Abstract
Primary intrinsic and/or acquired multidrug resistance (MDR) is the main obstacle to successful cancer treatment. Functional molecular imaging of MDR in cancer using single photon or positron emitters may be helpful to identify multidrug-resistant tumours and predict not only those patients who are resistant to treatment, with a clinically unfavourable prognosis, but also those who are susceptible to the development of drug toxicity or even certain tumours . Variations in the mdr1 gene product may directly affect the therapeutic effectiveness, and single nucleotide polymorphisms for the mdr1 gene may be associated with altered oral bioavailability of MDR1 substrates, drug resistance, and a susceptibility to some human diseases. The challenge of translating the concept of MDR modulation in vivo involves a complex cellular interplay between both malignant and normal cells. Integration and correlation of functional single photon emission tomography or positron emission tomography imaging findings with mdr1 genotype and clinical data may contribute to efficient management by selecting cancer patients with the appropriate molecular phenotype for maximal individual therapeutic benefit, as well as those who are non-responders. This review describes a role for functional imaging of classical mechanisms of MDR with an emphasis on readily available [99mTc]MIBI scintigraphy. MIBI scintigraphy has been shown to be a non-invasive cost-effective in vivo assay of ATP-binding cassette transporters associated with MDR in cancer, including P-glycoprotein, multidrug-resistant protein 1 and breast cancer resistant protein. New imaging agents for molecular targets such as vascular endothelial growth factor and HER2 receptors, may potentially be combined with MDR imaging substrates to more accurately predict the therapeutic response to anticancer drugs, guiding individualised treatment while minimising the economic health costs of ineffective therapy in an era of personalised medicine.
Keywords: Cancer, multidrug resistance, ABC transporters, P-glycoprotein, imaging
Background
Primary intrinsic and/or acquired drug resistance is the main underlying cause of failure in cancer treatment. Resistance of tumour cells to several structurally unrelated classes of natural products, including anthracyclines, taxanes, and epipodophyllotoxines, is often referred as multidrug resistance (MDR)[1].
In tumour cell lines, MDR is often associated with an ATP-dependent decrease in cellular drug accumulation and is attributed to the over-expression of certain ATP-binding cassette (ABC) transporter proteins. ABC transporters belong to the largest transporter gene family and generally use energy derived from ATP hydrolysis for translocation of different substrates across biological membranes. ABC transporters are classified into 7 subfamilies based on phylogenetic analysis and designated as ABCA to ABCG[2]. ABC proteins that confer drug resistance include (but are not limited to) P-glycoprotein (P-gp) (gene symbol ABCB1), the multidrug resistance protein 1 (MRP1, gene symbol ABCC1), MRP2 (gene symbol ABCC2), and breast cancer resistance protein (BCRP, gene symbol ABCG2).
In addition to their role in drug resistance, there is substantial evidence that these efflux pumps have overlapping physiologic functions in tissue defence. Collectively, these proteins are capable of transporting a vast and chemically diverse array of toxins, including bulky lipophilic cationic, anionic, and neutrally charged molecules and many clinical drugs, as well as conjugated organic anions, that encompass dietary and environmental carcinogens, pesticides, metals, metalloids, and lipid peroxidation products[3].
Single nucleotide polymorphisms (SNPs) in ABC drug efflux pumps may play a role in responses to drug therapy and disease susceptibility. The effects of various genotypes and haplotypes (combinations of SNPs) on the expression and function of these proteins are not yet entirely clear, and their true impact remains controversial[4]. The ABCB1 multidrug resistance gene 1 (mdr1) encodes P-gp, which has been addressed as a molecular hydrophobic vacuum cleaner, pulling substrates from the membrane and expelling them to promote MDR[5]. This 170-kDa plasma membrane protein functions as an energy-dependent adenosine-5′-triphosphate (ATP) efflux pump[6].
P-gp has an important role in normal physiology by protecting tissues from toxic xenobiotics and endogenous metabolites. It also regulates the transport of various structurally unrelated substrates, such as anticancer agents and toxins[7]. Many tissues express P-gp, including the bronchopulmonary epithelium, hepatobiliary epithelium, renal tubular epithelium, gastrointestinal tract, blood–brain barrier and choroid plexus, which are all strategically located to protect against the passage of xenobiotics. The differential expression of P-gp in many localised tissues and cells of the hematopoietic system, natural killer cells, antigen-presenting dendritic cells, human peripheral blood mononuclear cells (PBMCs) and subpopulations of T and B lymphocytes implies diverse physiologic and pharmacologic roles[8,9]. P-gp in the apical border of foetus-derived epithelial cells faces the maternal circulation and is therefore optimally placed to protect the foetus against toxins[8].
Genetically determined differences in P-gp expression
The variability of P-gp expression between individuals is linked to C3435T polymorphism of the human mdr1 gene which is located on the long arm of 7th chromosome at q21.1 band position. It plays a significant role in ADME processes (absorption, distribution, metabolism, and excretion) and drug–drug interaction. Variations in the mdr1 gene product can directly affect therapeutic effectiveness, with over-expression of P-gp resulting in increased efflux of anticancer drugs and development of drug resistance. The mdr1 gene is highly polymorphic and numerous SNPs have been identified of which some are known to influence MDR1 expression levels[7].
Polymorphism in exon 26 at C3435T (silent polymorphism) is known to influence the expression of P-gp. The C/C genotype is associated with increased P-gp expression; individuals with T/C genotype show intermediate P-gp expression and individuals who are homozygous carriers of T/T show functionally restrained P-glycoprotein. Individuals carrying the C/C genotype showed higher P-gp expression levels (2-fold) compared with T/T individuals[7]. The concentration of P-gp in intestinal epithelial cells and in a subset of lymphoid cells is substantially lower in people with the T/T genotype than in those with the CC genotype[10].
In the analysis of MDR1 variant genotype distribution in a large sample of white subjects, Cascorbi et al.[11] first demonstrated that C3435T occurred in 53.9% of subjects heterozygously (T/C), 28.6% of individuals were homozygous (T/T) carriers and 17.5% of the individuals were homozygous (C/C) carriers. In general, the prevalence of the T/T genotype in whites has been shown to be between 24% and 29%[11,10].
A role of MDR1 as a modulator of health and disease
T/T genotype: link with drug toxicity and susceptibility to P-gp mediated disease
It has been shown that C3435T/T polymorphism is associated with low P-gp expression, and hence lower protection against specific P-gp-dependent xenobiotics and carcinogens and with a reduced efficiency to eliminate toxins, resulting in higher intracellular concentrations of mutagens or toxins, leading to DNA damage and accumulation of mutations. Potential implications of this reduced mechanism of detoxification may also have implications for disease risk and therapeutic outcome due to the development of drug toxicity. Individuals with T/T genotype were found to be at increased risk of chronic myeloid leukaemia (CML)[7], acute childhood lymphoblastic leukaemia (ALL)[12], renal epithelial tumours[13], colorectal cancer, glioblastoma, breast cancer[7] and inflammatory bowel disease[10].
When data were compared with respect to gender, the T/T genotype was more frequent in males. Exposure to carcinogens was found to be higher in males as compared to females. An association with male glioblastoma and T/T genotype and a greater risk of developing CML in males has also been reported [7].
C/C genotype: link with multidrug resistance and poor risk prognosis
Increased C/C genotype is shown to be associated with multidrug resistance and hence potentially linked with poor disease prognosis. In cancer therapy, high expression and activity of MDR1 causes cancer cells to become refractory to treatment with many agents that are P-gp substrates.
The functional significance of MDR1 C3435T polymorphism with respect to imatinib treatment was studied in terms of haematologic and cytogenetic response. When data were analysed in relation to these 2 types of responses, the frequency of the C/C genotype was increased significantly in cytogenetic non-responders and the increase was found to be inversely proportional to the degree of cytogenetic response. The C/C genotype has also been demonstrated to be associated with poor prognosis in ALL and acute myelocytic leukaemia, as a result of multidrug resistance[7,12].
The influence of ABCB1 polymorphism on drugs that are P-gp substrates has also been shown to vary among races[14]. Racial variability within C3434T has also been demonstrated. Thus a significantly higher frequency of the C/C genotype is present in West Africans and African Americans (83% and 61%, respectively[15]), compared with whites (17.5%[11] to 26%; p < 0.0001[15]). These findings could affect the use of drugs that are P-gp substrates (such as HIV-1 protease inhibitors and cyclosporin) and anticancer drugs in African populations[15] due to a potentially higher rate of drug resistance.
The development of multidrug resistance not only reflects multiple genetic and epigenetic changes that occur inside cells under cytotoxic conditions, but is also a normal physiologic response of cells in their struggle for survival. The challenge of translating the concept of MDR modulation in vivo involves a complex cellular interplay between both malignant and normal cells[16].
In vivo imaging of ABC transporters in multidrug resistance
Multidrug resistance and specific ABC transporters can be imaged using radiopharmaceuticals that are themselves MDR substrates or inhibitors. The first and most studied of these is [99mTc]sestamibi (hexakis-methoxy-isobutyl isonitrile) or MIBI, which is a substrate for P-gp, MRP1, MRP2 and BCRP and can therefore be used to image their expression in vivo[17], as described in further detail below.
[99mTc]tetrofosmin and several other [99mTc]Q complexes that are closely related to MIBI in terms of their use in nuclear medicine are also transport substrates for P-gp and MRP[1]. The properties of tetrofosmin are similar but not identical to those of MIBI. The available data suggest that clinical studies involving imaging of MDR function and in vivo modulation of MDR function can be performed with both tetrofosmin and MIBI, but the 2 should probably not be used interchangeably[18]. Recent experiments with the positron-emitter [94mTc]MIBI parallel previous studies with [99mTc]MIBI, showing essentially identical performance and thereby providing biological validation for the use of micro-positron emission tomography (PET)[19].
Several 11C-labelled P-gp avid radioligands have been developed for PET, including [11C]colchicine, [11C]verapamil, [11C]daunorubicin, [11C]paclitaxel, and [11C]loperamide. All of these compounds have been evaluated in animals, but only [11C]verapamil and [11C]loperamide[20] have been extended to humans, not only for investigating MDR, but also P-gp expression at the blood–brain barrier[20,21]. Other compounds that have been developed include ([67/68Ga]-3-ethoxy-ENBDMPI)(+) tracers[22], 4-[18F]fluoropaclitaxel[23] and the positron labelled P-gp inhibitor, [11C]tariquidar[24] for microPET.
Although current functional imaging is focusing on P-gp, other ABC drug transporters are also attracting interest. For example, [99mTc]HIDA is transported only by MRP1,2. Hepatic P-gp and MRP1,2 could therefore be assessed by sequential use of both MIBI and HIDA. Leukotrienes are substrates for MRP, so N-[11C]acetyl leukotriene E4 may provide an opportunity to study MRP function noninvasively[1]. The imaging techniques, tracers and their relation to relevant ABC substrates and genotypes are summarised in Table 1.
Table 1.
SPECT and PET ABC substrates (s) and inhibitors (i) and their relationships to genes
| Imaging modality | Radiopharmaceuticals | ABC transporter | Gene symbol |
|---|---|---|---|
| SPECT | [99mTc]MIBI (s) | P-gp | ABCB1 (mdr1) |
| MRP1,2 BCRP |
ABCC1,2 |
||
| ABCG2 | |||
| [99mTc]Tetrofosmin (s) | P-gp | ABCB1 (mdr1) | |
| MRP1,2 | ABCC1,2 | ||
| [99mTc]HIDA (s) | MRP1,2 | ABCC1,2 | |
| [67Ga]3-ethoxy- ENBDMPI (s) | P-gp | ABCB1 (mdr1) | |
| MRP1 | ABCC1 | ||
| PET and microPET (µ) | [94mTc]MIBI (s, µ) | P-gp | ABCB1 (mdr1) |
| MRP1,2 |
ABCC1,2 |
||
| BCRP | ABCG2 | ||
| [11C]Colchicines (i, µ) | P-gp | ABCB1 (mdr1) | |
| [11C]Verapamil (i) | P-gp | ABCB1 (mdr1) | |
| [11C]Loperamide (i) | P-gp | ABCB1 (mdr1) | |
| [11C]Paclitaxel (i, µ) | P-gp | ABCB1 (mdr1) | |
| [11C]Daunorubicin (i, µ) | P-gp | ABCB1 (mdr1) | |
| 4-[18F]Fluoropaclitaxel (i, µ) | P-gp | ABCB1 (mdr1) | |
| [11C]Tariquidar (i, µ) | P-gp | ABCB1 (mdr1) | |
| [68Ga]3-ethoxy-ENBDMPI (s) | P-gp, MRP1 | ABCB1 (mdr1);ABCC1 | |
| N-[11C]Acetyl leukotriene E4 (s) | MRP 2 | ABCC2 |
[99mTc]MIBI as an in vivo assay of ABC transporters
[99mTc]MIBI is a lipophilic cationic radiotracer originally introduced for imaging myocardial perfusion. Chemical analysis of ground state [99mTc]sestamibi reveals a stable monovalent cation with a central Tc(I) core surrounded by 6 identical MIBI ligands, coordinated through the isonitrile carbons in an octahedral geometry[6].
[99mTc]MIBI is taken up by passive diffusion into cytoplasm and accumulates in mitochondria. Its tissue uptake rate following intravenous injection is variable but broadly dependent on tissue blood flow and cellularity. Cellular transport of [99mTc]MIBI is affected by apoptosis, cell proliferation and angiogenesis. MIBI is therefore used as an imaging biomarker for cellular metabolism in tumours[25,26]. Tissue retention is variable and markedly influenced by tissue expression of P-gp[6,27,28]. The mechanism of MIBI cellular uptake clearly differs from its mechanism of elimination, which specifically reflects activity of drug transporters, such as P-gp.
MIBI has been validated as a transport substrate for P-gp in cultured multidrug-resistant rodent[28,29] and human tumour cells[6,30], as well as in cells over-expressing the recombinant human mdr1 gene[31]. Piwnica-Worms et al.[6] first demonstrated that [99mTc]MIBI is a substrate of P-gp and that it can be used as a functional imaging agent for P-gp in tumour xenografts in nude mice. They and others have shown that tumour retention of MIBI correlates inversely with the degree of P-gp expression and can be modified in vitro by the presence of P-gp antagonists[32].
In rodent models, faster clearance of MIBI is observed in tumours that express P-gp compared with those that do not[28,30]. The hepatic and renal excretion pathways of [99mTc]MIBI are mediated by P-gp, and modulation of P-gp transport function can be detected in humans when this radiopharmaceutical is used with cytotoxic drugs. Intravenous administration of a P-gp modulator increased retention of MIBI in the liver and kidney in patients investigated for MDR[33]. In vitro MIBI studies have shown that P-gp inhibitors, such as verapamil and cyclosporin, can reverse P-gp expression in adenocarcinoma cells if given shortly before the administration of cytotoxic drug[34].
Additional mechanisms of cell resistance, mainly involving alterations of apoptosis, may also affect [99mTc]MIBI uptake in tumours. In particular, over-expression of the anti-apoptotic protein, Bcl-2, prevents tumour cells entering apoptosis and inhibits tracer accumulation in mitochondria. Therefore, although absent or reduced early tracer uptake in large breast carcinomas reflects the existence of a defective apoptotic programme, enhanced tracer clearance in [99mTc]MIBI-positive lesions reflects the activity of drug transporters, such as P-gp. The existence of 2 different mechanisms underlying the predictive role of [99mTc]MIBI scan may be important to establish whether individual patients may benefit from P-gp inhibitors or Bcl-2 antagonists[35].
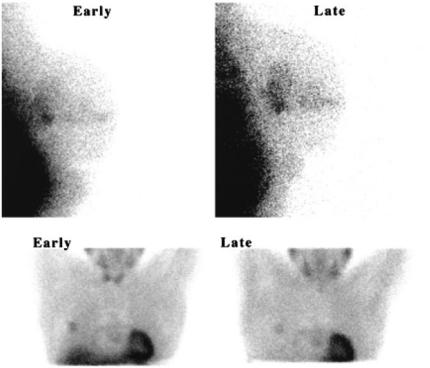
It has been demonstrated that preoperative washout rates of MIBI from primary breast tumours correlated with levels of P-gp found in the surgically resected specimens (Figure 1).
Figure 1.
[99mTc]Sestamibi images of breast cancer obtained 20 min (early) and 120 min (late) after injection of radiotracer. (A) A patient with tumour displaying immunohistochemically negative P-gp expression showing tumour/background (T/B) that increased from 1.65 to 1.99. (B) A patient with tumour displaying strongly positive P-gp expression showing T/B that decreased from 2.25 to 1.52. Reprinted by permission of the Society of Nuclear Medicine from Mubashar M, Harrington KJ, Chaudhary KS, et al. 99mTc-Sestamibi imaging in the assessment of toremifene as a modulator of multidrug resistance in patients with breast cancer. J Nucl Med 2002; 43: 519–25, Figure 2).
Scintigraphic data in patients with breast cancer demonstrated that [99mTc]MIBI washout rates from breast cancers over-expressing P-gp are threefold faster than those from cancers not expressing P-gp[36,37].
MIBI has also been used for imaging multidrug resistance in lung cancer[38], brain tumours[39,40], gastric cancer[41], head and neck cancer[42], hepatobiliary cancer[43] and haematologic malignancies[44].
In lung cancer, the sensitivity, specificity and accuracy of [99mTc]MIBI for identifying responders to chemotherapy are 94%, 90% and 92%, respectively[38]. There is evolving evidence that MIBI is cost-effective in predicting the response to chemotherapy in patients with lung cancer[38] and also for diagnosing breast cancer in patients with indeterminate mammography and dense breasts[45].
In thyroid imaging, MIBI scintigraphy can be used to reliably exclude thyroid cancer when ultrasound-guided fine-needle aspiration cytology is reported as non-diagnostic and hence avoid the need for more invasive surgical procedures and influence the cost-effectiveness profile[46]. However, it has only recently been demonstrated that semi-quantitative [99mTc]MIBI scintigraphy may preoperatively predict the malignant behaviour of non-oncocytic follicular thyroid nodules indeterminate at fine-needle aspiration biopsy, with a potential impact on clinical management. Moreover, a good correlation was found between immunohistochemical apical expression of MRP1 and MIBI scintigraphic findings. A negative MIBI retention index correlated strongly with those cases with high MRP1 expression. So MIBI scintigraphy may provide information on the molecular mechanisms responsible for abnormal [99mTc]MIBI thyroid scintigraphy[47].
A potential role for MIBI scintigraphy has been extensively investigated in the management of haematologic malignancies, particularly multiple myeloma (MM). It has been shown that MIBI washout can predict response to chemotherapy in patients with MM. Patients showing disease progression at restaging had higher washout (19.3 ± 9.8% vs 12.8 ± 6.9%, p < 0.05) than patients in remission . Disease-free survival was significantly better in patients with lower MIBI washout. When patients treated with melphalan were excluded from the analysis, 87.5% of patients in remission had slow washout[48].
Patients whose tumours showed MIBI uptake responded well to chemotherapy in multiple malignancies, whereas those whose tumours showed little or no uptake or quick MIBI washout (indicative of MDR) did not respond well[20].
Genetically determined responses to some anticancer drugs may also influence anticancer treatment. It has been shown that MIBI hepatic scanning may be used as a pre-treatment indicator of ABCB1-mediated drug clearance from the liver in cancer patients. MIBI hepatic elimination (kH) was significantly reduced in patients with SNPs in exons 21 and 26. The mean MIBI kH was 1.90 times and 2.21 times higher in subjects homozygous for the wild-type alleles than in those homozygous for these SNPs, respectively[49].
ABC drug transporters and imaging: past, present and future
Past
Because of the hypothesis in the 1980s and 1990s that blockade of ATP-dependent drug efflux pumps would improve the effect of chemotherapy, there has been intense search for compounds able to reverse MDR in cultured cells, animal models, and ultimately patients. Extensive effort has been made to visualise these pumps using both single photon emission tomography (SPECT) and PET tracers. Unfortunately, the addition of P-gp or MRP1 modulators has not shown significant clinical benefit in patient outcome, mainly due to their toxicity (first generation) or interaction with anticancer drugs and alteration in pharmacokinetics of the chemotherapy agents (second generation). These MDR tracers have not, therefore, been routinely used in clinical practice.
Present
ABC imaging can accurately predict chemotherapy response in a range of cancers and the use of ABC tracers can reduce costs related to ineffective chemotherapy by identifying non-responders[38]. Nevertheless, imaging MDR remains limited in routine clinical practice.
Promising clinical trials were conducted on acute myeloid leukaemia, breast carcinoma, and non-Hodgkin lymphoma, malignancies that are known to express P-gp. Paradoxically, many studies investigated the reversal of MDR in cancers whose major mode of resistance may not be P-gp-mediated. Clinical trials that yielded negative results were often conducted in cancers in which P-gp expression is generally lower, such as small-cell lung cancer and non-small-cell lung cancer. In effect, clinical trials such as the phase III trials of tariquidar in non-small-cell lung cancer were seeking to measure how effective P-gp inhibitors were in tumours that did not necessarily express the pharmacologic target[20].
The major reasons for recent negative results in clinical trials using third generation P-gp modulators are poor study design, regarding either dosing regimens or patient selection, and genetic polymorphism of P-gp. The 2 major phase III trials of tariquidar conducted in patients with non-small-cell lung cancer terminated prematurely due to toxicity, but used higher doses of chemotherapy than was recommended. On the other hand, the prevalence of various genetic polymorphisms of P-gp may have influenced results (both negatively and positively). Some SNPs and haplotypes of the mdr1 gene have been shown to alter P-gp expression and activity both in vitro and in vivo. For example, patients with ovarian cancer who express the wild-type allele for P-gp had a mean progression-free survival of 19 months when treated with chemotherapy, whereas those expressing the G1199A polymorphism had a mean progression-free survival of only 2 months[20]. Other studies have shown a connection with C3435T polymorphism. All the above-mentioned factors may influence imaging outcome in an individual patient, leading to controversial results, and hence functional imaging of MDR has not yet been fully utilised in clinical practice.
Future
Clinical trials better tailored to tumour types, genetic polymorphism and adequate dose regimens should be conducted. Thus, imaging may be useful in selecting patients whose cancers express MDR primarily though ABC-mediated mechanisms. For a proper assessment of P-gp levels in tumours, patients should undergo 2 scans with a P-gp radioligand: at baseline and again after P-gp inhibition. Patients whose tumours show enhanced uptake of the radioligand following P-gp blockade would be suitable candidates for P-gp inhibitor trials. Two studies using [99mTc]sestamibi following administration of tariquidar or valspodar have shown the utility of this approach. Another ongoing study is using MIBI to monitor progress throughout the trial[20].
Prolonged exposure of cells to a P-gp inhibitor may cause physiologic up-regulation of P-gp[50]. As many inhibitors are also modulators, initial down-regulation may be followed by up-regulation of P-gp expression, resulting in acquired drug resistance to P-gp inhibitors. Functional imaging may be used to monitor physiologic prolonged P-gp response to P-gp inhibitors in addition to an acute response.
Ongoing research has led to the development of a third generation of MDR modulators, some of which have demonstrated encouraging results compared with earlier generation MDR modulators. They are less toxic, more P-gp specific and do not affect the pharmacokinetics of anticancer drugs. Significant numbers of natural products have also been identified for their effectiveness in reversing MDR in a manner similar to the MDR modulation. Some MDR-reversing strategies aim to destroy mRNAs for ABC drug transporters or inhibit transcription of ABC transporter genes, or block ABC transporter activity using antibodies. There is an optimistic view that much more can be achieved in developing reversing agents for ABC transporters[51]. It is therefore likely that imaging agents that are analogues of ABC transporters will evolve with the development of more potent P-gp inhibitors.
In the post-genomic era of individualised medicine, ABC imaging may be helpful to adjust the treatment dose in individual patients. More research is needed to apply imaging techniques for the identification of patients susceptible to drug toxicity side effects and to provide information concerning dose adjustment by allowing better decision making when considering therapy with anticancer drugs that are substrates for ABC transporters and less toxic MDR modulators.
Evidence has emerged that combinations of chemotherapeutic drugs involved in MDR with the so-called targeted agents may improve patient outcome. Molecular imaging can be used to visualise the targets for these agents, such as HER2/neu and angiogenic factors such as vascular endothelial growth factor. Visualisation of molecular drug targets in the tumour could function as biomarkers to support treatment decisions for the individual patient[1]. Simultaneous combined imaging using both MDR and target analogues may also evolve initially in the clinical trials setting and this potential role is yet to be explored.
Conclusion
Functional imaging of MDR in cancer by single photon or positron emitters may be helpful in identifying drug-resistant tumours, thereby predicting not only non-responders, but also those who are susceptible to the development of certain malignancies or drug toxicity. Variations in the mdr1 gene product can directly affect therapeutic effectiveness. SNPs for the mdr1 gene may be associated with altered oral bioavailability of P-gp/MDR1 substrates, drug resistance, and a susceptibility to some human diseases. [99mTc]MIBI is a known ABC transporter substrate and has been shown to be a non-invasive, cost-effective predictive tool for identifying MDR in tumours but nevertheless underutilised in clinical practice, although readily available. Molecular functional imaging may guide individual treatment doses, thereby decreasing drug toxicity side effects or avoiding ineffective treatments in drug-resistant tumours and reducing economic health costs of potentially ineffective therapy in individual patients.
Footnotes
This paper is available online at http://www.cancerimaging.org. In the event of a change in the URL address, please use the DOI provided to locate the paper.
References
- 1.Nagengast W, Oude Munnink T, Dijkers E, et al. Multidrug resistance in oncology and beyond: from imaging of drug efflux pumps to cellular drug targets. Methods Mol Biol. 2010;596:15–31. doi: 10.1007/978-1-60761-416-6_2. . PMid:19949918. [DOI] [PubMed] [Google Scholar]
- 2.Lockhart A, Tirona L, Kim R. Pharmacogenetics of ATP-binding cassette transporters in cancer and chemotherapy. Mol Cancer Ther. 2003;2:685–98. PMid:12883042. [PubMed] [Google Scholar]
- 3.Leslie E, Deeley R, Cole S. Multidrug resistance proteins: role of P-glycoprotein, MRP1, MRP2, and BCRP (ABCG2) in tissue defense. Toxicol Appl Pharmacol. 2005;1:216–37. doi: 10.1016/j.taap.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 4.Sharom F. ABC multidrug transporters: structure, function and role in chemoresistance. Pharmacogenomics. 2008;9:105–27. doi: 10.2217/14622416.9.1.105. . PMid:18154452. [DOI] [PubMed] [Google Scholar]
- 5.Aller S, Yu J, Ward A, et al. Structure of P-glycoprotein reveals a molecular basis for poly-specific drug binding. Science. 2009;323:1718–22. doi: 10.1126/science.1168750. . PMid:19325113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piwnica-Worms D, Chiu M, Budding M, Kronauge J, Kramer R, Croop J. Functional imaging of multidrug-resistant p-glycoprotein with an organotechnetium complex. Cancer Res. 1993;53:977–84. PMid:8094997. [PubMed] [Google Scholar]
- 7.Sailaja K, Surekha D, Nageswara Rao D, Raghunadha Rao D, Vishnupriya S. ABCB1 (MDR1, P-glycoprotein) C3435T gene polymorphism and its possible association with chronic myeloid leukemia prognosis. Curr Trends Biotechnol Pharmacy. 2008;2:514–22. [Google Scholar]
- 8.Mizutani T, Masuda M, Nakai E, et al. Genuine functions of P-glycoprotein (ABCB1) Curr Drug Metab. 2008;9:167–74. doi: 10.2174/138920008783571756. . PMid:18288958. [DOI] [PubMed] [Google Scholar]
- 9.Chaudhary P, Mechetner E, Roninson I. Expression and activity of the multidrug resistance P-glycoprotein in human peripheral blood lymphocytes. Blood. 1992;11:2735–9. [PubMed] [Google Scholar]
- 10.Fromm M. Genetically determined differences in P-glycoprotein function: implications for disease risk. Toxicology. 2002;27:299–303. doi: 10.1016/s0300-483x(02)00297-4. [DOI] [PubMed] [Google Scholar]
- 11.Cascorbi I, Gerloff T, Johne A, et al. Frequency of single nucleotide polymorphisms in the P-glycoprotein drug transporter MDR1 gene in white subjects. Clin Pharmacol Ther. 2001;69:169–74. doi: 10.1067/mcp.2001.114164. . PMid:11240981. [DOI] [PubMed] [Google Scholar]
- 12.Jamroziak K, Mlynarski W, Balcerczak E, et al. Functional C3435T polymorphism of MDR1 gene: an impact on genetic susceptibility and clinical outcome of childhood acute lymphoblastic leukemia. Eur J Haematol. 2004;72:314–21. doi: 10.1111/j.1600-0609.2004.00228.x. . PMid:15059065. [DOI] [PubMed] [Google Scholar]
- 13.Sigesmund M, Brinkmann U, Schaffeler E, et al. Association of the P-glycoprotein transporter MDR1C3435T polymorphism with the susceptibility to renal epithelial tumours. J Am Soc Nephrol. 2002;13:1847–54. doi: 10.1097/01.ASN.0000019412.87412.BC. [DOI] [PubMed] [Google Scholar]
- 14.Seo T, Ishitsu T, Ueda N, et al. ABCB1 polymorphisms influence the response to antiepileptic drugs in Japanese epilepsy patients. Pharmacogenomics. 2006;7:551–61. doi: 10.2217/14622416.7.4.551. . PMid:16753003. [DOI] [PubMed] [Google Scholar]
- 15.Schaeffeler E, Eichelbaum M, Brinkmann U, et al. Frequency of C3435T polymorphism of MDR1 gene in African people. Lancet. 2001;358:383–4. doi: 10.1016/S0140-6736(01)05579-9. . PMid:11502320. [DOI] [PubMed] [Google Scholar]
- 16.Shukla S, Wu C, Ambudkar S. Development of inhibitors of ATP-binding cassette drug transporters: present status and challenges. Expert Opin Drug Metab Toxicol. 2008;4:205–23. doi: 10.1517/17425255.4.2.205. . PMid:18248313. [DOI] [PubMed] [Google Scholar]
- 17.Ghibellini G, Vasist L, Heizer W, Kowalsky R, Brouwer K. Quantitation of Tc-99m-sestamibi bibliary exretion in humans. Clin Pharmacol Therapeutics. 2005;79:19. doi: 10.1016/j.clpt.2005.12.069. [DOI] [Google Scholar]
- 18.Ballinger J. 99mTc-tetrofosmin for functional imaging of P-glycoprotein modulation in vivo. J Clin Pharmacol. 2001:39–47. [PubMed] [Google Scholar]
- 19.Bigott H, Prior J, Piwnica-Worms D, Welch M. Imaging multidrug resistance P-glycoprotein transport function using microPET with technetium-94m-sestamibi. Mol Imaging. 2005;4:30–9. doi: 10.1162/15353500200504166. PMid:15967124. [DOI] [PubMed] [Google Scholar]
- 20.Kannan P, John C, Zoghbi S, et al. Imaging the function of P-glycoprotein with radiotracers: pharmacokinetics and in vivo applications. Clin Pharmacol Ther. 2009;86:368–77. doi: 10.1038/clpt.2009.138. . PMid:19625998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muzi M, Mankoff D, Link J, et al. Imaging of cyclosporine inhibition of p-glycoprotein activity using 11C-verapamil in the brain: studies of healthy humans. J Nucl Med. 2009;50:1267–75. doi: 10.2967/jnumed.108.059162. . PMid:19617341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharma V, Prior J, Belinsky M, Kruh G, Piwnica-Worms D. Characterization of a 67Ga/68Ga radiopharmaceutical for SPECT and PET of MDR1 P-glycoprotein transport activity in vivo: validation in multidrug-resistant tumors and at the blood-brain barrier. J Nucl Med. 2005;46:354–64. PMid:15695797. [PubMed] [Google Scholar]
- 23.The MICAD Research Team. 4-[18F]Fluoropaclitaxel. Molecular Imaging and Contrast Agent Database [Internet]] 2006 [Google Scholar]
- 24.Bauer F, Mairinger S, Dorner B, et al. Synthesis and µPET of the radiolabelled P-glycoprotein inhibitor 11-C-tariquidar. Eur J Nucl Med Mol Imaging. 2009;36:S222. [Google Scholar]
- 25.Piwnica-Worms D, Kronauge J, Chiu M. Uptake and retention of hexakis (2-methoxyisobutyl isonitrile) technetium(I) in cultured chick myocardial cells: mitochondrial and plasma membrane potential dependence. Circulation. 1190;82:1826–38. doi: 10.1161/01.cir.82.5.1826. PMid:2225379. [DOI] [PubMed] [Google Scholar]
- 26.Piwnica-Worms D, Kronauge J, LeFurgey A. Mitochondrial localization and characterization of 99mTc-SESTAMIBI in heart cells by electron probe X-ray microanalysis and 99Tc-NMR spectroscopy. Magn Reson Imaging. 1994;12:641–52. doi: 10.1016/0730-725X(94)92459-7. . PMid:8057769. [DOI] [PubMed] [Google Scholar]
- 27.Kabasakal L, Halac M, Nisli C. The effect of P-glycoprotein function inhibition with cyclosporine A on the biodistribution of Tc-99m-sestamibi. Clin Nucl Med. 2000;25:20–3. doi: 10.1097/00003072-200001000-00005. . PMid:10634525. [DOI] [PubMed] [Google Scholar]
- 28.Marian T, Balkay L, Szabo G. Biphasic accumulation kinetics of [99mTc]-hexakis-2-methoxyisobutyl isonitrile in tumour cells and its modulation by lipophilic P-glycoprotein ligands. Eur J Pharm Sci. 2005;25:201–9. doi: 10.1016/j.ejps.2005.02.010. PMid:15911215. [DOI] [PubMed] [Google Scholar]
- 29.Ballinger J, Sheldon K, Boxen I. Differences between accumulation of 99mTc-MIBI and 201Tl-thallous chloride in tumour cells: role of P-glycoprotein. Q J Nucl Med. 1995;39:122–8. PMid:8574806. [PubMed] [Google Scholar]
- 30.Joseph D, Bhargava K, Malhi H. Sestamibi is a substrate for MDR1 and MDR2 P-glycoprotein genes. Eur J Nucl Med Mol Imaging. 2003;30:1024–31. doi: 10.1007/s00259-002-1111-z. . PMid:12536246. [DOI] [PubMed] [Google Scholar]
- 31.Koomaji R, Stammler G, Manegold C, Mattern J, Volm M. Expression of resistance-related proteins in tumoral and peritumoral tissues of patients with lung cancer. Cancer Lett. 1996;110:129–136. doi: 10.1016/S0304-3835(96)04471-0. [DOI] [PubMed] [Google Scholar]
- 32.Chen C, Meadows B, Regis J, et al. Detection of in vivo P-glycoprotein inhibition by PSC 833 using Tc-99m sestamibi. Clin Cancer Res. 1997;3:545–52. PMid:9815718. [PubMed] [Google Scholar]
- 33.Luker G, Fracasso P, Dobkin J, Piwnica-Worms D. Modulation of the multidrug resistance P-glycoprotein: detection with technetium-99m-sestamibi in vivo. J Nucl Med. 1997;38:369–72. PMid:9074520. [PubMed] [Google Scholar]
- 34.Casalta-Lopez J, Abrantes A, Rio J, et al. MDR and MDR reversal kinetics in humana adenocarcinoma cell lines. Eur J Nucl Med Mol Imaging. 2009;36:S242. [Google Scholar]
- 35.Vecchio S, Zannetti A, Salvatore B, Paone G, Fonti R, Salvatore M. Functional imaging of multidrug resistance in breast cancer. Phys Med. 2006;21:24–7. doi: 10.1016/S1120-1797(06)80019-0. . PMid:17645989. [DOI] [PubMed] [Google Scholar]
- 36.Ciarmiello A, Del Vecchio S, Silvestro P, et al. Tumor clearance of technetium 99m-sestamibi as a predictor of response to neoadjuvant chemotherapy for locally advanced breast cancer. J Clin Oncol. 1998;16:1677–83. doi: 10.1200/JCO.1998.16.5.1677. PMid:9586878. [DOI] [PubMed] [Google Scholar]
- 37.Mubashar M, Harrington K, Chaudhary K, et al. 99mTc-sestamibi imaging in the assessment of toremifene as a modulator of multidrug resistance in patients with breast cancer. J Nucl Med. 2002;43:519–25. PMid:11937596. [PubMed] [Google Scholar]
- 38.Mohan H, Miles K. Cost-effectiveness of 99mTc-sestamibi in predicting response to chemotherapy in patients with lung cancer: systematic review and meta-analysis. J Nucl Med. 2009;50:376–81. doi: 10.2967/jnumed.108.055988. . PMid:19223414. [DOI] [PubMed] [Google Scholar]
- 39.Bleichner-Perez S, Le Jeune F, Dubois F, Steinling M. 99mTc-MIBI brain SPECT as an indicator of the chemotherapy response of recurrent, primary brain tumors. Nucl Med Commun. 2007;28:888–94. doi: 10.1097/MNM.0b013e3282f1646c. . PMid:18090213. [DOI] [PubMed] [Google Scholar]
- 40.Sasajima T, Shimada N, Naitoh Y, et al. 99m)Tc-MIBI imaging for prediction of therapeutic effects of second-generation MDR1 inhibitors in malignant brain tumors. Int J Cancer. 2007;121:2637–45. doi: 10.1002/ijc.23011. . PMid:17708555. [DOI] [PubMed] [Google Scholar]
- 41.Kawata K, Kanai M, Sasada T, Iwata S, Yamamoto N, Takabayashi A. Usefulness of 99mTc-sestamibi scintigraphy in suggesting the therapeutic effect of chemotherapy against gastric cancer. Clin Cancer Res. 2004;10:3788–93. doi: 10.1158/1078-0432.CCR-1072-3. . PMid:15173086. [DOI] [PubMed] [Google Scholar]
- 42.Mubashar M, Harrington K, Chaudhary K, Lalani eN, Stamp G, Peters A. Differential effects of toremifene on doxorubicin, vinblastine and Tc-99m-sestamibi in P-glycoprotein-expressing breast and head and neck cancer cell lines. Acta Oncol. 2004;43:443–52. doi: 10.1080/02841860410031048. . PMid:15360048. [DOI] [PubMed] [Google Scholar]
- 43.Wang H, Chen X, Qiu F. Correlation of expression of multidrug resistance protein and messenger RNA with 99mTc-methoxyisobutyl isonitrile (MIBI) imaging in patients with hepatocellular carcinoma. World J Gastroenterol. 2004;10:1281–5. doi: 10.3748/wjg.v10.i9.1281. PMid:15112342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kostakoglu L. Noninvasive detection of multidrug resistance in patients with hematological malignancies: are we there yet? Clin Lymphoma. 2002;2:242–8. doi: 10.3816/CLM.2002.n.006. . PMid:11970764. [DOI] [PubMed] [Google Scholar]
- 45.Chen Y, Wang W, Chan T, Sun S, Kao A. A review of the cost-effectiveness of Tc-99m sestamibi scintimammography in diagnosis of breast cancer in Taiwanese women with indeterminate mammographically dense breast. Surg Oncol. 2002;11:151–5. doi: 10.1016/S0960-7404(02)00030-0. . PMid:12356511. [DOI] [PubMed] [Google Scholar]
- 46.Giovanella L, Suriano S, Maffioli M, Ceriani L, Spriano G. (99m)Tc-sestamibi scanning in thyroid nodules with nondiagnostic cytology. Head Neck. 2010;32:607–11. doi: 10.1002/hed.21229. PMid:19693945. [DOI] [PubMed] [Google Scholar]
- 47.Saggiorato E, Angusti T, Rosas R, et al. 99mTc-MIBI imaging in the presurgical characterization of thyroid follicular neoplasms: relationship to multidrug resistance protein expression. J Nucl Med. 2009;50:1785–93. doi: 10.2967/jnumed.109.064980. . PMid:19837765. [DOI] [PubMed] [Google Scholar]
- 48.Pace L, Catalano L, Del Vecchio S, et al. Washout of [99mTc] sestamibi in predicting response to chemotherapy in patients with multiple myeloma. Q J Nucl Med Mol Imaging. 2005;49:281–5. PMid:16172574. [PubMed] [Google Scholar]
- 49.Wong M, Evans S, Rivory L, et al. Hepatic technetium Tc 99m-labeled sestamibi elimination rate and ABCB1 (MDR1) genotype as indicators of ABCB1 (P-glycoprotein) activity in patients with cancer. Clin Pharmacol Ther. 2005;77:33–42. doi: 10.1016/j.clpt.2004.09.002. . PMid:15637529. [DOI] [PubMed] [Google Scholar]
- 50.Koziolek M, Riess R, Geiger H, Thevenod F, Hauser I. Expression of multidrug resistance P-glycoprotein in kidney allografts from cyclosporine A-treated patients. Kidney Int. 2003;60:156–66. doi: 10.1046/j.1523-1755.2001.00782.x. . PMid:11422747. [DOI] [PubMed] [Google Scholar]
- 51.Lee C. Reversing agents for ATP-binding cassette drug transporters. Methods Mol Biol. 2010;596:325–40. doi: 10.1007/978-1-60761-416-6_14. . PMid:19949930. [DOI] [PubMed] [Google Scholar]