Abstract
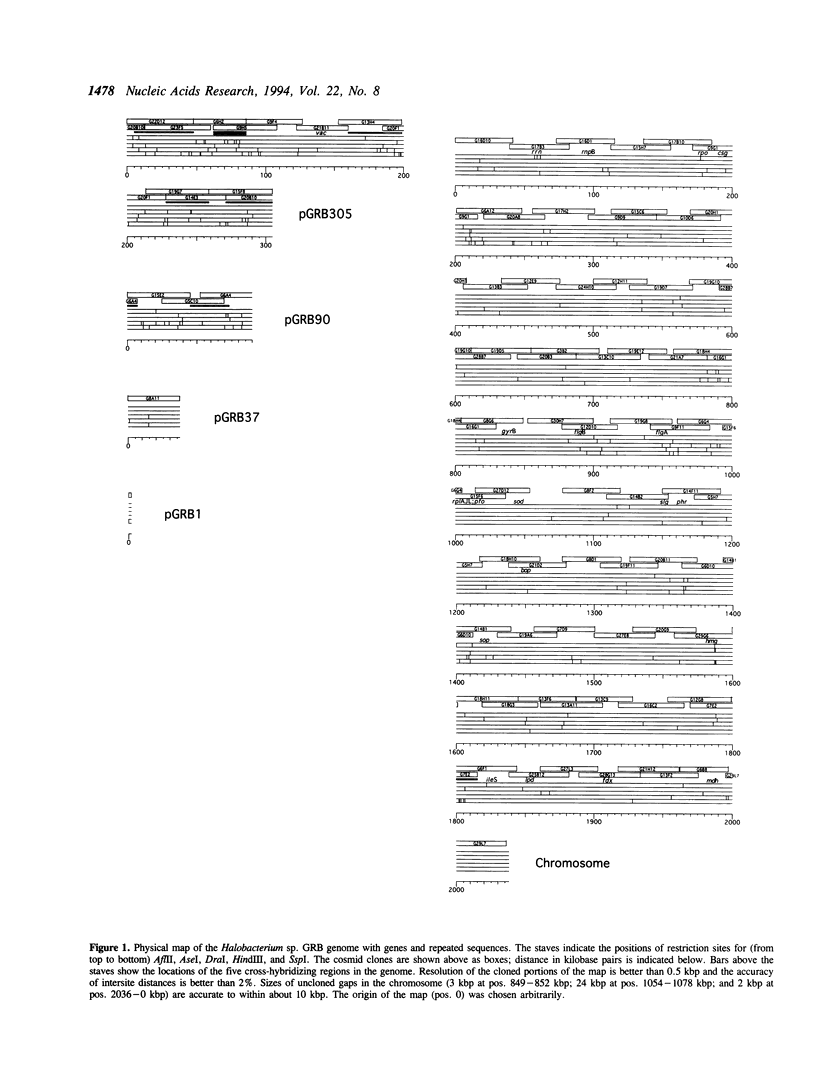
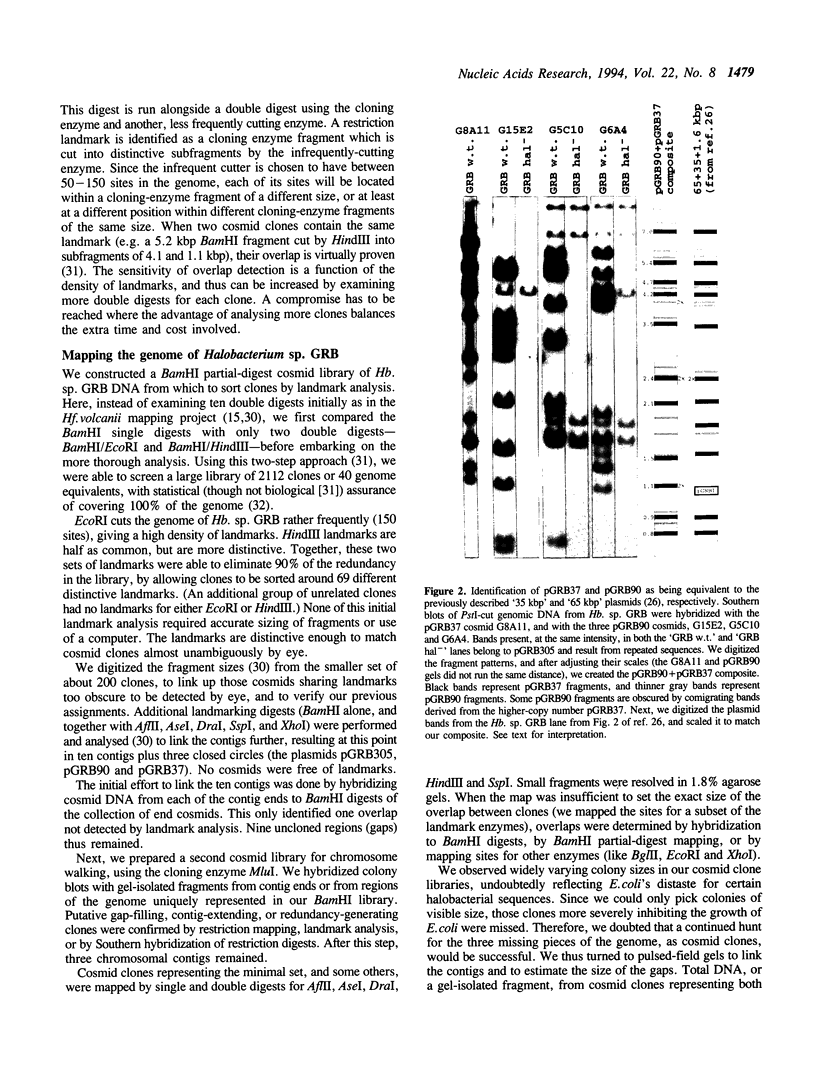
We have constructed a complete, five-enzyme restriction map of the genome of the archaeon Halobacterium sp. GRB, based on a set of 84 overlapping cosmid clones. Fewer than 30 kbp, in three gaps, remain uncloned. The genome consists of five replicons: a chromosome (2038 kbp) and four plasmids (305, 90, 37, and 1.8 kbp). The genome of Halobacterium sp. GRB is similar in style to other halobacterial genomes by being partitioned among multiple replicons and by being mosaic in terms of nucleotide composition. It is unlike other halobacterial genomes, however, in lacking multicopy families of insertion sequences.
Full text
PDF




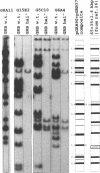
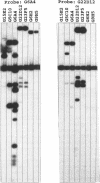
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akhmanova A. S., Kagramanova V. K., Mankin A. S. Heterogeneity of small plasmids from halophilic archaea. J Bacteriol. 1993 Feb;175(4):1081–1086. doi: 10.1128/jb.175.4.1081-1086.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betlach M., Pfeifer F., Friedman J., Boyer H. W. Bacterio-opsin mutants of Halobacterium halobium. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1416–1420. doi: 10.1073/pnas.80.5.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanck A., Oesterhelt D., Ferrando E., Schegk E. S., Lottspeich F. Primary structure of sensory rhodopsin I, a prokaryotic photoreceptor. EMBO J. 1989 Dec 20;8(13):3963–3971. doi: 10.1002/j.1460-2075.1989.tb08579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cendrin F., Chroboczek J., Zaccai G., Eisenberg H., Mevarech M. Cloning, sequencing, and expression in Escherichia coli of the gene coding for malate dehydrogenase of the extremely halophilic archaebacterium Haloarcula marismortui. Biochemistry. 1993 Apr 27;32(16):4308–4313. doi: 10.1021/bi00067a020. [DOI] [PubMed] [Google Scholar]
- Charlebois R. L., Hofman J. D., Schalkwyk L. C., Lam W. L., Doolittle W. F. Genome mapping in halobacteria. Can J Microbiol. 1989 Jan;35(1):21–29. doi: 10.1139/m89-004. [DOI] [PubMed] [Google Scholar]
- Charlebois R. L., Schalkwyk L. C., Hofman J. D., Doolittle W. F. Detailed physical map and set of overlapping clones covering the genome of the archaebacterium Haloferax volcanii DS2. J Mol Biol. 1991 Dec 5;222(3):509–524. doi: 10.1016/0022-2836(91)90493-p. [DOI] [PubMed] [Google Scholar]
- Churchill G. A., Daniels D. L., Waterman M. S. The distribution of restriction enzyme sites in Escherichia coli. Nucleic Acids Res. 1990 Feb 11;18(3):589–597. doi: 10.1093/nar/18.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline S. W., Doolittle W. F. Efficient transfection of the archaebacterium Halobacterium halobium. J Bacteriol. 1987 Mar;169(3):1341–1344. doi: 10.1128/jb.169.3.1341-1344.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A., Lam W. L., Charlebois R. L., Doolittle W. F., Schalkwyk L. C. Localizing genes on the map of the genome of Haloferax volcanii, one of the Archaea. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1602–1606. doi: 10.1073/pnas.89.5.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DasSarma S., Damerval T., Jones J. G., Tandeau de Marsac N. A plasmid-encoded gas vesicle protein gene in a halophilic archaebacterium. Mol Microbiol. 1987 Nov;1(3):365–370. doi: 10.1111/j.1365-2958.1987.tb01943.x. [DOI] [PubMed] [Google Scholar]
- DasSarma S. Mechanisms of genetic variability in Halobacterium halobium: the purple membrane and gas vesicle mutations. Can J Microbiol. 1989 Jan;35(1):65–72. doi: 10.1139/m89-010. [DOI] [PubMed] [Google Scholar]
- Gerl L., Sumper M. Halobacterial flagellins are encoded by a multigene family. Characterization of five flagellin genes. J Biol Chem. 1988 Sep 15;263(26):13246–13251. [PubMed] [Google Scholar]
- Hackett N. R., Krebs M. P., DasSarma S., Goebel W., RajBhandary U. L., Khorana H. G. Nucleotide sequence of a high copy number plasmid from Halobacterium strain GRB. Nucleic Acids Res. 1990 Jun 11;18(11):3408–3408. doi: 10.1093/nar/18.11.3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofman J. D., Schalkwyk L. C., Doolittle W. F. ISH51: a large, degenerate family of insertion sequence-like elements in the genome of the archaebacterium, Halobacterium volcanii. Nucleic Acids Res. 1986 Sep 11;14(17):6983–7000. doi: 10.1093/nar/14.17.6983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes M. L., Nuttall S. D., Dyall-Smith M. L. Construction and use of halobacterial shuttle vectors and further studies on Haloferax DNA gyrase. J Bacteriol. 1991 Jun;173(12):3807–3813. doi: 10.1128/jb.173.12.3807-3813.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne M., Englert C., Pfeifer F. Two genes encoding gas vacuole proteins in Halobacterium halobium. Mol Gen Genet. 1988 Aug;213(2-3):459–464. doi: 10.1007/BF00339616. [DOI] [PubMed] [Google Scholar]
- Itoh T. Complete nucleotide sequence of the ribosomal 'A' protein operon from the archaebacterium, Halobacterium halobium. Eur J Biochem. 1988 Sep 15;176(2):297–303. doi: 10.1111/j.1432-1033.1988.tb14281.x. [DOI] [PubMed] [Google Scholar]
- Joshi P., Dennis P. P. Characterization of paralogous and orthologous members of the superoxide dismutase gene family from genera of the halophilic archaebacteria. J Bacteriol. 1993 Mar;175(6):1561–1571. doi: 10.1128/jb.175.6.1561-1571.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagramanova V. K., Derckacheva N. I., Mankin A. S. Unusual nucleotide sequence heterogeneity of small multicopy pHSB plasmid from Halobacterium strain SB3, an archaebacterium. Can J Microbiol. 1989 Jan;35(1):160–163. doi: 10.1139/m89-024. [DOI] [PubMed] [Google Scholar]
- Lam W. L., Doolittle W. F. Mevinolin-resistant mutations identify a promoter and the gene for a eukaryote-like 3-hydroxy-3-methylglutaryl-coenzyme A reductase in the archaebacterium Haloferax volcanii. J Biol Chem. 1992 Mar 25;267(9):5829–5834. [PubMed] [Google Scholar]
- Lander E. S., Waterman M. S. Genomic mapping by fingerprinting random clones: a mathematical analysis. Genomics. 1988 Apr;2(3):231–239. doi: 10.1016/0888-7543(88)90007-9. [DOI] [PubMed] [Google Scholar]
- Lechner J., Sumper M. The primary structure of a procaryotic glycoprotein. Cloning and sequencing of the cell surface glycoprotein gene of halobacteria. J Biol Chem. 1987 Jul 15;262(20):9724–9729. [PubMed] [Google Scholar]
- Leffers H., Gropp F., Lottspeich F., Zillig W., Garrett R. A. Sequence, organization, transcription and evolution of RNA polymerase subunit genes from the archaebacterial extreme halophiles Halobacterium halobium and Halococcus morrhuae. J Mol Biol. 1989 Mar 5;206(1):1–17. doi: 10.1016/0022-2836(89)90519-6. [DOI] [PubMed] [Google Scholar]
- López-García P., Abad J. P., Smith C., Amils R. Genomic organization of the halophilic archaeon Haloferax mediterranei: physical map of the chromosome. Nucleic Acids Res. 1992 May 25;20(10):2459–2464. doi: 10.1093/nar/20.10.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May B. P., Tam P., Dennis P. P. The expression of the superoxide dismutase gene in Halobacterium cutirubrum and Halobacterium volcanii. Can J Microbiol. 1989 Jan;35(1):171–175. doi: 10.1139/m89-026. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Mevarech M., Werczberger R. Genetic transfer in Halobacterium volcanii. J Bacteriol. 1985 Apr;162(1):461–462. doi: 10.1128/jb.162.1.461-462.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore R. L., McCarthy B. J. Characterization of the deoxyribonucleic acid of various strains of halophilic bacteria. J Bacteriol. 1969 Jul;99(1):248–254. doi: 10.1128/jb.99.1.248-254.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng W. L., DasSarma S. Minimal replication origin of the 200-kilobase Halobacterium plasmid pNRC100. J Bacteriol. 1993 Aug;175(15):4584–4596. doi: 10.1128/jb.175.15.4584-4596.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwlandt D. T., Haas E. S., Daniels C. J. The RNA component of RNase P from the archaebacterium Haloferax volcanii. J Biol Chem. 1991 Mar 25;266(9):5689–5695. [PubMed] [Google Scholar]
- Pfeifer F., Betlach M. Genome organization in Halobacterium halobium: a 70 kb island of more (AT) rich DNA in the chromosome. Mol Gen Genet. 1985;198(3):449–455. doi: 10.1007/BF00332938. [DOI] [PubMed] [Google Scholar]
- Pfeifer F., Blaseio U., Horne M. Genome structure of Halobacterium halobium: plasmid dynamics in gas vacuole deficient mutants. Can J Microbiol. 1989 Jan;35(1):96–100. doi: 10.1139/m89-015. [DOI] [PubMed] [Google Scholar]
- Pfeifer F., Griffig J., Oesterhelt D. The fdx gene encoding the [2Fe--2S] ferredoxin of Halobacterium salinarium (H. halobium). Mol Gen Genet. 1993 May;239(1-2):66–71. doi: 10.1007/BF00281602. [DOI] [PubMed] [Google Scholar]
- Pfeifer F., Weidinger G., Goebel W. Characterization of plasmids in halobacteria. J Bacteriol. 1981 Jan;145(1):369–374. doi: 10.1128/jb.145.1.369-374.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer F., Weidinger G., Goebel W. Genetic variability in Halobacterium halobium. J Bacteriol. 1981 Jan;145(1):375–381. doi: 10.1128/jb.145.1.375-381.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaga W., Lottspeich F., Oesterhelt D. Improved purification, crystallization and primary structure of pyruvate:ferredoxin oxidoreductase from Halobacterium halobium. Eur J Biochem. 1992 Apr 1;205(1):391–397. doi: 10.1111/j.1432-1033.1992.tb16792.x. [DOI] [PubMed] [Google Scholar]
- Rosenshine I., Tchelet R., Mevarech M. The mechanism of DNA transfer in the mating system of an archaebacterium. Science. 1989 Sep 22;245(4924):1387–1389. doi: 10.1126/science.2818746. [DOI] [PubMed] [Google Scholar]
- Sapienza C., Doolittle W. F. Unusual physical organization of the Halobacterium genome. Nature. 1982 Feb 4;295(5848):384–389. doi: 10.1038/295384a0. [DOI] [PubMed] [Google Scholar]
- Sapienza C., Rose M. R., Doolittle W. F. High-frequency genomic rearrangements involving archaebacterial repeat sequence elements. Nature. 1982 Sep 9;299(5879):182–185. doi: 10.1038/299182a0. [DOI] [PubMed] [Google Scholar]
- Simon R. D. Halobacterium strain 5 contains a plasmid which is correlated with the presence of gas vacuoles. Nature. 1978 May 25;273(5660):314–317. doi: 10.1038/273314a0. [DOI] [PubMed] [Google Scholar]
- Soppa J., Oesterhelt D. Halobacterium sp. GRB: a species to work with!? Can J Microbiol. 1989 Jan;35(1):205–209. doi: 10.1139/m89-032. [DOI] [PubMed] [Google Scholar]
- Takao M., Kobayashi T., Oikawa A., Yasui A. Tandem arrangement of photolyase and superoxide dismutase genes in Halobacterium halobium. J Bacteriol. 1989 Nov;171(11):6323–6329. doi: 10.1128/jb.171.11.6323-6329.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vettakkorumakankav N. N., Stevenson K. J. Dihydrolipoamide dehydrogenase from Haloferax volcanii: gene cloning, complete primary structure, and comparison to other dihydrolipoamide dehydrogenases. Biochem Cell Biol. 1992 Aug;70(8):656–663. doi: 10.1139/o92-101. [DOI] [PubMed] [Google Scholar]
- Weidinger G., Klotz G., Goebel W. A large plasmid from Halobacterium halobium carrying genetic information for gas vacuole formation. Plasmid. 1979 Jul;2(3):377–386. doi: 10.1016/0147-619x(79)90021-0. [DOI] [PubMed] [Google Scholar]