-reported history of physician-diagnosed diabetes, hypertension or cardiovascular disease (CVD), and medication use were obtained from the questionnaire. Non-fasting blood specimens were obtained for measurement of serum glucose, plasma glycated hemoglobin, serum C-reactive protein, total cholesterol and high-density lipoprotein (HDL) cholesterol.
Education was categorized as less than high school, high school, or beyond high school. Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters. Hypertension was defined as systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or self-reported diagnosed hypertension or use of antihypertensive medications. Diabetes mellitus was defined as self-reported diagnosed diabetes, use of glucose-lowering medication, or being newly diagnosed with diabetes based on the presence of a casual blood sugar value ≥200 mg/dL or a glycated hemoglobin value that was >2 standard deviations above the mean for a given age/gender group.27
Statistical analysis
We performed two sets of analyses, first, examining incident CKD and longitudinal change in eGFR as the outcome, and second, examining incident retinal microvascular changes (arteriolar narrowing and venular widening) and longitudinal change in retinal microvascular diameter as the outcome.
For the first analysis, examining incident low eGFR as the outcome, the exposures of interest, including retinal arteriolar and venular diameters, were analyzed as quartiles. We calculated selected baseline characteristics of the participants by quartiles of retinal arteriolar diameter with the statistical significance of differences across quartiles determined using the chi-square tests or analysis of variance. We calculated the hazard ratio and 95% confidence intervals of incident low eGFR associated with quartiles of baseline retinal arteriolar and venular diameters in proportional hazards models with discrete handling of ties. We used two nested models: an age and sex-adjusted model, and a multivariable-adjusted model, additionally adjusted for education status, smoking, alcohol intake, BMI, diabetes, glycated hemoglobin, hypertension, C-reactive protein, total cholesterol and HDL cholesterol. As previous studies have shown that retinal arteriolar narrowing8;9 and retinal venular widening11 are associated with CKD, we used the widest quartile of retinal arteriolar diameter (quartile 4) as the reference category in the retinal arteriolar narrowing analysis, and the narrowest quartile of retinal venular diameter (quartile 1) as the reference category in the retinal venular widening analysis.
We examined the association between baseline retinal arteriolar diameters and the annual change in eGFR over the 15 year follow-up period using linear regression using PROC MIXED in SAS to account for the correlation within subjects due to repeated measures.28 We restricted this analysis to only those who had all four creatinine measurements (baseline and the 3 follow ups, n=1492). The explanatory variables included age, sex, education status, smoking, alcohol intake, BMI, diabetes, glycated hemoglobin, hypertension, C-reactive protein, total cholesterol, and LDL cholesterol. Time was entered as a linear term. The unit for slope for eGFR as the dependent variable was ml/min/1.73m2 per year, and the unit for slopes for retinal arteriolar and venular diameters (i.e. CRAE and CRVE) was μm per year.
For the second analysis, examining incident retinal microvascular changes (arteriolar narrowing and venular widening) and longitudinal change in retinal microvascular diameter as the outcome, the exposure of interest, baseline eGFR, was analyzed as a categorical (<45, 45-59, 60-74, 75-89, ≥90 ml/min/1.73 m2) variable.29 We estimated the hazard ratio and 95% confidence intervals of incident retinal microvascular changes associated with eGFR categories in proportional hazards models. We also examined the association between baseline eGFR and the annual change in retinal vascular diameters over the 15 year follow-up period using linear regression. We restricted this analysis to only those who had all four retinal vessel measurements (baseline and the 3 follow ups, n= 1382).
To test the robustness of our findings, we conducted several sets of supplementary analyses. First, following suggestions by Liew et al.,30 we additionally adjusted for the fellow vessel diameter in the multivariable-adjusted model; second, we repeated the main multivariable model in a time-varying covariate analysis, incorporating updated information from each follow-up examination; third, we examined the association between retinal microvascular changes and the incidence of cystatin C ≥1 mg/dL among those with cystatin C levels <1 mg/dL at the baseline; fourth, we repeated the main multivariable model employing incident eGFR <45 mL/min/1.73 m2 and a concurrent decrease in eGFR of at least 25% over the follow-up period as an alternative outcome; fifth, we repeated the main multivariable model using a composite end point of incident low eGFR (n=181) or death due to CKD (n=8) as an alternative outcome; sixth, we examined the association between baseline eGFR and retinal arteriolar and venular diameters additionally adjusting for average BP across all three follow ups.
RESULTS
Table 1a shows the baseline characteristics of the study population by quartiles of retinal arteriolar diameter. Compared to those in the highest retinal arteriolar diameter quartile (quartile 4), those in the lowest quartile (quartile 1) were more likely to be older, heavy drinkers; had higher levels of systolic and diastolic blood pressure, higher body mass index and lower levels of eGFR; were less likely to be current smokers. Table 1b shows the baseline characteristics of the study population by categories of eGFR. Compared to those with eGFR>90 mL/min/1.73m2, those with eGFR<45 mL/min/1.73m2 were more likely to be older, female, less than high school educated; had higher prevalence of previous history of cardiovascular disease, higher levels of systolic and diastolic blood pressure, higher body mass index and higher total cholesterol; were less likely to be current smokers.
Table 1A.
Baseline characteristics of the study population by categories of retinal arteriolar diameter
| Quartiles of retinal arteriolar diameter (μm) |
|||||
|---|---|---|---|---|---|
| Characteristics | First quartile (98.3-141.2) n=822 | Second quartile (141.3-149.8) n=828 | Third quartile (149.9-158.4) n=822 | Fourth quartile (158.5-199.7) n=830 | p value* |
| Age, mean (SD), y | 60.0 (10.0) | 60.6 (10.1) | 58.7 (9.8) | 57.6 (10.0) | <0.001 |
| Female, % | 55.7 | 51.2 | 54.6 | 57.4 | 0.08 |
| Less than high school education, % | 20.4 | 25.4 | 22.8 | 24.7 | 0.08 |
| Current smoking, % | 11.9 | 15.8 | 21.5 | 31.7 | <0.001 |
| Heavy drinker, % | 11.6 | 8.3 | 6.5 | 5.6 | <0.001 |
| Diabetes mellitus, % | 8.0 | 8.5 | 9.0 | 8.6 | 0.9 |
| Hypertension, % | 56.8 | 51.7 | 44.4 | 29.7 | <0.001 |
| Glycosylated hemoglobin, % | 5.9 (1.3) | 6.1 (1.5) | 6.0 (1.6) | 6.0 (1.8) | 0.5 |
| Systolic blood pressure, mean (SD), mm Hg | 136.5 (19.9) | 132.6 (18.7) | 128.2 (18.0) | 123.6 (17.7) | <0.001 |
| Diastolic blood pressure, mean (SD), mm Hg | 81.1 (10.3) | 79.2 (10.3) | 77.1 (10.1) | 75.1 (9.6) | <0.001 |
| Body mass index, mean (SD), kg/m2 | 29.1 (5.3) | 29.0 (5.5) | 28.7 (5.4) | 28.1 (5.2) | 0.0008 |
| Total cholesterol, mean (SD), mg/dL | 233.9 (42.2) | 229.8 (38.9) | 231.7 (46.3) | 231.0 (40.6) | 0.3 |
| HDL cholesterol, mean (SD), mg/dL | 53.8 (17.6) | 52.6 (17.8) | 51.9 (16.9) | 52.3 (17.6) | 0.2 |
| Baseline eGFR, mean (SD), ml/min/1.73m | 84.4 (12.5) | 84.3 (12.5) | 85.5 (12.9) | 87.2 (13.0) | <0.001 |
Abbreviations: HDL, High density lipoprotein; eGFR, estimated glomerular filtration rate; SD, standard deviation
P value represents difference in characteristic by retinal arteriolar diameter quartiles, using analysis of variance or chi square test.
Table 1B.
Baseline characteristics of the study population by categories of estimated glomerular filtration rate
| Estimated glomerular filtration rate, ml/min/1.73m2 |
p value* |
|||||
|---|---|---|---|---|---|---|
| Characteristics | 15-44 (n=50) | 45-59 (n=194) | 60-74 (n=579) | 75-89 (n=876) | >90 (n=987) | |
| Age, mean (SD), y | 71.7 (8.9) | 67.5 (10.2) | 64.4 (9.8) | 60.1 (9.5) | 54.1 (7.7) | <0.001 |
| Female, % | 74.0 | 65.0 | 57.9 | 53.4 | 54.8 | 0.002 |
| Less than high school education, % | 56.0 | 35.1 | 31.4 | 23.9 | 18.4 | <0.001 |
| Current smoking, % | 10.0 | 11.9 | 16.6 | 20.2 | 30.1 | <0.0001 |
| Heavy drinker, % | 0 | 4.1 | 6.2 | 6.1 | 7.5 | 0.1 |
| Diabetes mellitus, % | 14.0 | 10.3 | 9.0 | 7.4 | 9.2 | 0.3 |
| Hypertension, % | 78.0 | 55.1 | 49.7 | 42.2 | 35.5 | <0.001 |
| Glycosylated hemoglobin, % | 6.4 (1.5) | 6.0 (1.5) | 6.0 (1.3) | 6.0 (1.5) | 6.1 (1.8) | 0.3 |
| Previous history of cardiovascular disease, % | 20.8 | 20.5 | 13.8 | 10.6 | 8.3 | <0.001 |
| Systolic blood pressure, mean (SD), mm Hg | 138.0 (19.0) | 131.4 (20.0) | 131.7 (19.5) | 127.6 (18.7) | 125.8 (17.5) | <0.001 |
| Diastolic blood pressure, mean (SD), mm Hg | 73.4 (11.9) | 74.2 (10.6) | 76.3 (10.0) | 77.0 (18.7) | 77.6 (9.8) | <0.001 |
| Body mass index, mean (SD), kg/m2 | 30.3 (5.5) | 29.3 (5.3) | 28.8 (5.2) | 28.8 (5.4) | 28.3 (5.5) | 0.02 |
| Total cholesterol, mean (SD), mg/dL | 259.1 (51.1) | 242.9 (46.9) | 235.9 (39.6) | 232.6 (41.1) | 226.9 (43.4) | <0.001 |
| HDL cholesterol, mean (SD), mg/dL | 47.0 (15.1) | 51.0 (18.2) | 52.0 (18.1) | 52.1 (17.2) | 52.4 (17.1) | 0.3 |
| Baseline retinal arteriolar diameter, (μm) | 157.3 (10.4) | 155.4 (10.3) | 154.4 (9.0) | 155.0 (9.7) | 156.5 (10.2) | 0.002 |
| Baseline retinal venular diameter, (μm) | 234.5 (19.5) | 232.5 (19.8) | 234.5 (19.8) | 234.6 (19.5) | 237.1 (18.8) | 0.005 |
Abbreviations: HDL, High density lipoprotein; SD, standard deviation
P value represents difference in characteristic by categories of estimated glomerular filtration rate, using analysis of variance or chi square test.
Baseline retinal vessel diameters and incident CKD
Of the 3,302 participants at risk, 181 developed the primary kidney disease outcome of low eGFR accompanied by a 25% decrease in eGFR over the 15-year follow-up period. In unadjusted models, baseline retinal arteriolar diameter (149.9 vs. 148.7 μm, p= 0.3) and retinal venular diameter (230.5 vs. 230.2 μm, p= 0.8) was similar in participants who did and did not develop low eGFR.
Table 2 shows the association between retinal arteriolar and venular diameters and 15-year risk of developing low eGFR using Cox proportional hazards models. Retinal arteriolar narrowing was not associated with incident low eGFR(p-trend=0.75). Similarly, retinal venular widening was also not associated with incident low eGFR (p-trend=0.99).
Table 2.
Association between retinal vessel diameters and the incidence of low eGFR over the 15 year follow-up period
| No. at risk | Incident low eGFR | Age, sex- adjusted hazard ratio (95% confidence interval) | Multivariable-adjusted hazard ratio (95% confidence interval)* | |
|---|---|---|---|---|
| Retinal arteriolar diameter (CRAE), μm | ||||
| Quartile 4 (158.5-199.7) | 830 | 50 | 1 (referent) | 1 (referent) |
| Quartile 3 (149.9-158.4) | 822 | 43 | 1.29 (0.84-1.99) | 1.24 (0.81-1.92) |
| Quartile 2 (141.3-149.8) | 828 | 50 | 1.05 (0.67-1.64) | 1.00 (0.64-1.58) |
| Quartile 1 (98.3-141.2) | 822 | 38 | 1.19 (0.78-1.83) | 1.15 (0.74-1.80) |
| p-trend | 3302 | 181 | 0.7 | 0.8 |
| Per unit decrease in CRAE | 1.00 (0.99-1.01) | 1.00 (0.99-1.01) | ||
| Retinal venular diameter (CRVE), μm | ||||
| Quartile 1 (157.9- 216.2) | 824 | 43 | 1 (referent) | 1 (referent) |
| Quartile 2 (216.3-230.0) | 825 | 53 | 1.36 (0.90-2.06) | 1.38 (0.91-2.09) |
| Quartile 3 (230.1-244.0) | 815 | 45 | 1.18 (0.77-1.82) | 1.20 (0.77-1.85) |
| Quartile 4 (244.1-339.7) | 838 | 40 | 1.04 (0.67-1.61) | 1.05 (0.67-1.67) |
| p-trend | 3302 | 181 | 0.9 | 0.9 |
| Per unit increase in CRVE | 1.00 (1.00-1.01) | 1.00 (0.99-1.01) |
Adjusted for age (years), sex (men, women), education categories (<high school, high school, >high school), smoking (never, former, current), alcohol intake (never, former, current), body mass index (kg/m2), diabetes (absent, present), glycated hemoglobin (%), hypertension (absent, present), C-reactive protein (mg/dL), serum total cholesterol (mg/dL), and high-density lipoprotein cholesterol (mg/dl)
Table 3 shows the association between retinal arteriolar diameter, venular diameter and annual change in eGFR over the 15 year follow-up period using multivariable linear regression models. Similar to the Cox regression models, no significant association was detected between baseline retinal vascular diameters and eGFR.
Table 3.
Relationship of retinal vessel diameters with annual change in estimated glomerular filtration rate (eGFR) over the 15 year follow-up period
| Mean annual change (95% CI) in eGFR, ml/min/1.73m2 | |||
|---|---|---|---|
| |
|||
| Sample size | Age, sex- adjusted model | Multivariable-adjusted Model* | |
| Retinal arteriolar diameter (CRAE), μm | |||
| Quartile 4 (158.5-199.7) | 364 | 0 (Referent) | 0 (Referent) |
| Quartile 3 (149.9-158.4) | 364 | -0.14 (-0.36 to 0.07) | -0.21 (-0.43 to 0.01) |
| Quartile 2 (141.3-149.8) | 399 | -0.0008 (-0.21 to 0.21) | -0.03 (-0.25 to 0.19) |
| Quartile 1 (98.3-141.2) | 365 | -0.1 (-0.17 to 0.17) | -0.12 (-0.32 to 0.09) |
| p-trend | 1492 | 0.4 | 0.1 |
| Per unit decrease in CRAE | 0.004 (-0.002 to 0.009) | 0.006 (-0.00002 to 0.01) | |
| Retinal venular diameter (CRVE), μm | |||
| Quartile 1 (157.9- 216.2) | 380 | 0 (Referent) | 0 (Referent) |
| Quartile 2 (216.3-230.0) | 381 | -0.12 (-0.33 to -0.09) | -0.10 (-0.31 to 0.11) |
| Quartile 3 (230.1-244.0) | 372 | -0.10 (-0.31 to 0.11) | -0.06 (-0.28 to 0.15) |
| Quartile 4 (244.1-339.7) | 359 | -0.02 (-0.23 to 0.20) | 0.02 (-0.21 to 0.25) |
| p-trend | 1492 | 0.9 | 0.8 |
| Per unit increase in CRVE | -0.001 (-0.004 to 0.003) | -0.00007 (-0.004 to 0.004) | |
Adjusted for age (years), sex (men, women), education categories (<high school, high school, >high school), smoking (never, former, current), alcohol intake (never, former, current), body mass index (kg/m2), diabetes (absent, present), glycated hemoglobin (%), hypertension (absent, present), C-reactive protein (mg/dL), serum total cholesterol (mg/dL), and high-density lipoprotein cholesterol (mg/dl)
Baseline eGFR categories and incident retinal vessel changes
Table 4 shows the association between categories of eGFR and incident retinal arteriolar narrowing and incident retinal venular widening using Cox proportional hazards models. eGFR was not associated with either retinal arteriolar narrowing or retinal venular widening.
Table 4.
Association between estimated glomerular filtration rate (eGFR) and incident retinal arteriolar narrowing (<144.0 μm) and incident retinal venular widening (>243.8μm)
| eGFR, ml/min/1.73m2 | No. at risk | Incident cases | Age, sex- adjusted hazard ratio (95% confidence interval) | Multivariable-adjusted hazard ratio (95% confidence interval)* |
|---|---|---|---|---|
| Incident retinal arteriolar (CRAE) narrowing |
||||
| >90 | 987 | 250 | 1 (referent) | 1 (referent) |
| 75-89 | 876 | 270 | 1.21 (1.00-1.47) | 1.21 (1.00-1.47) |
| 60-74 | 579 | 130 | 0.85 (0.67-1.08) | 0.83 (0.65-1.07) |
| 45-59 | 194 | 46 | 0.94 (0.66-1.34) | 0.91 (0.63-1.30) |
| 15-44 | 50 | 17 | 1.45 (0.83-2.57) | 1.66 (0.93-2.96) |
| p-trend | 2686 | 713 | 0.6 | 0.6 |
| Per unit decrease in baseline eGFR | 1.00 (1.00-1.01) | 1.00 (1.00-1.01) | ||
|
Incident retinal venular (CRVE) widening |
||||
| >90 | 910 | 110 | 1 (referent) | 1 (referent) |
| 75-89 | 930 | 88 | 0.76 (0.57-1.03) | 0.84 (0.62-1.14) |
| 60-74 | 589 | 69 | 1.02 (0.73-1.43) | 1.11 (0.79-1.57) |
| 45-59 | 232 | 20 | 0.81 (0.48-1.35) | 0.92 (0.54-1.55) |
| 15-44 | 62 | 3 | 0.50 (0.15-1.63) | 0.60 (0.17-1.85) |
| p-trend | 2723 | 290 | 0.4 | 0.8 |
| Per unit decrease in baseline eGFR | 1.00 (1.00-1.01) | 1.00 (1.00-1.01) | ||
Adjusted for age (years), sex (men, women), education categories (<high school, high school, >high school), smoking (never, former, current), alcohol intake (never, former, current), body mass index (kg/m2), diabetes (absent, present), glycated hemoglobin (%), hypertension (absent, present), C-reactive protein (mg/dL), serum total cholesterol (mg/dL), high-density lipoprotein cholesterol (mg/dl), and history of cardiovascular disease
Table 5 shows the association between lower eGFR categories and annual change in retinal arteriolar and venular diameters over the 15 year follow-up period using multivariable linear regression models among those with all four retinal vessel measurements. Similar to the Cox regression models, neither retinal arteriolar narrowing nor retinal venular widening shows a significant association with eGFR.
Table 5.
Relationship of estimated glomerular filtration rate (eGFR) with annual change in retinal vessel diameters over the 15 year follow-up period
| Mean annual change in retinal vessel diameters, μm |
|||
|---|---|---|---|
| Sample size | Age, sex- adjusted model | Multivariable-adjusted Model* | |
| eGFR, ml/min/1.73m2 | Mean change in retinal arteriolar diameter (CRAE) (95% CI), μm |
||
| >90 | 549 | 0 (Referent) | 0 (Referent) |
| 75-89 | 489 | -0.09 (-0.22 to 0.04) | -0.09 (-0.22 to 0.04) |
| 60-74 | 256 | 0.05 (-0.11 to 0.21) | 0.03 (-0.13 to 0.20) |
| 45-59 | 74 | 0.41 (0.15 to 0.68) | 0.35 (0.09 to 0.62) |
| 15-44 | 14 | -0.28 (-0.86 to 0.29) | -0.42 (-1.00 to 0.16) |
| p-trend | 1382 | 0.1 | 0.9 |
| Per unit decrease in eGFR | -0.004 (-0.08 to 0.0002) | -0.003 (-0.007 to 0.001) | |
|
Mean change in retinal venular diameter (CRVE) (95% CI), μm |
|||
| >90 | 549 | 0 (Referent) | 0 (Referent) |
| 75-89 | 489 | -0.11 (-0.33 to 0.11) | -0.08 (-0.30 to 0.15) |
| 60-74 | 256 | 0.29 (0.02 to 0.57) | 0.34 (0.06 to 0.62) |
| 45-59 | 74 | -0.02 (-0.46 to 0.42) | 0.06 (-0.38 to 0.51) |
| 15-44 | 14 | -0.86 (-1.81 to -0.08) | -0.92 (-1.88 to 0.04) |
| p-trend | 1382 | 0.6 | 0.4 |
| Per unit decrease in eGFR | -0.001 (-0.008 to 0.005) | -0.003 (-0.01 to 0.004) | |
Adjusted for age (years), sex (men, women), education categories (<high school, high school, >high school), smoking (never, former, current), alcohol intake (never, former, current), body mass index (kg/m2), diabetes (absent, present), glycated hemoglobin (%), hypertension (absent, present), C-reactive protein (mg/dL), serum total cholesterol (mg/dL), high-density lipoprotein cholesterol (mg/dl), and history of cardiovascular disease
Supplementary analyses
We performed several supplementary analyses to test the robustness of our findings. First, additional adjustment for fellow vessel diameter in the multivariable model in Table 2 did not materially change the results (data not shown). Second, when employing time-dependent covariates, results were found to be essentially similar to our main findings. For example, for retinal arteriolar diameter, compared to the highest quartile (referent), the HR (95% CI) of low eGFR was 1.17 (0.84-1.62) in quartile 3, 1.04 (0.75-1.44) in quartile 2, and 1.01 (0.72-1.40) in quartile 1.
Third, when we repeated the analyses using an alternate definition of CKD based on cystatin C, the results were unchanged. For retinal arteriolar diameter, compared to quartile 4 (referent), the HR (95% CI) of CKD was 0.87 (0.58-1.31) in quartile 3, 0.79 (0.53-1.20) in quartile 2, and 0.97 (0.64-1.47) in quartile 1. For retinal venular diameter, compared to quartile 1 (referent), the HR (95% CI) was 1.28 (0.86-1.89) in quartile 2, 1.12 (0.74-1.68) in quartile 3, and 1.12 (0.74-1.70) in quartile 1.Fourth, we repeated the multivariable models using eGFR<45 mL/min/1.73m2 (n=143) as the outcome. For retinal arteriolar diameter, compared to quartile 4 (referent), the HR (95% CI) of CKD was 1.75 (0.87-3.50) in quartile 3, 1.42 (0.69-2.93) in quartile 2, and 1.27 (0.60-2.69) in quartile 1. Similarly for retinal venular diameter, compared to quartile 1 (referent), the HR (95% CI) of CKD was 0.83 (0.43-1.61) in quartile 2, 1.00 (0.53-1.88) in quartile 3, and 0.59 (0.29-1.21) in quartile 4. Fifth, using a composite end point of incident low eGFR or death due to CKD as the outcome (n=189), the results were essentially similar to the main multivariable analysis in Table 2. Sixth, when we repeated the multivariable models (retinal vascular diameters as the outcome) additionally adjusting for average BP across all three follow ups, the results were essentially similar to the analysis in Table 4. There were no significant interactions in the association between retinal arteriolar or venular diameters with the incidence of low eGFR by age or sex (all p>0.1).
DISCUSSION
In a population-based prospective study of adults, we found no evidence supporting an association between retinal arteriolar or venular diameters and the 15-year risk of developing a low eGFR. Similarly, in a parallel analysis, examining the association between baseline eGFR and incident retinal vascular changes, eGFR was associated with neither incident retinal arteriolar narrowing nor retinal venular widening. Adjustment for potential confounders including age, sex, education, smoking, alcohol intake, body mass index, diabetes, glycated hemoglobin, hypertension, C-reactive protein, serum total cholesterol, HDL cholesterol and previous history of cardiovascular disease did not alter these relationships.
Retinal microvascular signs, including arteriolar narrowing4-6 and venular widening31-33 have been shown to be associated with the development of CKD risk factors including diabetes,5 and hypertension.34;35 Previous cross-sectional studies have documented an association between retinal arteriolar narrowing and CKD.8;9;32 However, the results from prospective studies have been mixed. Among subjects with diabetes, retinal venular widening was shown to be associated with the risk of developing renal insufficiency and nephropathy.11;36 Among middle-aged subjects from the general population studied in the ARIC Study, lower AVR was not associated with incident renal dysfunction, including a hospitalization discharge or death coded for renal disease, but was associated with a longitudinal increase in serum creatinine.12 In contrast, among elderly individuals from the general population studied in the CHS, lower AVR was not associated with a longitudinal decrease in eGFR.10 However, previous prospective studies included subjects with both normal GFR as well as CKD subjects at baseline and defined change in creatinine or GFR as the outcome. Therefore these studies may not be able to clarify the direction of association between kidney disease and retinal microvascular disease.
In this context, our population-based study examining the putative association between retinal vessel diameters and kidney function is unique in that we had over 15 years of follow-up data, including up to four retinal vessel and serum creatinine measurements on the same set of subjects, enabling us to examine the bidirectional association between retinal vessel diameters and kidney function. The results of our study when considered together with previous literature may inform medicine in the following way: We found that retinal vessel changes do not precede the incidence of kidney disease and vice versa. However, previous cross-sectional and longitudinal studies showed that retinal vessel diameter changes and higher creatinine/low eGFR are mutually associated, without clarifying the direction of the association. Therefore it is possible that retinal vessel diameters and eGFR may run together through shared mechanisms but are not causally related. However, this being the first study examining incident kidney disease in relation to retinal vessel diameters, there is a need to confirm or disprove our findings in future longitudinal studies in other populations.
The major strengths of the study include its large sample size, prospective design, long duration of follow-up, precise measurements of retinal vessel diameters and eGFR, and information on confounders. Among its potential limitations, first, loss to follow-up might have resulted in selective mortality of those with retinal arteriolar narrowing, retinal venular widening and eGFR<60 mL/min/1.73 m2. This might have masked the association between retinal vessel diameters and low eGFR. Second, we do not have information on albuminuria, a potential confounder in the association between retinal vessel diameters and eGFR. Further, inclusion of a homogeneous white population may have affected generalizability of the study findings to other ethnic populations.
In conclusion, the results of this population-based prospective study do not support an association between retinal vessel diameters and eGFR decline in either direction.
ACKNOWLEDGEMENTS
Conflict of interest: There are no conflicts of interest related to this manuscript.
Ethical Approval: This study followed the recommendations of Declaration of Helsinki and was approved by the Human Subjects Committee of the University of Wisconsin School of Medicine and Public Health, Madison, WI. Written, informed consent was obtained from all participants.
Details of funding: Supported by National Institutes of Health grant EYO6594 (RK, BEK), NIA grant AG11099 (KJC), NIDDK grant DK73217 (RK, AS), and American Heart Association National Clinical Research Program (AS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–266. [PubMed] [Google Scholar]
- 2.Kang DH, Kanellis J, Hugo C, et al. Role of the microvascular endothelium in progressive renal disease. J Am Soc Nephrol. 2002;13:806–816. doi: 10.1681/ASN.V133806. [DOI] [PubMed] [Google Scholar]
- 3.Liew G, Wang JJ, Mitchell P, et al. Retinal Vascular Imaging: A New Tool in Microvascular Disease Research. Circulation: Cardiovascular Imaging. 2008;1:156–161. doi: 10.1161/CIRCIMAGING.108.784876. [DOI] [PubMed] [Google Scholar]
- 4.Wong TY, Klein R, Sharrett AR, et al. Retinal arteriolar narrowing and risk of diabetes mellitus in middle-aged persons. JAMA. 2002;287:2528–2533. doi: 10.1001/jama.287.19.2528. [DOI] [PubMed] [Google Scholar]
- 5.Wong TY, Shankar A, Klein R, et al. Retinal arteriolar narrowing, hypertension, and subsequent risk of diabetes mellitus. Arch Intern Med. 2005;165:1060–1065. doi: 10.1001/archinte.165.9.1060. [DOI] [PubMed] [Google Scholar]
- 6.Sharrett AR, Hubbard LD, Cooper LS, et al. Retinal arteriolar diameters and elevated blood pressure: the Atherosclerosis Risk in Communities Study. Am J Epidemiol. 1999;150:263–270. doi: 10.1093/oxfordjournals.aje.a009997. [DOI] [PubMed] [Google Scholar]
- 7.Ikram MK, Witteman JC, Vingerling JR, et al. Retinal vessel diameters and risk of hypertension: the Rotterdam Study. Hypertension. 2006;47:189–194. doi: 10.1161/01.HYP.0000199104.61945.33. [DOI] [PubMed] [Google Scholar]
- 8.Sabanayagam C, Shankar A, Koh D, et al. Retinal Microvascular Caliber and Chronic Kidney Disease in an Asian Population. Am J Epidemiol. 2008 doi: 10.1093/aje/kwn367. [DOI] [PubMed] [Google Scholar]
- 9.Sabanayagam C, Tai ES, Shankar A, et al. Retinal arteriolar narrowing increases the likelihood of chronic kidney disease in hypertension. J Hypertens. 2009 doi: 10.1097/HJH.0b013e328330141d. [DOI] [PubMed] [Google Scholar]
- 10.Edwards MS, Wilson DB, Craven TE, et al. Associations between retinal microvascular abnormalities and declining renal function in the elderly population: the Cardiovascular Health Study. Am J Kidney Dis. 2005;46:214–224. doi: 10.1053/j.ajkd.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 11.Wong TY, Shankar A, Klein R, et al. Retinal vessel diameters and the incidence of gross proteinuria and renal insufficiency in people with type 1 diabetes. Diabetes. 2004;53:179–184. doi: 10.2337/diabetes.53.1.179. [DOI] [PubMed] [Google Scholar]
- 12.Wong TY, Coresh J, Klein R, et al. Retinal microvascular abnormalities and renal dysfunction: the atherosclerosis risk in communities study. J Am Soc Nephrol. 2004;15:2469–2476. doi: 10.1097/01.ASN.0000136133.28194.E4. [DOI] [PubMed] [Google Scholar]
- 13.Shankar A, Klein R, Klein BE, et al. Association between glycosylated hemoglobin level and 16-year incidence of chronic kidney disease in type 1 diabetes. Exp Clin Endocrinol Diabetes. 2007;115:203–206. doi: 10.1055/s-2007-956170. [DOI] [PubMed] [Google Scholar]
- 14.Klein R, Klein BE, Linton KL, et al. The Beaver Dam Eye Study: visual acuity. Ophthalmology. 1991;98:1310–1315. doi: 10.1016/s0161-6420(91)32137-7. [DOI] [PubMed] [Google Scholar]
- 15.Linton KL, Klein BE, Klein R. The validity of self-reported and surrogate-reported cataract and age-related macular degeneration in the Beaver Dam Eye Study. Am J Epidemiol. 1991;134:1438–1446. doi: 10.1093/oxfordjournals.aje.a116049. [DOI] [PubMed] [Google Scholar]
- 16.Cruickshanks KJ, Wiley TL, Tweed TS, et al. Prevalence of hearing loss in older adults in Beaver Dam, Wisconsin. The Epidemiology of Hearing Loss Study. Am J Epidemiol. 1998;148:879–886. doi: 10.1093/oxfordjournals.aje.a009713. [DOI] [PubMed] [Google Scholar]
- 17.Cruickshanks KJ, Tweed TS, Wiley TL, et al. The 5-year incidence and progression of hearing loss: the epidemiology of hearing loss study. Arch Otolaryngol Head Neck Surg. 2003;129:1041–1046. doi: 10.1001/archotol.129.10.1041. [DOI] [PubMed] [Google Scholar]
- 18.Wong TY, Klein R, Klein BE, et al. Retinal vessel diameters and their associations with age and blood pressure. Invest Ophthalmol Vis Sci. 2003;44:4644–4650. doi: 10.1167/iovs.03-0079. [DOI] [PubMed] [Google Scholar]
- 19.Hubbard LD, Brothers RJ, King WN, et al. Methods for evaluation of retinal microvascular abnormalities associated with hypertension/sclerosis in the Atherosclerosis Risk in Communities Study. Ophthalmology. 1999;106:2269–2280. doi: 10.1016/s0161-6420(99)90525-0. [DOI] [PubMed] [Google Scholar]
- 20.Knudtson MD, Lee KE, Hubbard LD, et al. Revised formulas for summarizing retinal vessel diameters. Curr Eye Res. 2003;27:143–149. doi: 10.1076/ceyr.27.3.143.16049. [DOI] [PubMed] [Google Scholar]
- 21.Wong TY, Klein R, Nieto FJ, et al. Retinal microvascular abnormalities and 10-year cardiovascular mortality: a population-based case-control study. Ophthalmology. 2003;110:933–940. doi: 10.1016/S0161-6420(03)00084-8. [DOI] [PubMed] [Google Scholar]
- 22.Myers GL, Miller WG, Coresh J, et al. Recommendations for improving serum creatinine measurement: a report from the Laboratory Working Group of the National Kidney Disease Education Program. Clin Chem. 2006;52:5–18. doi: 10.1373/clinchem.2005.0525144. [DOI] [PubMed] [Google Scholar]
- 23.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bash LD, Coresh J, Kottgen A, et al. Defining incident chronic kidney disease in the research setting: The ARIC Study. Am J Epidemiol. 2009;170:414–424. doi: 10.1093/aje/kwp151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shlipak MG, Katz R, Sarnak MJ, et al. Cystatin C and prognosis for cardiovascular and kidney outcomes in elderly persons without chronic kidney disease. Ann Intern Med. 2006;145:237–246. doi: 10.7326/0003-4819-145-4-200608150-00003. [DOI] [PubMed] [Google Scholar]
- 26.Kirsztajn GM, Suassuna JH, Bastos MG. Dividing stage 3 of chronic kidney disease (CKD): 3A and 3B. Kidney Int. 2009;76:462–463. doi: 10.1038/ki.2009.178. [DOI] [PubMed] [Google Scholar]
- 27.Wong TY, Knudtson MD, Klein R, et al. A prospective cohort study of retinal arteriolar narrowing and mortality. Am J Epidemiol. 2004;159:819–825. doi: 10.1093/aje/kwh119. [DOI] [PubMed] [Google Scholar]
- 28.Littell RC, Milliken GA, Stroup WW, et al. The SAS system for mixed-models. SAS Institute; Cary (NC): 1996. [Google Scholar]
- 29.Chronic Kidney Disease Prognosis Consortium Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010 doi: 10.1016/S0140-6736(10)60674-5. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liew G, Sharrett AR, Kronmal R, et al. Measurement of retinal vascular caliber: issues and alternatives to using the arteriole to venule ratio. Invest Ophthalmol Vis Sci. 2007;48:52–57. doi: 10.1167/iovs.06-0672. [DOI] [PubMed] [Google Scholar]
- 31.Klein R, Klein BE, Moss SE, et al. Retinal vascular caliber in persons with type 2 diabetes: the Wisconsin Epidemiological Study of Diabetic Retinopathy: XX. Ophthalmology. 2006;113:1488–1498. doi: 10.1016/j.ophtha.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 32.Klein R, Klein BE, Moss SE, et al. Retinal vascular abnormalities in persons with type 1 diabetes: the Wisconsin Epidemiologic Study of Diabetic Retinopathy: XVIII. Ophthalmology. 2003;110:2118–2125. doi: 10.1016/S0161-6420(03)00863-7. [DOI] [PubMed] [Google Scholar]
- 33.Ikram MK, Janssen JA, Roos AM, et al. Retinal vessel diameters and risk of impaired fasting glucose or diabetes: the Rotterdam study. Diabetes. 2006;55:506–510. doi: 10.2337/diabetes.55.02.06.db05-0546. [DOI] [PubMed] [Google Scholar]
- 34.Wong TY, Klein R, Sharrett AR, et al. Retinal arteriolar diameter and risk for hypertension. Ann Intern Med. 2004;140:248–255. doi: 10.7326/0003-4819-140-4-200402170-00006. [DOI] [PubMed] [Google Scholar]
- 35.Wong TY, Shankar A, Klein R, et al. Prospective cohort study of retinal vessel diameters and risk of hypertension. BMJ. 2004;329:79. doi: 10.1136/bmj.38124.682523.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klein R, Klein BE, Moss SE, et al. Retinal vessel caliber and microvascular and macrovascular disease in type 2 diabetes: XXI: the Wisconsin Epidemiologic Study of Diabetic Retinopathy. Ophthalmology. 2007;114:1884–1892. doi: 10.1016/j.ophtha.2007.02.023. [DOI] [PubMed] [Google Scholar]