Abstract
Background
5-HT1B autoreceptors regulate release of serotonin from terminals of dorsal raphe nucleus (DRN) projections. 5-HT1B expression in the DRN correlates with behavioral measures of emotion, and viral-mediated overexpression of 5-HT1B receptors in the middle DRN reduces measures of fear and anxiety in unstressed rats. Since the caudal subregion of the DRN is important in translating stress into emotional dysregulation, we explored behavioral functions of 5-HT1B autoreceptors in the caudal DRN.
Methods
We manipulated 5-HT1B autoreceptor function in rats using either viral-mediated gene transfer into the caudal DRN or systemic injections of the 5-HT1B agonist 3-(1,2,5,6-tetrahydro-4-pyridyl)-5-propoxypyrrolo[3,2-b]pyridine (CP-94,253). Rats were tested in forced swim test, open field test, and contextual fear conditioning.
Results
5-HT1B overexpression in the caudal DRN increased swimming in the forced swim test. It did not alter locomotion or thigmotaxis in the open field test, but did reduce conditioned freezing. Freezing was reduced when 5-HT1B overexpression was present only during testing, but not training. CP-94,253 exerted an inverted U-shaped dose response curve on conditioned freezing, with most pronounced effects seen at 1 mg/kg. At this dose, CP-94,253 administered before a fear retention test reduced freezing both during that session and in subsequent drug-free testing, but only when drug was paired with re-exposure to the fear context.
Conclusions
5-HT1B autoreceptors originating in the caudal DRN regulate behavioral expression of helplessness and fear. Since systemic pharmacological treatment with a 5-HT1B agonist facilitates reductions in fear, 5-HT1B receptors may be a target for the treatment of certain anxiety disorders.
Keywords: serotonin, forced swim test, fear conditioning, open field test, HSV
Introduction
The neurotransmitter 5-hydroxytryptamine (5-HT; serotonin) has been widely implicated in the development and expression of psychiatric disease states such as depression and anxiety disorders (1). The dorsal raphe nucleus (DRN), a discrete cluster of serotonergic cell bodies in the midbrain, is the major source of forebrain 5-HT (2). Synaptic 5-HT is regulated in part by 5-HT1B autoreceptors, which are Gi/o-coupled receptors found on serotonergic terminals (3). 5-HT1B autoreceptors respond to extracellular 5-HT by inhibiting further release (4) and synthesis (5) while enhancing reuptake (6), thus functioning as a negative feedback system to fine-tune synaptic 5-HT concentrations. Levels of basal 5-HT1B autoreceptor mRNA in the DRN correlate with behavioral measures of anxiety (7, 8), and are dynamically regulated by emotion-arousing events such as stress, exercise, and chemical antidepressant treatment (8-10). Studies with 5-HT1B knockout mice demonstrate a role of 5-HT1B receptors in regulating emotional and cognitive processes (9). However, it is difficult to attribute these effects to 5-HT1B autoreceptors on serotonergic terminals or 5-HT1B heteroreceptors on non-serotonergic terminals. Although autoreceptor- and heteroreceptor-containing terminals are intermingled throughout the brain, the cell bodies that express the respective mRNAs are anatomically distinguishable, with autoreceptor mRNA restricted to the midbrain raphe nuclei and heteroreceptor mRNA found throughout the forebrain, most prominently in striatum and hippocampus (10). Our laboratory has developed a viral-mediated strategy to selectively increase 5-HT1B autoreceptor mRNA at the level of the cell body via stereotaxic injection of viral vector into the DRN (11).
In previous 5-HT1B overexpression studies we targeted the ventromedial region of the DRN at midrostrocaudal levels - the DRN subregion with the greatest density of serotonergic neurons (12) and highest basal expression of 5-HT1B mRNA (13). However, emerging evidence suggests that the DRN contains subregional heterogeneity with respect to anatomical connectivity and function (14). The caudal DRN, compared to other DRN subregions, is particularly sensitive to uncontrollable stress (15, 16), uniquely regulates active escape behavior in rodent models of “learned helplessness” (19), and is molecularly dysregulated in the brains of human suicide victims (20). Here we tested the effects of increased 5-HT1B expression in the caudal DRN in rodent behavioral models of fear, anxiety, and depression. Additionally, we tested whether systemic treatment with a selective 5-HT1B agonist reduced measures of conditioned fear.
Methods and Materials
Animal care
All animal procedures were approved by The University of Washington's animal care committee and carried out in accordance with National Institutes of Health guidelines. Care was taken to minimize animal discomfort. Adult male Sprague-Dawley rats (Charles River Laboratories; Hollister, CA) were housed 2-3 per cage with access to food and water available ad libitum. Rats were housed on a 12 / 12 h light cycle with lights on at 06:00. All experiments were performed during the rats' light cycle. Rats weighed 275-350 g at the time of behavioral testing. With the exception of the open field test / fear conditioning experiment (Figure 3), each rat was used in a single test of behavior.
Figure 3.
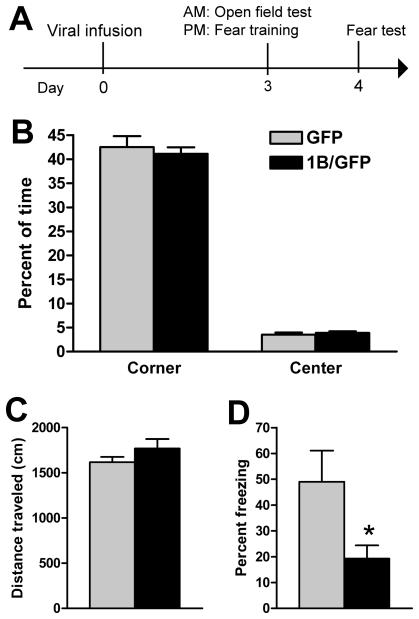
5-HT1B overexpression in caudal DRN reduces conditioned fear without altering anxiety or locomotion. A, Animals were injected with GFP (n=7) or 1B/GFP (n=9) viral vector and exposed to open field test, followed by contextual fear conditioning. B, Groups did not differ in anxiety, as measured by percent of time spent in the center and corner of the open field. C, Total distance traveled in the open field did not differ. D, 5-HT1B overexpression reduced percent of observations that rats spent freezing during a test of conditioned fear. Data shown as mean + SEM. * p < 0.05 vs GFP.
Stereotaxic surgery and viral gene transfer
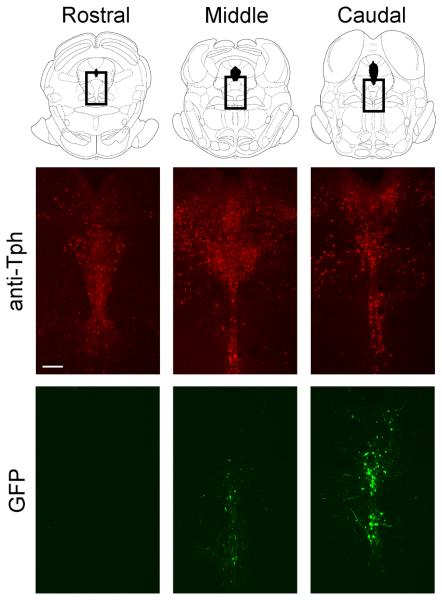
Experimental viral vector expressed hemagglutinin-tagged 5-HT1B and GFP from separate transcriptional cassettes (1B/GFP), whereas a vector expressing GFP alone was used as a control. We have shown that recombinant 5-HT1B receptors possess normal 5-HT1B-like function in vitro (13, 21) and in vivo (13, 22). Additionally, we recently found that overexpression of 5-HT1B autoreceptors enhances 5-HT reuptake in the hippocampus (Hagan, McDevitt, and Neumaier, in preparation). 1B/GFP and GFP viral vectors were prepared and injected with general surgical procedures carried out essentially as previously described (13). Briefly, a 27-gauge needle was stereotaxically directed into the caudal DRN (−8.4 mm bregma) at an angle of 25° off vertical. Because of the small size of the caudal DRN, injection volumes were restricted to 1 μl (~105 infective units) to minimize spread. 1B/GFP or GFP virus was injected over 5 min, after which the needle was left in place for 5 min, then withdrawn slowly. Post-surgical pain was managed with buprenorphine (0.05 mg/kg, s.c.). To confirm injection site, brains were post-fixed in 4% paraformaldehyde, sectioned, and examined for GFP fluorescence. The extent of transgene expression due to spread of viral particles across the rostrocaudal axis within the DRN was measured in a randomly chosen subset of 10 brains as 738 ± 59 μm (mean ± SEM). For illustrative purposes, tissue from one rat was prepared for tryptophan hydroxylase (Tph) immunohistochemistry. Immunohistochemistry was performed using standard methods, with incubation in sheep anti-Tph (1:500; Chemicon, Temecula,CA) followed by donkey anti-sheep Alexa 568 (1:500; Molecular Probes, Eugene, OR). Because rats in the fear acquisition experiment were sacrificed at a time point too late to observe HSV-mediated expression (23, 24) injection sites were assessed by cresyl violet staining and visualization of needle tracks. In all tissue analyses, an investigator blind to treatment conditions assessed DRN boundaries, defined by published atlases of the rat brain and of DRN gene expression (13, 17). For a rat to be included in data analysis, more than 50% of GFP-positive neurons (for cresyl violet stains, tip of the needle track) had to be within the caudal dorsal raphe nucleus (−8.0 to −9.3 mm bregma).
Drugs
Rats were injected intraperitoneally with saline or 3-(1,2,5,6-tetrahydro-4-pyridyl)-5-propoxypyrrolo[3,2-b]pyridine (CP-94,253; Tocris Bioscience, Ellisville, MO) dissolved in water. In the initial dose-response experiment, several waves were performed in which injection volumes were 1 ml/kg or 2 ml/kg. Comparison between waves suggested better efficacy of CP-94,253 when dissolved at 2 ml/kg (data not shown); therefore all subsequent drug experiments used injection volumes of 2 ml/kg. All injections were performed 30 min prior to behavioral testing. To minimize any influence of stress from the parenteral injection procedure on 5-HT1B receptor function, rats were gradually habituated to injection procedures. Following several days of routine handling, habituation began with rats being held briefly in a “bear claw” crip while in experimenter's arms. The next day, rats were held in this grip and cradled vertically against the experimenter's body while having their belly gently touched with a finger. Finally on the day before the experiment, rats were held and injected with 1 ml/kg saline.
Behavioral procedures
Forced swim test
A modified forced swim test was performed as previously described (13). Rats were videotaped from overhead and scored offline by experimenters blind to treatment group, using a time-sampling method (26) in which every 5 sec behavior was assessed as swimming, climbing, or immobility.
Open field test
Testing occurred in an arena previously described (18). Rats were placed in a small chamber attached to the open field by a guillotine door that could be opened from an adjacent room. After a 2 min acclimation period in the start box, the guillotine door was held open until the rat fully emerged into the open field, where it remained for 10 min under recording and digital tracking with SMART software (San Diego Instruments, San Diego, CA).
Contextual fear conditioning
Training and testing occurred in a shuttle box (Med Associates) in a sound-attenuated cubicle. The shuttlebox center door remained closed, restricting rats to a chamber 20 × 16 × 21 cm. The floor consisted of stainless steel rods 4.8 mm in diameter, with centers spaced 16 mm apart. Scrambled shocks were delivered through a constant current generator connected to a computer interface. Unless otherwise noted, rats were trained with one 2 sec, 1.0 mA footshock after a 2 min acclimation period; 24 h later, rats were placed back in the chamber for a 5 min test. Training and test sessions were recorded with a digital video camera. Videos were scored offline by experimenters blind to treatment, using a time sampling procedure (28) in which rats were observed every 10 sec and judged whether or not to be in a freezing posture, defined as the absence of all movement except that required for respiration.
Statistical analyses
Analyses were performed with SPSS (IBM; Chicago, IL) with alpha set at 0.05. For the forced swim test, a standard statistical approach was followed (19) in which the three classes of behaviors were analyzed separately. Swimming, climbing, and immobility were each analyzed with two-way repeated measures analysis of variance (ANOVA) using time as a within-subjects factor and virus treatment as a between-subjects factor. Post-hoc comparisons of GFP and 1B/GFP virus groups were made using two-tailed Student's t-tests. For the open field test, we used a standard approach (14) of separately analyzing each dependent variable, including center time, corner time, and total distance traveled, using two-tailed Student's t-tests. In fear conditioning experiments, the dependent variable used for analysis was freezing during test sessions. Although post-shock freezing during training sessions (or during a pretest) was often recorded, these values were used to assign animals to groups, and thus were not included in main analysis as a dependent measure. However, for the sake of demonstrating that groups were assigned without bias, group baseline freezing values were compared using either a Student's t-test or one-way ANOVA, depending on the number of groups compared. Values for freezing during the test session were compared with two tailed Student's t-test in experiments comparing two groups at a single time point (Figs 3D, 4A, 4B, 5C). The CP-94,253 experiment testing multiple doses over multiple time points (Figure S1 in the Supplement) was analyzed with two-way repeated measures ANOVA using time as a within-subjects factor and dose as a between-subjects factor. Dunnett's post-hoc comparisons were used to compare various doses of drug to a saline control group on any given day of testing. The CP-94,253 experiment using a single dose of drug with multiple time points (Fig 5B) was compared using repeated measures ANOVA with time as a within-subjects factor and dose as a between-subjects factor. Post-hoc comparisons between CP-94,253 and saline groups were made using two-tailed Student's t-tests. No animals were excluded as outliers in any analyses.
Figure 4.
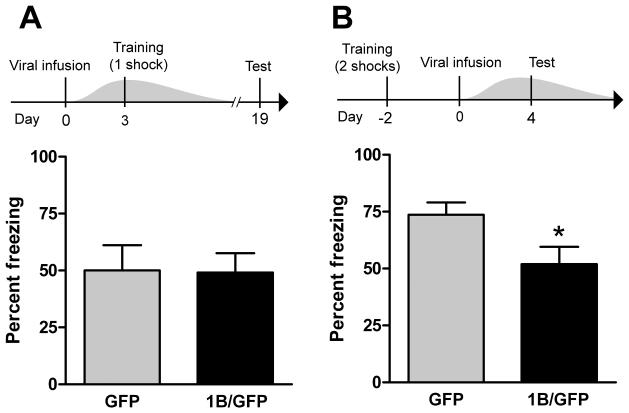
5-HT1B overexpression in caudal DRN reduces expression, but not acquisition, of conditioned fear. A, 5-HT1B overexpression does not alter acquisition of conditioned fear. Animals were injected with GFP (n=8) or 1B/GFP (n=11) viral vector and trained with 1 footshock in the fear chamber on day 3, at which time viral overexpression is maximal - grey wave in experimental design panel represents approximate time course of viral-mediated 5-HT1B expression (23). Fear testing occurred on day 19, when viral overexpression had dissipated. Thus, 5-HT1B expression was elevated during the acquisition, but not expression, of conditioned fear. B, 5-HT1B overexpression reduces expression of conditioned fear. Prior to viral infusion, rats were trained with 2 footshocks (to ensure sufficiently high post-surgery conditioning; see Methods section) in the fear chamber. Animals were then injected with GFP (n=12) or 1B/GFP (n=12) viral vector. Fear testing occurred at peak viral expression. Thus, 5-HT1B expression was elevated during the expression, but not acquisition, of contextual fear conditioning. Graphs in (A) and (B) depict mean + SEM of percent of observations that rats spent freezing during a test of conditioned fear. * p < 0.05 vs GFP.
Figure 5.
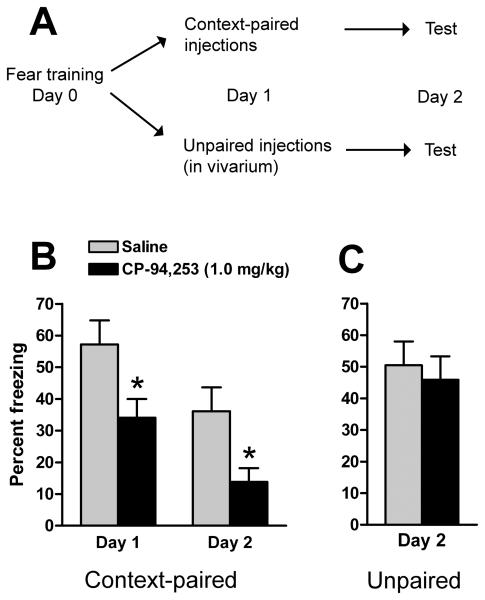
The selective 5-HT1B agonist CP-94,253 (1.0 mg/kg, i.p.) reduces conditioned fear only when paired with presentation of the conditioned stimulus. A, rats trained in contextually conditioned fear (n=13 per group) were assigned to one of two experiments in which drug was paired with reexposure to fear context or unpaired. B, CP-94,253 paired with context re-exposure reduced measures of fear on the day of injection (Day 1) and during a subsequent, drug-free trial (Day 2). C, CP-94,253 given in a neutral environment (Day 1) did not affect expression conditioned fear 24 h later (Day 2). Graphs depict mean +SEM percent of observations that rats spent freezing during tests of conditioned fear. * p<0.05 vs. saline.
Results
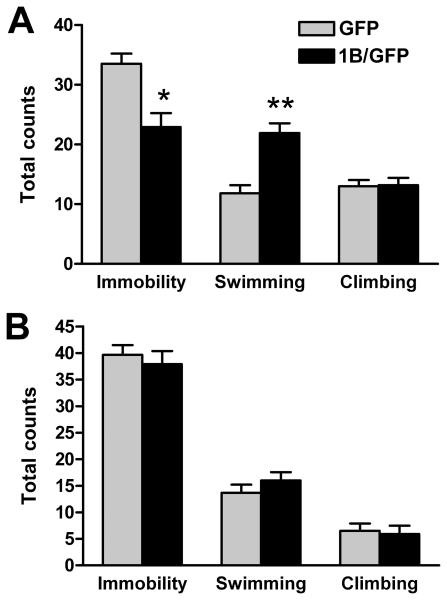
Rats were injected with GFP (n=12) or 1B/GFP (n=11) viral vector into the caudal DRN (Figure 1), and exposed to forced swim sessions on days 3 and 4 following surgery (Figure 2). Analysis of behavior in the second swim session is commonly used to screen for antidepressant-like effects of drugs administered between sessions (20). The effects of chronic antidepressants are apparent in a single 15 min swim session, with effects most visible during the first 5 min (19). Accordingly, we analyzed behavior during the first 5 min of the first swim and the full 5 min second swim (complete data in Table S1 in the Supplement). Climbing, swimming, and immobility were each separately analyzed with repeated measures ANOVA, with post-hoc comparisons performed using Student's t-tests. Analysis of immobility revealed an interaction between virus and day [F(1,21)=7.07, p=0.015]. Post-hoc comparisons showed that GFP and 1B/GFP groups differed in immobility during the first swim session (p=0.0014) but not the second (p=0.57). Analysis of swimming revealed a significant interaction between virus and day [F(1,21)=16.32, p<0.001]. Post-hoc tests indicated that GFP and 1B/GFP groups differed in swimming during the first swim (p<0.001) but not the second (p=0.31). There was no effect of virus on climbing.
Figure 1.
Viral-mediated expression of GFP in caudal DRN. Top row, schematics from Paxinos and Watson (17) depicting DRN tissue; boxes indicate location of tissue in images below. Middle and bottom rows, 40 um slices of tissue from the same animal showing spread of viral infection (via GFP) relative to serotonergic cell bodies (immunohistochemistry for Tph). Scale bar = 200 um.
Figure 2.
5-HT1B overexpression in caudal DRN increases swimming in the forced swim test. Animals received injections of GFP (n=12) or 1B/GFP (n=11) viral vector and were subjected to a standard forced swim test procedure on days 3 and 4 after surgery. A, 5-HT1B overexpression reduced immobility and increased swimming in the first 5 min of the first day swim. B, There was no effect of overexpression during the second swim session. Graphs represents mean counts + SEM of behavior. * p<0.05 vs GFP, ** p<0.001 vs GFP.
To test the role of 5-HT1B autoreceptors in behavioral models of anxiety and fear, rats were injected with GFP (n=7) or 1B/GFP (n=9) viral vector into the caudal DRN. On day 3 after surgery rats were subjected to the open field test in the morning and 4 h later were trained in fear. Fear retention testing occurred 24 h later. 5-HT1B overexpression did not alter locomotion (Figure 3C), percent time in the center or corners (Figure 3B), or number of entries into the center region during the first 3 min (data not shown), a measure previously used (13). In contrast, 5-HT1B overexpression significantly reduced freezing (p=0.027; Student's t-test) in a test of conditioned fear (Figure 3D). Evidence of innate fear of the context, defined as freezing during the 2 min acclimation period before footshock, was very low and did not differ between groups (data not shown).
To test the role of 5-HT1B autoreceptors in the acquisition of conditioned fear, we performed an experiment in which 5-HT1B expression was present during fear training, but not fear testing (Figure 4A). Rats were injected with GFP (n=8) or 1B/GFP (n=11) viral vector into the caudal DRN. Rats were trained on day 3 after surgery, and remained undisturbed in the vivarium until testing on day 19 after surgery. This is sufficient time for the HSV IE 4/5 promoter to inactivate and transgenic 5-HT1B protein to degrade (23, 24). Freezing in the test session did not differ between groups. We next tested the role of 5-HT1B autoreceptors in the expression of conditioned fear. Intact rats were trained, and later tested in the presence of 5-HT1B overexpression (Figure 4B). Preliminary testing suggested that surgery caused a partial reduction in the strength of fear conditioning (data not shown), as has been observed elsewhere (30). To ensure a sufficiently high retention of conditioning post-surgery, rats were trained with two footshocks. Post-shock freezing did not differ between groups (p=0.67; Student's t-test). After 2 d, rats were injected with GFP (n=12) or 1B/GFP (n=12) viral vector into the caudal DRN. In a test session on day 4 after surgery, 5-HT1B overexpression significantly reduced freezing (p=0.03; Student's t-test).
In an initial CP-94,253 dose-response experiment (0.3 - 3 mg/kg, i.p.) we found maximal reduction of conditioned freezing at 1.0 mg/kg (Figure S1 in the Supplement). To establish that these effects are context-specific, rats were trained and assigned to groups with balanced post-shock freezing, [F(3,48)=0.04, p=0.99]. Rats were administered CP-94,253 (1.0 mg/kg, i.p.) or saline in one of two contexts, either paired with reexposure to the conditioned stimulus or in their homecages in the vivarium (n=13 per group). Because the different pairings involved different numbers of observed test sessions, they were analyzed separately. Rats that received drug in vivarium (Figure 5C) showed no difference in freezing tested 24 h later (p=0.66; Student's t-test). In contrast, rats that received drug paired with reexposure to the fear chamber (Figure 5B) showed reduced freezing both at the time of injection and 24 h later. Repeated measures ANOVA revealed an overall effect of drug [F(1,24)=6.78, p=0.016] and time [F(1,24)=54.4, p<0.0001].
Discussion
Our laboratory has previously studied 5-HT1B autoreceptors in mid-rostral DRN neurons, where overexpression in unstressed rats reduces anxiety and conditioned fear without affecting behavior in the forced swim test (11). In the present study, 5-HT1B overexpression in the caudal DRN resulted in a different pattern of behavioral results, reducing conditioned fear and helplessness without affecting anxiety. These results suggest a rostrocaudal gradient of DRN effects on various emotional states, with rostral DRN affecting anxiety, caudal DRN affecting helplessness, and the full DRN axis influencing conditioned fear.
The forced swim test is highly responsive to chemical antidepressant treatment, and thus can be viewed as having predictive validity in modeling certain aspects of depression (21). 5-HT1B overexpression in the caudal (Figure 2), but not the mid-rostral DRN (11), increased swimming and reduced immobility in the forced swim test. Because overexpression did not alter measures of general locomotor activity in the open field test, we interpret this result as an antidepressant-like effect. This is consistent with evidence for a selective role of the caudal DRN in two distinct behavioral models of helplessness and depression. First, measures of immobility during a forced swim session correlate with changes in extracellular 5-HT in target regions of the caudal, but not rostral, DRN (22, 23). Second, pharmacological stimulation of caudal, but not rostral, DRN can substitute for stress in causing deficits in shuttlebox escape behavior (19). Thus, the influence of serotonin on helplessness behavior may occur in brain regions that receive serotonergic innervation preferentially from caudal versus rostral DRN – for example, lateral septum, nucleus accumbens, and hippocampus (32-34). We presume that overexpression of 5-HT1B autoreceptors in the caudal DRN serves to limit 5-HT release in target areas, and that this produces the antidepressant-like behavioral effect observed. It may seem counterintuitive that reductions in serotonin release could lead to the same behavioral effects as treatment with a serotonin reuptake inhibitor. In the lateral septum, however, fluoxetine pretreatment has complex bimodal effects on extracellular 5-HT: while baseline concentrations are elevated, there is a magnification in the decrease in 5-HT that occurs during a forced swim test session (22). Inhibition of the caudal DRN by direct infusion of a 5-HT1A agonist is sufficient to recapitulate the behavioral effects of antidepressant treatments in the forced swim test (24) and active shuttlebox escape (25). The specificity of 5-HT1B overexpression effect on swimming versus climbing is consistent with studies showing that these responses are selectively influenced by serotonin and norepinephrine, respectively (26, 37, 38). Antidepressant-like effects of CP-94,253 in mice have been reported in the forced swim test (26). The lack of effect of 5-HT1B overexpression on swimming behavior on the second day of testing suggests that exposure to uncontrollable stress may disrupt 5-HT1B function for at least 24 h. Similar stress effects have been observed with respect to physiological (27) and behavioral (28) measures of 5-HT1B function.
The open field test is a model of anxiety in which the benzodiazepine class of anxiolytics reduces avoidance of the field's center (“thigmotaxis”). While this test is not sensitive to chronic treatment with serotonin reuptake inhibitors, it is responsive to drugs that target specific 5-HT receptors (29). Thigmotaxis is altered by 5-HT1B overexpression in the mid-rostral (13), but not caudal, DRN (Figure 3B). Similarly, we have shown that measures of thigmotaxis correlate with basal 5-HT1B mRNA levels in the middle, but not caudal, DRN (30). Thus, the form of anxiety modeled by this test may be regulated by brain regions that receive serotonergic projections preferentially from the rostral and middle DRN – for example, the basolateral amygdala, frontal cortex, and dorsal striatum (32-34). Although exposure to behavioral tests of anxiety alone do not appear to strongly activate the caudal DRN, the combination of test exposure with a history of traumatic stress (31) or administration of corticotropin-releasing factor (32) robustly activates DRN neurons projecting to the prefrontal cortex and amygdala.
We found that conditioned fear was reduced by 5-HT1B overexpression in both the caudal (Figure 3D) and mid-rostral DRN (28). Within the caudal DRN, 5-HT1B overexpression reduced expression, but not acquisition, of conditioned freezing. In contrast, increased systemic serotonin function at the time of fear training is reported to enhance fear acquisition (33). These effects are likely mediated by serotonergic projections originating from neurons outside of the caudal DRN. However, it is possible that our behavioral procedures were less sensitive than those of Burghardt and colleagues (33) in detecting changes in fear acquisition. An additional possibility is that acquisition of conditioned fear can be altered by increases but not decreases in serotonergic function, regardless of methodology used for serotonergic manipulation or behavioral testing. The reduction of freezing that we observed after 5-HT1B autoreceptor overexpression, which presumably reduces extracellular serotonin at terminals, is consistent with numerous studies showing 5-HT to enhance various behavioral measures of expression of learned fear (41-46).
It is important to note certain technical limitations of the viral-mediated strategy used here. The virus and promoter system we used is not specific for a particular neuron type. Therefore, injections into the DRN would be expected to infect a variety of neuron types. The DRN as a whole is approximately 50% serotonergic, also containing neurons identified as GABAergic and dopaminergic. However, dopaminergic neurons are not found at caudal levels of the DRN, and most of the GABAergic neurons are located lateral to the midline caudal DRN subregion that we targeted (47-49). Therefore, while infected cells may represent different neuron types, we would predict on the basis of anatomy that most were serotonergic. This interpretation is supported by the convergence of our overexpression results with administration of CP-94,253 at a dose that is known to reduce extracellular 5-HT in terminal regions (34), as well as studies using other methods to manipulate 5-HT function (referenced earlier). In addition to projections throughout the forebrain, neurons of the DRN also send axon collaterals to other DRN neurons. Overexpression of 5-HT1B autoreceptors would thus be expected to alter release of 5-HT both in distal brain regions and within the DRN itself. Similarly, although most 5-HT1B receptors are actively transported to axon terminals (11, 35) we cannot exclude the possibility that some viral-mediated 5-HT1B receptors are present on dendrites. Such receptors might reduce dendritic 5-HT release within the DRN, reducing 5-HT1A-mediated autoinhibition of serotonergic neurons and thereby functioning to increase activity of serotonergic DRN neurons. Although we do not presently have electrophysiological or microdialysis data to directly measure these effects, the net behavioral effects of 5-HT1B autoreceptor overexpression were consistent with other methods of inhibiting 5-HT function.
The ability of 5-HT1B autoreceptors to reduce expression of fear is an attractive feature with regards to clinical application, since treatment is generally more available at the time of symptom presentation than during development. We therefore tested the effects of systemic treatment with the selective 5-HT1B agonist, CP-94,253. Although systemic treatment is not specific for 5-HT1B autoreceptors originating in the caudal DRN, it is more easily translated to human application – particularly since agonists for this receptor are routinely used for treatment of migraine headaches (36). The dose range we used is reported not to alter measures of general behavioral activity (37-39), suggesting that the effects on freezing are specific to conditioned fear. Furthermore, the fact that the drug-induced reduction in freezing persisted into a drug-free state suggests that its effects were due to reduction of fear, rather than temporary interference with context recognition or ability to perform a freezing response. Interestingly, this pattern of results differs from the benzodiazepine class of anxiolytics, which acutely reduce expression of fear but interfere with extinction (51, 52) by creating state-dependent extinction learning (53). The ability of CP-94,253 to induce long-term reductions in conditioned fear responses resembles recent findings with the NMDA partial agonist D-cycloserine (54). Our results suggest that, like Dcycloserine, acute pairing of CP-94,253 with exposure therapy may facilitate long-term reduction of fear associated with discrete cues. Because 5-HT1B autoreceptor function is inhibited by uncontrollable stress, the ability of 5-HT1B agonists to reduce fear in patients with posttraumatic stress disorder would depend on the return of 5-HT1B autoreceptor function.
CP-94,253 had an inverted U-shaped dose-response curve on conditioned freezing. CP-94,253 is selective for 5-HT1B receptors, with a 25-fold selectivity over 5-HT1D receptors (40). The doses that we tested (0.3 – 3.0 mg/kg) are within the range that causes behavioral effects that are reversible with selective 5-HT1B antagonists (41-43) or genetic deletion (59, 60). Thus, the biphasic effects of CP-94,253 on conditioned freezing are likely due to distinct populations of 5-HT1B receptors which are differentially recruited at low and high doses of agonist. 5-HT1B autoreceptor activity in synaptosomes is preferentially recruited at lower concentrations of both 5-HT and synthetic agonist, compared to 5-HT1B heteroreceptors (44). In vivo administration of CP-94,253 at 1 mg/kg may preferentially activate 5-HT1B autoreceptors - an interpretation that would be consistent with our findings that viral-mediated overexpression of 5-HT1B autoreceptors in the DRN exert similar behavioral effects on conditioned fear. Similar U-shaped dose-response curves, mediated by differential autoreceptor versus heteroreceptor sensitivities, have been described for 5-HT1A (25, 45) and D2 (46-48) receptors. The apparent loss of effect on fear at 3 mg/kg CP-94,253 may be driven by anxiogenic (39) and aversive (49, 50) effects of drug that occur at and above 3 mg/kg, but not at lower doses. If these effects are mediated by 5-HT1B heteroreceptors, agonists with greater selectivity for autoreceptors versus heteroreceptors may be more useful in clinical applications.
Supplementary Material
Acknowledgements
This work was supported by GM07108 (to RAM) and MH63303 (to JFN). We are grateful to Dr. Michele Kelly, William Brennan, and Timothy Sexton for virus preparation, Dr. Blair Hoplight for assistance with statistical analyses, and Simina Popa for critical reading of the manuscript.
Financial disclosures: The authors reported no biomedical financial interests or potential conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ressler KJ, Nemeroff CB. Role of serotonergic and noradrenergic systems in the pathophysiology of depression and anxiety disorders. Depress Anxiety. 2000;12(Suppl 1):2–19. doi: 10.1002/1520-6394(2000)12:1+<2::AID-DA2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 2.Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol Rev. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- 3.Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- 4.Hjorth S, Tao R. The putative 5-HT1B receptor agonist CP-93,129 suppresses rat hippocampal 5-HT release in vivo: comparison with RU 24969. Eur J Pharmacol. 1991;209:249–252. doi: 10.1016/0014-2999(91)90177-r. [DOI] [PubMed] [Google Scholar]
- 5.Hjorth S, Suchowski CS, Galloway MP. Evidence for 5-HT autoreceptor-mediated, nerve impulse-independent, control of 5-HT synthesis in the rat brain. Synapse. 1995;19:170–176. doi: 10.1002/syn.890190304. [DOI] [PubMed] [Google Scholar]
- 6.Daws LC, Gould GG, Teicher SD, Gerhardt GA, Frazer A. 5-HT(1B) receptor-mediated regulation of serotonin clearance in rat hippocampus in vivo. J Neurochem. 2000;75:2113–2122. doi: 10.1046/j.1471-4159.2000.0752113.x. [DOI] [PubMed] [Google Scholar]
- 7.Kaiyala KJ, Vincow ES, Sexton TJ, Neumaier JF. 5-HT1B receptor mRNA levels in dorsal raphe nucleus: inverse association with anxiety behavior in the elevated plus maze. Pharmacol Biochem Behav. 2003;75:769–776. doi: 10.1016/s0091-3057(03)00152-7. [DOI] [PubMed] [Google Scholar]
- 8.Neumaier JF, Edwards E, Plotsky PM. 5-HT(1B) mRNA regulation in two animal models of altered stress reactivity. Biol Psychiatry. 2002;51:902–908. doi: 10.1016/s0006-3223(01)01371-3. [DOI] [PubMed] [Google Scholar]
- 9.Stark KL, Hen R. Knockout Corner - 5-HT1B receptor knockout mice: a review. Int J Neuropsychopharmacol. 1999;2:145–150. doi: 10.1017/S146114579900142X. [DOI] [PubMed] [Google Scholar]
- 10.Bruinvels AT, Landwehrmeyer B, Gustafson EL, Durkin MM, Mengod G, Branchek TA, et al. Localization of 5-HT1B, 5-HT1D alpha, 5-HT1E and 5-HT1F receptor messenger RNA in rodent and primate brain. Neuropharmacology. 1994;33:367–386. doi: 10.1016/0028-3908(94)90067-1. [DOI] [PubMed] [Google Scholar]
- 11.Clark MS, Sexton TJ, McClain M, Root D, Kohen R, Neumaier JF. Overexpression of 5-HT1B receptor in dorsal raphe nucleus using Herpes Simplex Virus gene transfer increases anxiety behavior after inescapable stress. J Neurosci. 2002;22:4550–4562. doi: 10.1523/JNEUROSCI.22-11-04550.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Descarries L, Watkins KC, Garcia S, Beaudet A. The serotonin neurons in nucleus raphe dorsalis of adult rat: a light and electron microscope radioautographic study. J Comp Neurol. 1982;207:239–254. doi: 10.1002/cne.902070305. [DOI] [PubMed] [Google Scholar]
- 13.Clark MS, McDevitt RA, Neumaier JF. Quantitative mapping of tryptophan hydroxylase-2, 5-HT1A, 5-HT1B, and serotonin transporter expression across the anteroposterior axis of the rat dorsal and median raphe nuclei. J Comp Neurol. 2006;498:611–623. doi: 10.1002/cne.21073. [DOI] [PubMed] [Google Scholar]
- 14.Bouwknecht JA, Spiga F, Staub DR, Hale MW, Shekhar A, Lowry CA. Differential effects of exposure to low-light or high-light open-field on anxiety-related behaviors: relationship to cFos expression in serotonergic and non-serotonergic neurons in the dorsal raphe nucleus. Brain Res Bull. 2007;72:32–43. doi: 10.1016/j.brainresbull.2006.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evans AK, Heerkens JL, Lowry CA. Acoustic stimulation in vivo and corticotropin-releasing factor in vitro increase tryptophan hydroxylase activity in the rat caudal dorsal raphe nucleus. Neurosci Lett. 2009;455:36–41. doi: 10.1016/j.neulet.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 16.Grahn RE, Will MJ, Hammack SE, Maswood S, McQueen MB, Watkins LR, et al. Activation of serotonin-immunoreactive cells in the dorsal raphe nucleus in rats exposed to an uncontrollable stressor. Brain Res. 1999;826:35–43. doi: 10.1016/s0006-8993(99)01208-1. [DOI] [PubMed] [Google Scholar]
- 17.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 2nd ed. Academic Press; Sydney ; Orlando: 1986. [Google Scholar]
- 18.Hoplight BJ, Vincow ES, Neumaier JF. The effects of SB 224289 on anxiety and cocaine-related behaviors in a novel object task. Physiol Behav. 2005;84:707–714. doi: 10.1016/j.physbeh.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 19.Cryan JF, Page ME, Lucki I. Differential behavioral effects of the antidepressants reboxetine, fluoxetine, and moclobemide in a modified forced swim test following chronic treatment. Psychopharmacology (Berl) 2005;182:335–344. doi: 10.1007/s00213-005-0093-5. [DOI] [PubMed] [Google Scholar]
- 20.Detke MJ, Rickels M, Lucki I. Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology (Berl) 1995;121:66–72. doi: 10.1007/BF02245592. [DOI] [PubMed] [Google Scholar]
- 21.Cryan JF, Markou A, Lucki I. Assessing antidepressant activity in rodents: recent developments and future needs. Trends Pharmacol Sci. 2002;23:238–245. doi: 10.1016/s0165-6147(02)02017-5. [DOI] [PubMed] [Google Scholar]
- 22.Kirby LG, Lucki I. Interaction between the forced swimming test and fluoxetine treatment on extracellular 5-hydroxytryptamine and 5-hydroxyindoleacetic acid in the rat. J Pharmacol Exp Ther. 1997;282:967–976. [PubMed] [Google Scholar]
- 23.Waselus M, Galvez JP, Valentino RJ, Van Bockstaele EJ. Differential projections of dorsal raphe nucleus neurons to the lateral septum and striatum. J Chem Neuroanat. 2006;31:233–242. doi: 10.1016/j.jchemneu.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 24.Schreiber R, De Vry J. Neuroanatomical basis for the antidepressant-like effects of the 5-HT(1A) receptor agonists 8-OH-DPAT and ipsapirone in the rat forced swimming test. Behav Pharmacol. 1993;4:625–636. [PubMed] [Google Scholar]
- 25.Maier SF, Grahn RE, Watkins LR. 8-OH-DPAT microinjected in the region of the dorsal raphe nucleus blocks and reverses the enhancement of fear conditioning and interference with escape produced by exposure to inescapable shock. Behav Neurosci. 1995;109:404–412. doi: 10.1037//0735-7044.109.3.404. [DOI] [PubMed] [Google Scholar]
- 26.Adell A, Celada P, Artigas F. The role of 5-HT1B receptors in the regulation of serotonin cell firing and release in the rat brain. J Neurochem. 2001;79:172–182. doi: 10.1046/j.1471-4159.2001.00550.x. [DOI] [PubMed] [Google Scholar]
- 27.Bolanos-Jimenez F, Manhaes de Castro RM, Seguin L, Cloez-Tayarani I, Monneret V, Drieu K, et al. Effects of stress on the functional properties of pre- and postsynaptic 5-HT1B receptors in the rat brain. Eur J Pharmacol. 1995;294:531–540. doi: 10.1016/0014-2999(95)00590-0. [DOI] [PubMed] [Google Scholar]
- 28.Clark MS, Vincow ES, Sexton TJ, Neumaier JF. Increased expression of 5-HT1B receptor in dorsal raphe nucleus decreases fear-potentiated startle in a stress dependent manner. Brain Res. 2004;1007:86–97. doi: 10.1016/j.brainres.2004.01.070. [DOI] [PubMed] [Google Scholar]
- 29.Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol. 2003;463:3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- 30.Hiroi R, Neumaier JF. Estrogen decreases 5-HT1B autoreceptor mRNA in selective subregion of rat dorsal raphe nucleus: inverse association between gene expression and anxiety behavior in the open field. Neuroscience. 2009;158:456–464. doi: 10.1016/j.neuroscience.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Christianson JP, Ragole T, Amat J, Greenwood BN, Strong PV, Paul ED, et al. 5-hydroxytryptamine 2C receptors in the basolateral amygdala are involved in the expression of anxiety after uncontrollable traumatic stress. Biol Psychiatry. 2010;67:339–345. doi: 10.1016/j.biopsych.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meloni EG, Reedy CL, Cohen BM, Carlezon WA., Jr. Activation of raphe efferents to the medial prefrontal cortex by corticotropin-releasing factor: correlation with anxiety-like behavior. Biol Psychiatry. 2008;63:832–839. doi: 10.1016/j.biopsych.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burghardt NS, Sullivan GM, McEwen BS, Gorman JM, LeDoux JE. The selective serotonin reuptake inhibitor citalopram increases fear after acute treatment but reduces fear with chronic treatment: a comparison with tianeptine. Biol Psychiatry. 2004;55:1171–1178. doi: 10.1016/j.biopsych.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 34.Knobelman DA, Kung HF, Lucki I. Regulation of extracellular concentrations of 5-hydroxytryptamine (5-HT) in mouse striatum by 5-HT(1A) and 5-HT(1B) receptors. J Pharmacol Exp Ther. 2000;292:1111–1117. [PubMed] [Google Scholar]
- 35.Ghavami A, Stark KL, Jareb M, Ramboz S, Segu L, Hen R. Differential addressing of 5-HT1A and 5-HT1B receptors in epithelial cells and neurons. J Cell Sci. 1999;112(Pt 6):967–976. doi: 10.1242/jcs.112.6.967. [DOI] [PubMed] [Google Scholar]
- 36.Ramadan NM, Buchanan TM. New and future migraine therapy. Pharmacol Ther. 2006;112:199–212. doi: 10.1016/j.pharmthera.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 37.Bell R, Donaldson C, Gracey D. Differential effects of CGS 12066B and CP-94,253 on murine social and agonistic behaviour. Pharmacol Biochem Behav. 1995;52:7–16. doi: 10.1016/0091-3057(95)00077-a. [DOI] [PubMed] [Google Scholar]
- 38.Fish EW, Faccidomo S, Miczek KA. Aggression heightened by alcohol or social instigation in mice: reduction by the 5-HT(1B) receptor agonist CP-94,253. Psychopharmacology (Berl) 1999;146:391–399. doi: 10.1007/pl00005484. [DOI] [PubMed] [Google Scholar]
- 39.Lin D, Parsons LH. Anxiogenic-like effect of serotonin(1B) receptor stimulation in the rat elevated plus-maze. Pharmacol Biochem Behav. 2002;71:581–587. doi: 10.1016/s0091-3057(01)00712-2. [DOI] [PubMed] [Google Scholar]
- 40.Koe BK, Nielsen JA, Macor JE, Heym J. Biochemical and behavioral studies of the 5-HT1B receptor agonist, CP-94,253. Drug Dev Res. 1992;26:241–250. [Google Scholar]
- 41.Lee MD, Kennett GA, Dourish CT, Clifton PG. 5-HT1B receptors modulate components of satiety in the rat: behavioural and pharmacological analyses of the selective serotonin1B agonist CP-94,253. Psychopharmacology (Berl) 2002;164:49–60. doi: 10.1007/s00213-002-1162-7. [DOI] [PubMed] [Google Scholar]
- 42.Przegalinski E, Golda A, Frankowska M, Zaniewska M, Filip M. Effects of serotonin 5-HT1B receptor ligands on the cocaine- and food-maintained self-administration in rats. Eur J Pharmacol. 2007;559:165–172. doi: 10.1016/j.ejphar.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 43.Heisler LK, Jobst EE, Sutton GM, Zhou L, Borok E, Thornton-Jones Z, et al. Serotonin reciprocally regulates melanocortin neurons to modulate food intake. Neuron. 2006;51:239–249. doi: 10.1016/j.neuron.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 44.Sarhan H, Fillion G. Differential sensitivity of 5-HT1B auto and heteroreceptors. Naunyn Schmiedebergs Arch Pharmacol. 1999;360:382–390. doi: 10.1007/s002109900067. [DOI] [PubMed] [Google Scholar]
- 45.Hjorth S, Magnusson T. The 5-HT 1A receptor agonist, 8-OH-DPAT, preferentially activates cell body 5-HT autoreceptors in rat brain in vivo. Naunyn Schmiedebergs Arch Pharmacol. 1988;338:463–471. doi: 10.1007/BF00179315. [DOI] [PubMed] [Google Scholar]
- 46.Eilam D, Szechtman H. Biphasic effect of D-2 agonist quinpirole on locomotion and movements. Eur J Pharmacol. 1989;161:151–157. doi: 10.1016/0014-2999(89)90837-6. [DOI] [PubMed] [Google Scholar]
- 47.Skirboll LR, Grace AA, Bunney BS. Dopamine auto- and postsynaptic receptors: electrophysiological evidence for differential sensitivity to dopamine agonists. Science. 1979;206:80–82. doi: 10.1126/science.482929. [DOI] [PubMed] [Google Scholar]
- 48.Zhou QY, Palmiter RD. Dopamine-deficient mice are severely hypoactive, adipsic, and aphagic. Cell. 1995;83:1197–1209. doi: 10.1016/0092-8674(95)90145-0. [DOI] [PubMed] [Google Scholar]
- 49.Cervo L, Rozio M, Ekalle-Soppo CB, Carnovali F, Santangelo E, Samanin R. Stimulation of serotonin1B receptors induces conditioned place aversion and facilitates cocaine place conditioning in rats. Psychopharmacology (Berl) 2002;163:142–150. doi: 10.1007/s00213-002-1145-8. [DOI] [PubMed] [Google Scholar]
- 50.De Vry J, Eckel G, Kuhl E, Schreiber R. Effects of serotonin 5-HT(1) and 5-HT(2) receptor agonists in a conditioned taste aversion paradigm in the rat. Pharmacol Biochem Behav. 2000;66:797–802. doi: 10.1016/s0091-3057(00)00248-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.