Abstract
Combined hepatocellular carcinoma-cholangiocarcinoma (cHCC-CC) is a rare entity comprising 1–14.2% of all primary liver carcinomas. In this report, we present a case of rapid progression of cHCC-CC, a rare tumor in a 77-year-old Caucasian male patient with hepatitis B-induced cirrhosis, moderately elevated alpha fetoprotein, and imaging and pathologic features of a mixed liver tumor. There was no evidence of metastatic disease in the chest, abdomen or pelvis by computed tomography (CT) scan at the time of diagnosis. Needle biopsy of the segment 8 lesion revealed two discrete histologic components to the tumor: well-differentiated HCC and poorly differentiated adenocarcinoma, consistent with intrahepatic CC.The patient rapidly developed metastatic disease after initial local therapy with hepatic arterial chemoembolization and percutaneous cryoablation, dying within 5 months of diagnosis. Radiofrequency ablation, cryoablation and radioembolization with yttrium-90 microspheres remain possible treatment strategies for patients with cHCC-CC unable to undergo surgical resection. The diagnosis and treatment of cHCC-CC can be challenging due to clinical, imaging and histological features that overlap with pure HCC and CC.
Keywords: Combined hepatocellular carcinoma and cholangiocarcinoma, MRI
Introduction
Combined hepatocellular carcinoma and cholangiocarcinoma (cHCC-CC) is a rare entity comprising 1–14.2% of all primary liver carcinomas[1,2]. In this report, we present a case of rapid progression of cHCC-CC in an asymptomatic Caucasian male patient with hepatitis B-induced cirrhosis, moderately elevated alpha fetoprotein (AFP), and imaging and pathologic features of a mixed tumor.
Case report
A 77-year-old Caucasian male with a history of hepatitis B-induced cirrhosis presented to his gastroenterologist with a newly elevated serum AFP level (389.5 ng/ml) on routine screening. He was residing in North America and had no significant travel history to the East. He had no abdominal discomfort, jaundice or change in his bowel habits. Further review of systems was negative. His past medical history was remarkable for hypertension, type 2 diabetes mellitus, thrombocytopenia, chronic renal insufficiency requiring hemodialysis due to diabetic nephropathy, and congestive heart failure. He had stopped tobacco smoking and alcohol consumption 30 years previously. On physical examination, the abdomen was soft, non-tender, non-distended without palpable hepatosplenomegaly, masses, or evidence for ascites. On further laboratory testing, albumin was 3.8 g/dl, total bilirubin 1.2 mg/dl, platelets 161,000 cells/mm3, and international normalized ratio (INR) 1.1.
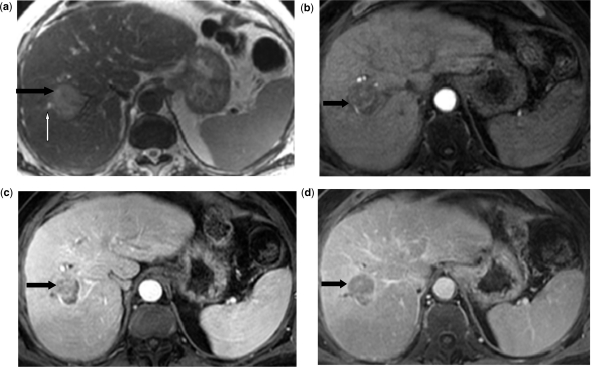
Abdominal magnetic resonance imaging (MRI) revealed a 4.0 × 3.8 cm mass in segment 8 of the liver, which was mildly hyperintense on T2-weighted images (Fig. 1a), hypointense on T1-weighted images, with early continuous peripheral rim enhancement (Fig. 1b) and revealed heterogeneous fill-in on venous (Fig. 1c) and delayed post-contrast images (Fig. 1d). There was segmental biliary dilatation distal to the mass without evidence of vascular invasion or thrombosis. An adjacent 1.1 × 0.9 cm satellite nodule in the right lobe demonstrated similar signal intensity and enhancement characteristics. Morphologic changes of cirrhosis were present. There was no evidence of metastatic disease in the chest, abdomen or pelvis by computed tomography (CT) scan.
Figure 1.
Abdominal MRI reveals a 4.0 × 3.8 cm mass in segment 8 of the liver which is mildly hyperintense on T2-weighted images (a) (black arrow), hypointense on T1-weighted images, with continuous peripheral rim enhancement on arterial phase (b) (black arrow), and heterogeneous fill-in on venous (c) and delayed post-contrast images (d) (black arrows). There is segmental biliary dilatation distal to the mass (long white arrow) without evidence of vascular invasion or thrombosis.
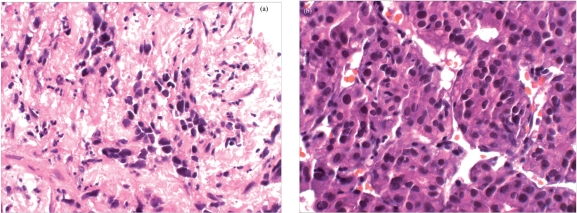
Needle biopsy of the segment 8 lesion revealed two discrete histologic components to the tumor (Fig. 2a,b): well-differentiated hepatocellular carcinoma (HCC) and poorly differentiated adenocarcinoma, consistent with intrahepatic cholangiocarcinoma (CC). Immunohistochemical analysis of the adenocarcinoma component showed positive staining for CK7, CK20, and carcinoembryonic antigen, and negative staining for HepPar-1 and synaptophysin. The background revealed established cirrhosis. On the basis of these two histologic patterns of tumor, a diagnosis of combined hepatocellular/cholangiocarcinoma (cHCC-CC) was established.
Figure 2.
Needle biopsy of the segment 8 liver lesion revealed two discrete histologic components to the tumor (a,b); well-differentiated HCC and poorly differentiated adenocarcinoma, consistent with intrahepatic CC. The background reveals established cirrhosis.
Due to the patient’s extensive comorbidities, underlying cirrhosis, and multifocal intrahepatic tumor, he was not considered a surgical candidate. After exploration of treatment options, he underwent right hepatic arterial chemoembolization with mitomycin, adriamycin and cisplatin along with percutaneous cryoablation of the segment 8 lesion. Post-procedure imaging demonstrated good coverage of the treated area. The patient tolerated the procedures well, although in the ensuing several weeks he developed generalized weakness, diminished appetite and a weight gain of 4.5 kg due to fluid retention. His liver function tests remained normal, though his AFP rapidly increased to 2200 ng/ml over the course of 2 months.
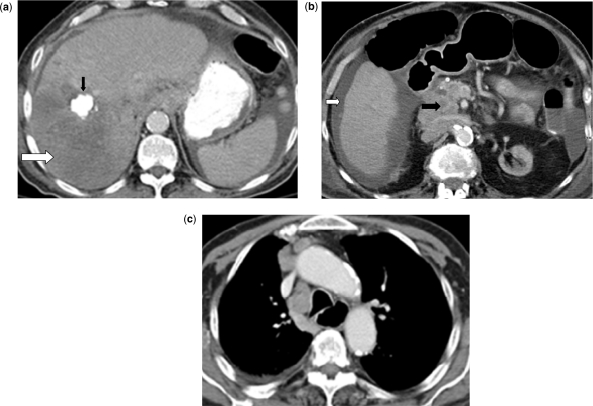
Abdominal CT scan revealed marked interval progression of disease, with a 13 × 10 cm recurrent liver mass that replaced most of the right lobe (Fig. 3a), new liver lesions, new portal vein thrombosis, metastatic lung nodules and metastatic adenopathy in the mesentery, retroperitoneum (Fig. 3b), and mediastinum (Fig. 3c). He died 5 months after his initial diagnosis due to progressive metastatic disease.
Figure 3.
Abdominal CT scan reveals a 13 × 10 cm recurrent hypodense liver mass that replaced most of the right lobe (a) (white arrow), a calcified nodular lesion in the right lobe due to prior chemoembolization (black arrow), ascites (b) (white arrow), metastatic adenopathy in the mesentery, retroperitoneum (b) (black arrow). Chest CT scan reveals metastatic mediastinal adenopathy (c).
Discussion
This case highlights some of the distinct clinical, imaging and pathologic features of cHCC-CC. cHCC-CC was first classified in 1949 on a histopathological basis by Allen and Lisa[1] into three subtypes: type A (dual cancer) wherein HCC and CC are present in different locations within the liver; type B (combined type) which shows HCC and CC components in the same location; and type C (mixed type), which contains an intimate mixture of HCC and CC within the confines of the tumor mass.
Several reports have documented both similarities and differences of cHCC-CC relative to either pure HCC or pure CC. In Western patients, Jarnagin et al.[3] reported large tumors in patients with cHCC-CC, and a low association with cirrhosis and chronic infection by hepatitis B or C, similar to patients with pure CC. The lower frequency of cirrhosis in the cHCC-CC population permitted more curative surgical resections in this group of patients. In contrast, other case series report a high incidence of viral hepatitis and cirrhosis among Eastern patients with cHCC-CC[4–7], similar to patients with pure HCC.
Combined tumors with mixed features of both HCC and CC can be difficult to diagnose on imaging. Findings at contrast-enhanced CT or MR imaging may include classic features of HCC or cholangiocarcinoma, or a combination of the two. Three CT appearances of combined tumors have been described[8]. One relatively large series of 30 patients with cHCC-CC reported imaging features of both HCC and CC, with masses containing areas of arterial hyperenhancement and subsequent washout, similar to pure HCC, as well as areas of delayed enhancement, similar to CC[9]. In another series, a diagnosis of cHCC-CC was suggested prospectively by CT imaging in only one-third of the patients[10]. On MRI, HCC, CC and combined tumors are typically moderately hyperintense compared with adjacent liver on T2-weighted imaging, with enhancement patterns similar to those seen by CT[11–16]. Additional findings seen in each of the three types of primary liver cancers include satellite nodules, biliary ductal dilation, portal or hepatic venous involvement and adenopathy[4,5,11,17].
In non-cirrhotic patients, the treatment of choice for cHCC-CC is hepatic resection with hilar lymph node dissection[18]. Median reported disease-free survival for patients with resectable HCC-CC is 10 months, with median overall survival time of 20 months[4,6]. Poor prognostic factors include the presence of vascular or bile duct invasion[3,4,18], satellite lesions, multiple tumors[5,18], tumor necrosis, regional lymph node involvement, and ascites[18]. Remnant liver is the most common site of recurrence after resection of cHCC-CC, followed by lymph node, lung, bone and adrenal glands[4,14,19]. Median survival is less than 1 year in unresected stage III cHCC-CC and less than 6 months in patients with metastatic disease[17].
Radiofrequency ablation (RFA), cryoablation and radioembolization with yttrium-90 microspheres remain possible treatment strategies for patients with cHCC-CC unable to undergo surgical resection[18,20]. Unresectable cHCC-CCs treated with transarterial chemoembolization appear to show a poorer response relative to pure HCC, which is thought to be due to prominent fibrosis and decreased vascularity in combined tumors, with median survival of 4 months in hypovascular and 16 months in hypervascular type of cHCC-CC[20]. Nevertheless, few reports are available using these local therapies to guide treatment decisions specifically in patients with cHCC-CC.
The appropriate systemic treatment is unclear for patients with advanced cHCC-CC. Patients with locally advanced or metastatic cholangiocarcinoma are commonly treated with gemcitabine-based chemotherapy programs[21]. Recently, the randomized, phase III Advanced Biliary Cancer-02 study demonstrated improvements in progression-free and overall survival for patients with cholangiocarcinoma or gallbladder carcinoma, who received gemcitabine plus cisplatin versus gemcitabine alone[22]. In contrast, cytotoxic chemotherapy has been relatively ineffective in the treatment of advanced HCC[23] including treatment with gemcitabine[24]. For patients with advanced HCC and Child–Pugh class A liver disease, sorafenib, an oral multikinase inhibitor, has been shown to prolong progression-free and overall survival times, compared with placebo[25]. Decisions regarding systemic therapy for patients with cHCC-CC must be made after careful consideration of the clinicopathological features of each individual case.
In this report, the patient rapidly developed metastatic disease after initial local therapy with hepatic arterial chemoembolization and percutaneous cryoablation for non-resectable cHCC-CC, dying within 5 months of diagnosis. The diagnosis and treatment of cHCC-CC can be challenging due to clinical, imaging and histological features that overlap with pure HCC and CC.
Footnotes
This paper is available online at http://www.cancerimaging.org. In the event of a change in the URL address, please use the DOI provided to locate the paper.
References
- 1.Allen RA, Lisa JR. Combined liver cell and bile duct carcinoma. Am J Pathol. 1949;25:647–55. PMid:18152860. [PMC free article] [PubMed] [Google Scholar]
- 2.Goodman ZD, Usa MM, Ishak KG, et al. Combined hepatocellular-cholangiocarcinoma: A histologic and immunohistochemical study. Cancer. 1985;55:124–35. doi: 10.1002/1097-0142(19850101)55:1<124::AID-CNCR2820550120>3.0.CO;2-Z. . PMid:2578078. [DOI] [PubMed] [Google Scholar]
- 3.Jarnagin WR, Weber S, Tickoo SK, et al. Combined hepatocellular and cholangiocarcinoma: demographic, clinical, and prognostic factors. Cancer. 2002;94:2040–6. doi: 10.1002/cncr.10392. . PMid:11932907. [DOI] [PubMed] [Google Scholar]
- 4.Yano Y, Yamamoto J, Kosuge T, et al. Combined hepatocellular and cholangiocarcinoma: a clinicopathologic study of 26 resected cases. Jpn J Clin Oncol. 2003;33:283–7. doi: 10.1093/jjco/hyg056. . PMid:12913082. [DOI] [PubMed] [Google Scholar]
- 5.Koh KC, Lee H, Choi MS. Clinicopathologic features and prognosis of combined hepatocellular cholangiocarcinoma. Am J Surg. 2005;189:120–5. doi: 10.1016/j.amjsurg.2004.03.018. . PMid:15701504. [DOI] [PubMed] [Google Scholar]
- 6.Liu CL, Fan ST, Lo CM, et al. Hepatic resection for combined hepatocellular and cholangiocarcinoma. Arch Surg. 2003;138:86–90. PMid:12511158. [PubMed] [Google Scholar]
- 7.Ng IO, Shek TW, Nicholls J, et al. Combined hepatocellular-cholangiocarcinoma: a clinicopathological study. J Gastroenterol Hepatol. 1998;13:34–40. doi: 10.1111/j.1440-1746.1998.tb00542.x. . PMid:9737569. [DOI] [PubMed] [Google Scholar]
- 8.Aoki K, Takayasu K, Kawano T, et al. Combined hepatocellular carcinoma and cholangiocarcinoma: clinical features and computed tomographic findings. Hepatology. 1993;18:1090–5. doi: 10.1002/hep.1840180512. . PMid:7693572. [DOI] [PubMed] [Google Scholar]
- 9.Fukukura Y, Taguchi J, Nakashima O, et al. Combined hepatocellular and cholangiocarcinoma: correlation between CT findings and clinicopathologic features. J Comput Assist Tomogr. 1997;21:52–8. doi: 10.1097/00004728-199701000-00011. . PMid:9022770. [DOI] [PubMed] [Google Scholar]
- 10.Nishie A, Yoshimitsu K, Asayam Y, et al. Detection of combined hepatocellular and cholangiocarcinomas on enhanced CT: comparison with histologic findings. Am J Roentgenol. 2005;184:1157–62. doi: 10.2214/ajr.184.4.01841157. [DOI] [PubMed] [Google Scholar]
- 11.Ebied O, Federle MP, Blachar A, et al. Hepatocellular-cholangiocarcinoma: helical computed tomography findings in 30 patients. J Comput Assist Tomogr. 2003;27:117–24. doi: 10.1097/00004728-200303000-00003. . PMid:12702999. [DOI] [PubMed] [Google Scholar]
- 12.Yuichi S, Kazuhiro Y, Hiroyuki I. Comparison of CT enhancement patterns and histologic features in hepatocellular carcinoma up to 2 cm: assessment of malignant potential with claudin-10 immunohistochemistry. Oncol Rep. 2007;17:1177–82. [PubMed] [Google Scholar]
- 13.Hashimoto T, Nakamura H, Hori S, et al. MR imaging of mixed hepatocellular and cholangiocellular carcinoma. Abdom Imaging. 1994;19:430–2. doi: 10.1007/BF00206932. . PMid:7950820. [DOI] [PubMed] [Google Scholar]
- 14.Yong EC, Myeong JK, Young NP, et al. Varying appearances of cholangiocarcinoma: radiologic–pathologic correlation. RadioGraphics. 2009;29:683–700. doi: 10.1148/rg.293085729. [DOI] [PubMed] [Google Scholar]
- 15.Kelekis NL, Semelka RC, Worawattanakul S, et al. Hepatocellular carcinoma in North America: a multiinstitutional study of appearance on T1-weighted, T2-weighted, and serial gadolinium-enhanced gradient echo images. Am J Roentgenol. 1998;170:1005–13. doi: 10.2214/ajr.170.4.9530051. [DOI] [PubMed] [Google Scholar]
- 16.Krinsky GA, Lee VS. MR imaging of cirrhotic nodules. Abdom Imaging. 2000;25:471–82. doi: 10.1007/s002610000015. . PMid:10931980. [DOI] [PubMed] [Google Scholar]
- 17.Lin G, Toh CH, Wu RC, et al. Combined hepatocellular cholangiocarcinoma: prognostic factors investigated by computed tomography/magnetic resonance imaging. Int J Clin Pract. 2008;62:1199–205. doi: 10.1111/j.1742-1241.2007.01291.x. . PMid:17537192. [DOI] [PubMed] [Google Scholar]
- 18.Kassahun WT, Hauss J. Management of combined hepatocellular and cholangiocarcinoma. Int J Clin Pract. 2008;62:1271–8. doi: 10.1111/j.1742-1241.2007.01694.x. . PMid:18284443. [DOI] [PubMed] [Google Scholar]
- 19.Shin CI, Lee JM, Kim SH, et al. Recurrence patterns of combined hepatocellular cholangiocarcinoma on enhanced computed tomography. J Comput Assist Tomogr. 2007;31:109–15. doi: 10.1097/01.rct.0000235072.34808.9b. . PMid:17259842. [DOI] [PubMed] [Google Scholar]
- 20.Jin HK, Hyun KY, Gi YK, et al. Nonresectable combined hepatocellular carcinoma and cholangiocarcinoma: analysis of the response and prognostic factors after transcatheter arterial chemoembolization. Radiology. 2010;255:270–7. doi: 10.1148/radiol.09091076. [DOI] [PubMed] [Google Scholar]
- 21.Hezel AF, Zhu AX. Systemic therapy for biliary tract cancers. Oncologist. 2008;13:415–23. doi: 10.1634/theoncologist.2007-0252. . PMid:18448556. [DOI] [PubMed] [Google Scholar]
- 22.Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–81. doi: 10.1056/NEJMoa0908721. . PMid:20375404. [DOI] [PubMed] [Google Scholar]
- 23.Thomas MB, Jaffe D, Choti MM, et al. Hepatocellular carcinoma: consensus recommendations of the National Cancer Institute Clinical Trials Planning Meeting. J Clin Oncol. 2010;28:3994–4005. doi: 10.1200/JCO.2010.28.7805. . PMid:20679622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fuchs CS, Clark JW, Ryan DP, et al. A phase II trial of gemcitabine in patients with advanced hepatocellular carcinoma. Cancer. 2002;94:3186–91. doi: 10.1002/cncr.10607. . PMid:12115351. [DOI] [PubMed] [Google Scholar]
- 25.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–90. doi: 10.1056/NEJMoa0708857. . PMid:18650514. [DOI] [PubMed] [Google Scholar]