Abstract
INTRODUCTION
Gastrointestinal (GI) perforation is a common surgical presentation. In recent years, computed tomography (CT) has been shown to be accurate for predicting the site of GI perforation, and has become the investigation of choice. However the signs may be subtle or only indirectly related to the site or aetiology of perforation.
SUBJECTS AND METHODS
A MEDLINE and PubMed search was performed for journals before June 2009 with MeSH major terms ‘CT’ and ‘perforation’. Non-English speaking literature was excluded.
RESULTS
Examples of GI perforation of various aetiologies are reviewed (inflammatory, neoplastic, traumatic and iatrogenic) high-lighting characteristic CT appearances as well as pitfalls in diagnosis. Features of perforation include the presence of free gas or fluid within the supra- and/or inframesocolic compartments, segmental bowel wall thickening, bowel wall discontinuity, stranding of the mesenteric fat and abscess formation.
CONCLUSIONS
These differentiating features facilitate accurate multidisciplinary pre-operative evaluation, necessary to plan patient management and potential surgical approach.
Keywords: GI perforation, Computed tomography, Pre-operative evaluation
Breach of gastrointestinal (GI) tract wall can be due to peptic ulcer disease, inflammatory disease, blunt or penetrating trauma, iatrogenic factors, a foreign body or a neoplasm.1–7 It is important to identify the presence, location, and cause of the perforation correctly for appropriate management and surgical planning. The clinical diagnosis of the site of GI tract perforation is difficult as the symptoms may be non-specific.
The presence of free intraperitoneal gas on a routine radiograph usually indicates bowel perforation. Experimental studies8 have shown that as little as 1 ml of gas can be detected below the right hemidiaphragm on properly exposed erect chest radiographs. Despite being the first line of investigation, plain film radiography (erect chest and abdominal radiographs) is sensitive in only 50-70% of cases9–12 and the site of perforation is almost never elucidated. A left lateral decubitus film can also be used in the detection of small amounts of free air that may be interposed between the free edge of the liver and the lateral wall of the peritoneal cavity. When interpreting a right lateral decubitus, gas within the stomach or colon may obscure small amounts of free air.
Other modalities include ultrasound which may be particularly useful in patient groups where radiation burden should be limited notably children and pregnant women. However, it should not be considered definitive in excluding a pneumoperitoneum.13
Computed tomography (CT) is useful in detecting extralumi-nal gas.2,14–17 Multi-detector CT (MDCT) is superior to single helical or conventional CT as it is able to provide rapid, high-volume coverage and diagnostic images even in patients unable to perform prolonged breath holds.18,19 A study of MDCT showed 86% accuracy in predicting the site of perforation.20
We aim to review and illustrate the salient features of various GI tract perforations.
Subjects and Methods
A MEDLINE and PubMed search was performed for journals before June 2009 with MeSH major terms ‘radiograph’, ‘CT’ and ‘perforation’. Non-English speaking literature was excluded.
Reults
Radiological anatomy
The peritoneal cavity is divided into supra- and inframesocolic compartments by the transverse mesocolon and this distinction can be useful in radiological differentiation of upper and lower GI perforations. On CT, the transverse mesocolon is identified as the fatty plane extending from the pancreas, particularly at the level of uncinate process, to the transverse colon and has the middle colic vessels coursing through it.21
Subsequently, upper GI tract perforation (stomach or duodenal bulb) would result in supramesocolic compartment gas and distal small and large bowel perforation in inframesocolic compartment gas.
Sections of the GI tract, such as stomach, first part of duodenum (5 cm), jejunum, ileum, caecum, appendix, transverse colon, sigmoid colon and upper third rectum are found within the peritoneal cavity, and are usually mobile.
The second and third parts of the duodenum, ascending and descending colon and middle third of rectum are retroperitoneal and fixed; therefore, they may present with gas within the retroperitoneal compartment, usually the anterior pararenal space.
Radiological free gas signs
Various radiological descriptions are used for specific distribution of free intraperitoneal gas. For example, on plain radiography there is the Rigler sign (gas outlining both sides of the bowel), football sign (oval shaped peritoneal gas), increased lucency in the right upper quadrant (gas accumulating anterior to the liver) and triangle sign (triangular gas pocket between three loops of bowel).
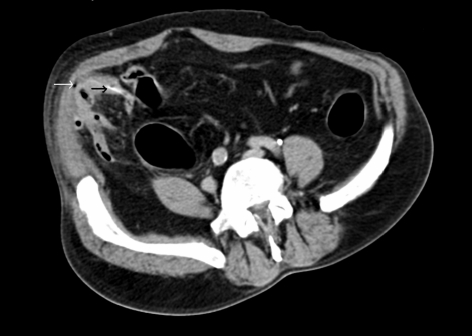
Likewise on CT, free gas may be seen outlining the intrahepatic fissure and ligamentum teres, called the ‘ligamentum teres sign’, often due to perforation of the duodenal bulb or stomach.11,12 The periportal free gas sign (PPFG; Fig. 1), when present, strongly suggests upper GI tract perforation.22
Figure 1.
CT scan showing ligamentum teres sign (white arrow) – free gas confined in the intra-hepatic fissure or ligamentum teres – and periportal free gas (PPFG) sign (black arrow) with gas tracking posterior to the main portal vein. Both are seen in a case of upper GI perforation.
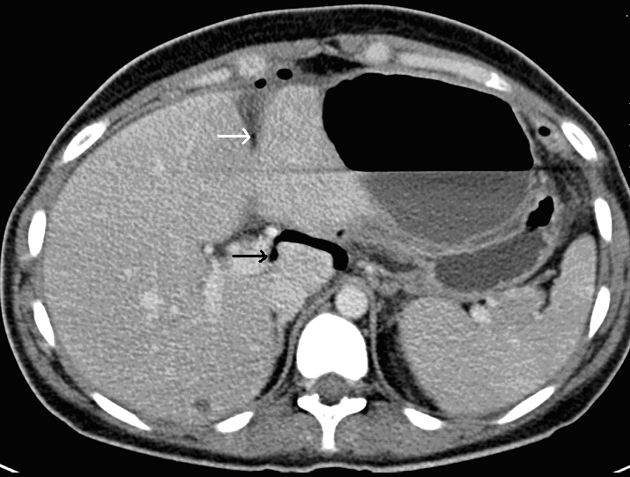
Another sign, ‘falciform ligament sign’ (Fig. 2) is free gas or a gas-fluid level crossing the mid-line and accentuating the falciform ligament, which is seen more in perforation of the proximal GI tract (stomach, duodenum, jejunum, and ileum).14
Figure 2.
A case of large bowel perforation: CT shows free gas crossing the midline, which outlines and accentuates the falciform ligament (white arrow) and produces the falciform ligament sign. Distended loops of transverse colon are also visible, which extend to an obstructing distal descending colon tumour.
Gastro-oesophageal junction
The causes of perforation near the gastro-oesophageal junction (GOJ) can be spontaneous, inflammatory (peptic ulcer disease), neoplastic, iatrogenic, traumatic or related to a foreign body.
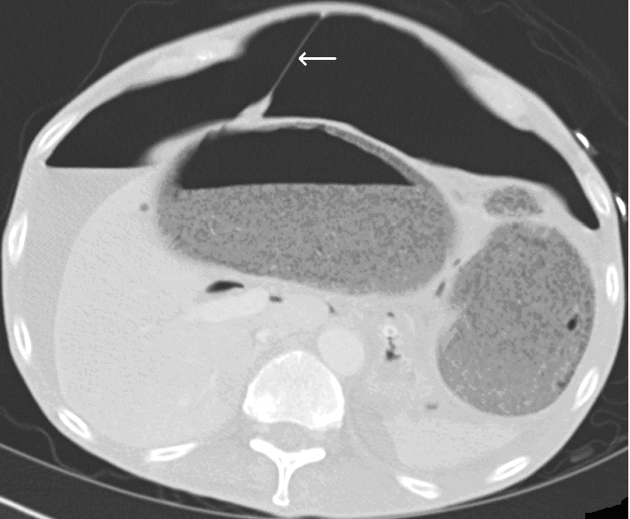
Spontaneous idiopathic oesophageal perforation also known as ‘Boerhaave syndrome’,23 usually occurs on the left side of distal oesophagus just above the GOJ as this site is not protected by supporting mediastinal structures. The patients are extremely ill with sepsis, mediastinitis and shock following an episode of severe retching. CT is useful to determine the extent of extraluminal collection of fluid and gas in the neck and mediastinum (Fig. 3), but may not be able to determine the site of perforation.24
Figure 3.
A case of presumed perforation of the oesophagus: CT shows gas within the mediastinum (thick white arrow) and subcutaneous tissues on the left side of thorax (thin white arrow). The exact site of perforation was not demonstrated.
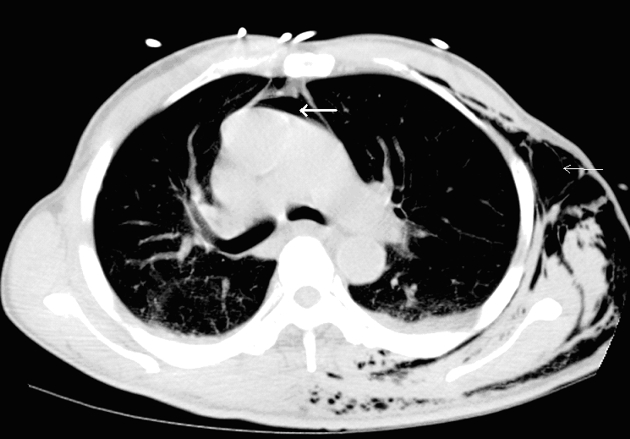
Iatrogenic perforation due to endoscopy occurs in approximately 1 in 3000 procedures usually affecting the cervical oesophagus or hypopharynx. Lower oesophageal perforations can occur (Fig. 4), usually related to intervention, a stricture or diverticulum.
Figure 4.
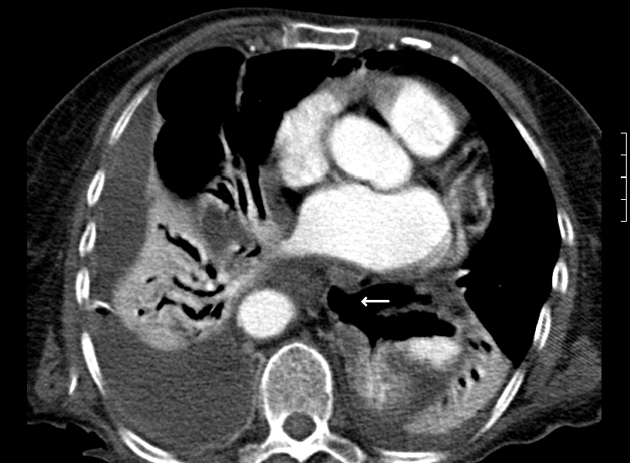
A case of gastro-oesophageal perforation following complicated endoscopy: CT shows extraluminal gas located adjacent to the site of oesophageal perforation and there is a defect in the oesophageal wall (white arrow). There are associated bibasal effusions and collapse/consolidation.
Gastroduodenal
Gastroduodenal perforation commonly occurs with peptic ulcer disease, neoplasia and postoperative anastomotic leaks. Penetrating ulcers of the anterior wall of the stomach or duodenum may perforate directly into the peritoneal cavity, whereas posterior stomach or duodenal ulcers often cause a walled-off or confined perforation. Duodenal ulcers are often located on the anterior bulb of the duodenum and are, therefore, a common cause of peritonitis. Studies using a water-soluble contrast agent may demonstrate extraluminal contrast material leakage as a direct sign of bowel perforation. However, the reported sensitivity of extravasation of oral contrast material on plain radiography varies from 19-42%.25
In a series of thin section spiral CTs in 10 patients with acute gastroduodenal peptic ulcer perforation, two important CT findings were indicative of the site of perforation – discontinuity in the gastroduodenal wall and/or tiny gas bubbles in close proximity to the bowel wall. This was in addition to evidence of wall thickening, enhancement and perigastroduodenal inflammatory change.3
It is possible, as a result of the anatomical relationship between the portal tract and the gastric antrum or duodenal bulb, that in upper GI tract perforations, free gas accumulates more frequently around the portal tract. This gives the peri-portal free gas sign (PPFG), which is considered the most significant finding in distinguishing upper from lower GI tract perforation.22
Other studies demonstrate that distribution of gas within the peritoneum is not very specific for the site of perforation. Indirect signs like perigastroduodenal fluid, stranding and gas bubbles in close proximity to the wall were considered more useful signs. Indeed, fluid between duodenum and pancreatic head is strongly associated with local perforation (Fig. 5).
Figure 5.
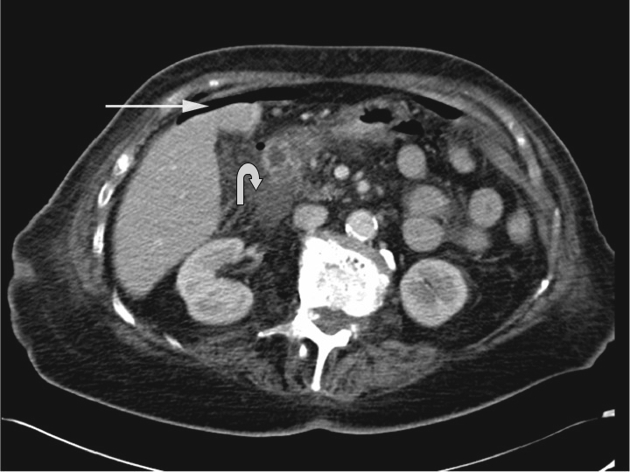
A case of duodenal perforation: CT shows there is a small amount of supramesocolic free gas (arrow) and periduodenal free fluid (curved arrow), associated with duodenal wall thickening and mesenteric stranding.
Small bowel
Small bowel perforation is due to obstruction, inflammatory conditions, ischaemia, infarction, trauma, neoplasia or can be iatrogenic.
The most common CT findings in small bowel perforation secondary to obstruction are hyperaemic bowel wall thickening, ascites and extraluminal gas or contrast medium. The absence of pneumoperitoneum does not exclude perforation of dilated fluid filled small bowel loops (Fig. 6). Even a small amount of peritoneal fluid may be the only sign indicating intestinal perforation.4
Figure 6.
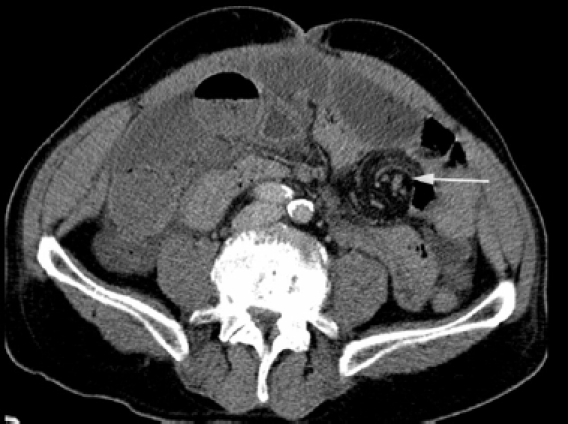
A case of small bowel obstruction: CT shows dilated loops of obstructed small bowel secondary to volvulus. There is twisting of the mesenteric vessels – the ‘whirl sign’ (white arrow). Free fluid was present within the abdomen and pelvis without evidence of free gas. Small bowel perforation was confirmed at surgery.
Acute inflammatory aetiologies, like Crohn's disease or diverticulitis, may lead to perforation with peritonitis or walled-off perforation with abscess formation. Inflammatory stranding in the small bowel mesentery adjacent to a thick-walled segment of bowel is not specific for perforation in patients with Crohn's disease.26 Diverticulitis affects 20% of those with Meckel's diverticulum, and may be associated with intestinal obstruction. Perforated Meckel's diverticulum is rare but should be considered in cases presenting with supra- or inframesocolic gas, hyperaemic bowel wall thickening and mesenteric oedema (Fig. 7), albeit often indistinguishable from other causes of small bowel perforation.
Figure 7.
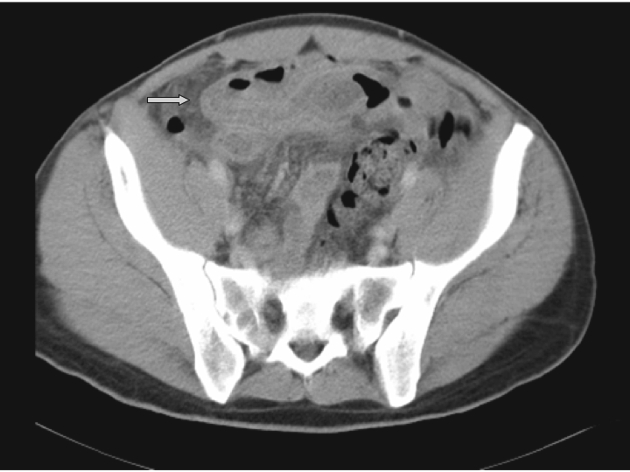
A case of perforated Meckel's diverticulum: CT shows thick-walled hyperaemic small bowel and mesenteric oedema (arrow), in the region of the perforated diverticulum.
Jejunal diverticulitis is an even rarer cause of perforation and MDCT may reveal a focal area of asymmetric small bowel wall thickening at the site of a focal out-pouching on the mesenteric side of the bowel.27
Blunt abdominal trauma may result in intestinal perforation. Shearing from rapid deceleration injury may occur, for example at the duodenojejunal flexure. CT findings (Fig. 8) include bowel wall thickening, pneumatosis, free gas or a focal fluid collection adjacent to an injured small bowel loop.28
Figure 8.
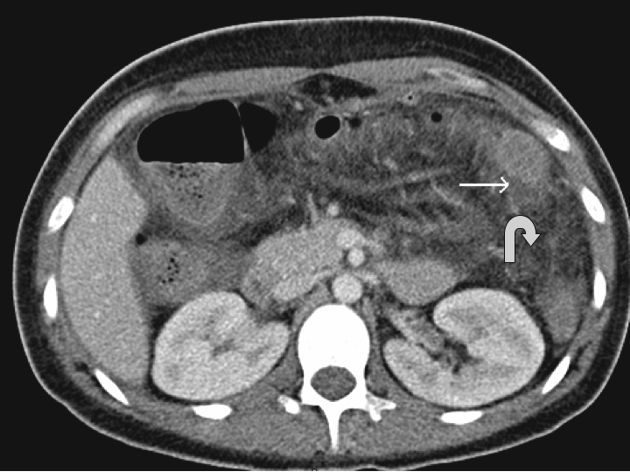
A case of perforation following blunt abdominal trauma: CT shows jejunal perforation, with jejunal wall thickening and focal fluid collection (white arrow) and mesenteric stranding (curved arrow).
Intestinal perforation is rare (< 1 %) after ingestion of a foreign body. However, perforations tend to occur at narrowed or angulated segments of bowel, most occurring within the ileum. The CT findings (Fig. 9) include a focal fluid collection adjacent to the injured bowel loop and bubbles of extraluminal gas.
Figure 9.
A case of a perforated terminal ileum following ingestion of a chicken bone (black arrow): CT shows a small bubble of extra-luminal gas (white arrow) and surrounding inflammatory change extending to the anterior abdominal wall. Note that there is no free gas.
Iatrogenic causes of small bowel injury can be related to laparoscopic surgery,29 although may go unrecognised at the time of the procedure, resulting in postoperative peritonitis which may develop after the patient is discharged. Interestingly, adhesions or a previous laparotomy may be risk factors.
Small bowel is one of many sites susceptible to late-stage, post-transplant, lymphoproliferative disorder (PTLD). Jejunum is most frequently affected and may present with perforation as a consequence of neoplastic infiltration.30
Appendix
Appendiceal perforation is mostly associated with underlying inflammation of the appendix. Perforated acute appendicitis may produce phlegmon31,32 and there may be associated wall thickening and abscess formation (Fig. 10). Recognition of the swollen appendix with peri-appendiceal infiltration, ascites, abscess and appendicolith, if present, enable the diagnosis of appendiceal rupture to be made with confidence.
Figure 10.
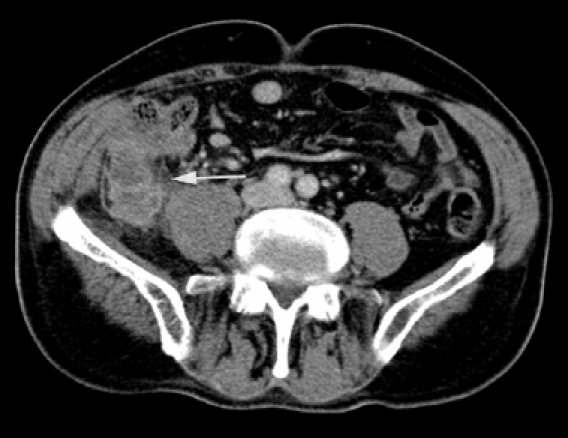
A case of appendiceal perforation: CT shows a right iliac fossa peri-appendiceal abscess (white arrow) surrounding a perforated appendix. No free gas was apparent.
Colonic perforation
Colonic perforation is a life-threatening condition requiring early recognition and treatment. Perforations can occur at the site of a localised pathological process (such as diverti-culitis, a neoplasm, a foreign body, or an iatrogenic cause) or within the proximal large bowel, usually caecum, secondary to distal colonic obstruction.
Colonic perforation, like gastroduodenal perforation, can appear as massive pneumoperitoneum with free gas throughout the abdomen and pelvis. If free gas is present only in the pelvis, the colon, and not small bowel, is the usual site of perforation. The reverse is true for suprameso-colic free gas. However, exceptions occur, as sigmoid perforations may have free gas only in the supramesocolic compartment, in which case focal signs such as wall thickening and peri-colonic stranding may be the only signs pointing towards the site of perforation (Fig. 11). Other CT findings observed in colonic perforation are of a complex mass, inflammatory change, an extraluminal fluid collection, and bowel wall thickening around the perforation site.2,33 Occasionally, a sigmoid diverticulum can perforate into the mesosigmoid, with gas tracking into the retroperitoneum. Free retroperitoneal gas, often in the anterior pararenal space, may also be caused by colonoscopic perforations of the posterior walls of the sigmoid, ascending and descending colon. Presentation is delayed unlike in intraperitoneal colonoscopic perforations where there is massive pneumoperitoneum due to procedural gas insufflation. These iatrogenic perforations occur in approximately 1 in 1000 patients. The rate of occurrence of symptomatic luminal perforation in CT colonography is four times lower than for colonoscopy.34
Figure 11.
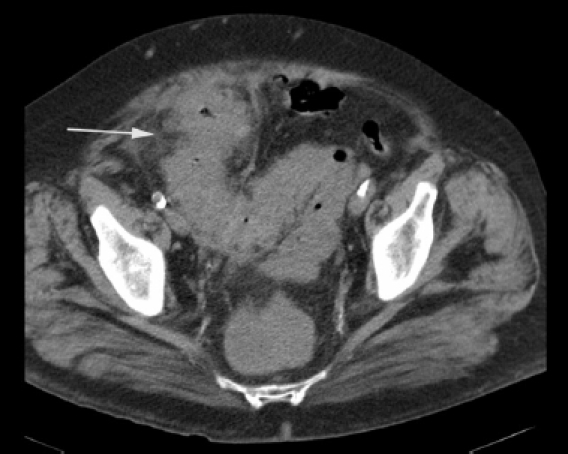
A case of perforated diverticulitis. CT shows bowel wall thickening within the sigmoid colon with pericolic inflammatory change (white arrow).
Recently, the distinction between benign pneumatosis and perforation has been clarified with CT colonography. Pneumatosis (gas contained within the bowel wall) is generally a self-limiting process, has usually resulted from the use of automated carbon dioxide insufflation and has a right-sided colonic distribution.35 Transmural perforation results in free peritoneal or retroperitoneal gas, although this is also usually indolent or asymptomatic; only 1 in 9 cases required surgical treatment.34 It is likely that similar cases of subclinical perforation occur following optical colonoscopy.36
Colonic carcinoma results in diffuse or focal bowel wall thickening and pericolonic stranding. Once tumour invades the serosal fat, there is increased possibility of perforation with abscess formation and gas leak (Fig. 12).
Figure 12.
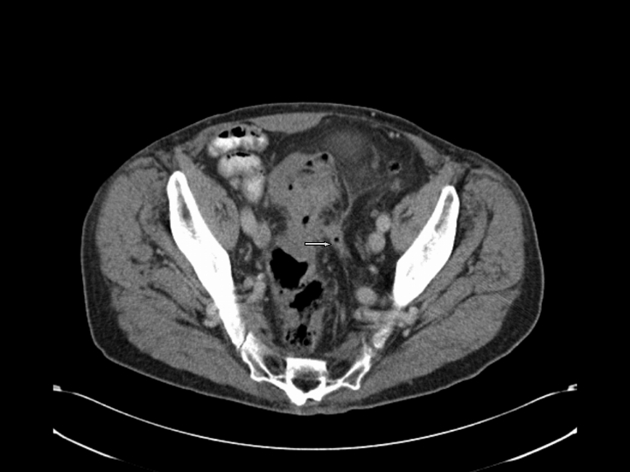
A case of sigmoid cancer within a segment of diverticular disease: CT shows a localised perforation (arrow) with stranding to the lateral side-wall.
Anorectal
The perforation of the extraperitoneal portion of rectum and anal canal is most commonly seen following trauma or surgery (anastomotic breakdown). Extraperitoneal emphysema is a rare clinical finding with anorectum perforation.37 History usually indicates the site of perforation. Cross-sectional imaging evaluates the extent of injury and the involvement of the sphincter, which determines the management.38 Sphincter anatomy is particularly well delineated at MRI scan.
Conclusions
CT precisely determines the presence of free intra-abdomi-nal gas, although the CT signs of perforation may be subtle and only indirectly related to the source. Findings such as a focal defect in the bowel wall, segmental bowel-wall thickening and concentrated bubbles of extraluminal gas in close proximity to the bowel wall have a high predictive value for indicating the site of perforation. A multidiscipli-nary approach to the evaluation of these acutely ill patients ensures early diagnosis and timely management.
References
- 1.Ghahremani GG. Radiologic evaluation of suspected gastrointestinal perforations. Radiol Clin North Am. 1993;31:1219–34. [PubMed] [Google Scholar]
- 2.Maniatis V, Chryssikopoulos H, Roussakis A, Kalamara C, Papadopoulos A, et al. Perforation of the alimentary tract: evaluation with computed tomography. Abdom Imaging. 2000;25:373–9. doi: 10.1007/s002610000022. [DOI] [PubMed] [Google Scholar]
- 3.Ongolo-Zogo P, Borson O, Garcia P, Grune L, Valette P. Acute gastroduodenal peptic ulcer perforation: contrast-enhanced and thin-section spiral CT findings in 10 patients. Abdom Imaging. 1999;24:329–32. doi: 10.1007/s002619900509. [DOI] [PubMed] [Google Scholar]
- 4.Sherck J, Shatney C, Sensaki K, Selivanov V. The accuracy of computed tomog raphy in the diagnosis of blunt small-bowel perforation. Am J Surg. 1994;168:670–5. doi: 10.1016/s0002-9610(05)80142-4. [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez JG, Gonzalez RR, Patino JV, Garcia AT, Alvarez CP, Pedrosa CS. CT findings in gastrointestinal perforation by ingested fish bones. J Comput Assist Tomogr. 1988;12:88–90. doi: 10.1097/00004728-198801000-00016. [DOI] [PubMed] [Google Scholar]
- 6.Lohrmann C, Ghanem N, Pache G, Makowiec F, Kotter E, Langer M. CT in acute perforated sigmoid diverticulitis. Eur J Radiol. 2005;56:78–83. doi: 10.1016/j.ejrad.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 7.Furukawa A, Sakoda M, Yamasaki M, Kono N, Tanaka T, et al. Gastrointestinal tract perforation: CT diagnosis of presence, site, and cause. Abdom Imaging. 2005;30:524–34. doi: 10.1007/s00261-004-0289-x. [DOI] [PubMed] [Google Scholar]
- 8.Miller RE, Nelson SW. The roentgenologic demonstration of tiny amounts of free intraperitoneal gas: experimental and clinical studies. AJR Am J Roentgenol. 1971;112:574–85. doi: 10.2214/ajr.112.3.574. [DOI] [PubMed] [Google Scholar]
- 9.Cho KC, Baker SR. Extraluminal air: diagnosis and significance. Radiol Clin North Am. 1994;32:829–44. [PubMed] [Google Scholar]
- 10.Levine MS, Scheiner JD, Rubesin SE, Laufer I, Herlinger H. Diagnosis of pneumoperitoneum on supine abdominal radiographs. AJR Am J Roentgenol. 1991;156:731–5. doi: 10.2214/ajr.156.4.2003436. [DOI] [PubMed] [Google Scholar]
- 11.Phatak MG, Frank SJ, Ellis JJ. Computed tomography of bowel perforation. Gastrointest Radiol. 1984;9:133–5. doi: 10.1007/BF01887819. [DOI] [PubMed] [Google Scholar]
- 12.Rice RP, Thompson WM, Gedgaudas RK. The diagnosis and significance of extraluminal gas in the abdomen. Radiol Clin North Am. 1982;20:819–37. [PubMed] [Google Scholar]
- 13.Chen SC, Wang HP, Chen WJ. Selective use of ultrasonography for the detection of pneumoperitoneum. Acad Emerg Med. 2002;9:643–5. doi: 10.1111/j.1553-2712.2002.tb02307.x. [DOI] [PubMed] [Google Scholar]
- 14.Glazer GM, Buy JN, Moss AA, Goldberg HI, Federle MP. CT detection of duode nal perforation. AJR Am J Roentgenol. 1981;137:333–6. doi: 10.2214/ajr.137.2.333. [DOI] [PubMed] [Google Scholar]
- 15.Jeffrey RB, Federle MP, Wall S. Value of computed tomography in detecting occult gastrointestinal perforation. J Comput Assist Tomogr. 1983;7:825–7. doi: 10.1097/00004728-198310000-00013. [DOI] [PubMed] [Google Scholar]
- 16.Stapakis JC, Thickman D. Diagnosis of pneumoperitoneum: abdominal CT vs. upright chest film. J Comput Assist Tomogr. 1992;16:713–6. [PubMed] [Google Scholar]
- 17.Yeung KW, Chang MS, Hsiao CP, Huang JF. CT evaluation of gastrointestinal tract perforation. Clin Imaging. 2004;28:329–33. doi: 10.1016/S0899-7071(03)00204-3. [DOI] [PubMed] [Google Scholar]
- 18.Vrtiska TJ, Fletcher JG, McCollough CH. State-of-the-art imaging with 64-channel multidetector CT angiography. Perspect Vasc Surg Endovasc Ther. 2005;17:3–8. doi: 10.1177/153100350501700102. discussion 9–10. [DOI] [PubMed] [Google Scholar]
- 19.Nikolaou K, Flohr T, Knez A, Rist C, Wintersperger B, et al. Advances in cardiac CT imaging: 64-slice scanner. IntJ Cardiovasc Imaging. 2004;20:535–40. doi: 10.1007/s10554-004-7015-1. [DOI] [PubMed] [Google Scholar]
- 20.Hainaux B, Agneessens E, Bertinotti R, De Maertelaer V, Rubesova E, et al. Accuracy of MDCT in predicting site of gastrointestinal tract perforation. AJR Am J Roentgenol. 2006;187:1179–83. doi: 10.2214/AJR.05.1179. [DOI] [PubMed] [Google Scholar]
- 21.Meyers MA. Intraperitoneal spread of malignancies. In: Meyers MA, editor. Dynamic radiology of the abdomen: normal and pathologic anatomy. 5th edn. New York: Springer; 2000. pp. 152–6. [Google Scholar]
- 22.Cho HS, Yoon SE, Park SH, Kim H, Lee Y, Yoon K. Distinction between upper and lower gastrointestinal perforation: Usefulness of the periportal free air sign on computed tomography. Eur J Radiol. 2009;69:108–13. doi: 10.1016/j.ejrad.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 23.Derbes VJ, Mitchel RE. Herman Boerhaave's ‘Atrocinec descripti prius, morbid historia’: first translation (from original Latin, 1724) of the classic case report of rupture of the oesophagus, with annotations. Bull Med Libr Assoc. 1955;43:217. [PMC free article] [PubMed] [Google Scholar]
- 24.Rubesin SE, Levine MS. Radiologic diagnosis of gastrointestinal perforation. Radiol Clin North Am. 2003;41:1095–115. doi: 10.1016/s0033-8389(03)00100-3. [DOI] [PubMed] [Google Scholar]
- 25.Becker CD, Mentha G, Schmidlin F, Terrier F. Blunt abdominal trauma in adults: role of CT in the diagnosis and management of visceral injuries. Eur Radiol. 1998;8:772–80. doi: 10.1007/s003300050471. [DOI] [PubMed] [Google Scholar]
- 26.Pickhardt PJ, Bhalla S, Balfe DM. Acquired gastrointestinal fistulas: classifica tion, etiologies, and imaging evaluation. Radiology. 2002;224:9–23. doi: 10.1148/radiol.2241011185. [DOI] [PubMed] [Google Scholar]
- 27.Macari M, Faust M, Liang H, Pachter HL. CT of jejunal diverticulitis: imaging findings, differential diagnosis, and clinical management. Clin Radiol. 2007;62:73–7. doi: 10.1016/j.crad.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 28.Winton TL, Girotti MJ, Manley PN, Sterns EE. Delayed intestinal perforation after non-penetrating intestinal trauma. Can J Surg. 1985;28:437–9. [PubMed] [Google Scholar]
- 29.Van der Voort M, Heijnsdijk EA, Gouma DJ. Bowel injury as a complication of laparoscopy. Br J Surg. 2004;91:1253–8. doi: 10.1002/bjs.4716. [DOI] [PubMed] [Google Scholar]
- 30.Pickhardt PJ, Siegel MJ. Abdominal manifestations of post transplant lymphoproliferative disorder. AJR Am J Roentgenol. 1999;171:1007–13. doi: 10.2214/ajr.171.4.9762986. [DOI] [PubMed] [Google Scholar]
- 31.Rao PM, Rhea JT, Novelline RA, McCabe CJ, Lawrason JN, et al. Helical CT technique for the diagnosis of appendicitis: prospective evaluation of a focused appendix CT examination. Radiology. 1997;202:139–44. doi: 10.1148/radiology.202.1.8988203. [DOI] [PubMed] [Google Scholar]
- 32.Horrow MM, White DS, Horrow JC. Differentiation of perforated from nonperforated appendicitis at CT. Radiology. 2003;227:46–51. doi: 10.1148/radiol.2272020223. [DOI] [PubMed] [Google Scholar]
- 33.Miki T, Ogata S, Uto M, Nakazono T, Urata M, et al. Multidetector-row CT findings of colonic perforation: direct visualization of ruptured colonic wall. Abdom Imaging. 2004;29:658–62. doi: 10.1007/s00261-003-0159-y. [DOI] [PubMed] [Google Scholar]
- 34.Burling D, Halligan S, Slater A, Noakes M, Taylor S. Potentially serious adverse events at CT colonography in symptomatic patients: national survey of the United Kingdom. Radiology. 2006;239:464–71. doi: 10.1148/radiol.2392051101. [DOI] [PubMed] [Google Scholar]
- 35.Pickhardt PJ, Kim DH, Taylor AJ. Asymptomatic pneumatosis at CT colonogra phy: a benign self-limited imaging finding distinct from perforation. AJR Am J Roentgenol. 2008;190:W112–7. doi: 10.2214/AJR.07.2843. [DOI] [PubMed] [Google Scholar]
- 36.Pickhardt PJ. Incidence of colonic perforation at CT colonography: review of existing data and implications for screening of asymptomatic adults. Radiology. 2006;239:313–6. doi: 10.1148/radiol.2392052002. [DOI] [PubMed] [Google Scholar]
- 37.Thomas PR. Ano-rectal injury causing extraperitoneal and subcutaneous emphy sema. Injury. 1987;18:426–7. doi: 10.1016/0020-1383(87)90299-3. [DOI] [PubMed] [Google Scholar]
- 38.Bostick PJ, Johnson DA, Heard JF, Islas JT, Sims EH, et al. Management of extraperitoneal rectal injuries. J Natl Med Assoc. 1993;85:460–3. [PMC free article] [PubMed] [Google Scholar]