Abstract
This study tested whether protein kinase M zeta (PKMζ) inhibition in the amygdala permanently disrupts fear memory by testing retention at various intervals after PKMζ blockade. Although the expression of fear memory was disrupted when the inhibitor was applied shortly before testing, it had no effect when rats were tested with longer retention intervals. These results suggest that PKMζ inhibition does not erase memory, but temporarily disrupts expression of memory.
Considerable effort has gone into determining the cellular events that take place shortly following learning that are important for memory consolidation, but relatively little is known about how memories are permanently represented in the brain. Prior work has demonstrated that the maintenance of late-phase long term potentiation can be blocked by disruption of PKMζ, implying that memories may be maintained by action of this persistently active kinase1,2. Accordingly, several studies have now shown that inhibition of PKMζ can disrupt memory for various tasks3–5. However, many of these experiments assessed memory shortly after application of the drug but not at later time points, raising the possibility that apparent memory erasure may instead reflect a disruption in retrieval of the memory or an interruption in the ability of the animals to produce the behavioral response from which memory is inferred. Other studies have assessed memory at longer time points after infusion, but some of these have used repeated testing6 which can have a profound impact on the stability of memory over time7,8. If blockade of PKMζ reverses the mechanism that maintains memory, then performance should be disrupted at any time point after infusion. We sought to test this idea by infusing the PKMζ inhibitor, zeta pseudosubstrate inhibitory peptide (ZIP), into the amygdala at various time points before testing memory for olfactory fear conditioning.
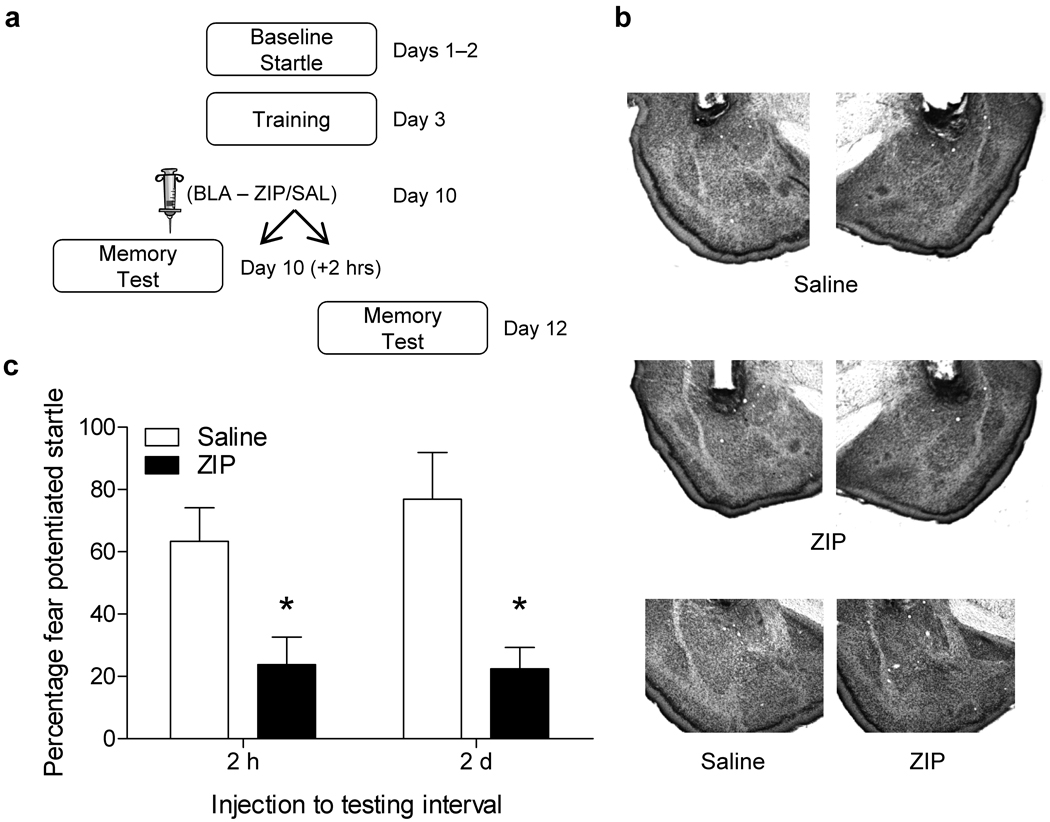
In accordance with protocols approved by the Animal Care and Use Committee at Emory University, rats were given five pairings of the odor acetophenone and foot shock during training for all experiments. Animals were infused with ZIP, saline, or scrambled ZIP (SCR ZIP) into the amygdala at varying time points after training and before testing. Memory was tested by assessing the potentiation of the acoustic startle reflex in the presence of acetophenone versus startle alone trials (Supplementary methods)9,10. In the first experiment (Fig. 1a), rats were infused with ZIP or saline into the amygdala (Fig. 1b) one week after training and tested 2 hours or 2 days later. PKMζ inhibition in the amygdala disrupted the expression of fear memory when tested 2 hours after the infusion (Fig. 1c). Similar results have been reported in other studies with the same infusion to testing interval5,11. Similarly, we observed a significant disruption in fear memory in rats treated with ZIP and tested 2 days later (Fig. 1c). A subset of these animals were given a reminder shock 2 days later and retested. There was very little evidence of recovery in the ZIP treated rats indicating that the deficit is not the result of enhanced extinction learning (Supplementary Fig. 1).
Figure 1.
Effect of PKMζ inhibition in the amygdala on olfactory-mediated fear potentiated startle. Rats were infused with ZIP (10nmol/µl, .5µl/side) or saline into the amygdala 2 hours or 2 days (a) before a memory test. (b) Nissl stained images (2x magnification) showing representative placements in rats infused with saline or ZIP. The bottom panel shows a 4x view of the amygdala in a saline and ZIP infused rat. No obvious signs of toxicity were observed. (c) Rats infused with ZIP (n=11) showed significantly less (p< .05, t–test) fear potentiated startle than controls (n=9) when tested 2 hours (c, left side) or 2 days (ZIP, n=15; SAL, n=14) (c, right side) after infusion. These and the subsequent graphs show means +/− SEM.
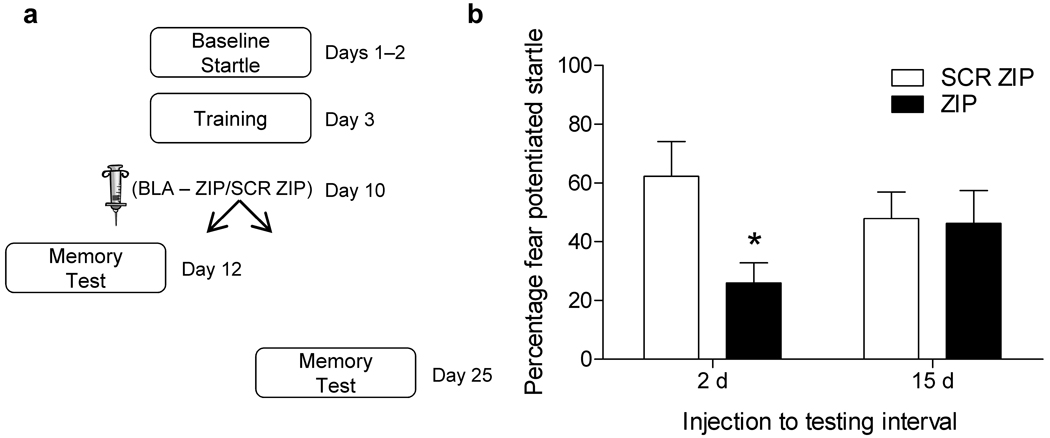
In the next experiment we tested if memory would be disrupted at even longer time points following infusion. Rats were infused with ZIP or SCR ZIP into the amygdala 1 week after training and were tested 15 days later, while another set of rats were run in parallel with the memory test occurring 2 days after infusion (Fig. 2a). Both sets of rats were trained on the same day and infused on the same day from the same aliquot of drug. Once again we found that ZIP disrupted the expression of fear memory when tested 2 days later (Fig. 2b, left side). However, rats that were tested 15 days after infusion exhibited intact memory (Fig. 2b, right side). The same pattern of results was seen in a separate experiment when rats were tested 12 days following infusion (Supplementary Fig. 2). These data indicate that the disruption in fear memory following PKMζ inhibition in the amygdala does not reflect a permanent erasure of memory.
Figure 2.
Temporary disruption of fear memory following PKMζ blockade in the amygdala. (a) Rats were infused with ZIP or SCR ZIP and tested 2 (n=20 SCR-ZIP, n=17 ZIP) or 15 days (n=10 SCR-ZIP, n=9 ZIP) later. ZIP infusion caused a significant disruption (p < .05, t–test) of fear memory when tested 2 days after infusion (b, left side), but not in rats tested 15 days after infusion.
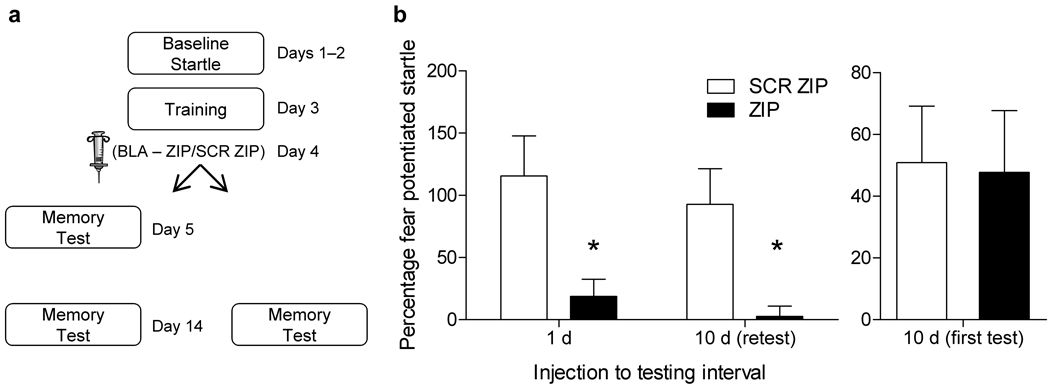
One prior study6 showed that infusion of ZIP in the amygdala disrupted the expression of fear memory when animals were tested one day following infusion and again 10 days later. This experiment differs from ours in that the infusion is closer to the time of training and the same rats were tested at both time points. As a direct comparison, we infused 2 groups of rats with ZIP or SCR ZIP into the amygdala 1 day after training and in one set of rats tested them 1 and 10 days after training, whereas the other group was tested only at 10 days (Fig. 3a). Rats infused with ZIP showed a disruption in the expression of fear memory when tested 1 day later, and this deficit was maintained when the same group was retested 10 days after training (Fig. 3b). In contrast, there was no difference between groups in the rats tested only at 10 days after training (Fig. 3b). These results indicate that if rats are tested soon after infusion of ZIP, the deficit tends to be stable over time. However, together with our prior results, ZIP does not disrupt memory if testing occurs only at relatively long intervals after infusion.
Figure 3.
PKMζ blockade in the amygdala only disrupts memory at long retention intervals if rats are tested shortly after infusion. (a) Rats were infused with ZIP (n=7) or SCR ZIP (n=7) 1 day following training and tested 1 and 10 days later. (b) ZIP infusion caused a significant disruption (p < .05, t–test) of fear memory expression at both time points. Another set of rats were infused with ZIP (n=6) or SCR ZIP (n=8) and tested only at 10 days after infusion. No difference was seen between groups in those rats tested only at 10 days (b, right side).
The demonstration of intact memory at later time points could be explained by decreasing dependence on PKMζ as the memory ages, and perhaps an increased reliance on more stable synaptic changescf.12. Alternatively, although there may be evidence that the amygdala permanently stores fear memories13, it is possible that expression of fear memory long after training may no longer depend on the amygdala. In either case, these ideas would predict that infusions of ZIP into the amygdala closer to the long-term test should not disrupt memory. However, when we tested this by infusing ZIP or SCR ZIP into the amygdala 20 days after training and 2 days prior to testing ZIP treated rats exhibited a significant disruption of potentiated startle at test (Supplementary Fig. 3). These data provide strong evidence that the apparent disruption of memory maintenance we see when rats are tested shortly after infusion represent some type of performance effect, rather than a blockade of memory maintenance.
In conclusion, spared fear memory following PKMζ inhibition in the amygdala was seen when testing occurred at time points long after application of the drug. While much of the work studying the role of PKMζ in memory has probed for memory soon after application of the inhibitor, studies of taste aversion learning have shown that memories are disrupted for as long as 1 month after infusions of ZIP into the insular cortex4. Our data contrast with these findings, but the disparate results may be related to differences in the task and the brain area targeted. It is possible that the maintenance of fear memories requires activity outside of the amygdala in cortical areas. Recent work lends credibility to this notion by showing that relatively old fear memories can be disrupted by ZIP infusion into secondary sensory cortical areas corresponding to the modality of the cue. One study6 showed that disruption of freezing behavior can last at least 10 days following infusion of ZIP in the amygdala. We performed a very similar experiment and showed that the presence of a deficit 10 days after infusion depended on whether or not the rats were also tested 1 day after infusions. The observation that ZIP has a long term effect in our experiments only if the rats are also administered an earlier test could mean that retrieving the memory at a short interval after ZIP infusion makes it more amenable to a permanent disruption. There is considerable evidence showing that initial memory tests can influence performance on subsequent ones7,8. This appears to be the case in the current study, but how this happens is unknown. What is clear from our results is that when testing occurs long after PKMζ blockade in the amygdala, fear memory can still be expressed. These results do not support the suggestion that PKMζ activity underlies permanent storage of fear memories in all parts of the brain.
Supplementary Material
Acknowledgments
This research was supported by grants R37 MH047840 (to M.D.) and F32 MH090700 (to R.G.P.) from the U.S. National Institutes of Health. Support was also provided by the Center for Behavioral Neuroscience (National Science Foundation agreement IBN-987675), and by an NIH/National Center for Research Resources base Grant (P51RR000165) to Yerkes National Primate Research Center. We thank G. Gafford for comments on this manuscript.
Footnotes
Author Contributions
R.G.P. designed and carried out the experiments, analyzed the results, wrote the initial manuscript and was involved in the revisions. M.D. was involved in the experimental design, helped interpret the data, and contributed to writing and revising the manuscript.
Competing Financial Interests
The authors declare no competing financial interests.
Reference List
- 1.Sacktor TC, et al. Proc. Natl. Acad. Sci. U. S. A. 1993;90:8342–8346. doi: 10.1073/pnas.90.18.8342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ling DS, et al. Nat. Neurosci. 2002;5:295–296. doi: 10.1038/nn829. [DOI] [PubMed] [Google Scholar]
- 3.Pastalkova E, et al. Science. 2006;313:1141–1144. doi: 10.1126/science.1128657. [DOI] [PubMed] [Google Scholar]
- 4.Shema R, Sacktor TC, Dudai Y. Science. 2007;317:951–953. doi: 10.1126/science.1144334. [DOI] [PubMed] [Google Scholar]
- 5.Serrano P, et al. PLoS. Biol. 2008;6:2698–2706. doi: 10.1371/journal.pbio.0060318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Migues PV, et al. Nat. Neurosci. 2010;13:630–634. doi: 10.1038/nn.2531. [DOI] [PubMed] [Google Scholar]
- 7.Estes WK. Psychol. Rev. 1997;104:148–169. doi: 10.1037/0033-295x.104.1.148. [DOI] [PubMed] [Google Scholar]
- 8.Stafford JM, Lattal KM. Learn. Mem. 2009;16:494–503. doi: 10.1101/lm.1452209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones SV, Heldt SA, Davis M, Ressler KJ. Behav. Neurosci. 2005;119:329–335. doi: 10.1037/0735-7044.119.1.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paschall GY, Davis M. Behav. Neurosci. 2002;116:4–12. doi: 10.1037//0735-7044.116.1.4. [DOI] [PubMed] [Google Scholar]
- 11.Kwapis JL, Jarome TJ, Lonergan ME, Helmstetter FJ. Behav. Neurosci. 2009;123:844–850. doi: 10.1037/a0016343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sossin WS. Trends Neurosci. 2008;31:170–175. doi: 10.1016/j.tins.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gale GD, et al. J. Neurosci. 2004;24:3810–3815. doi: 10.1523/JNEUROSCI.4100-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sacco T, Sacchetti B. Science. 2010;329:649–656. doi: 10.1126/science.1183165. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.