Abstract
Extracranial application of diffusion-weighted magnetic resonance imaging (MRI) has gained increasing importance in recent years. As a result of technical advances, this new non-invasive functional technique has also been applied in head and neck radiology for several clinical indications. In cancer imaging, diffusion-weighted MRI can be performed for tumour detection and characterization, monitoring of treatment response as well as the differentiation of recurrence and post-therapeutic changes after radiotherapy. Even for lymph node staging promising results have been reported recently. This review article provides overview of potential applications of diffusion-weighted MRI in head and neck with the main focus on its applications in oncology.
Keywords: Diffusion-weighted MRI, head and neck
Introduction
Diffusion-weighted magnetic resonance imaging (DWI) is already an established magnetic resonance imaging (MRI) method that is routinely used in most institutions for the diagnosis of an acute stroke[1]. It has been increasingly performed in recent years for extracranial applications in experimental settings and even in daily clinical practice as a result of fast imaging sequences[2]. However, physiological motion from respiration and cardiac movement remain a constant technical challenge. As in other organ sites, the main applications of diffusion-weighted MRI in head and neck radiology include functional imaging[3], tumour detection and characterization, monitoring of treatment response, differentiation of recurrence and post-therapeutic changes after radiochemotherapy and lymph node staging. The present article provides a short introduction of the technical considerations to perform DWI and focuses on oncologic applications in head and neck radiology.
Background and technical requirements
Diffusion-weighted MRI is an MR imaging technique that depicts molecular diffusion, which is the Brownian motion of the water protons in biological tissues[4]. Quantification is performed using the so-called apparent diffusion coefficient (ADC), which combines the effects of capillary perfusion and water diffusion in the extracellular extravascular space. The ADC value varies strongly with the underlying chosen b-values. When only low b-values are used, the corresponding ADC reflects mainly microperfusion and microcirculation and has only limited influence of diffusion, whereas the choice of only high b-values is reflected in an ADC that approximates true diffusion of the tissue. This choice is an important prerequisite for the correct application and interpretation of diffusion-weighted MRI. When comparing ADC values with those published in the literature, it is important to always look at the applied b-values. Furthermore, when performing follow-up studies, identical b-values have to be applied in order to compare the resulting ADC values.
The ADC value provides information on the microstructure of the underlying tissue. Hypercellular tumour tissue leads to an impeded diffusion and subsequent low ADC value, whereas necrotic or responding tumour tissue shows increased diffusivity and a high ADC value.
The technical requirements for performing DWI include a standardized imaging approach especially during follow-up studies. Parallel imaging has the advantage of shortening the acquisition time. The shortest echo time achievable allows the signal to noise ratio to be increased. Optimized fat suppression limits artefacts thereby increasing diagnostic image quality. The diffusion gradients (b-values) should be applied in three orthogonal directions in order to minimize the influence of anisotropy. For ADC calculation, images with at least two b-values (e.g. 0 and 1000 s/mm2) should be acquired, but if one wants to exclude the influence of perfusion, higher b-values (e.g. 100–1000 s/mm2) should be applied. Morphological images using the same geometry as diffusion-weighted images have to be performed in order to compare morphologic and functional images, allowing exact localization of the lesion and avoiding necrotic areas for region of interest delineation.
Tumour detection and characterization
Diffusion-weighted MRI allows tumours in the head and neck region to be detected as a high signal intensity lesion on high b-value diffusion-weighted images (Fig. 1) corresponding to a low signal intensity on the calculated ADC map. Several reports have shown the potential of DWI to differentiate between benign and malignant lesions as well as the separation of squamous cell carcinoma and lymphoma based on the measured ADC value.
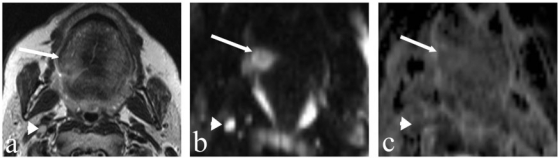
Figure 1.
A 54-year-old man with cancer of the posterior part of the tongue on the right. (a) T2-weighted axial MR image showing a slightly hyperintense lesion on the right-side of the tongue (arrow). (b) DW image at a b-value of 1000 s/mm2 clearly depicts the tongue tumour as a hyperintense lesion (arrow) without crossing the midline. (c) The same lesion is slightly hypointense on the calculated ADC map (arrow, ADC = 1.03×10−3 mm2/s). Note the small lymph node (4 mm) at level II on the right (arrowhead) which is bright in (b) and dark in (c) with an ADC value of 0.74×10−3 mm2/s suspicious for the presence of a metastasis, which has been confirmed by histology.
The differentiation between squamous cell carcinoma and malignant lymphoma of the head and neck was shown in a study including 39 patients with squamous cell carcinoma and 14 patients with lymphoma[5]. Using an ADC threshold value of 0.76 × 10−3 mm2/s DWI was able to make this distinction with an accuracy of 98%, meaning that 52 out of 53 lesions were correctly classified. The differentiation of benign and malignant head and neck lesions is another frequently encountered problem, especially in the absence of necrotic areas. A study including 33 patients (17 benign, 16 malignant lesions) performed on a 3-T MR unit using b-values of 0 and 800 s/mm2 was able to differentiate benign and malignant lesions using a threshold value of 1.3 × 10−3 mm2/s[6]. These results were confirmed in a study performed on 78 paediatric patients on a 1.5-T MR unit. In these studies b-values of 0, 500 and 1000 s/mm2 were applied and the ADC value for malignant tumours was 0.93 ± 0.18 × 10−3 mm2/s, 1.57 ± 0.26 × 10−3 mm2/s for benign solid masses and 2.01 ± 0.21 × 10−3 mm2/s for cystic lesions. Using a threshold ADC value of 1.25 × 10−3 mm2/s, an accuracy of 92.8%, sensitivity of 94.4%, specificity of 91.2%, positive predictive value of 91% and a negative predictive value of 94.2% were reported[7].
Another diagnostic issue in daily clinical routine including computed tomography (CT) and MRI is the evaluation of incidental thyroid nodules. In general, lower ADC values were reported in malignant lesions compared with benign lesions. With an ADC cut-off value of 0.98 × 10−3 mm2/s, a sensitivity of 97.5% and a specificity of 91.7% has been reported when applying b-values of 0, 250 and 500 s/mm2[8].
Salivary gland tumours are common and MRI may be used to assess these lesions or they may be detected incidentally during investigation for other pathological processes in the head and neck. Most of these salivary gland tumours are benign, being either pleomorphic adenomas or Warthin's tumours, but some are malignant[9]. DWI studies have shown that there is a significant difference in the mean ADC values of these three groups of tumours. Pleomorphic adenomas (which contain myxoid tissue) have the highest ADC value, being substantially greater than that of either malignant tumours or Warthin's tumours (which contain lymphoid tissue and have the lowest ADC value of any of the three groups)[10–12]. Initial promising results using ADC thresholds to differentiate benign from malignant parotid gland lesions in 45 histologically proven tumours[13] could not be confirmed by the same group in a larger scale study evaluating 136 parotid tumours[12]. Although DWI of pleomorphic adenomas and myoepithelial adenomas could be distinguished from malignant tumours, a final differentiation between benign and malignant parotid gland tumours based on ADC values was not possible because of an overlap between Warthin's tumour and malignant lesions.
In summary: malignant tumours have significantly lower ADC values than benign lesions provided that necrotic areas are excluded from image analysis.
Monitoring of treatment response
Non-surgical organ preservation therapy is the standard of care in most head and neck tumours because it allows preservation of functionality (swallowing and speech) while maintaining the same survival rate. Therefore, a surrogate biomarker for early treatment response in head and neck squamous cell carcinoma patients is needed. The evaluation of response to therapy by physical examination, endoscopy and by cross-sectional imaging (CT or MRI) is based on volumetric changes[14] that take place often relatively late in the time course of treatment. Therefore, early evaluation of treatment response may provide prognostic information about treatment outcome and eventually allow subsequent tailoring of treatment based upon individual response[15]. The potential of DWI for monitoring disease in the head and neck has been initially demonstrated in an animal model followed by promising results in patients with head and neck squamous cell carcinoma as shown in recent studies. Early detection of treatment response might change therapeutic strategies in the case of non-responders, whereby unnecessary toxicity and negative side effects might be avoided and individualized treatment would be possible with an ultimate goal of increasing long-term survival and saving money by avoiding ineffective treatment. In an animal study different treatment regimens (chemotherapy, radiotherapy, combined radiochemotherapy and controls) have been studied before treatment and at days 3, 5 and 7 of treatment looking at the change in ADC values. During combination therapy animals exhibited a much more abrupt increase in ADC, in contrast to the other three groups 5 days after treatment and this difference continued to increase with time[16]. The authors of this animal study concluded that DWI as an imaging biomarker has potential for early evaluation of the response to chemoradiation treatment in squamous cell carcinoma of the head and neck.
Initial clinical results looking at 33 patients with head and neck squamous cell carcinoma and metastatic cervical lymph nodes measured ADC values in the metastatic lymph nodes before, 1 week after start of chemoradiation therapy and at the end of treatment[17]. The median tumour volume of the partial responder group was significantly higher than that of the complete responder group; however, no relative differences in size were found at any time point. In contrast, significant differences between median ADC in complete responders and partial responders before treatment were observed with a significantly higher increase in ADC in the complete responder group at 1 week and after treatment. These results suggest that the ADC can be used as a marker for prediction and early detection of response to concurrent chemoradiation therapy in cervical metastases of squamous cell carcinoma. Primary head and neck squamous cell carcinoma tumours and cervical lymph node metastases have also been studied in 15 patients[18] who underwent chemoradiotherapy. MRI was performed before and 3 weeks after the start of treatment and the evaluation of response (partial and complete response) was evaluated 6 months after the end of treatment by conventional imaging including CT and MRI. When performing ADC histograms, the change in ADC towards higher values was larger in the complete responder group compared with the partial responder group. However, using a parametric response map that allows a voxel-by-voxel analysis of the entire tumour, the complete responders showed a significantly higher number of voxels with a significant increase in ADC values after start of treatment. This so-called parametric response map has the potential to provide not only prognostic but also spatial information during non-surgical organ preservation therapy of head and neck cancer.
Another study focused on outcome prediction in 30 patients with head and neck tumours undergoing chemoradiotherapy. Imaging including DWI was performed before, 2 and 4 weeks into chemoradiotherapy, whereas remission and recurrence were evaluated by follow-up studies performed up to 2 years after the end of chemoradiotherapy. Compared with baseline values, the percentage ADC change at 2 and 4 weeks into treatment was significantly higher in the group with complete remission compared with the group with recurrence. However, when looking at the individual patient a certain overlap could be noted[19]. In another recent study a total of 50 patients with head and neck squamous cell carcinoma were examined before and during treatment by morphological MRI and DWI. In contrast to previous studies, four different ADC patterns were described. A continuous increase in ADC was defined as pattern A or B, whereas an initial increase followed by a drop in ADC was defined as pattern C and an initial drop in ADC followed by an increase in ADC was defined by pattern D. The single ADC measurements pre- or intra-treatment were not able to predict response, however patterns C and D with an early or late fall in ADC were able to identify local failure at an early time point[20].
In summary, DWI allows early evaluation of treatment response preceding morphological changes. A treatment response is usually correlated with an increase in ADC but should not be followed by a decrease in ADC because this would suggest recurrence.
Differentiation between post-therapeutic changes and recurrence
The differentiation between post-therapeutic changes and recurrent tumours in patients treated for head and neck cancer is a diagnostic dilemma both for the radiologists and the clinicians[21]. These diagnostic problems are because chemotherapy and radiotherapy lead to often extensive changes of the underlying tissue including soft tissue oedema, cartilage necrosis, fibrosis and perichondritis[22]. In daily clinical practice, CT and MRI are the imaging modalities that are routinely used for the evaluation of the post-therapeutic neck, but differentiating recurrence from chondroradionecrosis is often impossible based on imaging findings. Positron emission tomography (PET)-CT has shown promising results, however hypermetabolic lesions can be found in tumours as well as in inflammation and are therefore sometimes difficult to interpret in the individual patient. DWI can be very helpful to resolve this particular problem. Image analysis can be performed by qualitative or quantitative assessment. Qualitative evaluation is based on imaging findings of high b-value images and the corresponding ADC map. In general recurrent tumour is reflected as a high signal intensity lesion on high b-value images with a low signal intensity of the corresponding ADC map (Figs. 2 and 3), whereas post-therapeutic changes are reflected in a high or low signal on the high b-value images and always in a high signal on the corresponding ADC map. When measuring the underlying ADC values, those of post-therapeutic changes are usually significantly higher than those of recurrent cancer.
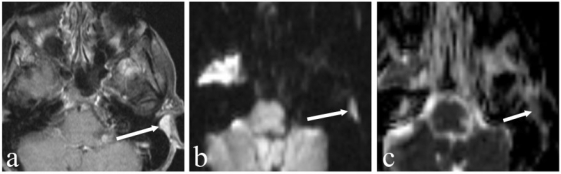
Figure 2.
A 61-year-old woman who underwent resection of a small retroauricular adenoid cystic carcinoma 6 months prior to imaging. (a) Axial T1-weighted contrast-enhanced fat saturated MR image shows post-treatment changes with an unchanged small retroauricular soft tissue mass (arrow) over time. (b) DW image at a b-value of 1000 s/mm2 depicts this lesion as hyperintense with a low signal on the corresponding ADC map (c) (ADC = 0.82×10−3 mm2/s) suspicious for residual tumour. This has been confirmed by histology.
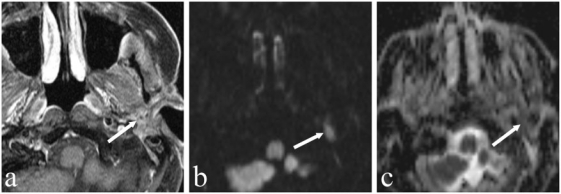
Figure 3.
A 79-year-old man who underwent left parotidectomy (arrow) for an acinic cell carcinoma. Imaging for newly developed facial nerve paralysis on the left. (a) Axial T1-weighted contrast-enhanced fat saturated MR image shows post-treatment changes after left parotidectomy. (b) DW image at a b-value of 1000 s/mm2 shows a circumscribed hyperintense lesion along the facial nerve (arrow) corresponding to a hypointense lesion on the ADC map with an ADC value of 0.75×10−3 mm2/s suspicious for recurrent tumour. Biopsy was performed and histology revealed recurrent acinic cell carcinoma.
Lymph node staging
Nodal metastasis is an adverse prognostic factor in patients with head and neck squamous cell carcinoma, therefore its detection is important for therapy planning[23]. To date, the diagnosis of lymph node metastases is mainly based on size criteria and morphology; however, micrometastases can also be observed in normal-sized nodes, and reactive nodes can also be enlarged. Promising results using DWI to detect cervical lymph node metastases and to differentiate benign from malignant enlarged lymph nodes have been reported[24–26]. In general the ADC values in nodal metastases of squamous cell carcinoma were significantly lower compared with benign lymph nodes[24,26–28]. Furthermore, DWI shows significant differences among malignant nodes of squamous cell carcinoma and lymphoma with significantly lower ADC values in lymphoma compared with squamous cell carcinoma[28] (Figs. 1 and 4). Although ADC threshold values can help to distinguish squamous cell carcinoma from lymphoma, when looking at nasopharyngeal carcinoma quite a big overlap between lymphoma and squamous cell carcinoma has been reported. In a previously published study of 87 enlarged cervical lymph nodes[24] the ADC values of lymph nodes involved by lymphoma were lower than those in metastatic lymph nodes of squamous cell carcinoma and in benign lymph nodes; however, there was an overlap between these groups for individual patients. Although these findings were quite promising, larger scale studies have to be performed to confirm these results and to allow DWI to differentiate benign and malignant lymph nodes in the individual patient.
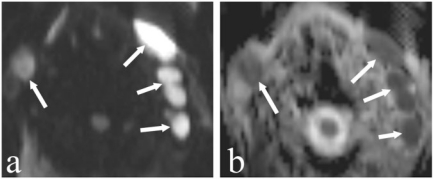
Figure 4.
An 83-year-old man with bilateral enlarged neck lymph nodes. (a) DW image at a b-value of 1000 s/mm2 shows enlarged hyperintense nodes (arrows) at level II on both sides. (c) These lymph nodes are dark on the corresponding ADC map (arrows) with a mean ADC value of 0.56×10−3 mm2/s suspicious for the presence of a lymphoma. Histology depicted a B-cell lymphoma.
Conclusion
DWI allows differentiation of benign and malignant head and neck masses in adults and children with significantly lower ADC values in malignant lesions compared with benign masses. In addition, the ADC values in lymphomas are significantly lower than those of squamous cell carcinoma.
DWI is helpful in predicting and monitoring treatment response in head and neck tumours, as changes in ADC precede changes in tumour size. An increase in ADC early after initiation of treatment without any decrease during treatment is correlated with complete response as shown in several studies. DWI might allow prediction of outcome at an early time point and might therefore be helpful in individualizing treatment. For the differentiation between recurrent tumour and post-therapeutic changes, qualitative and quantitative assessment using DWI seems to be helpful in resolving this diagnostic dilemma. For lymph node staging the ADC values of lymphomas were significantly lower compared with those of metastases of squamous cell carcinoma. The ADC values of malignant nodes were significantly lower than those reported in benign cervical nodes. A comparison with morphologic images is mandatory to exclude necrotic areas from measurement because these might lead to a false higher ADC values.
DWI in the head and neck has a wide variety of clinical applications with a special focus on oncology. However, attention has to be paid to the choice of b-values and has to be taken into account when comparing findings in the literature and when performing follow-up studies. A comparison between DWI findings and morphologic images is a prerequisite for correct image analysis and interpretation. When measuring the ADC, necrotic areas frequently encountered in squamous cell carcinomas of the head and neck have to be avoided.
To successfully perform DWI of the head and neck, a good collaboration between radiologists, physicists and clinicians is a prerequisites for the best management of the patient. In addition, larger scale studies for the different applications of DWI in the head and neck should be performed ideally in multicenter trials to confirm the promising results already published.
Acknowledgement
The author was supported by grant no. 320000-113512/1 from the Swiss National Foundation for Research and CARIGEST SA, Geneva, Switzerland. The author would also like to thank Frederik De Keyzer for his input in the preparation of this manuscript.
Footnotes
This article was presented at the ICIS Meeting 2010. It replaces the article at http://www.cancerimaging.org, doi: 10.1102/1470-7330.2010.9030, which was published in the Cancer Imaging Special Issue A in error. The publisher apologizes to the author, the Editor and ICIS.
References
- 1.Buckley BT, Wainwright A, Meagher T, Briley D. Audit of a policy of magnetic resonance imaging with diffusion-weighted imaging as first-line neuroimaging for in-patients with clinically suspected acute stroke. Clin Radiol. 2003;58:234–7. doi: 10.1016/S0009-9260(02)00463-4. . PMid:12639530. [DOI] [PubMed] [Google Scholar]
- 2.Thoeny HC, De Keyzer F. Extracranial applications of diffusion-weighted magnetic resonance imaging. Eur Radiol. 2007;17:1385–93. doi: 10.1007/s00330-006-0547-0. . PMid:17206421. [DOI] [PubMed] [Google Scholar]
- 3.Thoeny HC, De Keyzer F, Claus FG, Sunaert S, Hermans R. Gustatory stimulation changes the apparent diffusion coefficient of salivary glands: initial experience. Radiology. 2005;235:629–34. doi: 10.1148/radiol.2352040127. . PMid:15858103. [DOI] [PubMed] [Google Scholar]
- 4.Le Bihan D, Breton E, Lallemand D, Aubin ML, Vignaud J, Laval-Jeantet M. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology. 1988;168:497–505. doi: 10.1148/radiology.168.2.3393671. PMid:3393671. [DOI] [PubMed] [Google Scholar]
- 5.Maeda M, Kato H, Sakuma H, Maier SE, Takeda K. Usefulness of the apparent diffusion coefficient in line scan diffusion-weighted imaging for distinguishing between squamous cell carcinomas and malignant lymphomas of the head and neck. AJNR Am J Neuroradiol. 2005;26:1186–92. PMid:15891182. [PMC free article] [PubMed] [Google Scholar]
- 6.Srinivasan A, Dvorak R, Perni K, Rohrer S, Mukherji SK. Differentiation of benign and malignant pathology in the head and neck using 3T apparent diffusion coefficient values: early experience. AJNR Am J Neuroradiol. 2008;29:40–4. doi: 10.3174/ajnr.A0743. . PMid:17921228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abdel Razek AA, Gaballa G, Elhawarey G, Megahed AS, Hafez M, Nada N. Characterization of pediatric head and neck masses with diffusion-weighted MR imaging. Eur Radiol. 2009;19:201–8. doi: 10.1007/s00330-008-1123-6. . PMid:18704436. [DOI] [PubMed] [Google Scholar]
- 8.Razek AA, Sadek AG, Kombar OR, Elmahdy TE, Nada N. Role of apparent diffusion coefficient values in differentiation between malignant and benign solitary thyroid nodules. AJNR Am J Neuroradiol. 2008;29:563–8. doi: 10.3174/ajnr.A0849. . PMid:18039755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thoeny HC. Imaging of salivary gland tumours. Cancer Imaging. 2007;7:52–62. doi: 10.1102/1470-7330.2007.0008. . PMid:17485257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ikeda M, Motoori K, Hanazawa T, et al. Warthin tumor of the parotid gland: diagnostic value of MR imaging with histopathologic correlation. AJNR Am J Neuroradiol. 2004;25:1256–62. PMid:15313720. [PMC free article] [PubMed] [Google Scholar]
- 11.Yabuuchi H, Matsuo Y, Kamitani T, et al. Parotid gland tumors: can addition of diffusion-weighted MR imaging to dynamic contrast-enhanced MR imaging improve diagnostic accuracy in characterization? Radiology. 2008;249:909–16. doi: 10.1148/radiol.2493072045. . PMid:18941162. [DOI] [PubMed] [Google Scholar]
- 12.Habermann CR, Arndt C, Graessner J, et al. Diffusion-weighted echo-planar MR imaging of primary parotid gland tumors: is a prediction of different histologic subtypes possible? AJNR Am J Neuroradiol. 2009;30:591–6. doi: 10.3174/ajnr.A1412. . PMid:19131405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Habermann CR, Gossrau P, Graessner J, et al. Diffusion-weighted echo-planar MRI: a valuable tool for differentiating primary parotid gland tumors? Rofo. 2005;177:940–5. doi: 10.1055/s-2005-858297. PMid:15973595. [DOI] [PubMed] [Google Scholar]
- 14.Forastiere AA, Ang K, Brizel D, et al. Head and neck cancers. J Natl Compr Canc Netw. 2005;3:316–91. doi: 10.6004/jnccn.2005.0019. PMid:16002004. [DOI] [PubMed] [Google Scholar]
- 15.Thoeny HC, Ross BD. Predicting and monitoring cancer treatment response with diffusion-weighted MRI. J Magn Reson Imaging. 2010;32:2–16. doi: 10.1002/jmri.22167. . PMid:20575076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamstra DA, Lee KC, Moffat BA, Chenevert TL, Rehemtulla A, Ross BD. Diffusion magnetic resonance imaging: an imaging treatment response biomarker to chemoradiotherapy in a mouse model of squamous cell cancer of the head and neck. Transl Oncol. 2008;1:187–94. doi: 10.1593/tlo.08166. PMid:19043529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim S, Loevner L, Quon H, et al. Diffusion-weighted magnetic resonance imaging for predicting and detecting early response to chemoradiation therapy of squamous cell carcinomas of the head and neck. Clin Cancer Res. 2009;15:986–94. doi: 10.1158/1078-0432.CCR-08-1287. . PMid:19188170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galbán CJ, Mukherji SK, Chenevert TL, et al. A feasibility study of parametric response map analysis of diffusion-weighted magnetic resonance imaging scans of head and neck cancer patients for providing early detection of therapeutic efficacy. Transl Oncol. 2009;2:184–90. doi: 10.1593/tlo.09175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vandecaveye V, Dirix P, De Keyzer F, et al. Predictive value of diffusion-weighted magnetic resonance imaging during chemoradiotherapy for head and neck squamous cell carcinoma. Eur Radiol. 2010;20:1703–14. doi: 10.1007/s00330-010-1734-6. . PMid:20179939. [DOI] [PubMed] [Google Scholar]
- 20.King AD, Mo FK, Yu KH, et al. Squamous cell carcinoma of the head and neck: diffusion-weighted MR imaging for prediction and monitoring of treatment response. Eur Radiol. 2010;20:2213–20. doi: 10.1007/s00330-010-1769-8. . PMid:20309553. [DOI] [PubMed] [Google Scholar]
- 21.Zbären P, Weidner S, Thoeny HC. Laryngeal and hypopharyngeal carcinomas after (chemo)radiotherapy: a diagnostic dilemma. Curr Opin Otolaryngol Head Neck Surgery. 2008;16:147–53. doi: 10.1097/MOO.0b013e3282f702a9. [DOI] [PubMed] [Google Scholar]
- 22.Tshering Vogel DW, Zbaeren P, Thoeny HC. Cancer of the oral cavity and oropharynx. Cancer Imaging. 2010;16:62–72. doi: 10.1102/1470-7330.2010.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson JT. A surgeon looks at cervical lymph nodes. Radiology. 1990;175:607–10. doi: 10.1148/radiology.175.3.2188292. PMid:2188292. [DOI] [PubMed] [Google Scholar]
- 24.Abdel Razek AA, Soliman NY, Elkhamary S, Alsharaway MK, Tawfik A. Role of diffusion-weighted MR imaging in cervical lymphadenopathy. Eur Radiol. 2006;16:1468–77. doi: 10.1007/s00330-005-0133-x. . PMid:16557366. [DOI] [PubMed] [Google Scholar]
- 25.Sumi M, Sakihama N, Sumi T, et al. Discrimination of metastatic cervical lymph nodes with diffusion-weighted MR imaging in patients with head and neck cancer. AJNR Am J Neuroradiol. 2003;24:1627–34. PMid:13679283. [PMC free article] [PubMed] [Google Scholar]
- 26.Vandecaveye V, De Keyzer F, Vander Poorten V, et al. Head and neck squamous cell carcinoma: value of diffusion-weighted MR imaging for nodal staging. Radiology. 2009;251:134–46. doi: 10.1148/radiol.2511080128. . PMid:19251938. [DOI] [PubMed] [Google Scholar]
- 27.de Bondt RB, Hoeberigs MC, Nelemans PJ, et al. Diagnostic accuracy and additional value of diffusion-weighted imaging for discrimination of malignant cervical lymph nodes in head and neck squamous cell carcinoma. Neuroradiology. 2009;51:183–92. doi: 10.1007/s00234-008-0487-2. . PMid:19137282. [DOI] [PubMed] [Google Scholar]
- 28.King AD, Ahuja AT, Yeung DK, et al. Malignant cervical lymphadenopathy: diagnostic accuracy of diffusion-weighted MR imaging. Radiology. 2007;245:806–13. doi: 10.1148/radiol.2451061804. . PMid:17911539. [DOI] [PubMed] [Google Scholar]