Abstract
During transrectal ultrasound (TRUS)-guided prostate biopsies, the actual location of the biopsy site is rarely documented. Here, we demonstrate the capability of TRUS-magnetic resonance imaging (MRI) image fusion to document the biopsy site and correlate biopsy results with multi-parametric MRI findings. Fifty consecutive patients (median age 61 years) with a median prostate-specific antigen (PSA) level of 5.8 ng/ml underwent 12-core TRUS-guided biopsy of the prostate. Pre-procedural T2-weighted magnetic resonance images were fused to TRUS. A disposable needle guide with miniature tracking sensors was attached to the TRUS probe to enable fusion with MRI. Real-time TRUS images during biopsy and the corresponding tracking information were recorded. Each biopsy site was superimposed onto the MRI. Each biopsy site was classified as positive or negative for cancer based on the results of each MRI sequence. Sensitivity, specificity, and receiver operating curve (ROC) area under the curve (AUC) values were calculated for multi-parametric MRI. Gleason scores for each multi-parametric MRI pattern were also evaluated. Six hundred and 5 systemic biopsy cores were analyzed in 50 patients, of whom 20 patients had 56 positive cores. MRI identified 34 of 56 positive cores. Overall, sensitivity, specificity, and ROC area values for multi-parametric MRI were 0.607, 0.727, 0.667, respectively. TRUS-MRI fusion after biopsy can be used to document the location of each biopsy site, which can then be correlated with MRI findings. Based on correlation with tracked biopsies, T2-weighted MRI and apparent diffusion coefficient maps derived from diffusion-weighted MRI are the most sensitive sequences, whereas the addition of delayed contrast enhancement MRI and three-dimensional magnetic resonance spectroscopy demonstrated higher specificity consistent with results obtained using radical prostatectomy specimens.
Keywords: Prostate cancer, multi-parametric MR imaging, TRUS/MRI fusion tracking
Introduction
Prostate cancer is the most common solid-organ malignancy among American men with an estimated incidence and annual death rate of 217,730 and 32,050, respectively in 2010[1]. It is generally diagnosed with systematic transrectal ultrasound (TRUS)-guided core biopsies. However, the exact site of each biopsy is not routinely documented and thus, it can be difficult to determine the accuracy of other imaging studies such as magnetic resonance imaging (MRI) without a surgical specimen. This makes it difficult to study populations who do not undergo surgery such as those on active surveillance or those undergoing radiation therapy. Moreover, failure to document the location of a biopsy may lead to subsequent oversampling of some regions and undersampling of other regions of the prostate gland.
MRI has emerged as the best non-invasive imaging method for the detection of prostate cancer due to its superior soft tissue resolution and the ability to interrogate the prostate gland using a variety of different MRI parameters. It is therefore desirable to document the location of prostate biopsies on MRI. The introduction of TRUS-MRI fusion enables the localization of each biopsy site to be determined and correlated with multi-parametric MRI. From this, the relative accuracy of each MRI parameter can be assessed without requiring that patients undergo surgery to be evaluable. The feasibility of this approach has been documented with single parameter MRI (T2 weighting)[2]. Here, we use TRUS-MRI fusion to document the location of 12-core biopsy and validate the results from multi-parametric prostate MRI in a cohort of 50 patients.
Materials and methods
Study design and patient population
This prospective single institution study was approved by the local institutional review board and was compliant with Health Insurance Portability and Accountability Act; informed consent was obtained from each patient. Fifty consecutive patients who underwent multi-parametric prostate MRI and subsequent TRUS-MRI fusion guided prostate biopsy with electromagnetic (E-M) needle tracking were included in the study population. The median age of the patients was 61 years (mean 61.6 ± 8.4 years), and the median serum prostate-specific antigen (PSA) level was 5.8 ng/ml (mean 8.7; range 2.0–14.6 ng/ml).
MR imaging
MR imaging studies were performed using a combination of an endorectal coil (BPX-30, Medrad, Pittsburgh, PA, USA) and a 6-channel cardiac SENSE coil (Philips Healthcare, Best, Netherlands) on a 3-T magnet (Achieva, Philips Healthcare, Best, Netherlands) without prior bowel preparation. After digital rectal examination, the endorectal coil was inserted using a semi-anesthetic gel (Lidocaine, AstraZeneca, USA) while the patient was in the left lateral decubitus position. The balloon surrounding the coil was distended with perfluorocarbon (Fluorinert FC-770, 3 M, St. Paul, MN, USA) to a volume of approximately 50 ml to reduce susceptibility artifacts. The MR imaging protocol included T2-weighted (T2W) images in 3 planes (axial, coronal and sagittal), apparent diffusion coefficient (ADC) maps based on diffusion-weighted (DW) MRI, three-dimensional MR spectroscopy and dynamic contrast-enhanced (DCE) MRI; the imaging parameters are summarized in Table 1.
Table 1.
MRI parameters used in the current study
| MRI sequence | TR/TE (ms) | FOV (mm) | Resolution (mm) | Matrix | Flip angle | Slice thickness (mm) |
|---|---|---|---|---|---|---|
| Sagittal T2W TSE | 2659/120 | 140 | 0.46 × 0.6 × 3.0 | 304× 234 | 90 | 3 |
| Axial T2W TSE | 4434/120 | 140 | 0.46 × 0.6 × 3.0 | 304× 234 | 90 | 3 |
| Coronal T2W TSE | 2174/120 | 140 | 0.46 × 0.6 × 3.0 | 304× 234 | 90 | 3 |
| Axial DW MRIa | 5770/52 | 160 | 1.25 × 1.25 × 3.0 | 112× 108 | 90 | 3 |
| 3D MR PRESSb | 980/100 | 72 | 6.0 × 6.0 × 6.0 | 10× 10 | 90 | 6 |
| Axial pre-contrast T1 | 5.5/2.1 | 260 | 0.86 × 1.18 × 6.0 | 256× 186 | 5 | 6 |
| Axial 3D DCEc | 5.5/2.1 | 260 | 0.86 × 1.18 × 6.0 | 188× 96 | 15 | 6 |
FOV, field of view; TE, echo time; TR, repetition time; TSE, turbo spin echo.
aAxial DW multi-slice images with 20 slices taken with 5 evenly spaced B values from 0 to 750 s/mm2 and ADC maps were calculated.
b3D MR point resolved spectroscopy; water and fat signals were suppressed before data collection; each spectrum (1024 complex points) was obtained from a voxel size of 6 × 6 × 6 mm3 tissue with spectral width of 2000 Hz. Second-order shimming was used to maximize magnetic field homogeneity in the localized volume.
cAxial DCE images before, during, and after a single-dose injection of gadopentetate dimeglumine (Magnevist, Berlex, Wayne, NJ, USA) at a dose of 0.1 mmol/kg through a peripheral vein at a rate of 3 ml/s via a mechanical injector (Spectris MR Injection System, Medrad, Pittsburgh, PA, USA). The DCE acquisition consisted of a 10-slice, 3D T1W fast-field echo with a phase encoding direction from left to right without fat saturation. Four unenhanced sets (13 s) and approximately 96 contrast-enhanced sets of images were acquired sequentially without a delay between acquisitions. A total of 1000 images were obtained during DCE MRI (temporal resolution = 3.1 s).
TRUS-MRI fusion guided biopsy with E-M needle tracking
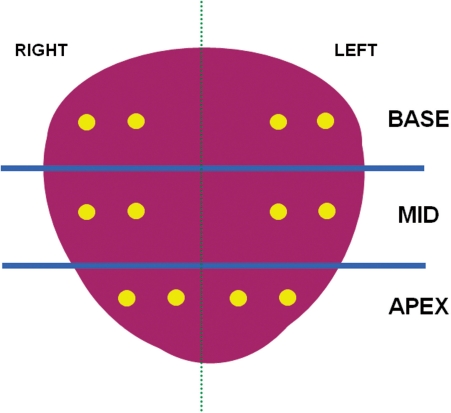
The median interval between diagnostic MRI studies and TRUS-guided biopsy procedures was 12 days (mean 30.2 days; range 3–133 days). The pre-operative MR images are imported directly from the picture archiving and communication systems (PACS). An electromagnetic field generator (Northern Digital Inc., Waterloo, Canada) is placed above the pelvis, which allows for real-time tracking of a custom biopsy needle guide (Civco Inc, Kalona IA, USA) embedded with a miniature electromagnetic tracking sensor (Philips Healthcare, Toronto, Canada). A two-dimensional axial TRUS sweep was performed from the base to the apex of the prostate to reconstruct a 3D volume of the prostate before each biopsy procedure. This volume was used as a reference for TRUS-MRI registration and motion compensation. Once registered to the MRI, TRUS-guided biopsies were performed using a navigation system that was previously developed for targeted prostate biopsy procedures[3–5]. Although it is possible to display the real-time ultrasound image and the superimposed fused MRI during biopsy, for this study only the TRUS image was visible to the operator. A disposable needle guide with two 5 degrees of freedom electromagnetic sensors (Traxtal Inc., Waterloo, Ontario, Canada) was attached to an end-firing endorectal probe (C9-5 Philips Healthcare, Bothell, WA, USA), allowing the probe to be tracked throughout the procedure with 6 degrees of freedom. The real-time TRUS images were captured using a frame grabber. The tracking information and the synchronized ultrasound video stream were recorded with a dedicated workstation. A 12-core biopsy (Fig. 1) was performed for each patient by a senior urologic surgeon (P.A.P.) and an interventional radiologist (B.J.W.) and the needle track for each biopsy core was also documented. Biopsies were performed blinded to pre-procedural MRI data.
Figure 1.
Coronal view of a 12-core prostate biopsy schema used in the current study (yellow dots represent biopsy sites).
Retrospective analysis of 12-core biopsy sites on MRI
The position of each biopsy specimen was annotated on the MRI by translating the 3 coordinates of the needle track from the TRUS to the MRI. The analysis first identifies the specimen location on TRUS and then transforms the coordinates of the specimen location from TRUS to MRI using image-based registration software (Philips Research North America, Briarcliff Manor, NY, USA) that allows for image fusion between MRI and TRUS. The software is customized from the software for TRUS-MRI guided targeted biopsy[2] by replacing real-time ultrasound images and probe tracking with the recorded data (Fig. 2). Previously, this method has proven accurate within 2.4 mm and lesions were determined on MRI based on the assumption that they were at least 5 mm in diameter, thereby insuring that the lesion would be targeted even with maximum error.
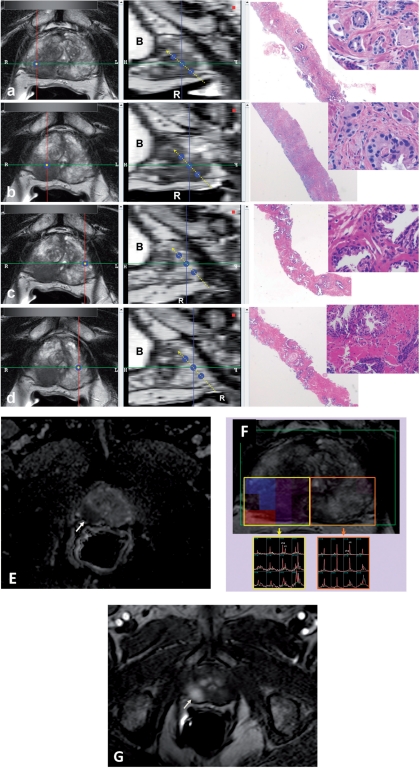
Figure 2.
The software used for MRI analysis at sextant specimen locations. Left column windows show multi-planar reconstructed images perpendicular to the biopsy core (single blue dot); the middle column windows show the sagittal views aligned with the tract of the biopsy core (triple blue dots with dashed yellow line) (a–d). T2W MRI findings of RML (a), RMM (b), LML (c) and LMM (d) peripheral zone biopsy core sites of which RML and RMM core sites show positive MRI findings for tumor, whereas LML and LMM core sites appear normal. The biopsy results for RML (a), RMM (b), LML (c) and LMM (d) are Gleason 4 + 4 (70%), Gleason 4 + 5 (50%), benign and benign, respectively. Right column windows show the corresponding hematoxylin/eosin stained biopsy images with 2× and 40× magnification. RML, right mid lateral; RMM, right mid medial; LML, left mid lateral; LMM, left mid medial; R, rectum; B, bladder. Multi-parametric MRI sequences (ADC maps of DW MRI (e), MRS (f) and DCE MRI (g)) localize the right sided tumor (arrows).
MRI analysis of the biopsy core sites was performed by 2 radiologists (B.T., P.L.C.) in consensus. Each MRI data set was evaluated separately and independently blinded to biopsy results. All MRI studies except 3D MR spectroscopy (MRS) were analyzed on customized in-house software, which enabled display of the multi-planar reconstructions (MPRs) of the MR images based on the position and orientation of each specimen, allowing the radiologist to browse the MR images along the angle of each biopsy core. Each biopsy core was modeled as a cylinder of 4 mm in diameter and 16 mm in length. If a biopsy core intersected an MRI-visible tumor, it was classified as positive for the sequence; otherwise it was classified as negative. On T2W MR images and ADC maps of DW MRI, the criterion for a visible lesion was a well-circumscribed, round ellipsoid, low signal intensity region within the prostate gland[6]. The 3D MRS analysis evaluated choline/citrate (Cho/Cit) ratios within voxels in the biopsy core sites. Voxels were considered abnormal when the (Cho/Cit) ratio was 3 or more standard deviations (SD) above the mean healthy Cho/Cit ratio value (≥0.373), which was defined as 0.13 ± 0.081 on the basis of results recorded from 433 healthy voxels from peripheral zone regions with negative biopsy results in 44 additional patients who were referred for prostate MR imaging and who had histologic confirmation[6]. DCE MR images were evaluated by direct visual interpretation of raw dynamic enhanced T1W images and the diagnostic criterion for prostate cancer was defined as a focus of early and intense enhancement with rapid wash out compared with the background[6].
Data analysis
The correlation between multi-parametric MR imaging and histopathologic findings was determined and sensitivity, specificity, and ROC AUC values were calculated. Additionally, Gleason scores for each multi-parametric MRI pattern were evaluated.
Results
MRI results
All 50 patients underwent successful endorectal coil MRI including T2W MRI, DW MRI, MRS and DCE MRI. All patients tolerated the procedure well. All patients had positive findings on MRI necessitating biopsy that was performed within 2 months of the MRI.
TRUS-guided biopsy with E-M needle tracking
TRUS-MRI registration was successful in all 50 patients. Six hundred and five systemic biopsy cores were obtained under TRUS guidance with E-M needle tracking in the 50 patients. Of 605 sextant biopsy cores, 56 (9.3%) were found to contain tumor (n = 21 Gleason 6, n = 19 Gleason 7, n = 11 Gleason 8, n = 2 Gleason 9 and n = 3 prostatic intraepithelial neoplasia). On a per patient basis, 20 of 50 (40%) had positive biopsy cores. Following the prostate biopsy, 37 patients preferred to have active surveillance and follow up, 10 had robotic prostatectomy and 3 had radiation treatment.
Retrospective analysis of MR imaging sequences
Independent retrospective analysis of each biopsy core was performed successfully for every modality in all patients. Sensitivity, specificity, and ROC AUC values in the PZ for T2W MRI, ADC maps of DW MRI, MR spectroscopy, DCE MRI were 0.571, 0.589, 0.268, 0.411 and 0.783, 0.796, 0.985, 0.956 and 0.677, 0.693, 0.627, 0.683, respectively (Table 2). Overall (when any of the 4 MRI sequences was positive), sensitivity, specificity and ROC area values for multi-parametric MRI in were 0.607, 0.727, 0.667, respectively (Table 2). Thirteen of 21 (62% false-negative) Gleason 6 biopsy cores were found to be negative in all 4 MRI sequences, whereas 9 of 19 (47.3% false-negative) Gleason 7 biopsy cores were evaluated as negative in all 4 MRI modalities. All 13 (0% false-negative) biopsy cores that were scored as Gleason 8 or 9 (100%) were detected with MRI (Table 3).
Table 2.
Results of the retrospective evaluation of 4 MRI sequences
| MRI sequence | Sensitivity | Specificity | ROC area | Positive predictive value | Negative predictive value |
|---|---|---|---|---|---|
| T2W MRI | 0.571 | 0.783 | 0.677 | 0.212 | 0.947 |
| ADC maps of DW MRI | 0.589 | 0.796 | 0.693 | 0.228 | 0.95 |
| MRS | 0.268 | 0.985 | 0.627 | 0.652 | 0.93 |
| DCE MRI | 0.411 | 0.956 | 0.683 | 0.489 | 0.941 |
| Overalla | 0.607 | 0.727 | 0.667 | 0.185 | 0.948 |
aWhen T2W MRI or ADC maps of DW MRI or MR spectroscopy or DCE MRI is positive.
Table 3.
MRI findings of detected tumor lesions according to their Gleason scores
| T2W–, ADC–, DCE–, 3D MRS– | T2W–, ADC+, DCE–, 3D MRS– | T2W+, ADC–, DCE–, 3D MRS– | T2W+, ADC+, DCE–, 3D MRS– | T2W–, ADC+, DCE+, 3D MRS– | T2W+, ADC+, DCE+, 3D MRS– | T2W+, ADC+, DCE+, 3D MRS+ | Total | |
|---|---|---|---|---|---|---|---|---|
| PIN | – | – | – | 1 | 1 | 1 | – | 3 |
| Gleason 6 | 13 | 1 | 1 | 3 | – | 2 | 1 | 21 |
| Gleason 7 | 9 | – | – | 4 | – | 4 | 2 | 19 |
| Gleason 8 | – | – | – | 1 | – | – | 10 | 11 |
| Gleason 9 | – | – | – | – | – | – | 2 | 2 |
| Total | 22 | 1 | 1 | 9 | 1 | 7 | 15 | 56 |
PIN, prostatic intraepithelial neoplasia.
Discussion
Validating MRI findings without requiring prostatectomy specimens is important in populations where patients are either followed or treated with radiation. However, to date, biopsy results have been considered too unreliable because the exact site of the biopsy relative to the MRI findings is uncertain. Using TRUS-MRI fusion we were able to confirm the location of the biopsy sample within ± 2.4 mm thus providing information that potentially could change clinical management. For instance, in a low-risk patient with a lesion measuring 1 cm or greater on MRI, a positive biopsy result from the lesion might lead the patient to choose definitive therapy over active surveillance. On the other hand, a negative biopsy might lead to a repeat biopsy of a different part of the prostate.
Results of this analysis reveal that TRUS-MRI fusion can be used to assess the accuracy of multi-parametric MRI in non-surgical patients. For instance, this study demonstrated that MRI can detect 49% (21/43) of low-intermediate risk lesions (Gleason 7 and below) based on positive T2W MRI or ADC maps of DW MRI[7]. For lesions with Gleason score 8 and above, multi-parametric MRI yielded a tumor detection rate of 100% (13/13) and 12/13 demonstrated positive findings on every MRI sequence. The results of our study suggest that T2W MR images and ADC maps of DW MRI are most sensitive for prostate cancers, whereas DCE MRI and 3D MRS have higher specificity. As the number of positive MRI sequences increased for a particular lesion, there was a greater likelihood of a higher Gleason score at biopsy.
The concept of localizing prostate biopsies has been used previously with saturation biopsies via a transperineal template, similar to the template used for placing brachytherapy seeds. Saturation template biopsy results enable systematic mapping of the prostate based on the presumed needle location. However, saturation biopsies have been criticized for their invasiveness (often involving >50 biopsies), protracted procedure time and their expense because each biopsy must be interpreted by a pathologist[8,9]. On the other hand, the biopsy system used in this study utilizes E-M tracking of a transrectal probe to map a limited number of sites for biopsy, and adds only 10 additional minutes to the regular biopsy procedure; Since it can be used with existing TRUS equipment, it could be widely utilized at minimal additional cost[2]. This method allows more accurate and complete prostate sampling at subsequent re-biopsy when the first biopsy is negative but the patient continues to have increasing PSA values. Moreover, prospective utilization of this system can be used to plan for focal therapy such as intensity modulation radiation therapy, placement of brachytherapy seeds, or investigational types of focal therapy such as cryotherapy, laser, high-intensity focused ultrasound or alcohol ablation.
This study has a number of limitations. First, it was assumed that the TRUS-MRI fusion used for retrospective analysis was accurate; however this system has known errors secondary to the deformation of the prostate gland due to the endorectal coil. Phantom and cadaver studies have demonstrated that the accuracy of the TRUS-MRI fusion system is approximately 2.4 ± 1.2 mm[5]. Additionally, the number of patients enrolled in the current study is relatively small and most of the patients were low-intermediate risk patients. However, this group constitutes most patients with prostate cancer who have undergone screening with annual serum PSA and therefore, may require TRUS-MRI fusion biopsy.
In conclusion, TRUS-MRI fusion is feasible for documenting the location of the biopsy sites and for validating the results of different pulse sequences on MRI without the need for whole-mount prostatectomy specimens. Using this method we have shown that T2W MRI and ADC maps of DW MRI are the 2 most reliable sequences for predicting the presence of cancer, whereas DCE MRI and 3D-MRS may be used to improve the accuracy of MRI diagnosis. Moreover, multi-parametric MRI can predict higher Gleason scores, especially when all sequences are positive.
Acknowledgments
NIH and Philips have intellectual property in the field. This study was supported in part by the Intramural Research Program of the NIH. NCI contract number HHSN261200800001E.
References
- 1. American Cancer Society. Cancer Facts & Figures 2010. Atlanta, GA: American Cancer Society; 2010.
- 2.Turkbey B, Xu S, Kruecker J, et al. Documenting the location of prostate biopsies with image fusion. BJU Int. 2011;107:53–7. doi: 10.1111/j.1464-410X.2010.09483.x. . PMid:20590543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wood BJ, Zhang H, Durrani A, et al. Navigation with electromagnetic tracking for interventional radiology procedures: a feasibility study. J Vasc Interv Radiol. 2005;16:493–505. doi: 10.1097/01.RVI.0000148827.62296.B4. PMid:15802449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh AK, Kruecker J, Xu S, et al. Initial clinical experience with real-time transrectal ultrasonography-magnetic resonance imaging fusion-guided prostate biopsy. BJU Int. 2008;101:841–5. doi: 10.1111/j.1464-410X.2007.07348.x. . PMid:18070196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu S, Kruecker J, Turkbey B, et al. Real-time MRI-TRUS fusion for guidance of targeted prostate biopsies. Comput Aided Surg. 2008;13:255–64. doi: 10.3109/10929080802364645. . PMid:18821344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turkbey B, Pinto PA, Mani H, et al. Prostate cancer: value of multiparametric MR imaging at 3T for detection-histopathologic correlation. Radiology. 2010;255:89–99. doi: 10.1148/radiol.09090475. . PMid:20308447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooperberg MR, Lubeck DP, Mehta SS, Carroll PR CaPSURE. Time trends in clinical risk stratification for prostate cancer: implications for outcomes (data from CaPSURE) J Urol. 2003;170:S21–5. doi: 10.1097/01.ju.0000095025.03331.c6. . PMid:14610406. [DOI] [PubMed] [Google Scholar]
- 8.Onik G, Barzell W. Transperineal 3D mapping biopsy of the prostate: an essential tool in selecting patients for focal prostate cancer therapy. Urol Oncol. 2008;26:506–10. doi: 10.1016/j.urolonc.2008.03.005. PMid:18774464. [DOI] [PubMed] [Google Scholar]
- 9.Merrick GS, Taubenslag W, Andreini H, et al. The morbidity of transperineal template-guided prostate mapping biopsy. BJU Int. 2008;101:1524–9. doi: 10.1111/j.1464-410X.2008.07542.x. . PMid:18325064. [DOI] [PubMed] [Google Scholar]