Abstract
This pictorial essay illustrates the imaging appearances of a wide variety of metastases to the pancreas as seen on computed tomography (CT), magnetic resonance imaging and positron emission tomography/CT. Key clinical and radiologic features (lesion distribution, non-contrast imaging appearance, enhancement pattern and pattern of spread) that may aid differentiation of primary from solitary secondary pancreatic malignancies are discussed.
Keywords: Computed tomography, positron emission tomography, magnetic resonance imaging, abdominal radiology, pancreas
Introduction
As in other organs, malignancies involving the pancreas can be broadly classified into primary and secondary tumors. Among the primary malignancies, pancreatic adenocarcinomas (PCa) are the most common, comprising more than 85% of cases. In contrast, neuroendocrine tumors (NET) that are derived from endocrine pancreas are much less common, comprising just over 1% of cases[1]. Secondary malignancies of the pancreas are divided based on the mode of spread: direct invasion by a primary malignancy arising from an adjacent organ, and hematogenous spread of metastases.
The learning objectives of this review were to identify the two main modes of tumor spread to the pancreas, list the common sources of hematogenous metastases to the pancreas, and compare the key imaging features that may differentiate solitary hematogenous metastases from primary malignancies of the pancreas.
Direct invasion of the pancreas
Extrapancreatic malignancies that directly invade the pancreas typically originate in the gastrointestinal tract. These include the stomach[2], duodenum[3],transverse colon[4], and gallbladder[5]. The location of the primary tumor influences the site of pancreatic invasion. For example, a tumor originating in the gallbladder spreads contiguously along the hepatoduodenal ligament to involve the pancreatic head (Fig. 1).
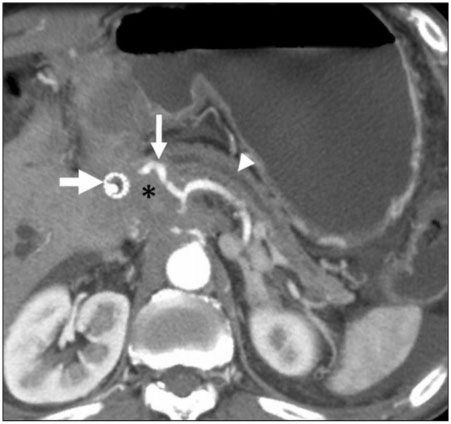
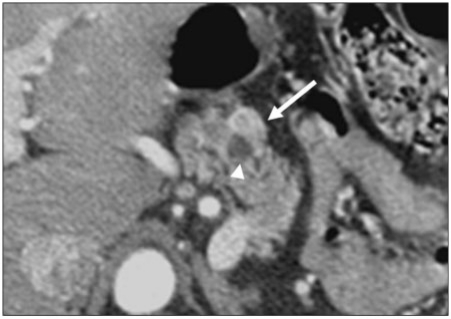
Figure 1.
A 45-year-old woman with locally advanced gallbladder cancer. Tumor has extended along the hepatoduodenal ligament. Axial contrast-enhanced CT image shows tumor (*) surrounding an endobiliary stent (bold arrow) and common hepatic artery (thin arrow). Note dilatation of the pancreatic duct (arrowhead), a sign that tumor has invaded the pancreas. The gallbladder was entirely replaced and is not shown.
Direct invasion of tumor into the pancreas confers a worse prognosis[4] and the survival rates for patients with duodenal adenocarcinoma invading the pancreas have been shown to be significantly worse than in those without (16% versus 78%)[3]. Similar findings were reported in patients with gastric cancer; the presence of pancreatic invasion was associated with increased vascular, nodal and hepatic involvement[2]. Direct invasion of the pancreas is an important consideration in surgical planning, since en bloc resection is feasible in selected patients[2], and a multivariate analysis in a series of 29 patients showed improved overall survival after complete clearance with a negative resection margin[4]. Direct invasion of the pancreas is usually clearly evident on cross-sectional imaging, and is not discussed further in this article.
Hematogenous metastases to the pancreas
Patients with metastases to the pancreas are usually identified in one of three ways: during initial metastatic workup, routine surveillance after resection of the primary lesion, and symptoms secondary to the lesion[6]. Jaundice (25%) and abdominal pain (20%) were the most common presenting symptoms of metastases to the pancreas[7].
Hematogenous spread of metastases to the pancreas is uncommon, and the prevalence is low (20 of 1050) at 1.9–2%[8,9]. The most commonly reported primary tumors metastatic to the pancreas are lung, gastrointestinal tract, kidney, breast tumors and melanoma[10]. Gynecological malignancies, gastrointestinal stromal tumors, sarcomas, lymphoma and adrenal cortical carcinoma are rare (Figs. 2–4).
Figure 2.
(a) A 49-year-old woman with a history of cervical cancer. Axial contrast-enhanced CT image showing hypovascular metastasis (arrow) in the pancreatic tail. A second deposit is demonstrated in the left kidney (arrowhead). The pancreatic metastasis is hypoattenuating, similar to pancreatic adenocarcinoma (PCa). Unlike PCa, this metastasis is relatively well defined, and is not associated with pancreatic ductal dilatation or regional vascular invasion. (b) Contrast-enhanced CT image at a more inferior position in the pancreas showing additional lesions (arrowheads). Metastases were homogeneously hypodense to pancreatic parenchyma. The superior mesenteric vein is intact even though one lesion (arrow) lies just adjacent to it. Lack of vascular invasion favors metastatic disease over PCa. However, multifocal pancreatic involvement is the strongest factor supportive of metastatic disease.
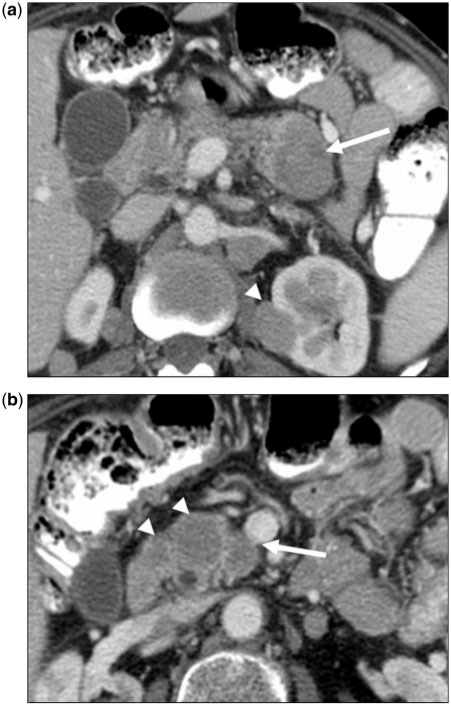
Figure 3.
(a) A 45-year-old man with a history of retroperitoneal sarcoma. Axial contrast-enhanced CT image of a patient with unclassified retroperitoneal sarcoma metastasizing to the pancreatic head. Large lesion (arrow) is heterogeneously hypodense to the rest of the pancreas, however does not cause pancreatic ductal dilation or occlusion of the superior mesenteric vein (arrowhead). (b) Axial fat-suppressed T2-weighted fast spin echo image of the same lesion showing minimally prominent caliber of the pancreatic duct (arrow). Imaging findings favor metastatic disease, as pancreatic adenocarcinoma of this size would be expected to cause significant biliary and pancreatic ductal dilatation.
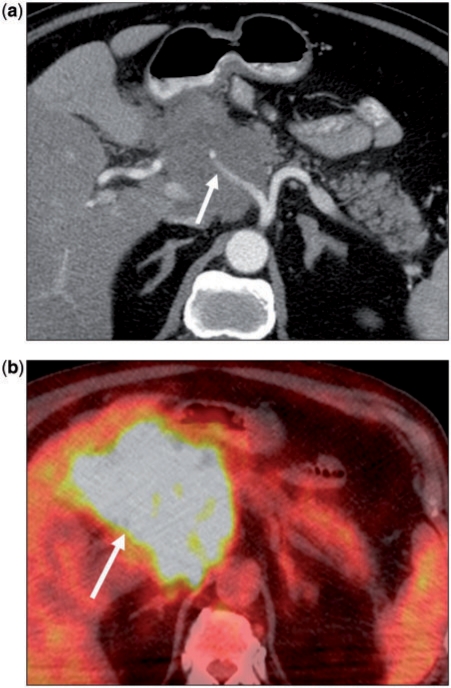
Figure 4.
(a) A 75-year-old woman with non-Hodgkin lymphoma. Axial contrast-enhanced CT image showing a hypodense mass in the pancreatic head. The common hepatic artery (arrow) is encased. Note there is no pancreatic ductal dilation. (b) Corresponding fusion positron emission tomography (PET)/CT image shows marked hypermetabolism in keeping with the primary diagnosis. However, such a finding is not specific, as even primary pancreatic adenocarcinoma would be expected to show increased metabolic activity.
In general, the presence of hematogenous metastases to the pancreas implies disseminated disease and a poor prognosis with a reported median survival of 8.7 months[9]. There may be a significantly long latency period between initial diagnosis of the primary tumor and subsequent detection of pancreatic metastases. For example, in a series of patients with renal cell carcinoma (RCC)[11], most (88%) metastases were detected metachronously, with an average lag time of 9.2 years. RCC metastases have been reported up to 21 years after treatment of the primary tumor[12]. Long latency periods have also been reported for patients with breast cancer[6].
Surgical resection of pancreatic metastases is usually not indicated in disseminated disease, except in select patients where tumor burden is low and limited to the pancreas. However, when metastases to the pancreas in such select patients are successfully resected, the prognosis may be significantly improved.
Features of hematogenous metastases to the pancreas
Clinical findings
In the setting of a known pancreatic mass, the sensitivity and specificity of carbohydrate antigen (CA) 19-9 for PCa, although approximately 81% and 89% respectively, is still considered limited[13]. Clinical and laboratory findings such as Zollinger–Ellison syndrome (in gastrinoma) and recurrent episodes of hypoglycemia (in insulinoma) can be extremely helpful in the diagnosis of primary functioning pancreatic NET. A history of extrapancreatic malignancy can be crucial for the diagnosis of metastatic disease to the pancreas.
Lesion distribution
In terms of the distribution of metastases within the pancreas, there is little difference: 35% occur in the head, 23% in the body and 35% in the tail[14]. Depiction of the sites of extrapancreatic disease, particularly when they are not typical for either PCa or NET, is an important key to the diagnosis (Fig. 5). More than 90% of patients with pathologically proven metastases to the pancreas may have extrapancreatic disease[15]. Multicentric disease within the pancreas also suggests metastatic disease[16] (Fig. 6). However, it should be noted that more than half of hematogenous metastases to the pancreas can occur singly (Fig. 7)[17]. Unlike PCa, most metastases tend to be well circumscribed although they may rarely present as diffusely infiltrative masses (Figs. 8 and 9).
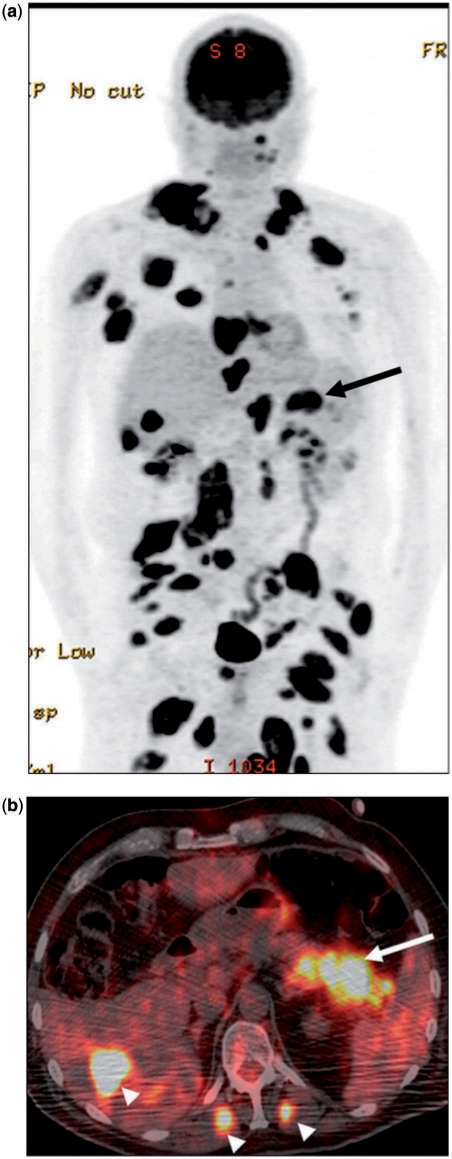
Figure 5.
(a) A 62-year-old man with known metastatic melanoma. Coronal PET image showing extensive deposits throughout the body. A large metastatic deposit was present in the pancreatic tail (arrow). (b) Corresponding fusion PET/CT image in the axial plane confirms the anatomic location of the lesion (arrow). Note the presence of extrapancreatic metastases (arrowheads) in the liver and bilateral erector spinae muscles. The latter are rarely seen in PCa, but a fairly frequent finding in metastatic melanoma.
Figure 6.
A 55-year-old woman with a history of gastrointestinal stromal tumor. Axial contrast-enhanced CT image shows multiple hypodense masses (arrows) throughout the pancreas. Multicentric disease is a sign of hematogenous metastases. Note the absence of pancreatic ductal dilatation and the presence of mass effect on the superior mesenteric vein–portal vein confluence (arrowhead).
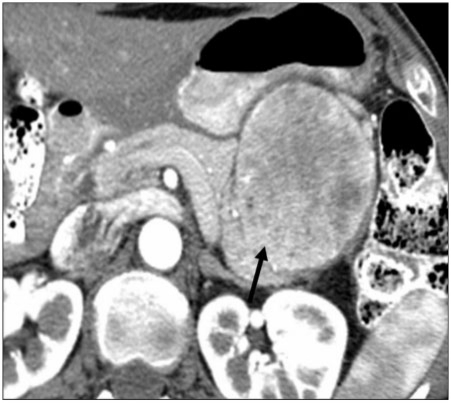
Figure 7.
A 56-year-old woman with a history of breast cancer. Axial contrast-enhanced CT image of the proximal pancreas showing single hypovascular metastasis (arrow). In general, metastatic disease to the pancreas most commonly manifests as a single focus rather than multiple. Furthermore, the well-circumscribed margins are highly atypical for PCa.
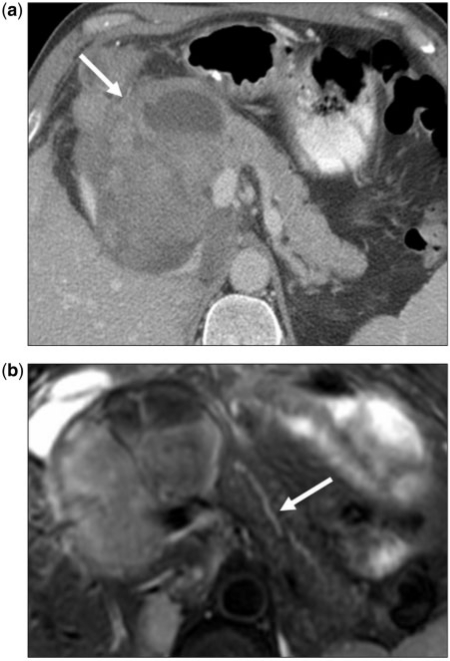
Figure 8.
A 61-year-old woman with a known history of breast cancer. Axial contrast-enhanced CT image of the pancreas shows an infiltrative large mass occupying the pancreatic body and tail (arrow). Despite the extent of disease, the superior mesenteric vein (arrowhead) is patent.
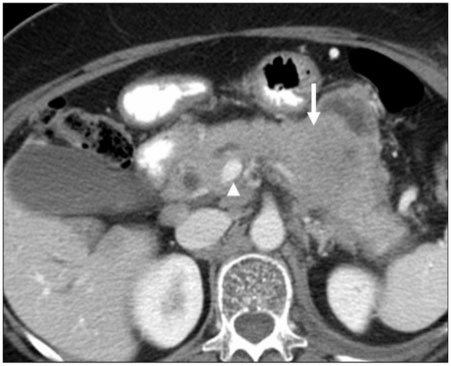
Figure 9.
A 55-year-old man with a history of renal cell carcinoma. Axial contrast-enhanced CT image showing the infiltrative pattern of a hypervascular metastasis (arrowhead) in the proximal pancreas. This metastasis was associatd with biliary obstruction (not shown), likely secondary to the relatively large size of the metastasis and its location. Biliary obstruction is more common with larger metastases. Overall, the frequency of associated biliary obstruction is less than that for primary pancreatic cancer. Note the atrophic distal pancreas with associated ductal dilatation (arrow), a sign of chronic obstruction.
Non-contrast imaging features
Relying on lesion attenuation (on CT) and signal intensity (on magnetic resonance imaging (MRI)) on non-contrast imaging is not helpful because significant overlap between primary and metastatic lesions exists. On CT, most metastases are hypoattenuating to normal pancreatic parenchyma. On T1-weighted MRI, due to the presence of enzymes, the normal pancreas shows an inherently high signal[18], whereas tumor is generally low in signal. On T2-weighted and diffusion-weighted images, malignancies are generally of higher signal intensity compared with the pancreas (Figs. 3 and 10).
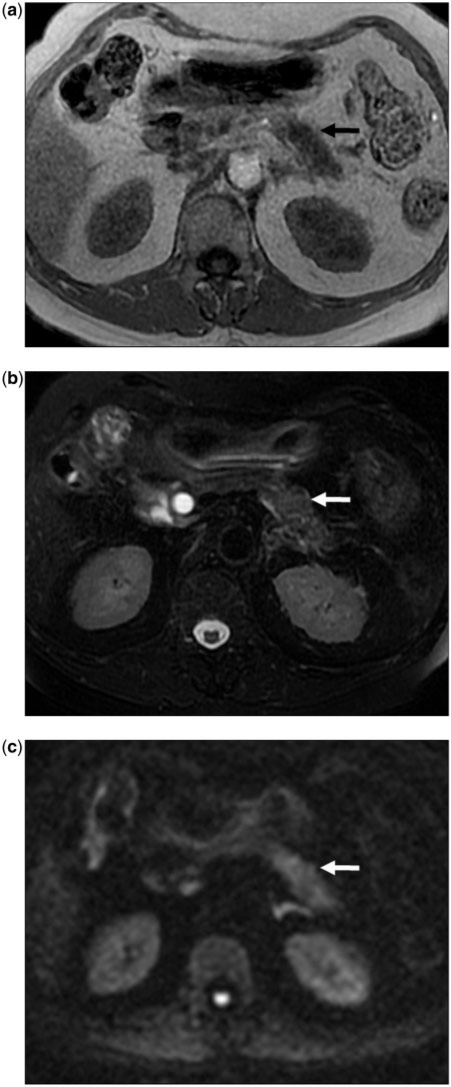
Figure 10.
(a) A 67-year-old man with typical non-contrast MRI features for PCa. Axial T1-weighted gradient recalled echo MR image at the level of the body of the pancreas showing the typical appearance for PCa. Tumor (arrow) is hypointense to the pancreas. (b) Axial fat-suppressed T2 fast spin echo MR image at the same level as (a) shows the same infiltrative mass as mildly hyperintense (arrow). The pancreas itself is not well visualized due to its relatively low signal. (c) Axial diffusion-weighted MR image (b-value 1000 s/mm2) showing heterogeneous increased signal in the body of the pancreas consistent with a tumor (arrow).
Contrast-enhanced imaging features
The enhancement pattern may be helpful in lesion characterization, particularly in hypervascular lesions, for which the list of differential diagnoses is smaller. Hypervascular lesions typically include NET (primary) (Fig. 11) and metastases (RCC, extrapancreatic NETs and medullary thyroid carcinomas) (Figs. 12 and 13). A commonly encountered feature among hypervascular metastases is that smaller tumors enhance homogenously and larger lesions show peripheral enhancement due to central necrosis, although this may not always be the case (Fig. 12).
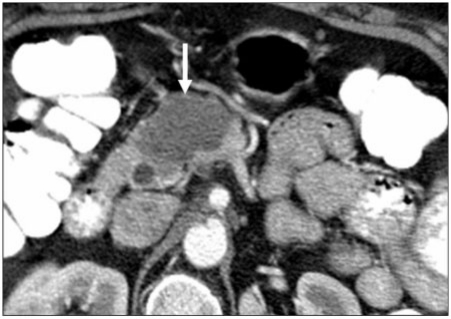
Figure 11.
A 54-year-old woman with a known primary neuroendocrine tumor of the pancreas. Axial contrast-enhanced CT image of the pancreatic tail showing the typical hypervascular nature of the tumor (arrow).
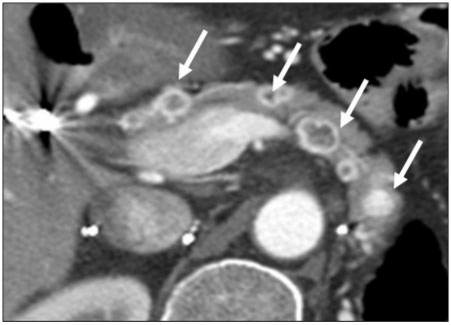
Figure 12.
A 46-year-old woman with a history of renal cell cancer. Axial contrast-enhanced CT image of a patient with a history of renal cell carcinoma showing the typical appearance of renal cell carcinoma metastases. In this case, multiple lesions (arrows) are present throughout pancreas, alluding to metastatic disease. The lesions all show central hypodensity with avid peripheral enhancement. However, there is no pancreatic ductal dilation.
Figure 13.
A 55-year-old woman with known medullary thyroid carcinoma. Axial contrast-enhanced CT image of the pancreatic neck showing small hypervascular metastasis (arrow). This lies just adjacent to the common bile duct (arrowhead), which is not invaded by tumor.
Most other metastases are typically hypovascular. The late arterial capillary phase (also known as the pancreatic phase) provides maximal pancreatic parenchymal enhancement and is optimal for lesion detection[19]. Without other associated features, it is not possible to differentiate metastatic disease from primary pancreatic malignancy based on lesion enhancement pattern.
Pattern of spread
Perineural invasion is a prominent feature of PCa, occurs in approximately 70% of cases[20], and is thought to be related to the presence of neurotropic growth factors[21]. It typically manifests as soft tissue infiltration along the peripancreatic neural plexus, which runs in tandem with the smaller branches of the peripancreatic vessels, such as the gastroduodenal and inferior pancreaticoduodenal arteries (Fig. 14)[22]. In our experience, such perineural changes are unusual for metastatic disease to the pancreas. Instead, they tend to be well circumscribed (Figs. 2 and 7).
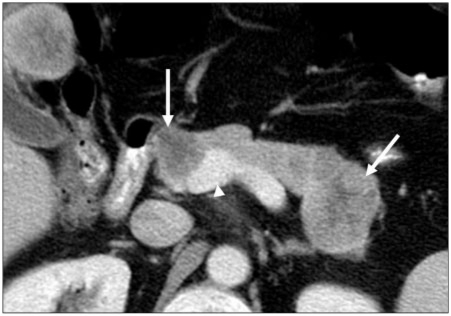
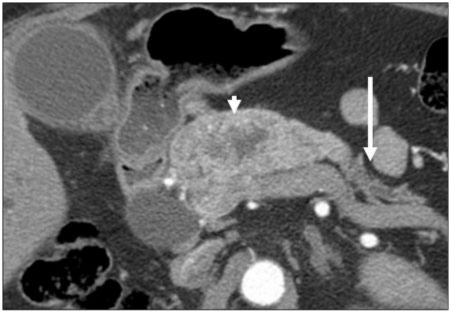
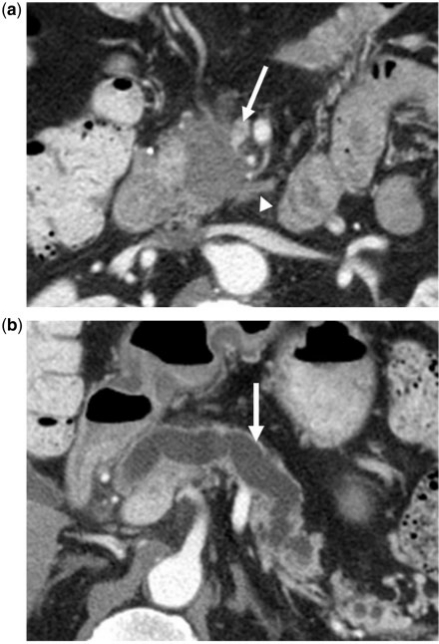
Figure 14.
(a) A 65-year-old man with a history of PCa. Axial contrast-enhanced CT image showing tumor presenting as an irregular hypodense mass in the pancreatic head. The tear-drop sign of the superior mesenteric vein (arrow) is consistent with vascular invasion. Perineural (arrowhead) invasion is likely present and involving the superior mesenteric plexus given tumor extension along the inferior pancreaticoduodenal artery. (b) Gross dilatation of the pancreatic duct (arrow) is also present and supportive of diagnosis of primary pancreatic cancer.
PCa tends to invade regional vessels, such as the superior mesenteric artery and vein, and primary NETs may demonstrate vascular invasion, with or without pancreatic ductal ingrowth. In contrast, metastatic lesions are not typically associated with such findings (Fig. 6).
Due to its ductal origin, biliary dilatation is a common feature of PCa. The exceptions to this are tumors located in the uncinate process, which do not cause biliary dilatation, thereby mimicking focal pancreatitis and other pancreatic neoplasms. In contrast, hematogenous metastases to the pancreas are not commonly associated with pancreatic main duct dilatation unless they are of relatively large size. Even in such cases, duct dilatation is often not as severe as with primary pancreatic cancer (Fig. 9).
Peripancreatic lymphadenopathy is uncommon in hematogenous metastases to the pancreas and is therefore regarded to be a sign of a primary malignancy[10].
Summary
A diverse range of metastases to the pancreas are known to occur from extrapancreatic primary malignancies. Through the use of illustrative examples, pertinent imaging findings are described, and some features that may differentiate them from primary malignancies of the pancreas are highlighted. With this knowledge, the radiologist can provide a more useful differential diagnosis.
Footnotes
This paper is available online at http://www.cancerimaging.org. In the event of a change in the URL address, please use the DOI provided to locate the paper.
References
- 1.Altekruse SF, Kosary CL, Krapcho M, et al. Cancer of the pancreas (invasive), percent distribution and counts by histology among histologically confirmed cases, 2003–2007. Bethesda, MD: National Cancer Institute; SEER cancer statistics review, 1975–2007. Available at: http://seer.cancer.gov/csr/1975_2007/browse_csr.php?section=22&page=sect_22_table.22.html, based on November 2009 SEER data submission (accessed 8 July 2010) [Google Scholar]
- 2.Maehara Y, Oiwa H, Tomisaki S, et al. Prognosis and surgical treatment of gastric cancer invading the pancreas. Oncology. 2000;59:1–6. doi: 10.1159/000012128. . PMid:10895058. [DOI] [PubMed] [Google Scholar]
- 3.Ohigashi H, Ishikawa O, Tamura S, et al. Pancreatic invasion as the prognostic indicator of duodenal adenocarcinoma treated by pancreatoduodenectomy plus extended lymphadenectomy. Surgery. 1998;124:510–5. doi: 10.1016/S0039-6060(98)70097-2. . PMid:9736903. [DOI] [PubMed] [Google Scholar]
- 4.Varker KA, Muscarella P, Wall K, Ellison C, Bloomston M. Pancreatectomy for non-pancreatic malignancies results in improved survival after R0 resection. World J Surg Oncol. 2007;5:145. doi: 10.1186/1477-7819-5-145. . PMid:18162131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirano S, Tanaka E, Shichinohe T, et al. Feasibility of en-bloc wedge resection of the pancreas and/or the duodenum as an alternative to pancreatoduodenectomy for advanced gallbladder cancer. J Hepatobiliary Pancreat Surg. 2007;14:149–54. doi: 10.1007/s00534-006-1109-1. . PMid:17384905. [DOI] [PubMed] [Google Scholar]
- 6.Showalter SL, Hager E, Yeo CJ. Metastatic disease to the pancreas and spleen. Semin Oncol. 2008;35:160–71. doi: 10.1053/j.seminoncol.2007.12.008. . PMid:18396201. [DOI] [PubMed] [Google Scholar]
- 7.Sweeney AD, Wu MF, Hilsenbeck SG, Brunicardi FC, Fisher WE. Value of pancreatic resection for cancer metastatic to the pancreas. J Surg Res. 2009;156:189–98. doi: 10.1016/j.jss.2009.01.017. . PMid:19375718. [DOI] [PubMed] [Google Scholar]
- 8.Volmar KE, Jones CK, Xie HB. Metastases in the pancreas from nonhematologic neoplasms: report of 20 cases evaluated by fine-needle aspiration. Diagn Cytopathol. 2004;31:216–20. doi: 10.1002/dc.20100. . PMid:15452907. [DOI] [PubMed] [Google Scholar]
- 9.Roland CF, van Heerden JA. Nonpancreatic primary tumors with metastasis to the pancreas. Surg Gynecol Obstet. 1989;168:345–7. PMid:2928909. [PubMed] [Google Scholar]
- 10.Dubois J. TxNxM1: the anatomy and clinics of metastatic cancer. Boston, MA: Kluwer; 2002. pp. 68–9. [Google Scholar]
- 11.Sellner F, Tykalsky N, De Santis M, Pont J, Klimpfinger M. Solitary and multiple isolated metastases of clear cell renal carcinoma to the pancreas: an indication for pancreatic surgery. Ann Surg Oncol. 2006;13:75–85. doi: 10.1245/ASO.2006.03.064. . PMid:16372157. [DOI] [PubMed] [Google Scholar]
- 12.Rypens F, Van Gansbeke D, Lambilliotte JP, Van Regemorter G, Verhest A, Struyven J. Pancreatic metastasis from renal cell carcinoma. Br J Radiol. 1992;65:547–8. doi: 10.1259/0007-1285-65-774-547. . PMid:1628191. [DOI] [PubMed] [Google Scholar]
- 13.Cwik G, Wallner G, Skoczylas T, Ciechanski A, Zinkiewicz K. Cancer antigens 19-9 and 125 in the differential diagnosis of pancreatic mass lesions. Arch Surg. 2006;141:968–73. doi: 10.1001/archsurg.141.10.968. discussion 974. PMid:17043274. [DOI] [PubMed] [Google Scholar]
- 14.Klein KA, Stephens DH, Welch TJ. CT characteristics of metastatic disease of the pancreas. Radiographics. 1998;18:369–78. doi: 10.1148/radiographics.18.2.9536484. PMid:9536484. [DOI] [PubMed] [Google Scholar]
- 15.Ferrozzi F, Bova D, Campodonico F, Chiara FD, Passari A, Bassi P. Pancreatic metastases: CT assessment. Eur Radiol. 1997;7:241–5. doi: 10.1007/s003300050144. . PMid:9038124. [DOI] [PubMed] [Google Scholar]
- 16.Wente MN, Kleeff J, Esposito I, et al. Renal cancer cell metastasis into the pancreas: a single-center experience and overview of the literature. Pancreas. 2005;30:218–22. doi: 10.1097/01.mpa.0000153337.58105.47. . PMid:15782097. [DOI] [PubMed] [Google Scholar]
- 17.Muranaka T, Teshima K, Honda H, Nanjo T, Hanada K, Oshiumi Y. Computed tomography and histologic appearance of pancreatic metastases from distant sources. Acta Radiol. 1989;30:615–9. doi: 10.3109/02841858909174725. . PMid:2631949. [DOI] [PubMed] [Google Scholar]
- 18.Megibow AJ, Lavelle MT, Rofsky NM. MR imaging of the pancreas. Surg Clin North Am. 2001;81:307–20. doi: 10.1016/S0039-6109(05)70119-5. ix–x. . PMid:11392418. [DOI] [PubMed] [Google Scholar]
- 19.McNulty NJ, Francis IR, Platt JF, Cohan RH, Korobkin M, Gebremariam A. Multi-detector row helical CT of the pancreas: effect of contrast-enhanced multiphasic imaging on enhancement of the pancreas, peripancreatic vasculature, and pancreatic adenocarcinoma. Radiology. 2001;220:97–102. doi: 10.1148/radiology.220.1.r01jl1897. PMid:11425979. [DOI] [PubMed] [Google Scholar]
- 20.Hirai I, Kimura W, Ozawa K, et al. Perineural invasion in pancreatic cancer. Pancreas. 2002;24:15–25. doi: 10.1097/00006676-200201000-00003. . PMid:11741178. [DOI] [PubMed] [Google Scholar]
- 21.Farrow B, Evers BM. Inflammation and the development of pancreatic cancer. Surg Oncol. 2002;10:153–69. doi: 10.1016/S0960-7404(02)00015-4. . PMid:12020670. [DOI] [PubMed] [Google Scholar]
- 22.Deshmukh SD, Willmann JK, Jeffrey RB. Pathways of extrapancreatic perineural invasion by pancreatic adenocarcinoma: evaluation with 3D volume-rendered MDCT imaging. AJR Am J Roentgenol. 2010;194:668–74. doi: 10.2214/AJR.09.3285. . PMid:20173143. [DOI] [PubMed] [Google Scholar]